Abstract
The pseudocereal buckwheat is one of the ancient domesticated crops. The aim of the present review was to outline the potential of buckwheat as an agricultural crop and brings studies on buckwheat into a new larger perspective combining current knowledge in agricultural history and practice, nutritional and sensory properties, as well as possible benefits to human health. Historically, buckwheat was an appreciated crop because of its short growth period, moderate requirements for growth conditions, and high adaptability to adverse environments. Nowadays, interest in buckwheat-based food has increased because of its nutritional composition and many beneficial properties for human health. Buckwheat is a rich course of proteins, dietary fibers, vitamins, minerals, and bioactive compounds, including flavonoids. Moreover, it contains no gluten and can be used in the production of gluten-free foods for individuals diagnosed with celiac disease, non-celiac gluten sensitivity, or wheat protein allergies. Buckwheat is traditionally used in the production of various foods and can be successfully incorporated into various new food formulations with positive effects on their nutritional value and attractive sensory properties. Further research is needed to optimize buckwheat-based food development and understand the mechanism of the health effects of buckwheat consumption on human well-being.
Introduction
Globally, the negative effects of agriculture on biodiversity are the result of both habitat elimination through land clearing, and extensive areas of monocultures, which are virtually biological deserts (Small Citation2017). Other reasons for biodiversity loss include pollution, soil and water degradation by fertilizers and pesticides. Exploring the potential of underutilized crops could increase resilience through a more diversified agricultural production system in sustaining food and nutritional security under climate change and biodiversity degradation.
In many countries, where agricultural production is based on a few crops only, biodiversity might dramatically decrease. Buckwheat (Fagopyrum sp.) is a promising environmentally friendly crop that can contribute to the balance between biodiversity benefits and crop yield and can be used as a cover crop for weed suppression and for the vegetation restoration of degraded ecosystems (Björkman and Shail Citation2013). Moreover, buckwheat is a rich source of a broad gene pool and can be used to both increase biodiversity and improve the quality of other crops in the food and biotechnology industries (Singh, Malhotra, and Sharma Citation2020). Buckwheat can increase the presence of pollinator species in agriculture (Taki et al. Citation2009). Finally, buckwheat is known for its high nutritional value and superior sensory qualities and has the potential to be a part of a healthy diet (Wijngaard and Arendt Citation2006).
Buckwheat is a pseudocereal belonging to the genus Fagopyrum of the family Polygonaceae. Although there are many buckwheat species grown across the world, only nine are used in agriculture and food industry (Krkošková and Mrázová Citation2005). Common buckwheat (F. esculentum) and Tartary buckwheat (F. tartaricum) are the two most commonly cultivated species of buckwheat. The wild species are mainly used as forage in Southern Asia and in traditional medicine (Luthar, Fabjan, and Mlinarič Citation2021). Buckwheat has been consumed for a long time in Nordic countries. Already in July 1749, when visiting Scania, Carl Linnaeus, noted the snow-white fields of buckwheat. During the eighteenth century, the cultivation of buckwheat was widespread, especially in southern Sweden. It was commonly used for porridge and gruel (Olsson et al. Citation2021).
This review outlines the potential of buckwheat as an agricultural crop, with a focus on recent research findings. In contrast to numerous recently published review articles on buckwheat, the present review brings studies on buckwheat into a new larger perspective combining current knowledge in agricultural history and practice, nutritional and sensory properties, as well as possible benefits to human health. A literature review was conducted by collecting, evaluating, and analyzing data from publications in peer-reviewed scientific journals from the Scopus, PubMed, and Web of Science databases written in English, Ukrainian, and Russian languages. Conference articles and reports were also included if relevant.
History and genetic diversity of buckwheat
The origin and wild progenitors of modern buckwheat species were largely unknown until recently. Several controversial hypotheses of buckwheat origin were suggested, including India, Siberia, or Tibet (Ohnishi Citation2004). Modern advanced technology and phylogenetic analyses pointed to several regions in China as the buckwheat origin (Fan et al. Citation2020). Modern palynological and archaeological records suggested that the cultivation history of buckwheat in China was initiated 4000–4500 years ago, although where exactly domestication started in China is still unknown (Hunt, Shang, and Jones Citation2018; Krzyzanska et al. Citation2022; Yao et al. Citation2023).
The expansion of cultivated buckwheat to Europe is also controversial. Common buckwheat is likely to derive to Europe from China through the Land Silk Road, but this needs to be further verified (Wang et al. Citation2022). According to Beug (Citation2011), buckwheat spread to Western Europe only in the late fifteenth century. However, in Eastern Europe, some modest amount of buckwheat pollen was recorded already during the Stone Age (Alenius, Mökkönen, and Lahelma Citation2013). Alenius, Mökkönen, and Lahelma (Citation2013) explained this modest amount by the fact that they might be carried up to 2 km by wind and insects.
Morphology, genetic diversity and geographical distribution investigations suggested that southwestern China is also the center of buckwheat diversity (Yao et al. Citation2023). High genetic diversity among buckwheat cultivars has been revealed with the help of allozyme analysis, Random Amplified Polymorphic DNA (RAPD), amplified fragment length polymorphism (AFLP), and simple sequence repeat analyses (Iwata et al. Citation2005; Kump and Javornik Citation1996). The modern powerful genomic technologies open up a broad new area and allow future research and breeding programs toward buckwheat cultivars with improved agronomic characteristics and superior nutritional qualities, such as reduced content of antinutrients or development of low-allergen lines.
Cultivation of buckwheat
Nowadays, buckwheat is commonly grown as a conventional food crop in many parts of the world, especially Asia, Central and Eastern Europe. In Sweden, buckwheat cultivation is increasing and has reached 500 ha in 2022. Bielski, Marks-Bielska, and Wiśniewski (Citation2022) provided an economic analysis of buckwheat production for Central Europe, comparing low-input and high-input production systems in Poland, and showed the potential of economic efficiency of buckwheat production. However, further research on energy use efficiency and greenhouse gas emissions in buckwheat production is needed. Grain yield greatly depends on genotype, applied growing technology as well as climate and soil quality.
Growing conditions
Historically, buckwheat was an appreciated crop because of its short growth period and high adaptability to adverse environments. Buckwheat has the ability to grow well in poor soils (Ikanović et al. Citation2013; Kreft Citation2007; Small Citation2017) and prefers sandy soil because of its thin roots. Buckwheat has a higher tolerance to soil acidity compared to cereals, but as with many other crops, it is salt sensitive. High salinity can lead to a decreased germination rate of buckwheat seeds and a lower fresh weight of sprouts (Lim et al. Citation2012).
Buckwheat is sensitive to frost, although it is tolerant to high temperatures (Pomeranz Citation1983). For example, photosynthetic processes remain active up to approximately 40 °C (Kajfeẑ-Bogataj and Gaberščik Citation1986), which indicated higher resistance to global warming than other crops (Germ and Gaberščik Citation2016). It should be noted that at extremely high temperatures and consequently dried soil, buckwheat could be exposed to water stress because of the thin root system. Common buckwheat is more sensitive to climate conditions compared to Tartary buckwheat (Germ and Gaberščik Citation2016), although there are large variations within species (Aubert and Quinet Citation2022).
The critical periods for buckwheat yield determination are not well established. Flowering is probably the most sensitive period since water stress can inhibit fertilization and the newly formed zygotes are aborted, leading to crop failure (Pomeranz Citation1983). Guglielmini, Forcat, and Miralles (Citation2019) described the critical period, during which the final yield can be reduced by 50%, from the appearance of the first open flowers to the first brown fruits.
The nutrient requirements of buckwheat are low, and intensive fertilization is not required because buckwheat can easily absorb macro- and microelements from the soil. However, it also easily accumulates toxic metals such as cadmium and lead (Domańska, Leszczyńska, and Badora Citation2021), which makes buckwheat useful even in non-food applications such as cleaning the soil from toxic metals.
Sowing
Buckwheat varieties used in the last 15 years have a growing season of 85 to 110 days. Seeds germinate in moist soil at a temperature of + 7–8 °C. The optimum soil moisture for buckwheat is at the level of 70–75% of the total field moisture capacity.
The sowing date varies with the geographical region because of the susceptibility of buckwheat to frost conditions. Buckwheat is sensitive to frost, at a temperature of −1.5 °C, the crops are damaged, and at −2.0 °C and below, they die. The best sowing date for buckwheat in Western Europe is from mid-May to July (Halbrecq, Romedenne, and Ledent Citation2005). Mariotti, Masoni, and Arduini (Citation2016) suggested early spring (mid-April) as an optimal sowing time in Mediterranean environments. In Northern India, buckwheat is sown in July, and in Nilgiris in April (Ratan and Kothiyal Citation2011). The sowing date is selected to avoid the risk of frost and avoid high temperatures during the period of seed formation. The recommended sowing depth of seeds also varies. For example, the recommended depth in Belarus for tetraploid cultivars is 4–5 cm, while for diploid cultivars it is 3–4 cm. In China, the optimum depth of 4 cm is recommended (Xiang et al. Citation2014). Generally recommended depth is therefore 4–6 cm (Farooq et al. Citation2016). On dry soils, the seeding depth is usually increased by 2 cm. On soils of light granulometric composition, rolling after sowing is mandatory.
Sowing rate is an important factor determining the grain yield. The highest average grain yield on carbonate meadow black soil in Serbia was obtained at the rate of 160 grains per m2 (Nikolic et al. Citation2019). It should be emphasized that in that study only slight differences between the rates of 120 and 160 grains per m2 were observed, thus, due to economy reasons, the sowing rate of 120 grains per m2 was recommended. Similar results were obtained in Croatia (Jukić et al. Citation2021). In Latvia, the highest yield was obtained at the sowing rate of 250 grains per m2 (Vilcāns, Volkova, and Gaile Citation2011). Higher density might be needed if the soil is too wet, cold, or poorly prepared for sowing. In a semiarid zone of Northern Kazakhstan, the optimal sowing rate was 300 germinated seeds/m2 (Syzdykova et al. Citation2016). Kumskova (Citation2004) suggested that with a continuous sowing method, the optimal sowing rate is 250–500 grains/m2 (80–120 kg/ha), and with a wide row sowing − 100–250 grains/m2 (25–60 kg/ha). Berdin, Straholis, and Klitsenko (Citation2018) suggested the optimal sowing rate from 200 to 300 grains/m2. The effectiveness greatly depends on the soil and buckwheat variety. The differences in recommendations are due to the fact that the sowing rate depends on the sowing method, buckwheat variety, and type of soil cultivation. Wide-row sowing with a row spacing of 45–60 cm is effective for tetraploid varieties, and middle-row spacing of 12–15 cm is commonly used when cultivating diploid varieties.
Harvest
Buckwheat is usually harvested approximately 10 wk after sowing (Farooq et al. Citation2016). Windrowing is one of the traditional methods. The crop is cut when approximately 70–80% of the seeds have turned brown and kept in windrows until the moisture content of the seeds reach 16–18%. Then, the bundles are made to minimize the shattering losses (Babu et al. Citation2018). It was suggested that the shattering is low during morning harvesting (Joshi et al. Citation2020). There were also numerous attempts to decrease shattering problems by genetic selection, and several shattering resistant genes were identified (Morishita, Hara, and Hara Citation2020). We believe that these new approaches could certainly be beneficial for buckwheat production; however, further research is required to develop new cultivars with desired characteristics and without negative effects on other production traits.
Direct combining is another common method to harvest buckwheat. This method, however, increases the risk of shattering during direct combining (Björkman Citation2009), and therefore morning harvesting is recommended.
The buckwheat seeds often contain higher water content, particularly in direct combining; therefore, it is important to reduce water content directly after the harvest to a common storage level for grain of 14%. Dehulling is another required postharvest procedure of buckwheat prior to final buckwheat product processing.
To summarize, the cultivation of buckwheat has multiple benefits for agriculture sustainability over other grain crops, as it requires low inputs and is well adapted to adverse environments.
Diseases
Buckwheat is usually considered a healthy crop. However, in the regions with intensive buckwheat cultivation, some damage has been observed. Infections of the fungal pathogens Fusarium oxysporum and Rhizoctonia solani might cause 50% loss due to wilting and root rot diseases, respectively (Agarwal et al. Citation2017). Buckwheat might also suffer from root lesion nematodes (Pratylynchus penetrans), and be susceptible to leaf spot (Ramularia sp.), stipple spot (Bipolaris Sorokiniana), aster yellows (phytoplasma), and sclerotinia stem rot (Sclerotinia sp.) diseases (Babu et al. Citation2018). A buckwheat burn virus, a highly virulent pathogen that belongs to the Rhabdoviridae family virus, was characterized in Ukraine (Yuzvenko et al. Citation2011). Buckwheat burn virus might cause up to 80% of crop losses, but some buckwheat varieties have higher resistance to this virus (Shevchuk, Demchenko, and Yuzvenko Citation2011; Sindarovska et al. Citation2014). It is obvious that varieties with high resistance can serve as valuable material to select varieties for production in regions with a high prevalence of the buckwheat burn virus.
Nutritional composition of buckwheat
The nutritional quality of food is one of the most important factors for human health and well-being. The growing popularity of buckwheat is largely due to its attractive nutritional quality and deficiency in gluten. Buckwheat is similar to conventional cereals in its usage and chemical composition. Environmental factors and genetic differences are mainly responsible for the differences in nutritional composition between common buckwheat versus Tartary buckwheat. Geographic region is also an important determinant of the nutritional composition of untreated grains, even within the same variety (Zhang and Xu Citation2017). The nutrient composition of final buckwheat-based products depends on the milling fraction and processing conditions (Liu et al. Citation2018; Steadman et al. Citation2001a, Citation2001b).
Proteins
Plant proteins are regarded as a sustainable alternative to animal proteins due to their lower carbon footprint and health benefits. Nowadays, buckwheat protein is underutilized by the food industry.
Although buckwheat does not provide as much protein as other pseudocereals, such as amaranth and quinoa, buckwheat seeds are generally richer in protein than rice and wheat (Yeşil and Levent Citation2022). The protein content of buckwheat grains varies, ranging from 7% to 34% (Alonso-Miravalles and O’Mahony Citation2018; Guo et al. Citation2007; Sinkovič et al. Citation2022) (). The differences in protein content may be due to cultivars variability and growing conditions, as well as sample preparation. Generally, protein content is similar in the common and Tartary buckwheat (Sinkovič et al. Citation2022).
Table 1. Protein content in buckwheat species in different studies.Table Footnotea
The nutritional quality of proteins depends on the amino acid composition and bioavailability of essential amino acids. A number of studies have demonstrated that buckwheat protein is of high biological value and has a balanced amino acid profile. In contrast to cereal proteins, lysine is abundantly present in the buckwheat protein fraction (5.2–5.9%) (Huda et al. Citation2021; Kowalski et al. Citation2022). This is two-fold higher than in wheat. Buckwheat protein is also rich in arginine and aspartic acid (Kowalski et al. Citation2022). Ration lysine/arginine in buckwheat is higher compared to many other plant proteins (Dzakhmisheva and Khokonova Citation2021). Some buckwheat varieties had undetectable levels of cysteine. Bonafaccia, Marocchini, and Kreft (Citation2003) reported cysteine as one of the limiting amino acids in Tartary buckwheat. A low content of cysteine (179–243 mg/100 g) was also reported in common buckwheat (Mota et al. Citation2016; Motta et al. Citation2019).
Variations in protein content due to sowing time are usually low (Domingos and Bilsborrow Citation2021). Sowing at early spring (mid-April) and late June–early July might result in slightly lower protein content compared to later sowing in Italy and Iran, respective, probably due to decreased roots development and efficiency of nitrogen absorption (Mariotti et al. Citation2015; Sobhani et al. Citation2014). Protein content also varies between years due to different weather conditions (Bárta et al. Citation2004). Thus, late-developed buckwheat seeds might result in a lower content of protein due to insufficient conversion of nitrogenous substances into proteins (Bárta et al. Citation2004). Nitrogen fertilization can also change protein yield and accumulation (Wang et al. Citation2023). Yet, knowledge of the effects of growing conditions and technology on buckwheat proteins is insufficient, and further research is warranted.
Buckwheat protein has a relatively lower digestibility (70–80%) than cereal protein (Jin, Ohanenye, and Udenigwe Citation2022; Luthar, Fabjan, and Mlinarič Citation2021). This is likely due to the presence of fibers and reduced susceptibility for the proteolytic enzymes to the protein fractions. Another explanation of lower digestibility is the interactions between proteins and phenolic substances in the intestine (Li et al. Citation2023; Luthar, Fabjan, and Mlinarič Citation2021) or the formation of denser aggregates from buckwheat starch during the gelatinization-cooling process (Du et al. Citation2022). Protein digestibility can be improved by appropriate processing, such as ultrasonic pretreatment (Jin et al. Citation2021).
Carbohydrates
The main carbohydrate in buckwheat groats is starch, which content varies from 68 to 73% (Kreft and Skrabanja Citation2002), with 19–34% of amylose and approximately 70% of amylopectin (Gao et al. Citation2016; Qin et al. Citation2010; Wang et al. Citation2021a). The amylose content of buckwheat starch is therefore comparable with or slightly lower than that of cereals, and similar in common buckwheat and Tartary buckwheat (Izydorczyk et al. Citation2014; Qin et al. Citation2010; Wijngaard and Arendt Citation2006). The amylose content in starch is an important parameter as it determines the digestibility of starch because amylose is more resistant to digestion. The starch granules in common buckwheat groats have rough surfaces and are mainly polygonal, which is associated with high stabilizing and thickening properties (Juan, Yan, and Zhengbiao Citation2009). Gelatinization and cooling of buckwheat starch result in the formation of a strong rigid gel with an elasticity modulus significantly higher than that of cereal starches (Izydorczyk et al. Citation2014). Environmental and genetic factors are important in the determination of the physicochemical properties of buckwheat starch, such as solubility, relative crystallinity, and gelatinization enthalpy (Wang et al. Citation2023). Total starch content is affected by the sowing date. For example, buckwheat sowed at earlier August in Iran contained a higher amount of starch (Sobhani et al. Citation2014). The application of nitrogen fertilizer also affects the total starch content by changing the activities of enzymes involved in starch synthesis (Gao et al. Citation2021).
Buckwheat is a rich source of dietary fibers. The total content of dietary fibers of six common buckwheat varieties with hulls was measured to be 20–26% (Dziadek et al. Citation2016) with brans containing more fibers than refined flour. After de-hulling, the content of insoluble fibers decreases. Dehulled common buckwheat seeds, grown and harvested in China, had 2.9% insoluble fiber and 2.4% of soluble fiber (Wefers and Bunzel Citation2015). The water-soluble fiber of buckwheat includes pectins, arabinogalactans, and xyloglucans (Izydorczyk et al. Citation2014).
Both of Tartary and common buckwheats are rich sources of D-chiro-inositol (DCI) and its galactosyl derivatives fagopyritols (Steadman et al. Citation2000). Generally, buckwheat, in contrast to most plant seeds, accumulates R-galactosyl D-chiro-inositols rather than the R-galactosyl sucrose series (raffinose, stachyose, and verbascose) (Horbowicz and Obendorf Citation2005). The concentrations of fagopyritols are generally higher than DCI in seeds (Steadman et al. Citation2000). Bioavailability of DCI is relatively low (Zieliński et al. Citation2019) but might be improved by processing. However, information on potential enhancers or inhibitors of DCI absorption is still limited.
Vitamins and minerals
Buckwheat is a rich source of certain vitamins. It is especially known for the contents of thiamin (vitamin B1), niacin (vitamin B3), pyridoxine (vitamin B6), vitamin E, and vitamin K (Gallo and Montesano Citation2023; Huda et al. Citation2021). Bonafaccia, Marocchini, and Kreft (Citation2003) reported that the concentrations of total vitamin B, thiamin, niacin, and pyridoxine were lower in common compared to Tartary buckwheat. On the other hand, common buckwheat contained higher concentrations of vitamin E than Tartary buckwheat with γ-tocopherol being the main component of vitamin E (Joshi et al. Citation2020; Kalinova, Triska, and Vrchotova Citation2006). Total tocopherol concentrations in buckwheat grains ranged from 14.3 to 21.7 mg/kg (Kim, Kim, and Park Citation2002).
Buckwheat grains contain a large number of essential minerals. The concentrations of minerals in buckwheat flour depend on the milling method (Liu et al. Citation2018). The greatest concentrations of most of the minerals were observed in stone-milled flour (Liu et al. Citation2018). The largest portion of minerals in buckwheat accounts for iron, manganese, copper, and zinc (Bonafaccia, Marocchini, and Kreft Citation2003; Dzakhmisheva and Khokonova Citation2021; Krupa-Kozak, Wronkowska, and Soral-Śmietana Citation2011). The contents of some minerals in Tartary buckwheat flours were the following: iron (1.75 to 17.21), manganese (0.08 to 2.72), copper (0.64 to 2.81), zinc (1.23 to 5.79), potassium (280.6 to 648.7), magnesium (66.90 to 362.90), and calcium (30.0 to 331 mg/100 g) (Bhinder et al. Citation2020). Thus, buckwheat foods can improve the intake of iron and manganese in the Swedish population, as the average intake of these minerals is below the daily recommended intake for adults in Sweden (Becker et al. Citation2011).
Bioactive compounds
Buckwheat contains a variety of bioactive compounds, which in addition to basic nutrients, contribute to positive health benefits. Until now, approximately 180 bioactive compounds were detected and identified in buckwheat (Huda et al. Citation2021). Buckwheat is particularly known for its high flavonoid content. Total phenolic content was higher in Tartary buckwheat than in common buckwheat (Liu et al. Citation2019). Rutin (quercetin-3-d-rutinosid) was the dominant phenolic compound in the flour from Tartary buckwheat grown in China (Liu et al. Citation2018). Fabjan et al. (Citation2003) found that higher rutin concentrations were observed in the Chinese variety when these in Slovenia, likely due to different growth conditions. Another study investigated three varieties of common buckwheat and identified rutin and epicatechin as dominant in buckwheat grains, whereas quercetin was not detected (Kalinova and Vrchotova Citation2011). In the flour from Tartary buckwheat, quercetin was detected in relatively high concentrations, especially if the flour was prepared by wet milling (Liu et al. Citation2018). This is due to the hydrolysis of rutin to quercetin endogenous rutin-degrading enzymes, such as rutinosidase (Yasuda and Nakagawa Citation1994). Generally, Tartary buckwheat has higher contents of rutin and quercetin than common buckwheat seeds (Zielińska et al. Citation2012), although rutin levels are highly dependent on cultivation conditions, such as sowing date and stand density (Kalinová and Dadáková Citation2013; Nikitina, Vagner, and Martynova Citation2020). Even though both rutin and quercetin have positive health effects, a new Tartary buckwheat variety with higher rutin than quercetin levels was recently bred in Japan (Luthar et al. Citation2020). This variety is characterized by lower rutinosidase activity and thus a lower degree of rutin degradation to quercetin. Food products from this variety are less bitter and more acceptable by consumers (Suzuki et al. Citation2014). Dehulling greatly affects rutin concentrations (Błaszczak et al. Citation2013; Klepacka and Najda Citation2021). The level of phenolic compounds is highly dependent on the roasting process, which affects the total content, and may cause severe losses in the levels of coumaric acid and rutin (Klepacka and Najda Citation2021).
Antinutrients
Buckwheat, similarly to cereals, contains common anti-nutrients, phytic acid and tannins that can bind to carbohydrates, proteins, some vitamins, and minerals, reducing their intestinal absorption.
Phytic acid content in untreated buckwheat grains is 1.1–1.4 g/100 g dry matter (Egli et al. Citation2002; Thakur et al. Citation2021). For comparison, untreated wheat grains contain 1.0 g/100 g dry matter of phytic acid (Egli et al. Citation2002). The highest concentration of phytic acid was found in the hull, up to 3.25 % (Kasar et al. Citation2021). Buckwheat flakes also had higher content (22 mg/g dry matter) of phytic acid compared to wheat flakes (11 mg/g dry matter) (Kiewlicz and Rybicka Citation2020). The apparent phytase activity of untreated buckwheat grains was 2.9 PUa/g dry matter (phytase unit (PU) equivalent to the enzymatic activity which liberates 1 µmol inorganic phosphate per min), which is comparable to the activity in wheat 3.1 PUa/g dry matter, but lower than in rye 6.9 PUa/g dry matter (Egli et al. Citation2002). Due to high phytase activity, buckwheat can be used as an ingredient in foods based on mixtures of cereals and legumes and provide sufficient enzymatic activity to degrade phytic acid in cereals and legumes with low phytase activity.
Buckwheat also contains condensed tannins, a group of polyphenols with antinutrient properties (Xiao et al. Citation2022). The concentrations of condensed tannins in common buckwheat ranged from 15 to 41 mg/100 g, and in a hybrid (F. esculentum × F. homotropicum) can be up to 220 mg/100 g (Olschläger et al. Citation2008). The content of tannins was the lowest in bran and the highest in the fine flour (Kasar et al. Citation2021; Steadman et al. Citation2001b). Buckwheat flakes had a slightly lower content of total tannins (7 mg/g dry matter) compared to wheat flakes (9 mg/g dry matter) (Kiewlicz and Rybicka Citation2020).
Approaches and sensory aspects of buckwheat and buckwheat food products
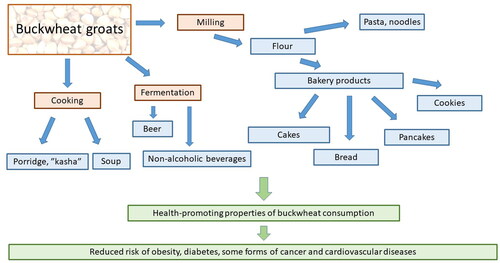
Due to its nutritional composition and sensory qualities, buckwheat is traditionally used in the production of various foods (Kreft et al. Citation2020) (). Cooked buckwheat groats (“kasha”) are an important part of traditional cuisine in central and Eastern Europe, including Slovenia, Croatia, Poland, Ukraine, Belarus, and Russia. In Japan, buckwheat is mainly consumed as noodle soba (Mikami, Motonishi, and Tsutsui Citation2018). Buckwheat flour is unmixed or mixed with wheat flour to prepare bakery products, including bread, blini, and cookies. Buckwheat is also used to make beverages (Kowalska and Ziarno Citation2020; Xu et al. Citation2019).
Sensory quality and consumer acceptance of buckwheat products were investigated using both common buckwheat and Tartary buckwheat as raw materials. These two varieties differ in the content of flavonoids (Liu et al. Citation2019), which greatly affects palatability (Ma et al. Citation2013). This led to recommendations that the use of Tartary buckwheat in different food products should not exceed 30% as the higher portion might lead to undesirable sensory properties such as bitterness and astringency (Appiani et al. Citation2021).
Aroma components of buckwheat
The number of studies evaluating aroma components of buckwheat and buckwheat products is limited, and the use of different methodology to identify these components complicates the between-studies comparison. A flavor analysis on Tartary buckwheat performed by gas chromatography-olfactometry-mass spectrometry (GC-O-MS) showed 49 aroma-active components, where the major flavor compounds were geranylacetone (green, fruity, woody), phenethyl alcohol (floral, rose-like), and β-damascone (fruity, floral) (Shi et al. Citation2021). In the same study, omission tests identified geosmin (fresh, earthy, musty), α-isomethylionone (floral, woody), α-methylionone (fruity, floral, woody), β-ionone (floral, woody), linalool (citrus, floral), β-damascone (fruity, floral), geranylacetone (green, fruity, woody), guaiacol (phenolic, smoky), ethyl hexanoate (fruity), geraniol (floral, fruity), vanillin, tetrahydrolinalool (fresh, floral), and 2,5-dimethyl-4-hydroxy-3-(2H)-furanone (caramel) as the key odorants. Interestingly, the aroma of common and Tartary buckwheat differs, mainly due to the presence of salicylaldehyde in common buckwheat (Janeš and Kreft Citation2008; Janeš, Prosen, and Kreft Citation2012). It was suggested that the presence of salicylaldehyde could be used to detect adulteration of Tartary buckwheat products (Janeš, Prosen, and Kreft Citation2012). The concentrations of aroma components differ between geographical regions. Thus, the concentrations of salicylaldehyde were higher in the common buckwheat from the Ukraine compared to Slovenian buckwheat (Janeš and Kreft Citation2008). Additionally, storage and milling conditions affect buckwheat aroma.
Buckwheat groats
Buckwheat groats are the hulled and roasted seeds of the buckwheat, which are commonly consumed after cooking in water as an alternative to rice or other cereals, or potatoes. To obtain the best nutritional value of buckwheat groats, the roasting process should be carried out at a temperature close to 100 °C at a time that will ensure the appropriate sensory attributes (Klepacka and Najda Citation2021). The recommended method of cooking buckwheat groats is boiling for 30 min in a ratio of 2:1 (water:groats) (Hęś et al. Citation2014). This method increased the content of polyphenolic compounds and did not alter the rutin content (Hęś et al. Citation2014). With a longer cooking time up to 60 min, rutin content significantly decreased (Kreft, Fabjan, and Yasumoto Citation2006). Cooked buckwheat is soft and has a pleasant aroma. Unfortunately, the main research on cooked buckwheat was focused on technological parameters, and sensory evaluation of the cooked buckwheat in comparison with cereals or other pseudocereals is still lacking. The preparation of buckwheat groats of high quality is highly dependent on the technological processes applied in buckwheat groats production since they affect the content of phenolic compounds and antioxidant activity of the groats.
Bread
Bread is the most common baked product and a staple food in many parts of the world. Buckwheat is an attractive ingredient in gluten-free breads, although there are numerous limitations in using buckwheat in the baking industry. Generally, the results on the sensory quality of bread made from buckwheat, with or without a combination with other flours, are conflicting. Gluten-free bread based on rice and buckwheat (husked and unhusked) with the proportions of rice:buckwheat: 90:10, 80:20, and 70:30, showed very similar rheological properties as wheat-based bread. All breads were sensory acceptable (Torbica, Hadnađev, and Dapčević Citation2010). Bread based on wheat and rye with an addition of 3, 5, 7, 10, and 15% of buckwheat flour also received high consumer acceptability scores, although the porosity and specific loaf volume of the bread were reduced with increasing buckwheat portion (Temnikova et al. Citation2021). Thus, the portion of buckwheat flour in bread correlates with the rheological properties of dough and consumer acceptability. The incorporation of a higher portion of buckwheat flour (above 20%) negatively affected the technological and sensory parameters of bread and increases crumb hardness (Wronkowska et al. Citation2020). On the contrary, Aguiar et al. (Citation2021) suggested that the maximum amount of buckwheat flour in bread can be up to 85% based the acceptance of appearance, color, odor and texture. The same study demonstrated that using 35%, 45%, or even up to 50% buckwheat in bread resulted in rheological and thermomechanical properties similar to conventional bread, and the specific volumes may even be higher than the conventional bread. Southgate et al. (Citation2017) used a blend of buckwheat, rice, and cassava flours and demonstrated that increasing the buckwheat flour portion from 15% to 45% caused an increase in insoluble fiber content, crumb cell circularity, and loaf specific volume. A sensory test showed that buckwheat flour had a positive impact on bread quality and bread was accepted by consumers (Southgate et al. Citation2017). It should, however, be noted that bread with the highest content of buckwheat flour received the lowest sensory acceptability scores. When fermenting rice-buckwheat or pure buckwheat dough, an addition of teff flour increased consumer preferences (Campo et al. Citation2016). The combination of teff (10%) with rice and/or buckwheat sourdough enhanced the bread aroma, increasing fruity, cereal, and toasty notes. However, high levels of teff (20%) induced a decrease on the loaf area. The visual appearance of breads with 20% teff was highly appreciated by consumers, while bread combining 10% teff was preferred in terms of flavor. The bitter taste of buckwheat sourdough was generally considered a negative attribute. However, a group of consumers liked bitter bread as they associated it with a traditional, artisan, “malty-like” product (Campo et al. Citation2016). Generally, Tartary buckwheat products have a bitter taste, because of the degradation of the high rutin content to quercetin. Hydrothermal processing directly on buckwheat flour it is possible to reduce the bitter taste in breads with Tartary buckwheat (Wang, Fan, and Zhang Citation2017).
Bilgiçli and İbanoğlu (Citation2015) pointed out that a blend of quinoa and buckwheat flour could replace 20% of wheat flour in the formulation of bread with maintained sensory acceptability. However, the addition of quinoa and buckwheat had a negative effect on the volume and the hardness of the breads.
In conventional bread based on refined wheat flour, a supplementation of buckwheat flour may increase the dough’s qualities. Moisture content, ash, proteins, lipids, and carbohydrates vary according to the particle sizes of the buckwheat. The medium-sized particle fraction is the richest in protein, lipid, and ash, which contribute to increased water and swelling properties and reduced volumetric density. Amylase activity increases with the particle size in composite flour. A decrease of particle size increases water absorption, dough viscosity during starch gelatinization and retrogradation, while the level of added buckwheat may increase the dough development time and gel stability as well as decrease the rate of protein weakening (Coțovanu and Mironeasa Citation2021).
A study on rolls with or without added buckwheat showed significant differences between the breads in the intensity of the sensory properties sweet odor, bread crust odor, crust and crumb color, pore distribution, “sand-feeling” texture, or buckwheat taste in buckwheat-enriched bakery products (Wronkowska et al. Citation2019). However, these differences did not affect consumer acceptance. Furthermore, it was shown that buckwheat ingredients may improve the microbial qualities of the bread during storage (Wronkowska et al. Citation2019). Consumers evaluated the acceptability of the appearance, color, aroma, flavor, texture and overall liking of the gluten-free yeast rolls where whole grain flours from buckwheat, rice, sorghum, millet, amaranth, or quinoa, were replacing the flour-starch base in the original recipe (Drub et al. Citation2021). The new yeast rolls were accepted in all sensory attributes, varying from 6.0 to 8.6 on a 9-point scale, and did not differ from the control gluten-free yeast rolls. In addition, brown rice or buckwheat flour presence in the yeast roll resulted in a product that can receive the claim of “fiber source” as it contains more than 3% of dietary fiber (Drub et al. Citation2021).
Discrepancies in the results from different studies concerning buckwheat qualities underline the importance to develop innovative approaches to enhance the sensory properties of buckwheat-based breads. The application of transglutaminase (TG), which modifies proteins through cross-linking, can improve the overall quality of a gluten-free bread, especially if used in buckwheat sourdough. The use of sourdough significantly reduces the specific bread volume, whereas the crumb becomes more cohesive and springier. When adding TG, the crumb is also more cohesive and springier, but has a lower density, similar to that of conventional bread. Moreover, the overall sensory qualities of buckwheat sourdough bread with added TG increase, as the characteristic bitter aftertaste of buckwheat is less noticeable (Diowksz and Sadowska Citation2021). The overall sensory acceptability can also be increased by the addition of calcium and sodium caseinates, as it was demonstrated in a study on rice-buckwheat bread (Burešová et al. Citation2016). More studies are needed to evaluate calcium and sodium caseinates on buckwheat bread quality.
Biscuits, sweet bread, cakes, and cookies
Sweet bread, biscuits, cakes, and cookies are popular products in many parts of the world and can also be produced from buckwheat. The incorporation of buckwheat and amaranth flour (33% and 50%) into muffins based on wheat had a positive effect on nutritional properties by substantially increasing the unsaturated fatty acid profile and fiber content (Antoniewska et al. Citation2018). Furthermore, the antioxidative capacity of those muffins increased, and shelf life could be prolonged. The incorporation of buckwheat significantly affected the sensory attributes by increasing the intensities of cereal and nut aroma and taste (Antoniewska et al. Citation2018).
The exchange of rice flour for buckwheat flour (10, 20, and 30%) in gluten-free cookies resulted in significantly higher mineral content (Sakač et al. Citation2016). The 20% buckwheat cookie was the most acceptable. Another study successfully replaced wheat flour with 20% buckwheat and 10% sprouts flour in baked buns (Sturza et al. Citation2020). The nutritional value increased without any negative consequences on sensory and texture properties. The addition of guar gum improved gumminess, springiness, and adhesiveness (Sturza et al. Citation2020). Kaur et al. (Citation2015) reported that gluten-free biscuits were sensorially improved if 1% of gums (guar gum, gum acacia, xanthan, or gum tragacanth) were added to the buckwheat flour. Among the gums, the addition of xanthan gum resulted in significant improvement in biscuit color, appearance, flavor, and overall acceptability. For the evaluation of the shelf life of rice-buckwheat cookies, it was found that sensory was a better method to use, than measurements of volatile aldehydes (Sakač et al. Citation2016).
Biscuits with gluten-free flours (buckwheat, sorghum, and lentil) and without rice and maize flours and starches, had an enhanced nutritional profile and were accepted by consumers, however, different consumers groups differed in preferences (Di Cairano et al. Citation2022). Hussain and Kaul (Citation2018) also carried out a study on biscuits. In that study, barley flour (10%) and buckwheat flour (10%, 20%, 30%, 40%, and 50%) were incorporated into wheat flour. The resulting biscuits were evaluated for sensory attributes as well as physicochemical and functional properties. All the blended samples exhibited high fiber, fat, ash, carbohydrate, and mineral contents when compared to those prepared from 100% wheat flour. Considering the taste, flavor, texture, and overall acceptability, the biscuit with 10% added buckwheat flour was found to be the most preferred. The incorporation of buckwheat flour increased the antioxidant potential and hence increased the functional property of the blended product. A reduction in negative oil aroma and the bitter after-taste in buckwheat-oat based biscuits was observed by the addition of herbs from the Lamiaceae family (sage, mint, rosemary, oregano, thyme) (Starowicz et al. Citation2020). It was noted that mint and rosemary significantly lowered the hexanal share, probably due to their antioxidative effects. The addition of rosemary increased functional properties and was the most effective in forming a positive aroma profile with high sensory acceptance (Starowicz et al. Citation2020).
Germinated buckwheat improved the liking of texture in rice crackers, which could be due to a decrease in starch retrogradation (Kim, In, and Rho Citation2017). Added buckwheat and corn flour to waffles did not have any negative impact on the sensory experience, although some changes in flavor (by a slight aroma of buckwheat) and color (browner with added buckwheat) occurred (Dorohovych, Hrytsevich, and Isakova Citation2018). A study on wheat-based crackers showed that the addition of buckwheat and sourdough could improve taste, texture, and chewiness (Selimović et al. Citation2017).
Farzana et al. (Citation2021) showed that cakes with up to 30% added buckwheat were highly accepted and had higher nutritional values due to the presence of fibers, proteins, and micronutrients. Another study on cookies with incorporated buckwheat flour at different levels (0, 20, 40, 60, 80, and 100%) into wheat flour found significant variation in the physicochemical and functional properties of the blended flour (Jan et al. Citation2015). The addition of buckwheat increased the antioxidant properties of blended flour proportionally, decreased hardness, and increased the non-enzymatic browning. The overall acceptability of cookies by sensory analysis was highest at 40% level of blending (Jan et al. Citation2015).
Pasta
Generally, the addition of buckwheat to pasta decreases the consumer acceptability. However, pretreatment of the buckwheat may improve the final product, for example, one study indicated that hydrothermal treatment seems to decrease bitterness and grittiness (Škrobot et al. Citation2022). The same authors suggested that maximum 20% of buckwheat can be added to maintain consumer acceptability. Sun et al. (Citation2018) concluded that pregelatinization treatment of buckwheat flours, including roasting, steaming, extrusion, boiling, and microwaving might be a potential way to enhance its use in food formulation as those treatments significantly influence the physiochemical, morphological, and functional properties of the flours. Sensory analysis showed that noodles based on pregelatinized buckwheat flour had higher scores than native buckwheat flour noodle in appearance, color, flavor, palatability, toughness, stickiness smoothness, taste, and overall acceptability (Sun et al. Citation2018). Using mixtures with different components might also be an attractive option. Pasta based on a mixture of rice-buckwheat flour showed good quality, specifically for high nutritional composition, low cooking loss and stickiness, and acceptable score for sensory attributes such as appearance, color, flavor, taste stickiness, and overall acceptability (Bouasla and Wójtowicz Citation2019). The texture was homogeneous and had a compact microstructure. Thus, gluten-free rice-buckwheat pasta would constitute a good alternative for persons diagnosed with celiac disease. Wang et al. (Citation2019) showed that extruded buckwheat flour greatly may improve the cooking, texture, and eating qualities (hardness, elasticity, and resilience) of buckwheat noodles. The dynamic viscosity of extruded buckwheat flour was significantly related to the molecular size of whole molecules.
A comparison of cooked pasta based on dried or fresh pasta with added buckwheat showed that fresh pasta exhibited more desirable cooking qualities, in the form of lower cooking loss and breakage ratio, and more elastic texture than dried pasta (Wang et al. Citation2021b). The dried pasta had a harder texture. This was explained by the higher mobility of water in the fresh pasta and a more dense inner structure in the dried.
Hoehnel et al. (Citation2022) evaluated the sensory characteristics of pasta with added flour from buckwheat, faba beans and/or lupin protein isolate and compared them with pasta made of 100% wheat. The most notable differences in the sensory profile between the wheat-based pasta and the pastas with buckwheat, faba beans and/or lupin protein isolate were observed for odor and color. The wheat-based pasta had a significantly stronger flour odor than the other kinds of pasta, while the legume odor was much more pronounced for the pastas with added flours. Furthermore, the perception of a beige color was much lower for the wheat-based pasta. No significant differences were detected in cooking loss, firmness, tensile strength, and stickiness. The pastas with added flour from buckwheat, faba beans and/or lupin protein isolate also scored high in overall sensory quality (Hoehnel et al. Citation2022). The addition of 20% buckwheat to gnocchi pasta based on potatoes was shown to increase the liking due to texture properties such as the increase of uniform structure and firm texture (Cappa et al. Citation2021).
Snacks and drinks
Ready-to-eat snacks are very popular foods and perceived by many consumers as tasty and convenient. Sensory tests have revealed that snacks made from buckwheat were liked to a similar or higher degree compared to the reference snack products, which shows commercial potential for developing buckwheat-containing snacks (Defries et al. Citation2018). Extruded snacks based on buckwheat and bread waste, such as dry bread milled to flour, were positively evaluated due to appearance, texture, mouthfeel, and overall sensory experiences (Iqbal et al. Citation2021). Another study focused on extruded protein-rich snacks based on a mix of pulses and pseudocereals, where a mix of blue lupin and buckwheat received the highest scores, especially regarding the texture attributes. The studied materials included lentil, lupin or faba bean mixed with quinoa, amaranth or buckwheat (Martin et al. Citation2022). Singh et al. (Citation2019) analyzed extrudates based on corn grit with added buckwheat at three levels (0, 10, 20, and 30%), at different temperatures (130, 150, and 170 °C), and with or without roasting (92 °C for 15 min) and found that roasting improved flavor and texture. Extrudates with 20% added buckwheat and a temperature of 150 °C yielded the highest sensory scores.
Ertugay, Yangılar, and Çebi (Citation2020) added buckwheat fibers to ice cream and found that both freezing and melting points significantly decreased with the increase in the fiber content. The evaluated sensory properties showed high acceptance among consumers regarding overall impression and attributes concerning taste/flavor, texture, and appearance. In fact, the overall acceptance was higher in samples with added fibers than without.
To develop nutritious and functional yogurt with added buckwheat, an encapsulation methodology was used to form capsules of Tartary buckwheat flavonoids using polymeric whey protein as wall material (Sun, Zhou, and Huang Citation2020). Beside the effective delivery of the flavonoids to the small intestine, the encapsulation also masked the bitter taste and enhanced the color of the yogurt.
To develop a lager beer with high rutin content, a bioflavonoid and a strong antioxidant (Holasova et al. Citation2002), and with desirable sensory characteristics, Tartary buckwheat malt can be used as a brewing adjunct (Deng et al. Citation2019). In comparison to conventional beer, the rutin content in the buckwheat beers was significantly higher. The total flavonoid content in buckwheat beers was strongly dependent on the mashing method. The rutin-rich beers also showed better oxidative stability during forced-aging and were found to be acceptable regarding flavor and taste (Deng et al. Citation2019).
Two buckwheat cultivars in Poland were both distilled in different ways to produce raw spirits. It was shown that pressure-thermal treatment was a beneficial method for starch liberation. However, both cultivars were given high sensory scores when using the pressure-thermal treatment (Ługowoj, Balcerek, and Pielech-Przybylska Citation2019).
To conclude, buckwheat-based products are generally accepted by consumers with varying degrees, although some challenges remain for product developers and technologists.
Health effects of buckwheat
Historically, in many countries, buckwheat was considered as a source of compounds with therapeutic potential and as a pool for the identification and development of new drugs. Consumption of buckwheat has been associated with various beneficial effects on health, such as reduced risk of obesity, diabetes, some forms of cancer, and cardiovascular diseases (CVD) (Kreft Citation2016) (). Buckwheat-based foods such as noodles and breads generally have a lower predicted glycaemic index compared to wheat foods. Due to the presence of resistant starch, buckwheat grains have prebiotic properties (Skrabanja and Kreft Citation1998). The effects of buckwheat consumption on health were recently reviewed by Kreft (Citation2016); thus, in the present review, we only briefly touch those aspects.
Type 1 diabetes mellitus
Generally, there is limited information about food intake and type 1 diabetes mellitus (T1DM) because this type of diabetes is an autoimmune disease that is not associated with lifestyle factors. However, hypoglycemia might be a problem for people with type 1 diabetes mellitus. Vetrani et al. (Citation2019) studied the effect of fiber-enriched buckwheat pasta on postprandial blood glucose in 8 women and 2 men, with T1DM and celiac disease using corn pasta as a control. The postprandial response to buckwheat pasta in that study was more stable, indicating a lower risk of hypoglycemia.
Type 2 diabetes mellitus
In a parallel randomized 4-week dietary intervention trial, Tartary buckwheat (110 g/d) has improved insulin resistance and lipid profile in patients with type 2 diabetes mellitus (T2DM) (Qiu et al. Citation2016). In that study, Tartary buckwheat as a whole food was used to replace a portion of dietary wheat or rice. Although the mechanism behind this improvement is not yet elucidated, there are indications that fagopyritols might be responsible for reduced symptoms of non-insulin dependent diabetes mellitus (Steadman et al. Citation2000). To understand the potential role of fagopyritols and DCI in glucose metabolism, Wu et al. (Citation2018) investigated the ability of fagopyritols and DCI to regulate glucose consumption in normal and insulin-resistant HepG2 cells. It was demonstrated that fagopyritols and DCI enhanced the glucose consumption in normal and insulin-resistant HepG2 cell, with fagopyritol B1 being more effective than DCI. The same study demonstrated that purified fagopyritols A1 and B1 suppressed the increase of blood glucose, improved the serum lipid profile, and enhanced insulin resistance in an insulin-resistant mice model after 6 wk.
Generally, the main indications of beneficial effects of buckwheat on health were observed in in vitro and in animal studies. Kawa, Taylor, and Przybylski (Citation2003) observed lower serum glucose after an acute dose of buckwheat concentrate in male Sprague − Dawley rats in the fed state. A decrease in the risk of T2DM development was also attributed to the presence of flavonoids, especially quercetin, which have been reported to inhibit the activity of α-glucosidase and α-amylase, thus reducing starch digestibility (Peng et al. Citation2019). The presence of fibers in buckwheat foods also contributes to health improvement in T2DM conditions. Supplementation with soluble dietary fibers from Tartary buckwheat bran at the levels of 0.5, 1, and 2% in the diet reduced levels of blood glucose and improved lipid profiles in diabetic mice (Wu et al. Citation2021). A buckwheat protein product was able to reduce serum cholesterol levels and the lithogenic index in rats. Both reductions were associated with enhanced excretion of fecal neutral sterols and fecal bile acid, respectively (Tomotake et al. Citation2007). The oral administration of buckwheat-albumin hydrolysates reduced the postprandial blood glucose, but not insulin, in rats after oral starch loading (Ninomiya et al. Citation2022). This effect was explained by inhibition of α-amylase in the gut (Ninomiya et al. Citation2018). Thus, buckwheat can potentially be used as a functional ingredient to prevent or improve diabetes mellitus and reduce associated healthcare costs.
Despite the positive effects of buckwheat observed in vitro and in animal studies, the results from clinical human studies are contradictory. Thus, Stringer et al. (Citation2013) did not observe any alterations in acute post-prandial glucose or insulin in healthy individuals or in patients with type 2 diabetes after buckwheat consumption. In contrast, Zhang et al. (Citation2007) suggested that buckwheat positively affected glucose metabolism and hyperglycemia in the pastureland Mongolian population in China.
Allergy
Besides the positive health effect of buckwheat consumption, there are potential negative effects. Buckwheat flour and meal might cause allergic reactions, including hypersensitivities such as asthma and gastrointestinal disorders (Norbäck and Wieslander Citation2021). The allergy to buckwheat proteins is one of the most common food allergies worldwide (Satoh, Jensen-Jarolim, and Teshima Citation2020). Several clinically relevant IgE-binding proteins in common buckwheat have been identified as allergens with the 22 kDa protein being the major allergen, with the molecular mass belonging to the globulin protein legumin-like β subunit (Wang et al. Citation2004). Interestingly, it was suggested that the allergenicity and surface functionalities of proteins can be altered by conjugation with the polysaccharides (Nakamura et al. Citation2008).
Peripheral nerve damage
To the best of our knowledge, only one study reported an association between toxic peripheral neuropathy and buckwheat consumption. Toxic peripheral neuropathy was reported in 5 male diabetic patients taking buckwheat composite tablets (Yang et al. Citation2014). It should be, however, noted that these patients used the tablets from the same batch and the exact source of the peripheral neuropathy is still unknown.
Conclusion
Buckwheat is an attractive but underutilized crop for food production that could contribute to higher biodiversity when used in mixed crop systems. Moreover, buckwheat has the ability to adjust to adverse climatic conditions. Buckwheat is an excellent source of protein with a well-balanced amino acid composition, dietary fibers, vitamins, minerals, and bioactive compounds. Buckwheat contains no gluten and can serve as valuable food for persons suffering from gluten intolerance. Consumption of buckwheat has been associated with various beneficial effects on health, such as reduced risk of obesity, diabetes, some forms of cancer, and CVD. Fagopyritols are of special interest for the treatment of non-insulin dependent diabetes mellitus. Buckwheat-based products are generally accepted by consumers and have a potential to be a part of a healthy diet. Thus, the use of buckwheat in agriculture and food industry can improve world food security and economic growth. Future research should be focused on breeding programs toward buckwheat cultivars with improved agronomic characteristics and superior nutritional qualities, such as reduced content of antinutrients or development of low-allergen lines. The long-term effects of buckwheat consumption of human health and buckwheat’s contribution to more resilient farming systems and promotion of biodiversity should also be further studied. Finally, more research is needed to facilitate the successful development of tasty and safe buckwheat-based foods with environmental and health benefits.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Agarwal, M., S. Dheeman, R. C. Dubey, P. Kumar, D. K. Maheshwari, and V. K. Bajpai. 2017. Differential antagonistic responses of Bacillus pumilus MSUA3 against Rhizoctonia solani and Fusarium oxysporum causing fungal diseases in Fagopyrum esculentum Moench. Microbiological Research 205:40–7. doi: 10.1016/j.micres.2017.08.012.
- Aguiar, E. V., F. G. Santos, A. C. L. S. Centeno, and V. D. Capriles. 2021. Influence of pseudocereals on gluten-free bread quality: A study integrating dough rheology, bread physical properties and acceptability. Food Research International (Ottawa, Ont.) 150 (Pt A):110762. doi: 10.1016/j.foodres.2021.110762.
- Alenius, T., T. Mökkönen, and A. Lahelma. 2013. Early farming in the Northern Boreal Zone: Reassessing the history of land use in Southeastern Finland through high-resolution pollen analysis. Geoarchaeology 28 (1):1–24. doi: 10.1002/gea.21428.
- Alonso-Miravalles, L., and J. A. O’Mahony. 2018. Composition, protein profile and rheological properties of pseudocereal-based protein-rich ingredients. Foods (Basel, Switzerland) 7 (5):73. doi: 10.3390/foods7050073.
- Antoniewska, A., J. Rutkowska, M. M. Pineda, and A. Adamska. 2018. Antioxidative, nutritional and sensory properties of muffins with buckwheat flakes and amaranth flour blend partially substituting for wheat flour. LWT 89:217–23. doi: 10.1016/j.lwt.2017.10.039.
- Appiani, M., N. S. Rabitti, C. Proserpio, E. Pagliarini, and M. Laureati. 2021. Tartary buckwheat: A new plant-based ingredient to enrich corn-based gluten-free formulations. Foods (Basel, Switzerland) 10 (11):2613. doi: 10.3390/foods10112613.
- Aubert, L., and M. Quinet. 2022. Comparison of heat and drought stress responses among twelve Tartary buckwheat (Fagopyrum tataricum) varieties. Plants 11 (11):1517. doi: 10.3390/plants11111517.
- Babu, S., G. S. Yadav, R. Singh, R. K. Avasthe, A. Das, K. P. Mohapatra, M. Tahashildar, K. Kumar, M. Prabha, M. Thoithoi Devi, et al. 2018. Production technology and multifarious uses of buckwheat (Fagopyrum spp.): A review. Indian Journal of Agronomy 63 (4):415–27.
- Bárta, J., J. Kalinová, J. Moudrý, and V. Čurn. 2004. Effects of environmental factors on protein content and composition in buckwheat flour. Cereal Research Communications 32 (4):541–8. doi: 10.1007/BF03543346.
- Becker, W., L. Jorhem, B. Sundström, and K. P. Grawé. 2011. Contents of mineral elements in Swedish market basket diets. Journal of Food Composition and Analysis 24 (2):279–87. doi: 10.1016/j.jfca.2010.10.001.
- Berdin, S. I., I. M. Straholis, and G. V. Klitsenko. 2018. Varietal reaction of buckwheat to methods and norms of seeding. Bulletin of Sumy National Agrarian University 3 (35):64–7.
- Beug, H.-J. 2011. Vegetation changes during the Slavic period, shown by a high resolution pollen diagram from the Maujahn peat bog near Dannenberg, Hanover Wendland, Germany. Vegetation History and Archaeobotany 20 (3):199–206. doi: 10.1007/s00334-011-0284-4.
- Bhinder, S., A. Kaur, B. Singh, M. P. Yadav, and N. Singh. 2020. Proximate composition, amino acid profile, pasting and process characteristics of flour from different Tartary buckwheat varieties. Food Research International (Ottawa, Ont.) 130:108946. doi: 10.1016/j.foodres.2019.108946.
- Bielski, S., R. Marks-Bielska, and P. Wiśniewski. 2022. Investigation of energy and economic balance and GHG emissions in the production of different cultivars of buckwheat (Fagopyrum esculentum Moench): A case study in Northeastern Poland. Energies 16 (1):17. doi: 10.3390/en16010017.
- Bilgiçli, N., and Ş. İbanoğlu. 2015. Effect of pseudo cereal flours on some physical, chemical and sensory properties of bread. Journal of Food Science and Technology 52 (11):7525–9. doi: 10.1007/s13197-015-1770-y.
- Björkman, T. 2009. Buckwheat Production Guide for the Northeast.em > Ver. 1.100716. Cornell University. http://hort.cornell.edu/bjorkman/lab/buck/guide/
- Björkman, T., and J. W. Shail. 2013. Using a buckwheat cover crop for maximum weed suppression after early vegetables. HortTechnology 23 (5):575–80. doi: 10.21273/HORTTECH.23.5.575.
- Błaszczak, W., D. Zielińska, H. Zieliński, D. Szawara-Nowak, and J. Fornal. 2013. Antioxidant properties and rutin content of high pressure-treated raw and roasted buckwheat groats. Food and Bioprocess Technology 6 (1):92–100. doi: 10.1007/s11947-011-0669-5.
- Bonafaccia, G., M. Marocchini, and I. Kreft. 2003. Composition and technological properties of the flour and bran from common and tartary buckwheat. Food Chemistry 80 (1):9–15. doi: 10.1016/S0308-8146(02)00228-5.
- Bouasla, A., and A. Wójtowicz. 2019. Rice-buckwheat gluten-free pasta: Effect of processing parameters on quality characteristics and optimization of extrusion-cooking process. Foods (Basel, Switzerland) 8 (10):496. doi: 10.3390/foods8100496.
- Burešová, I., L. Masaříková, L. Hřivna, S. Kulhanová, and D. Bureš. 2016. The comparison of the effect of sodium caseinate, calcium caseinate, carboxymethyl cellulose and xanthan gum on rice-buckwheat dough rheological characteristics and textural and sensory quality of bread. LWT - Food Science and Technology 68:659–66. doi: 10.1016/j.lwt.2016.01.010.
- Campo, E., L. del Arco, L. Urtasun, R. Oria, and A. Ferrer-Mairal. 2016. Impact of sourdough on sensory properties and consumers’ preference of gluten-free breads enriched with teff flour. Journal of Cereal Science 67:75–82. doi: 10.1016/j.jcs.2015.09.010.
- Cappa, C., M. Laureati, M. C. Casiraghi, D. Erba, M. Vezzani, M. Lucisano, and C. Alamprese. 2021. Effects of red rice or buckwheat addition on nutritional, technological, and sensory quality of potato-based pasta. Foods (Basel, Switzerland) 10 (1):91. doi: 10.3390/foods10010091.
- Coțovanu, I., and S. Mironeasa. 2021. Buckwheat seeds: Impact of milling fractions and addition level on wheat bread dough rheology. Applied Sciences 11 (4):1731. doi: 10.3390/app11041731.
- Defries, D. M., J. C. Petkau, T. Gregor, and H. Blewett. 2018. A randomized, controlled, crossover study of appetite-related sensations after consuming snacks made from buckwheat. Applied Physiology, Nutrition, and Metabolism = Physiologie Appliquee, Nutrition Et Metabolisme 43 (2):194–202. doi: 10.1139/apnm-2017-0295.
- Deng, Y., J. Lim, G.-H. Lee, T. T. H. Nguyen, Y. Xiao, M. Piao, and D. Kim. 2019. Brewing rutin-enriched lager beer with buckwheat malt as adjuncts. Journal of Microbiology and Biotechnology 29 (6):877–86. doi: 10.4014/jmb.1904.04041.
- Di Cairano, M., N. Condelli, F. Galgano, and M. C. Caruso. 2022. Experimental gluten-free biscuits with underexploited flours versus commercial products: Preference pattern and sensory characterisation by Check All That Apply Questionnaire. International Journal of Food Science & Technology 57 (4):1936–44. doi: 10.1111/ijfs.15188.
- Diowksz, A., and A. Sadowska. 2021. Impact of sourdough and transglutaminase on gluten-free buckwheat bread quality. Food Bioscience 43:101309. doi: 10.1016/j.fbio.2021.101309.
- Domańska, J., D. Leszczyńska, and A. Badora. 2021. The possibilities of using common buckwheat in phytoremediation of mineral and organic soils contaminated with Cd or Pb. Agriculture 11 (6):562. doi: 10.3390/agriculture11060562.
- Domingos, I. F. N., and P. E. Bilsborrow. 2021. The effect of variety and sowing date on the growth, development, yield and quality of common buckwheat (Fagopyrum esculentum Moench). European Journal of Agronomy 126:126264. doi: 10.1016/j.eja.2021.126264.
- Dorohovych, V., M. Hrytsevich, and N. Isakova. 2018. Effect of gluten-free flour on sensory, physico-chemical, structural and mechanical properties of wafer batter and waffles. Ukrainian Food Journal 7 (2):253–63. doi: 10.24263/2304-974X-2018-7-2-8.
- Drub, T. F., F. Garcia dos Santos, A. C. Ladeia Solera Centeno, and V. D. Capriles. 2021. Sorghum, millet and pseudocereals as ingredients for gluten-free whole-grain yeast rolls. International Journal of Gastronomy and Food Science 23:100293. doi: 10.1016/j.ijgfs.2020.100293.
- Du, J., H. Li, J. Huang, H. Tao, A. Hassane Hamadou, D. An, Y. Qi, and B. Xu. 2022. Insights into the reasons for lower digestibility of buckwheat-based foods: The structure-physical properties of starch aggregates. Journal of Cereal Science 107:103506. doi: 10.1016/j.jcs.2022.103506.
- Dzakhmisheva, I. S., and M. B. Khokonova. 2021. Functional properties of bucket granule. Proceedings of the Voronezh State University of Engineering Technologies 83 (3):86–91. doi: 10.20914/2310-1202-2021-3-86-91.
- Dziadek, K., A. Kopeć, E. Pastucha, E. Piątkowska, T. Leszczyńska, E. Pisulewska, R. Witkowicz, and R. Francik. 2016. Basic chemical composition and bioactive compounds content in selected cultivars of buckwheat whole seeds, dehulled seeds and hulls. Journal of Cereal Science 69:1–8. doi: 10.1016/j.jcs.2016.02.004.
- Egli, I., L. Davidsson, M. A. Juillerat, D. Barclay, and R. F. Hurrell. 2002. The influence of soaking and germination on the phytase activity and phytic acid content of grains and seeds potentially useful for complementary feedin. Journal of Food Science 67 (9):3484–8. doi: 10.1111/j.1365-2621.2002.tb09609.x.
- Ertugay, M. F., F. Yangılar, and K. Çebi. 2020. Ice cream with organic kavilca (buckwheat) fibre: Microstructure, thermal, physicochemical and sensory properties. Carpathian Journal of Food Science and Technology 12 (3):35–50. doi: 10.34302//crpjfst/2020.12.3.3.
- Fabjan, N., J. Rode, I. J. Kosir, Z. Wang, Z. Zhang, and I. Kreft. 2003. Tartary buckwheat (Fagopyrum tataricum Gaertn.) as a source of dietary rutin and quercitrin. Journal of Agricultural and Food Chemistry 51 (22):6452–5. doi: 10.1021/jf034543e.
- Fan, Y., M-q Ding, K-x Zhang, Y. Tang, W. Fang, K-l Yang, Z-w Zhang, J-p Cheng, and M-l Zhou. 2020. Overview and utilization of wild germplasm resources of the Genus Fagopyrum Mill. i China. Journal of Plant Genetic Resources 21 (6):1395–406. doi: 10.13430/j.cnki.jpgr.20200317002.
- Farooq, S., R. U. Rehman, T. B. Pirzadah, B. Malik, F. A. Dar, and I. Tahir. 2016. Chapter twenty three - Cultivation, agronomic practices, and growth performance of buckwheat. In Molecular breeding and nutritional aspects of buckwheat, ed. M. Zhou, I. Kreft, S.-H. Woo, N. Chrungoo, and G. Wieslander, 299–319. USA: Academic Press.
- Farzana, T., J. Fatema, F. B. Hossain, S. Afrin, and S. S. Rahma. 2021. Quality improvement of cakes with buckwheat flour, and its comparison with local branded cakes. Current Research in Nutrition and Food Science 9 (2):570–77. doi: 10.12944/CRNFSJ.9.2.20.
- Gallo, M., and D. Montesano. 2023. 2.11 - Buckwheat: Properties, beneficial effects and technological applications. In Sustainable food science - A comprehensive approach, ed. P. Ferranti, 150–64. USA: Elsevier. doi: 10.12944/B978-0-12-823960-5.00008-1.
- Gao, J., I. Kreft, G. Chao, Y. Wang, X. Liu, L. Wang, P. Wang, X. Gao, and B. Feng. 2016. Tartary buckwheat (Fagopyrum tataricum Gaertn.) starch, a side product in functional food production, as a potential source of retrograded starch. Food Chemistry 190:552–8. doi: 10.1016/j.foodchem.2015.05.122.
- Gao, L., M. Xia, C. Wan, Y. Jia, L. Yang, M. Wang, P. Wang, Q. Yang, P. Yang, X. Gao, et al. 2021. Analysis of synthesis, accumulation and physicochemical properties of Tartary buckwheat starches affected by nitrogen fertilizer. Carbohydrate Polymers 273:118570. doi: 10.1016/j.carbpol.2021.118570.
- Germ, M., and A. Gaberščik. 2016. Chapter twenty one - The Effect of Environmental Factors on Buckwheat. In Molecular breeding and nutritional aspects of buckwheat, ed. M. Zhou, I. Kreft, S.-H. Woo, N. Chrungoo, and G. Wieslander, 273–81. USA: Academic Press.
- Guglielmini, A. C., J. I. Forcat, and D. J. Miralles. 2019. The critical period for yield determination in common buckwheat (Fagopyrum esculentum Moench). European Journal of Agronomy 110:125933. doi: 10.1016/j.eja.2019.125933.
- Guo, Y.-Z., Q.-F. Chen, L.-Y. Yang, and Y.-H. Huang. 2007. Analyses of the seed protein contents on the cultivated and wild buckwheat Fagopyrum esculentum resources. Genetic Resources and Crop Evolution 54 (7):1465–72. doi: 10.1007/s10722-006-9135-z.
- Halbrecq, B., P. Romedenne, and J. F. Ledent. 2005. Evolution of flowering, ripening and seed set in buckwheat (Fagopyrum esculentum Moench): Quantitative analysis. European Journal of Agronomy 23 (3):209–24. doi: 10.1016/j.eja.2004.11.006.
- Hęś, M., K. Dziedzic, D. Górecka, A. Drożdżyńska, and E. Gujska. 2014. Effect of boiling in water of barley and buckwheat groats on the antioxidant properties and dietary fiber composition. Plant Foods for Human Nutrition (Dordrecht, Netherlands) 69 (3):276–82. doi: 10.1007/s11130-014-0425-x.
- Hoehnel, A., J. Bez, I. L. Petersen, R. Amarowicz, J. Juśkiewicz, E. Zannini, and E. K. Arendt. 2022. Combining high-protein ingredients from pseudocereals and legumes for the development of fresh high-protein hybrid pasta: Enhanced nutritional profile. Journal of the Science of Food and Agriculture 102 (12):5000–10. doi: 10.1002/jsfa.11015.
- Holasova, M., V. Fiedlerova, H. Smrcinova, M. Orsak, J. Lachman, and S. Vavreinova. 2002. Buckwheat—the source of antioxidant activity in functional foods. Food Research International 35 (2-3):207–11. doi: 10.1016/S0963-9969(01)00185-5.
- Horbowicz, M., and R. L. Obendorf. 2005. Fagopyritol accumulation and germination of buckwheat seeds matured at 15, 22, and 30 °C. Crop Science 45 (4):1264–70. doi: 10.2135/cropsci2004.0431.
- Huda, M. N., S. Lu, T. Jahan, M. Ding, R. Jha, K. Zhang, W. Zhang, M. I. Georgiev, S. U. Park, and M. Zhou. 2021. Treasure from garden: Bioactive compounds of buckwheat. Food Chemistry 335:127653. doi: 10.1016/j.foodchem.2020.127653.
- Hunt, H. V., X. Shang, and M. K. Jones. 2018. Buckwheat: A crop from outside the major Chinese domestication centres? A review of the archaeobotanical, palynological and genetic evidence. Vegetation History and Archaeobotany 27 (3):493–506. doi: 10.1007/s00334-017-0649-4.
- Hussain, A., and R. Kaul. 2018. Formulation and characterization of buckwheat-barley supplemented multigrain biscuits. Current Research in Nutrition and Food Science Journal 6 (3):873–81. doi: 10.12944/CRNFSJ.6.3.30.
- Ikanović, J., S. Rakić, V. Popović, S. Janković, and K. Glamočlijaborde. 2013. Agro-ecological conditions and morpho-productive properties of buckwheat. Biotechnology in Animal Husbandry 29 (3):555–62.
- Iqbal, S., M. P. Thanushree, M. L. Sudha, and K. Crassina. 2021. Quality characteristics of buckwheat (Fagopyrum esculentum) based nutritious ready-to-eat extruded baked snack. Journal of Food Science and Technology 58 (5):2034–40. doi: 10.1007/s13197-020-04940-2.
- Iwata, H., K. Imon, Y. Tsumura, and R. Ohsawa. 2005. Genetic diversity among Japanese indigenous common buckwheat (Fagopyrum esculentum) cultivars as determined from amplified fragment length polymorphism and simple sequence repeat markers and quantitative agronomic traits. Genome 48 (3):367–77. doi: 10.1139/g04-121.
- Izydorczyk, M. S., T. McMillan, S. Bazin, J. Kletke, L. Dushnicky, and J. Dexter. 2014. Canadian buckwheat: A unique, useful and under-utilized crop. Canadian Journal of Plant Science 94 (3):509–24. doi: 10.4141/cjps2013-075.
- Jan, U., A. Gani, M. Ahmad, U. Shah, W. N. Baba, F. A. Masoodi, S. Maqsood, A. Gani, I. A. Wani, and S. M. Wani. 2015. Characterization of cookies made from wheat flour blended with buckwheat flour and effect on antioxidant properties. Journal of Food Science and Technology 52 (10):6334–44. doi: 10.1007/s13197-015-1773-8.
- Janeš, D., and S. Kreft. 2008. Salicylaldehyde is a characteristic aroma component of buckwheat groats. Food Chemistry 109 (2):293–8. doi: 10.1016/j.foodchem.2007.12.032.
- Janeš, D., H. Prosen, and S. Kreft. 2012. Identification and quantification of aroma compounds of Tartary buckwheat (Fagopyrum tataricum Gaertn.) and some of its milling fractions. Journal of Food Science 77 (7):C746–C751. doi: 10.1111/j.1750-3841.2012.02778.x.
- Jin, J., I. C. Ohanenye, and C. C. Udenigwe. 2022. Buckwheat proteins: Functionality, safety, bioactivity, and prospects as alternative plant-based proteins in the food industry. Critical Reviews in Food Science and Nutrition 62 (7):1752–64. doi: 10.1080/10408398.2020.1847027.
- Jin, J., O. D. Okagu, A. E. A. Yagoub, and C. C. Udenigwe. 2021. Effects of sonication on the in vitro digestibility and structural properties of buckwheat protein isolates. Ultrasonics Sonochemistry 70:105348. doi: 10.1016/j.ultsonch.2020.105348.
- Joshi, D. C., K. Zhang, C. Wang, R. Chandora, M. Khurshid, J. Li, M. He, M. I. Georgiev, and M. Zhou. 2020. Strategic enhancement of genetic gain for nutraceutical development in buckwheat: A genomics-driven perspective. Biotechnology Advances 39:107479. doi: 10.1016/j.biotechadv.2019.107479.
- Juan, G., H. Yan, and G. Zhengbiao. 2009. Study on physico-chemicai properties of buckwheat starch. Food and Fermentation Industries 30 (11):104–8.
- Jukić, G., I. Beraković, I. Delić, I. Rukavina, I. Varnica, and K. Dugalić. 2021. Influence of buckwheat sowing rate on seed yield and quality. Agronomski Glasnik 81 (6):389–98. doi: 10.33128/ag.81.6.3.
- Kajfeẑ-Bogataj, L., and A. Gaberščik. 1986. Analysis of net photosynthesis response curve for buckwheat. Fagopyrum 6:6–8.
- Kalinová, J., and E. Dadáková. 2013. Influence of sowing date and stand density on rutin level in buckwheat. Cereal Research Communications 41 (2):348–58. doi: 10.1556/CRC.2012.0039.
- Kalinova, J., J. Triska, and N. Vrchotova. 2006. Distribution of vitamin E, squalene, epicatechin, and rutin in common buckwheat plants (Fagopyrum esculentum Moench). Journal of Agricultural and Food Chemistry 54 (15):5330–5. doi: 10.1021/jf060521r.
- Kalinova, J., and N. Vrchotova. 2011. The influence of organic and conventional crop management, variety and year on the yield and flavonoid level in common buckwheat groats. Food Chemistry 127 (2):602–8. doi: 10.1016/j.foodchem.2011.01.050.
- Kasar, C., M. P. Thanushree, S. Gupta, and A. A. Inamdar. 2021. Milled fractions of common buckwheat (Fagopyrum esculentum) from the Himalayan regions: Grain characteristics, functional properties and nutrient composition. Journal of Food Science and Technology 58 (10):3871–81. doi: 10.1007/s13197-020-04848-x.
- Kaur, M., K. S. Sandhu, A. Arora, and A. Sharma. 2015. Gluten free biscuits prepared from buckwheat flour by incorporation of various gums: Physicochemical and sensory properties. LWT - Food Science and Technology 62 (1):628–32. doi: 10.1016/j.lwt.2014.02.039.
- Kawa, J. M., C. G. Taylor, and R. Przybylski. 2003. Buckwheat concentrate reduces serum glucose in streptozotocin-diabetic rats. Journal of Agricultural and Food Chemistry 51 (25):7287–91. doi: 10.1021/jf0302153.
- Kiewlicz, J., and I. Rybicka. 2020. Minerals and their bioavailability in relation to dietary fiber, phytates and tannins from gluten and gluten-free flakes. Food Chemistry 305:125452. doi: 10.1016/j.foodchem.2019.125452.
- Kim, S. L., S. K. Kim, and C. H. Park. 2002. Comparisons of lipid, fatty acids and tocopherols of different buckwheat species. Food Science and Biotechnology 11 (4):332–6.
- Kim, Y. A., S. J. In, and J. Rho. 2017. Effect of germinated grain flours on physicochemical characteristics of rice cakes, Seolgitteok. Food Science and Biotechnology 26 (1):21–8. doi: 10.1007/s10068-017-0003-8.
- Klepacka, J., and A. Najda. 2021. Effect of commercial processing on polyphenols and antioxidant activity of buckwheat seeds. International Journal of Food Science & Technology 56 (2):661–70. doi: 10.1111/ijfs.14714.
- Kowalska, E., and M. Ziarno. 2020. Characterization of buckwheat beverages fermented with lactic acid bacterial cultures and bifidobacteria. Foods (Basel, Switzerland) 9 (12):1771. doi: 10.3390/foods9121771.
- Kowalski, S., A. Mikulec, B. Mickowska, and K. Buksa. 2022. Nutritional properties and amino acid profile of buckwheat bread. Journal of Food Science and Technology 59 (8):3020–30. doi: 10.1007/s13197-022-05518-w.
- Kreft, I. 2007. Buchweizen Slowenien. Das Buchweizen Buch: mit Rezepten aus aller Welt. 2. überarbeitete und erweiterte Aufl:71–9.
- Kreft, I., N. Fabjan, and K. Yasumoto. 2006. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chemistry 98 (3):508–12. doi: 10.1016/j.foodchem.2005.05.081.
- Kreft, I., and V. Skrabanja. 2002. Nutritional properties of starch in buckwheat noodles. Journal of Nutritional Science and Vitaminology 48 (1):47–50. doi: 10.3177/jnsv.48.47.
- Kreft, I., M. Zhou, A. Golob, M. Germ, M. Likar, K. Dziedzic, and Z. Luthar. 2020. Breeding buckwheat for nutritional quality. Breeding Science 70 (1):67–73. doi: 10.1270/jsbbs.19016.
- Kreft, M. 2016. Buckwheat phenolic metabolites in health and disease. Nutrition Research Reviews 29 (1):30–9. doi: 10.1017/s0954422415000190.
- Krkošková, B., and Z. Mrázová. 2005. Prophylactic components of buckwheat. Food Research International 38 (5):561–8. doi: 10.1016/j.foodres.2004.11.009.
- Krupa-Kozak, U., M. Wronkowska, and M. Soral-Śmietana. 2011. Effect of buckwheat flour on microelements and proteins contents in gluten-free bread. Czech Journal of Food Sciences 29 (2):103–8. doi: 10.17221/136/2010-CJFS.
- Krzyzanska, M., H. V. Hunt, E. R. Crema, and M. K. Jones. 2022. Modelling the potential ecological niche of domesticated buckwheat in China: Archaeological evidence, environmental constraints and climate change. Vegetation History and Archaeobotany 31 (4):331–45. doi: 10.1007/s00334-021-00856-9.
- Kump, B., and B. Javornik. 1996. Evaluation of genetic variability among common buckwheat (Fagopyrum esculentum Moench) populations by RAPD markers. Plant Science 114 (2):149–58. doi: 10.1016/0168-9452(95)04321-7.
- Kumskova, N. D. 2004. Buckwheat: Monography. Blagoveshchensk, Russia Dal GAU.
- Li, D., L. Zhu, Q. Wu, Y. Chen, G. Wu, and H. Zhang. 2023. Identification of binding sites for Tartary buckwheat protein-phenols covalent complex and alterations in protein structure and antioxidant properties. International Journal of Biological Macromolecules 233:123436. doi: 10.1016/j.ijbiomac.2023.123436.
- Lim, J.-H., K.-J. Park, B.-K. Kim, J.-W. Jeong, and H.-J. Kim. 2012. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chemistry 135 (3):1065–70. doi: 10.1016/j.foodchem.2012.05.068.
- Liu, F., C. He, L. Wang, and M. Wang. 2018. Effect of milling method on the chemical composition and antioxidant capacity of Tartary buckwheat flour. International Journal of Food Science & Technology 53 (11):2457–64. doi: 10.1111/ijfs.13837.
- Liu, Y., C. Cai, Y. Yao, and B. Xu. 2019. Alteration of phenolic profiles and antioxidant capacities of common buckwheat and tartary buckwheat produced in China upon thermal processing. Journal of the Science of Food and Agriculture 99 (12):5565–76. doi: 10.1002/jsfa.9825.
- Ługowoj, S., M. Balcerek, and K. Pielech-Przybylska. 2019. Buckwheat as an interesting raw material for agricultural distillate production. Polish Journal of Food and Nutrition Sciences 70 (2):125–37. doi: 10.31883/pjfns/113533.
- Luthar, Z., P. Fabjan, and K. Mlinarič. 2021. Biotechnological methods for buckwheat breeding. Plants (Basel) 10 (8):1547. doi: 10.3390/plants10081547.
- Luthar, Z., M. Germ, M. Likar, A. Golob, K. Vogel-Mikuš, P. Pongrac, A. Kušar, I. Pravst, and I. Kreft. 2020. Breeding buckwheat for increased levels of rutin, quercetin and other bioactive compounds with potential antiviral effects. Plants (Basel) 9 (12):1638. doi: 10.3390/plants9121638.
- Ma, Y. J., X. D. Guo, H. Liu, B. N. Xu, and M. Wang. 2013. Cooking, textural, sensorial, and antioxidant properties of common and tartary buckwheat noodles. Food Science and Biotechnology 22 (1):153–9. doi: 10.1007/s10068-013-0021-0.
- Mariotti, M., V. Andreuccetti, B. Tozzi, G. Liponi, B. Turchi, I. Arduini, and D. Gatta. 2015. Forage production and nutritional characteristics of buckwheat as affected by maturity and conservation method. Agrochimica 59 (2):137–54. doi: 10.12871/0021857201524.
- Mariotti, M., A. Masoni, and I. Arduini. 2016. Forage and grain yield of common buckwheat in Mediterranean conditions: Response to sowing time and irrigation. Crop and Pasture Science 67 (9):1000–8. doi: 10.1071/CP16091.
- Martin, A., V. Schmidt, R. Osen, J. Bez, E. Ortner, and S. Mittermaier. 2022. Texture, sensory properties and functionality of extruded snacks from pulses and pseudocereal proteins. Journal of the Science of Food and Agriculture 102 (12):5011–21. doi: 10.1002/jsfa.11041.
- Mikami, T., S. Motonishi, and S. Tsutsui. 2018. Production, uses and cultivars of common buckwheat in Japan: An overview. Acta Agriculturae Slovenica 111 (2):511–17. doi: 10.14720/aas.2018.111.2.23.
- Morishita, T., T. Hara, and T. Hara. 2020. Important agronomic characteristics of yielding ability in common buckwheat; ecotype and ecological differentiation, preharvest sprouting resistance, shattering resistance, and lodging resistance. Breeding Science 70 (1):39–47. doi: 10.1270/jsbbs.19020.
- Mota, C., M. Santos, R. Mauro, N. Samman, A. S. Matos, D. Torres, and I. Castanheira. 2016. Protein content and amino acids profile of pseudocereals. Food Chemistry 193:55–61. doi: 10.1016/j.foodchem.2014.11.043.
- Motta, C., I. Castanheira, G. B. Gonzales, I. Delgado, D. Torres, M. Santos, and A. S. Matos. 2019. Impact of cooking methods and malting on amino acids content in amaranth, buckwheat and quinoa. Journal of Food Composition and Analysis 76:58–65. doi: 10.1016/j.jfca.2018.10.001.
- Nakamura, S., Y. Suzuki, E. Ishikawa, T. Yakushi, H. Jing, T. Miyamoto, and K. Hashizume. 2008. Reduction of in vitro allergenicity of buckwheat Fag e 1 through the Maillard-type glycosylation with polysaccharides. Food Chemistry 109 (3):538–45. doi: 10.1016/j.foodchem.2007.12.075.
- Nikitina, V. I., V. V. Vagner, and O. V. Martynova. 2020. Dependence of the rutin content in buckwheat plants on the sowing method, variety and seeding rate. IOP Conference Series: Earth and Environmental Science 548:052037. August 01, 2020. doi: 10.1088/1755-1315/548/5/052037.
- Nikolic, O., M. Pavlovic, D. Dedic, and V. Sabados. 2019. The influence of sow density on productivity and moisture in buckwheat grain (Fagopyrum esculentum moench.) in condition of stubble sowing and irrigation. Agriculture and Forestry 65 (4):193–202. doi: 10.17707/AgricultForest.65.4.17.
- Ninomiya, K., S. Ina, A. Hamada, Y. Yamaguchi, M. Akao, F. Shinmachi, H. Kumagai, and H. Kumagai. 2018. Suppressive effect of the α-amylase inhibitor albumin from buckwheat (Fagopyrum esculentum Moench) on postprandial hyperglycaemia. Nutrients 10 (10):1503. doi: 10.3390/nu10101503.
- Ninomiya, K., Y. Yamaguchi, F. Shinmachi, H. Kumagai, and H. Kumagai. 2022. Suppression of postprandial blood glucose elevation by buckwheat (Fagpopyrum esculentum) albumin hydrolysate and identification of the peptide responsible to the function. Food Science and Human Wellness 11 (4):992–8. doi: 10.1016/j.fshw.2022.03.026.
- Norbäck, D., and G. Wieslander. 2021. A review on epidemiological and clinical studies on buckwheat allergy. Plants (Basel) 10 (3):607. doi: 10.3390/plants10030607.
- Ohnishi, O. 2004. On the origin of cultivated buckwheat. Paper read at Proceedings of the 9th International Symposium on Buckwheat, at Prague, Czech Republic
- Olschläger, C., I. Regos, F. J. Zeller, and D. Treutter. 2008. Identification of galloylated propelargonidins and procyanidins in buckwheat grain and quantification of rutin and flavanols from homostylous hybrids originating from F. esculentumxF. homotropicum. Phytochemistry 69 (6):1389–97. doi: 10.1016/j.phytochem.2008.01.001.
- Olsson, V., C. Feiff, and G. Petterson. 2021. Buckwheat – revival in a new foodscape. Extended abstract. In NAFS Workshop 2020 “Foodways at a crossroads: Sustainability, Gastronomy and Rethinking the traditions of how to Eat”, ed. K. Hult, I.M. Jonsson, H. Scander and L. Wellton, 20–22. Grythyttan, November 14, Örebro University. ISSN: 1652-2656 ISBN: 978-91-87789-44-1 (e-pub) ISBN: 978-91-87789-43-4, 2021
- Peng, L. X., L. J. Wei, Q. Yi, G. H. Chen, Z. D. Yao, Z. Y. Yan, and G. Zhao. 2019. In vitro potential of flavonoids from tartary buckwheat on antioxidants activity and starch digestibility. International Journal of Food Science & Technology 54 (6):2209–18. doi: 10.1111/ijfs.14131.
- Pomeranz, Y. 1983. Buckwheat: Structure, composition, and utilization. Critical Reviews in Food Science and Nutrition 19 (3):213–58. doi: 10.1080/10408398309527376.
- Qin, P., Q. Wang, F. Shan, Z. Hou, and G. Ren. 2010. Nutritional composition and flavonoids content of flour from different buckwheat cultivars. International Journal of Food Science & Technology 45 (5):951–8. doi: 10.1111/j.1365-2621.2010.02231.x.
- Qiu, J., Y. Liu, Y. Yue, Y. Qin, and Z. Li. 2016. Dietary tartary buckwheat intake attenuates insulin resistance and improves lipid profiles in patients with type 2 diabetes: A randomized controlled trial. Nutrition Research (New York, N.Y.) 36 (12):1392–401. doi: 10.1016/j.nutres.2016.11.007.
- Ratan, P., and P. Kothiyal. 2011. Fagopyrum esculentum Moench (common buckwheat) edible plant of Himalayas: A review. Asian Journal of Pharmacy and Life Science 1 (4):1–17.
- Sakač, M., M. Pestorić, A. Mandić, A. Mišan, N. Nedeljković, D. Jambrec, P. Jovanov, V. Lazić, L. Pezo, and I. Sedej. 2016. Shelf-life prediction of gluten-free rice-buckwheat cookies. Journal of Cereal Science 69:336–43. doi: 10.1016/j.jcs.2016.04.008.
- Satoh, R., E. Jensen-Jarolim, and R. Teshima. 2020. Understanding buckwheat allergies for the management of allergic reactions in humans and animals. Breeding Science 70 (1):85–92. doi: 10.1270/jsbbs.19051.
- Selimović, A., D. Miličević, A. Selimović, S. O. Žuljević, A. Jašića, and A. Vranac. 2017. Properties of crackers with buckwheat sourdough. Acta Chimica Slovaca 10 (2):152–8. doi: 10.1515/acs-2017-0025.
- Shevchuk, V., O. Demchenko, and L. Yuzvenko. 2011. Sensitivity evaluation within the collection Fagopyrum tataricum Gaertn of different ecological origin to the buckwheat burn virus. Scientific Bulletin Uzhgorod University (Ser. Biology ) 30:161–3.
- Shi, J., G. Tong, Q. Yang, M. Huang, H. Ye, Y. Liu, J. Wu, J. Zhang, X. Sun, and D. Zhao. 2021. Characterization of key aroma compounds in Tartary buckwheat (Fagopyrum tataricum Gaertn.) by means of sensory-directed flavor analysis. Journal of Agricultural and Food Chemistry 69 (38):11361–71. doi: 10.1021/acs.jafc.1c03708.
- Sindarovska, Y. R., O. I. Guzyk, L. V. Yuzvenko, O. A. Demchenko, L. F. Didenko, O. I. Grynevych, and M. Y. Spivak. 2014. Ribonuclease activity of buckwheat plant (Fagopyrum esculentum) cultivars with different sensitivities to buckwheat burn virus. The Ukrainian Biochemical Journal 86 (3):33–40. doi: 10.15407/ubj86.03.033.
- Singh, J. P., A. Kaur, B. Singh, N. Singh, and B. Singh. 2019. Physicochemical evaluation of corn extrudates containing varying buckwheat flour levels prepared at various extrusion temperatures. Journal of Food Science and Technology 56 (4):2205–12. doi: 10.1007/s13197-019-03703-y.
- Singh, M., N. Malhotra, and K. Sharma. 2020. Buckwheat (Fagopyrum sp.) genetic resources: What can they contribute towards nutritional security of changing world? Genetic Resources and Crop Evolution 67 (7):1639–58. doi: 10.1007/s10722-020-00961-0.
- Sinkovič, L., M. Deželak, R. Kopinč, and V. Meglič. 2022. Macro/microelements, nutrients and bioactive components in common and Tartary buckwheat (Fagopyrum spp.) grain and stone-milling fractions. LWT 161:113422. doi: 10.1016/j.lwt.2022.113422.
- Skrabanja, V., and I. Kreft. 1998. Resistant starch formation following autoclaving of buckwheat (Fagopyrum esculentum Moench) groats. An in vitro study. Journal of Agricultural and Food Chemistry 46 (5):2020–3. doi: 10.1021/jf970756q.
- Škrobot, D., L. Pezo, J. Tomić, M. Pestorić, M. Sakač, and A. Mandić. 2022. Insights into sensory and hedonic perception of wholegrain buckwheat enriched pasta. LWT 153:112528. doi: 10.1016/j.lwt.2021.112528.
- Small, E. 2017. 54. Buckwheat – the world’s most biodiversity-friendly crop? Biodiversity 18 (2-3):108–23. doi: 10.1080/14888386.2017.1332529.
- Sobhani, M. R., G. Rahmikhdoev, D. Mazaheri, and M. Majidian. 2014. Influence of different sowing date and planting pattern and N rate on buckwheat yield and its quality. Australian Journal of Crop Science 8 (10):1402–14.
- Southgate, A. N. N., P. M. Scheuer, M. F. Martelli, L. Menegon, and A. de Francisco. 2017. Quality properties of a gluten-free bread with buckwheat. Journal of Culinary Science & Technology 15 (4):339–48. doi: 10.1080/15428052.2017.1289134.
- Starowicz, M., E. Lelujka, E. Ciska, G. Lamparski, T. Sawicki, and M. Wronkowska. 2020. The application of Lamiaceae Lindl. promotes aroma compounds formation, sensory properties, and antioxidant activity of oat and buckwheat-based cookies. Molecules 25 (23):5626. doi: 10.3390/molecules25235626.
- Steadman, K. J., M. S. Burgoon, B. A. Lewis, S. E. Edwardson, and R. L. Obendorf. 2001a. Buckwheat seed milling fractions: Description, macronutrient composition and dietary fibre. Journal of Cereal Science 33 (3):271–8. doi: 10.1006/jcrs.2001.0366.
- Steadman, K. J., M. S. Burgoon, B. A. Lewis, S. E. Edwardson, and R. L. Obendorf. 2001b. Minerals, phytic acid, tannin and rutin in buckwheat seed milling fractions. Journal of the Science of Food and Agriculture 81 (11):1094–100. doi: 10.1002/jsfa.914.
- Steadman, K. J., M. S. Burgoon, R. L. Schuster, B. A. Lewis, S. E. Edwardson, and R. L. Obendorf. 2000. Fagopyritols, D-chiro-inositol, and other soluble carbohydrates in buckwheat seed milling fractions. Journal of Agricultural and Food Chemistry 48 (7):2843–7. doi: 10.1021/jf990709t.
- Stringer, D. M., C. G. Taylor, P. Appah, H. Blewett, and P. Zahradka. 2013. Consumption of buckwheat modulates the post-prandial response of selected gastrointestinal satiety hormones in individuals with type 2 diabetes mellitus. Metabolism: Clinical and Experimental 62 (7):1021–31. doi: 10.1016/j.metabol.2013.01.021.
- Sturza, A., A. Păucean, M. S. Chiș, V. Mureșan, D. C. Vodnar, S. M. Man, A. C. Urcan, I. E. Rusu, G. Fostoc, and S. Muste. 2020. Influence of buckwheat and buckwheat sprouts flours on the nutritional and textural parameters of wheat buns. Applied Sciences 10 (22):7969. doi: 10.3390/app10227969.
- Sun, X., W. Li, Y. Hu, X. Zhou, M. Ji, D. Yu, K. Fujita, E. Tatsumi, and G. Luan. 2018. Comparison of pregelatinization methods on physicochemical, functional and structural properties of tartary buckwheat flour and noodle quality. Journal of Cereal Science 80:63–71. doi: 10.1016/j.jcs.2018.01.016.
- Sun, Y., W. Zhou, and Y. Huang. 2020. Encapsulation of tartary buckwheat flavonoids and application to yoghurt. Journal of Microencapsulation 37 (6):445–56. doi: 10.1080/02652048.2020.1781943.
- Suzuki, T., T. Morishita, Y. Mukasa, S. Takigawa, S. Yokota, K. Ishiguro, and T. Noda. 2014. Breeding of ‘Manten-Kirari’, a non-bitter and trace-rutinosidase variety of Tartary buckwheat (Fagopyrum tataricum Gaertn.). Breeding Science 64 (4):344–50. doi: 10.1270/jsbbs.64.344.
- Syzdykova, G., A. Zhumakayev, S. Makhanova, A. Tleppayeva, N. Zhanbyrshina, N. Safronova, and K. Seidalina. 2016. Study of optimal sowing date and rate of buckwheat in the semiarid zone of Northern Kazakhstan. Journal of Engineering and Applied Sciences 11 (14):2998–3003. doi: 10.3923/jeasci.2016.2998.3003.
- Taki, H., K. Okabe, S. Makino, Y. Yamaura, and M. Sueyoshi. 2009. Contribution of small insects to pollination of common buckwheat, a distylous crop. Annals of Applied Biology 155 (1):121–9. doi: 10.1111/j.1744-7348.2009.00326.x.
- Temnikova, O. E., E. Y. Rudenko, O. V. Senchenko, and A. A. Ruzyanova. 2021. Technology of functional bread using buckwheat flour. IOP Conference Series: Earth and Environmental Science 640 (2):022002. doi: 10.1088/1755-1315/640/2/022002.
- Thakur, P., K. Kumar, N. Ahmed, D. Chauhan, Q. U. Eain Hyder Rizvi, S. Jan, T. P. Singh, and H. S. Dhaliwal. 2021. Effect of soaking and germination treatments on nutritional, anti-nutritional, and bioactive properties of amaranth (Amaranthus hypochondriacus L.), quinoa (Chenopodium quinoa L.), and buckwheat (Fagopyrum esculentum L.). Current Research in Food Science 4:917–25. doi: 10.1016/j.crfs.2021.11.019.
- Tomotake, H., N. Yamamoto, H. Kitabayashi, A. Kawakami, J. Kayashita, H. Ohinata, H. Karasawa, and N. Kato. 2007. Preparation of tartary buckwheat protein product and its improving effect on cholesterol metabolism in rats and mice fed cholesterol-enriched diet. Journal of Food Science 72 (7):S528–S33. doi: 10.1111/j.1750-3841.2007.00474.x.
- Torbica, A., M. Hadnađev, and T. Dapčević. 2010. Rheological, textural and sensory properties of gluten-free bread formulations based on rice and buckwheat flour. Food Hydrocolloids. 24 (6-7):626–32. doi: 10.1016/j.foodhyd.2010.03.004.
- Vetrani, C., L. Bozzetto, M. Giorgini, L. Cavagnuolo, E. Di Mattia, P. Cipriano, A. Mangione, A. Todisco, G. Inghilterra, A. Giacco, et al. 2019. Fibre-enriched buckwheat pasta modifies blood glucose response compared to corn pasta in individuals with type 1 diabetes and celiac disease: Acute randomized controlled trial. Diabetes Research and Clinical Practice 149:156–62. doi: 10.1016/j.diabres.2019.02.013.
- Vilcāns, M., J. Volkova, and Z. Gaile. 2011. Buckwheat yield depending on sowing type, time and rate. In Ražas svetki “Vecauce - 2011.” Jelgava, Latvia: Latvijas Lauksaimniecības Universitāte (LLU), 62–5.
- Wang, G., Q. Chen, Y. Yang, Y. Duan, and Y. Yang. 2022. Exchanges of economic plants along the land silk road. BMC Plant Biology 22 (1):619. doi: 10.1186/s12870-022-04022-9.
- Wang, J., Y. Wu, M. Han, X. Lei, J. Leng, Q. Yang, P. Yang, and J. Gao. 2023. Effect of environment and variety on the physicochemical properties of Tartary buckwheat starch. Journal of the Science of Food and Agriculture 103 (5):2413–24. doi: 10.1002/jsfa.12446.
- Wang, L., L. Wang, A. Wang, J. Qiu, and Z. Li. 2021a. Effects of superheated steam on starch structure and physicochemical properties of buckwheat flour during storage. Journal of Cereal Science 99:103221. doi: 10.1016/j.jcs.2021.103221.
- Wang, R., M. Li, S. Chen, Y. Hui, A. Tang, and Y. Wei. 2019. Effects of flour dynamic viscosity on the quality properties of buckwheat noodles. Carbohydrate Polymers 207:815–23. doi: 10.1016/j.carbpol.2018.09.048.
- Wang, R., M. Li, Y. Wei, B. Guo, M. Brennan, and C. S. Brennan. 2021b. Quality differences between fresh and dried buckwheat noodles associated with water status and inner structure. Foods (Basel, Switzerland) 10 (1):187. doi: 10.3390/foods10010187.
- Wang, X., D. Fan, and T. Zhang. 2017. Effects of hydrothermal processing on rutin retention and physicochemical properties of Tartary buckwheat enriched dough and Chinese steamed bread. International Journal of Food Science & Technology 52 (10):2180–90. doi: 10.1111/ijfs.13497.
- Wang, Z., Z. Zhang, Z. Zhao, G. Wieslander, D. Norbäck, and I. Kreft. 2004. Purification and characterization of a 24 kDa protein from tartary buckwheat seeds. Bioscience, Biotechnology, and Biochemistry 68 (7):1409–13. doi: 10.1271/bbb.68.1409.
- Wefers, D., and M. Bunzel. 2015. Characterization of dietary fiber polysaccharides from dehulled common buckwheat (Fagopyrum esculentum) seeds. Cereal Chemistry Journal 92 (6):598–603. doi: 10.1094/CCHEM-03-15-0056-R.
- Wijngaard, H. H., and E. K. Arendt. 2006. Buckwheat. Cereal Chemistry Journal 83 (4):391–401. doi: 10.1094/CC-83-0391.
- Wronkowska, M., A. Jarmułowicz, G. Lamparski, T. Jeliński, and C. M. Haros. 2020. Oat–buckwheat breads – technological quality, staling and sensory properties. Irish Journal of Agricultural and Food Research 59 (1):33–41. https://www.jstor.org/stable/27041754.
- Wronkowska, M., H. Zieliński, B. Szmatowicz, A. Ostaszyk, G. Lamparski, and A. Majkowska. 2019. Effect of roasted buckwheat flour and hull enrichment on the sensory qualities, acceptance and safety of innovative mixed rye/wheat and wheat bakery products. Journal of Food Processing and Preservation 43 (8):e14025. doi: 10.1111/jfpp.14025.
- Wu, W., Z. Li, F. Qin, and J. Qiu. 2021. Anti-diabetic effects of the soluble dietary fiber from tartary buckwheat bran in diabetic mice and their potential mechanisms. Food and Nutrition Research 65:4998. doi: 10.29219/fnr.v65.4998.
- Wu, W., L. Wang, J. Qiu, and Z. Li. 2018. The analysis of fagopyritols from tartary buckwheat and their anti-diabetic effects in KK-Ay type 2 diabetic mice and HepG2 cells. Journal of Functional Foods 50:137–46. doi: 10.1016/j.jff.2018.09.032.
- Xiang, D., L. Zou, L. Peng, G. Zhao, Y. Fan, S. Wei, C. Song, X. Liu, and J. Hailai. 2014. Appropriate mechanical sowing depth and soil-covering thickness improving seedling quality of tartary buckwheat. Nongye Gongcheng Xuebao/Transactions of the Chinese Society of Agricultural Engineering 30 (12):26–33. doi: 10.3969/j.issn.1002-6819.2014.12.003.
- Xiao, Y., K. Li, H. Zhang, Y. Li, L. Han, H. Liu, and M. Wang. 2022. The profile of buckwheat tannins based on widely targeted metabolome analysis and pharmacokinetic study of ellagitannin metabolite urolithin A. LWT 156:113069. doi: 10.1016/j.lwt.2022.113069.
- Xu, Q., L. Wang, W. Li, Y. Xing, P. Zhang, Q. Wang, H. Li, H. Liu, H. Yang, X. Liu, et al. 2019. Scented Tartary buckwheat tea: Aroma components and antioxidant activity. Molecules 24 (23):4368. doi: 10.3390/molecules24234368.
- Yang, F., S-y Yu, Y. Wang, R-f Wang, and F. Jing. 2014. Prospective induction of peripheral neuropathy by the use of Tartarian Buckwheat. Journal of the Neurological Sciences 347 (1-2):155–8. doi: 10.1016/j.jns.2014.09.037.
- Yao, Y.-F., X.-Y. Song, G. Xie, Y.-N. Tang, A. H. Wortley, F. Qin, S. Blackmore, C.-S. Li, and Y.-F. Wang. 2023. New insights into the origin of buckwheat cultivation in southwestern China from pollen data. The New Phytologist 237 (6):2467–77. doi: 10.1111/nph.18659.
- Yasuda, T., and H. Nakagawa. 1994. Purification and characterization of the rutin-degrading enzymes in tartary buckwheat seeds. Phytochemistry 37 (1):133–6. doi: 10.1016/0031-9422(94)85012-7.
- Yeşil, S., and H. Levent. 2022. The influence of fermented buckwheat, quinoa and amaranth flour on gluten-free bread quality. LWT 160:113301. doi: 10.1016/j.lwt.2022.113301.
- Yuzvenko, L. V., O. Lozova, O. Kvasko, Y. Sindarovska, L. F. Didenko, N. Y. Spivak, V. Shevchuk, and O. B. Veisz. 2011. Properties of buckwheat burn virus. Paper read at Climate change: Challenges and opportunities in agriculture. AGRISAFE Final Conference, at Budapest, Hungary.
- Zhang, H. W., Y. H. Zhang, M. J. Lu, W. J. Tong, and G. W. Cao. 2007. Comparison of hypertension, dyslipidaemia and hyperglycaemia between buckwheat seed-consuming and non-consuming Mongolian-Chinese populations in Inner Mongolia, China. Clinical and Experimental Pharmacology & Physiology 34 (9):838–44. doi: 10.1111/j.1440-1681.2007.04614.x.
- Zhang, Q., and J.-G. Xu. 2017. Determining the geographical origin of common buckwheat from China by multivariate analysis based on mineral elements, amino acids and vitamins. Scientific Reports 7 (1):9696. doi: 10.1038/s41598-017-08808-y.
- Zielińska, D., M. Turemko, J. Kwiatkowski, and H. Zieliński. 2012. Evaluation of flavonoid contents and antioxidant capacity of the aerial parts of common and Tartary buckwheat plants. Molecules (Basel, Switzerland) 17 (8):9668–82. doi: 10.3390/molecules17089668.
- Zieliński, H., J. Honke, N. Bączek, A. Majkowska, and M. Wronkowska. 2019. Bioaccessibility of D-chiro-inositol from water biscuits formulated from buckwheat flours fermented by lactic acid bacteria and fungi. LWT 106:37–43. doi: 10.1016/j.lwt.2019.02.065.