Abstract
Bacteriophages (phages), highly prevalent in aquatic and terrestrial environments, have emerged as novel antimicrobial agents in food and agricultural systems. Owing to their efficient and unique infection mechanism, phages offer an alternative to antibiotic therapy as they specifically target their host bacteria without causing antibiotic resistance. However, the real-world applications of phages as antimicrobials are still limited due to their low survivability under harsh conditions and reduced antimicrobial efficacy. There is an unmet need to understand the challenges of using phages in food and agricultural systems and potential strategies to enhance their stability and delivery. This review overviews the challenges of using phages, including acidic conditions, improper temperatures, UV-light irradiation, desiccation, and inefficient delivery. It also summarizes novel strategies such as encapsulation, embedding, and immobilization, which enable improved viability and enhanced delivery. The protein capsid and nucleic acid components of phages are delicate and sensitive to physicochemical stresses. Incorporating phages into biocompatible materials can provide a physical barrier for improving phage stability and enhancing phage delivery, resulting in a high antimicrobial efficacy. In conclusion, the development of phage delivery systems can significantly overcome the challenges associated with phage treatments and reduce the risk of foodborne diseases in the industry.
1. Introduction
A bacteriophage (also known as a phage) is a virus that can specifically infect its host bacteria. Phages are extremely abundant in aquatic and terrestrial environments, with an estimated population of more than 1031 phage particles contained on earth. They can be found anywhere bacteria thrive, such as in ocean water (Bonnain, Breitbart, and Buck Citation2016), lands (Takahashi et al. Citation2011), animals (Hammerl et al. Citation2021), humans (Kim et al. Citation2008), and foods (Pereira et al. Citation2007).
Since the first discovery of phages recorded in the nineteenth century by Hankin (Citation1896), phages have been characterized and categorized based on their host preference, genome types, morphology, and auxiliary structures (Ackermann Citation2009). Phages have three basic structural forms: an icosahedral head with a tail, an icosahedral head without a tail, and a filamentous form (Orlova Citation2012). The abundance of morphological distribution presents temporal and/or spatial patterns, but at least 96% of examined phages are tailed, constituting the order Caudovirales and three main families (Myoviridae, Siphoviridae, and Podoviridae) (reviewed in Ackermann Citation2003; Ackermann Citation2001). Their genomes are packaged in a prism-shaped head, surrounded by a protein capsid connected to the elongate sheath by a neck or collar region. The sheath creates a hollow tube used for injecting the viral genomes into the host cells. Furthermore, a baseplate located at the bottom of the sheath holds several contractile or noncontractile tail fibers (typically six), which facilitate the adhesion and attachment of phages to their host cells. Based on the way to infect host bacteria, phages can be classified into two categories. One is called lytic or virulent phages, which hijack the host to replicate DNA, synthesize proteins, assemble new phage particles, and immediately lysis bacteria to release progeny. A widely-studied example, coliphage, shows that each lytic cycle lasts around 40 mins and can end when the host cell bursts, producing about 100 new phages (Campbell Citation2003). The other group is called lysogenic phages or temperate phages. Instead of rapid lysis, lysogenic phages can integrate their genomes into the bacterial chromosome as a prophage or exist as a plasmid while not destroying their host bacteria. The incorporated phage genomes will be replicated along with the host bacterial genome, so a new generation of bacteria will inherit the viral genome. Such viral DNA transition can stably occur through multiple generations of host bacteria. Under certain conditions, however, lysogenic phages can be induced to trigger a lytic cycle, resulting in a release of fully assembled phages (Jancheva and Böttcher Citation2021).
Due to their unique constitution and replication mode, phages have potential roles in biotechnological exploitation and antimicrobial approaches. Basic phage research has contributed sophisticated tools with wide-ranging biotechnological utility, such as clustered regularly interspaced short palindromic repeats (CRISPR)-Cas systems (Guan et al. Citation2022), biosensor (Nanduri et al. Citation2007) and phage display (Smith Citation1985). Moreover, emerging antibiotic resistance, coupled with a bottleneck of discovering new antibiotics, has urged alternative antimicrobial strategies in medicine, agriculture, and food industries. Phage therapy using strictly lytic phages is one of the new therapeutic strategies for infectious diseases, showing promising progress in addressing antimicrobial resistance (Kolenda et al. Citation2022). In the food and agriculture systems, extensive use of antibiotics and synthetic antimicrobial chemicals are relatively effective in controlling foodborne diseases. However, both synthetic antibiotics and antimicrobial chemicals have caused antibiotic/biocide resistance that threatens human health and environmental sustainability (Rodriguez-Mozaz et al. Citation2015). Lytic phages have been recognized as an excellent alternative to synthetic antibiotics and antimicrobials because of many incomparable advantages. Firstly, phages possess high specificity and only inactivate their host bacteria. The infection process is triggered by specific recognition between phage receptor binding proteins located at the tip of tails and bacterial cell surface receptors, such as protein receptors, lipopolysaccharide receptors, and receptors at capsular polysaccharides, pili, and flagella (reviewed in Rakhuba et al., Citation2010). The high specificity allows a selective inactivation of target bacteria without disturbing the commensal beneficial microbiota, which is highly required to protect healthy intestinal microflora during animal treatments (Hu et al. Citation2018). Secondly, phages are endowed with the ability to evolve, circumventing bacterial antiviral mechanisms. The continuous adaptation of phages relieves the risk of developing anti-phage resistance in bacterial cells (Borin et al. Citation2021). The use of phage cocktails (a mixture of various phages) can also reduce phage-resistant mutants’ occurrence (Pereira et al. Citation2016; Yuan et al. Citation2019). Phages can self-replicate and self-limit, depending on the presence or absence of bacteria (Sillankorva, Oliveira, and Azeredo Citation2012). This characteristic provides phages not only with increased safety but also with enduring environmental and economic sustainability. Last but not least, phages can spread within biofilms if they carry the polysaccharide depolymerase, which disrupts those obstinate biofilms by degrading the extracellular polymeric substance (Lu and Collins Citation2007). In contrast, temperate phages are not only ineffective at killing bacteria but also bringing a potential risk for transferring viral genes among the bacterial population and creating new pathogenic strains (Moye, Woolston, and Sulakvelidze Citation2018). Therefore, this review will only focus on the lytic phage because it is more applicable to controlling foodborne pathogens.
The success in using phages as antimicrobial agents has led to numerous commercial products in food, agricultural, biomedical, and clinical applications. The Environmental Protection Agency (EPA) approved the safety and bactericidal effectiveness of Agriphage products that treat bacterial spots in different crops (Cole Citation2005). In addition, ListShield™ has been approved by the U.S. Food and Drug Administration (FDA) as a food additive and considered generally recognized as safe (GRAS) status, which is the first commercialized phage product for directly using on ready-to-eat (RTE) food (Intralytix, n.d). Furthermore, Phageguard L™ (former Listex™P100) has been approved as clean label processing aid in several countries, such as the USA, Canada, Australia, New Zealand, and Switzerland (Phageguard, n.d). More commercially available phage products are summarized in . It’s worth mentioning that phage production is cost-effective compared to the research and development of antibiotics. Antibiotic development often requires investments of millions of dollar s and substantial time for innovating new drugs. Developing a single targeted antibiotic is estimated to cost around US$1,500 million (Towse et al. Citation2017), whereas the process of isolating, propagating, and producing phages comes at a reduced cost. For instance, the production costs for producing a phage cocktail containing six Salmonella-specific phages used in the poultry industry were analyzed to be US$1,422 per dose and US$0.03 per chicken (Torres-Acosta et al. Citation2019). The use of lytic phages as cost-effective antimicrobials holds an appealing promise.
Table 1. Commercial bacteriophage products for biocontrol applications
Phage-based antimicrobial treatments can follow different routes, which involve applying/spraying phage suspension directly (Liu et al. Citation2020; Thung et al. Citation2017) or incorporating phages into various matrices such as carriers, coatings and packaging materials (Radford et al. Citation2017; Vonasek et al. Citation2018). Regarding agricultural systems, phages as an alternative to antibiotics have been used in the livestock farming industry to control bacterial disease, where phages are given through oral administration (Clavijo et al. Citation2019; Stanford et al. Citation2010). Although this is a practical approach to feeding animals with a relatively high concentration of phages, the treatment effectiveness still highly depends on phage stability and delivery efficiency in the gastrointestinal tract (GI tract) of animals (Atterbury et al. Citation2007). Regarding spray systems, some extrinsic factors, such as harsh environments and physical barriers, affect phage survivability and resistance in both food and agriculture systems. More details on the challenges of phage applications will be discussed in the following section.
This review provides an overview of the challenges of using phages as antimicrobial agents and summarizes the novel strategies to overcome these challenges and enable the efficient delivery of phages in complex food and agricultural systems. While we maintained our primary focus on food and agricultural production, we also covered some advanced techniques in other fields, such as biomedical applications.
2. Challenges in using phages as antimicrobial agents
2.1. Influence of the environmental factors on phage viability
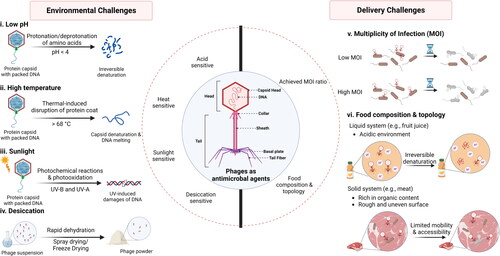
Despite the significant benefits of phage-based antimicrobial treatments, barriers hinder the application of phages in food and agriculture areas (). Typically, an entire virus particle consists of an outer protein shell called a capsid and an inner core of nucleic acid (either RNA or DNA). The core confers infectivity, and the capsid provides specificity to the virus. However, both components, protein capsid and nucleic acid, are delicate and sensitive to various physicochemical stresses (Burrell, Howard, and Murphy Citation2017). Typically, these stresses are environmental factors in the food and agricultural systems, such as low pH, high temperature, UV irradiation, moisture content, and so on ().
Table 2. Effect of pH, temperature and UV irradiation on the stability of phages of foodborne pathogen
2.1.1. Low pH
Environmental acidity is one of the harshest factors influencing phage stability. The optimal pH value for phages to maintain a high activity ranges from pH 7 to pH 10 (Jurczak-Kurek et al. Citation2016; Shahin et al. Citation2021). Although phages have varied tolerance to acids depending on their characteristics, such as phage morphology, most are susceptible when the pH value is below 4 (Jurczak-Kurek et al. Citation2016). Phage survivability depends highly on the capsid proteome, whose conformation can be modulated by changing pH conditions. The ionizable side chain of some amino acids, such as Asp, Glu, His, Lys and Arg, changes through protonation or deprotonation when the environmental pH is lower or more significant than their isoelectric point (pI). According to a review article by Zhou and Pang (Citation2018), such electrostatic interaction affects protein folding status, and enormous repulsion occurs between protein molecules when ample charged amino acids are protonated or deprotonated, resulting in an irreversible denaturation. For instance, it has been reported that the precipitation of T2 phages was observed when exposed to the buffer with a pH below 4, and rapid coagulation occurred at pH 2, indicating the protein denaturation in the presence of a high concentration of hydrogen ions (Sharp et al. Citation1946).
Such acid intolerance significantly limits the applications of phages in food processing. Many food systems may have a relatively low pH because of indigenous acids (e.g., citrate, malate and ascorbate) or added acids (e.g., hydrochloric acid, sulfuric acid and nitric acid) (Saltmarsh Citation2020). Most fermented foods and fruits have enough edible acids to have a pH below 4.6 (Price et al. Citation2020). Dairy products such as cheese are typical examples of acidic foods. Bueno et al. (Citation2012) reported that the viability of a phage cocktail (phi-IPLA35 and phi-IPLA88) dramatically decreased from 108 to 102 log (PFU/mL) because the pH dropped from 6 to 4 during fresh-type cheese manufacturing. In contrast, during the ripening of hard-type cheeses, the phage population was stable at around 107 PFU/mL because a constant acidic level was maintained at pH 5 (Bueno et al. Citation2012). Acidity is a vital constituent of fleshy fruit taste that is attributed to malic acid and citric acid, the major organic acids found in fruit (Tucker Citation1993). The respiratory rate determines the levels of acids accumulated in ripened fruits, while most edible fruits have pH values that fall from pH 3 to pH 5. High-acid fruit can weaken the antimicrobial efficacy of phages. Oliveira et al. (Citation2014) used Listex™P100 to treat microbial contamination on different fresh-cut fruits. The results suggest phage treatment was more effective on melon slices followed by pears but had no significant effect on apple slices. This was because melons had a higher pH value between 5.77-5.92 compared with 4.61-4.91 on pears and below 4 on apples (Oliveira et al. Citation2014). The different pH levels of fruits are caused by their different respiration behaviors (Tucker Citation1993). Moreover, the GI tract of humans and animals is also a typical acidic aqueous system, as the stomach is a reservoir of strong acids required to inactivate enzymes that facilitate digestion. The addition of phages into animal feeds as an alternative to antibiotics has attracted extensive interest. However, the acidity of the gastric environment of most animals (pH 2-2.5) is a major challenge for effectively delivering phages to the intestine, limiting the wide applications of phage-based feed supplements (Lorenzo-Rebenaque et al. Citation2021; Yin et al. Citation2021). The extremely low pH conditions can significantly reduce phage titer and proliferation, and an aqueous system facilitates phage diffusion and distribution, thus promoting acid-induced denaturation (Guenther et al. Citation2009). However, some in vivo studies suggest that phages may pass through the GI tract because of the buffering effect of a meal (Ma et al. Citation2016). Some other studies believe that survival of phages mainly depends on propagation, which outweighs phage loss during the GI tract, rather than surviving (Bardina et al. Citation2012; Dąbrowska Citation2019; Wagenaar et al. Citation2005). Ideally, the potential of phages to propagate in the gut can help phage recovery and compensate for viability loss due to stomach acidity (Dąbrowska Citation2019). However, many in vivo studies failed to present a significant efficacy by feeding phages to animals due to the absence of a suitable bacterial host in the GI tract (Bardina et al. Citation2012; Wagenaar et al. Citation2005) or insufficient phages being delivered to bacterial colonization locations (Lorenzo-Rebenaque et al. Citation2021; Ma et al. Citation2016). The propagation requires live phages having prolonged retention time within the GI tract and being delivered to the target site of a host because bacterial cells are usually lysed after 25-30 min of infection (Kutter, Guttman, and Carlson Citation1994). For example, the broiler crop with neutral pH condition acts as a reservoir, so the prolonged phage retention in this site can facilitate sustained exposure to a new susceptible host, allowing phage to propagate and continuously supplying the gut with more phages (Ma et al. Citation2016). Although factors leading to a failure treatment are abundant and interactive, the target delivery and controlled release are still critical for an efficient phage treatment.
In summary, the reduced survivability of phages under low pH conditions has caused a significant technical barrier for phage-based antimicrobial applications. Therefore, enhancing phage stability using rigid carriers as a physical barrier or using acidity neutralization compositions is urgently needed.
2.1.2. Temperature
Temperature is another important factor influencing phage viability because high temperature induces capsid denaturation and DNA melting. Jurczak-Kurek et al. (Citation2016) investigated the biological properties of diverse phages and suggested a significant reduction of phage titer occurring after 40 min exposure to a temperature higher than 62 °C. The thermal sensitivity of different phages reported in previous studies is summarized in . Generally, heat-induced denaturation of protein capsid follows the first-order transition from the native state to a more disordered state, characterized by unfolded protein structures (reviewed in Lepock Citation2004). Referring to Poland-Scheraga type models, DNA molecules compose alternating sequences of bound and denaturized states during the thermal denaturation, and the excluded-volume interactions between denatured loops are sufficient to drive the first-order transition (Kafri, Mukamel, and Peliti Citation2000). The hydrophobic effect from non-polar amino acids is an important driving force for protein folding, while buried hydrophobic residues are normally exposed after thermal denaturation, resulting in aggregation due to the increased protein-protein interactions (reviewed in Lepock Citation2004). The melting profiles of phage nucleoproteins have been recorded at the heating rate of 0.5 °C/min and present two structural transitions: the first transition takes place around 50-65 °C, and the second transition occurs at a higher temperature ranging from 83-90 °C (Egyeki et al. Citation2003; Tóth et al. Citation1984). The first transition phase is associated with an irreversible loss of the higher-order DNA structure due to the protein coat’s thermal-induced disruption, resulting in a subsequent release of DNA from the protein capsid head (Fekete et al. Citation1982). The denaturation of DNA helix-coil structure predominates the second transition phase (Egyeki et al. Citation2003; Fekete et al. Citation1982; Tóth et al. Citation1984). Recent studies reported that the DNA could escape from the protein capsid at 68 °C due to the disrupted phage tail, and the melting of the protein capsid occurs at 87 °C (Qiu Citation2012). These findings have been supported by many studies summarized in , which suggest that most phages can be eradicated even when the temperature rises to 60 °C.
The thermal instability of phages limits their applications in food processing, such as pasteurization, baking, and cooking. The conventional thermal pasteurization process refers to the heat treatment of liquid food below 100 °C (usually between 65-80 °C), which can destroy pertinent target pathogens and reduce the initial spoilage organisms (Fellows Citation2017). Furthermore, the recommended internal temperature for cooking food (especially meat products) should be higher than 60 °C to inactivate foodborne pathogens, such as Salmonella, E. coli O157:H7, Listeria monocytogenes, and so on (Jarvis et al. Citation2016; Kim et al. Citation2019). Since some commercial phage products have been approved to be used on food, applying phages as a preservative-free aid is attractive. They are considered a promising agent for suppressing the growth of spoilage bacteria in beverage and food matrices, thus extending product shelf-life (Brovko, Anany, and Griffiths Citation2012). However, this may require phages to be added to food products before processing. As one of the most frequently used food processing methods, thermal processing requires a high temperature that significantly diminishes the viability of the added phages. For example, Ahmadi et al. (Citation2017) investigated the stability of commercially available Listeria-specific phages in a heating-holding-cooling process that simulates the preparation procedure of RTE meats. The P100 phage titer showed about 9-log (PFU/mL) reduction during temperature raising from 4 °C to 71 °C, and the final phage concentration was below the detection limit (1 log PFU/mL) after 30 s of holding at 71 °C. The A511 phage viability decreased by about 6 log (PFU/mL) after the temperature was raised to 75 °C, and complete inactivation was recorded after 30 s holding time. Even though their activity showed partial recovery during the cooling stage (at 4 °C), when the heating temperature was increased to 85 °C and held for the same period, the phages failed to resume their activity after cooling (Ahmadi et al. Citation2017). Another example is the inactivation of HU1 phages in raw milk after pasteurization (63 °C for 30 min) (Tanaka et al. Citation2018).
In addition, the efficacy of phage-based antimicrobial treatment can be affected by low temperatures. This is because the occurrence of phage propagation prefers a narrow spectrum of temperatures, depending on a symbiotic relationship between phages and their host bacteria. Changes in the environmental temperature affect the host’s metabolism, thereby influencing DNA replication, protein synthesis, and virion assembly (Zaburlin, Quiberoni, and Mercanti Citation2017). In general, phages can be categorized into three physiological types based on the effect of temperature on their efficiency of plating: (a) high-temperature (HT) phages, plating at or above 25 °C; (b) low-temperature (LT) phages, plating at or below 30 °C; and (c) mid-temperature (MT) phages, plating in the range of 15-42 °C (Jurczak-Kurek et al. Citation2016; Seeley and Primrose Citation1980). It has been demonstrated that a temperature lower than optimum suitable for phage propagation will reduce the efficiencies of phage plaquing (Tokman et al. Citation2016) and infection of host bacteria (Zaburlin, Quiberoni, and Mercanti Citation2017). Guo et al. (Citation2021) compared the antimicrobial efficacy of phages against Salmonella spp. at 25 °C and 4 °C. The results demonstrate the slightly stronger antimicrobial activity of phages at 25 °C compared to 4 °C, because the metabolic activity of host bacteria was greatly suppressed at refrigeration temperature (Guo et al. Citation2021). Under lower temperatures, an extended period of phage treatment may be required to inactivate their host bacteria. For example, commercial phage (EcoShield™) suspension was used to treat fresh lettuce for 2 min, and the treated lettuce was stored at refrigerated condition (4 °C) for 7 days. The population of E. coli O157:H7 started to slowly decline 24 to 48 h after the phage treatment (Ferguson et al. Citation2013). Apart from suppressed metabolism of host cells, the low temperature can interfere with phage assembly, which blocks phage propagation. Many empty or partially filled capsids of T4 were observed in lysate at 19 °C, which implies that the process of DNA packaging can be inhibited at a relatively low temperature, resulting in fragmentary phages (Yanagida, Suzuki, and Toda Citation1984). Therefore, to achieve a high phage antimicrobial efficacy, the potential impediments come from both high and unfavorable temperatures.
In short, a narrow spectrum of temperature is preferred by phages. A high temperature significantly influences phage viability by causing protein denaturation and DNA melting, while a low temperature may reduce the treatment efficacy due to the decreased metabolic activity of host bacteria or inhibited phage assembly. Therefore, novel strategies would be needed to insulate fragile phages from the physiological stresses caused by temperature changes.
2.1.3. Ultraviolet irradiation
Among all the environmental factors affecting phage viability, sunlight is the most destructive because ultraviolet (UV) irradiation can inactivate phages within a few minutes (John Hudson et al. Citation2010; Kim et al. Citation2018; Ramirez et al. Citation2018). The reduced phage viability under UV irradiation is predominantly caused by DNA damage. Ultraviolet radiation can be separated into three wavelength ranges: UV-A (315-400 nm), UV-B (280-315 nm), and UV-C (200-280 nm). Generally, the shorter the wavelength, the more damage it can cause. It has been reported that photo-induced damages are more severe under UV-C irradiation (Masuma et al. Citation2013). However, most UV-C irradiation from the sunlight is absorbed by stratospheric gases (ozone), and very little can reach the earth’s surface. UV-B is a major ultraviolet portion of sunlight reaching the terrestrial surface, which is only partially absorbed by the ozone layer (Blaustein and Searle Citation2013). UV-B irradiation can cause DNA lesions by photochemical reactions of UV-B photons and components, resulting in conformational changes that break DNA strands (Slieman and Nicholson Citation2000). As the preferential target of UV-B-induced reactions on DNA, pyrimidine constituents can form two significant classes of detrimental photoproducts: cyclobutene pyrimidine dimers and pyrimidine (6-4) pyrimidone adducts (reviewed in Ravanat, Douki, and Cadet Citation2001). In addition, the UV-A portion from the sunlight can result in cellular damage caused by photooxidation reactions, although DNA bases cannot absorb UV-A light (reviewed in Ravanat, Douki, and Cadet Citation2001). The UV-A mediated DNA damage is predominated by the formation of singlet oxygen (1O2) and electroactive compounds (8-oxo-7,8-dihydro-2′-deoxyguanosine, 8-oxodGuo) (reviewed in Ravanat, Douki, and Cadet Citation2001). In addition to DNA damage, UV irradiation also causes damage to other components of phages, such as protein capsid and self-repairability. However, the mechanisms are still ambiguous (Rodriguez et al. Citation2014).
The low resistance of phages to UV irradiation has hampered their applications as biocontrol agents in agriculture, such as greenhouses and field applications. A field study inoculated 100 mL of Salmonella serovars (107 CFU/mL) onto the flowers of tomato plants in week 7 after planting, and screened for the presence of Salmonella on the surface and internal tissue after harvest during the early breaker stage, which evidenced pathogens could be deposited on a blossom and bacterial colonization occurred both externally and internally in the subsequent fruits (Shi et al. Citation2007). Moreover, 100 mL of Salmonella (107 CFU/mL) was inoculated onto the blossom of tomato in the presence or absence of a phage cocktail (107 PFU/mL). After 7 wk of development, the levels of pathogens and phages were determined from the fruits’ surface rinse and internal tissues (Ye et al. Citation2009). Their results suggested that Salmonella was found internally in 43% of the fruits from untreated plants but only 19% from the plants treated with phages. However, the bacteria were recovered from 92% and 83% of the surface rinse solutions taken from tomatoes derived from the untreated plants and the plants treated with phage. Although the prevalence of Salmonella inside tomatoes was reduced by the phage treatment, the effect of phage treatment on reducing surface contamination was very limited because of the UV radiation from sunlight that significantly influenced phage viability. The impact of sunlight on phage treatment has been highlighted by Flaherty et al. (Citation2000), where the best time for spraying phage suspensions to reduce the incidence of bacterial spots in tomato plants was designed to be at or before daybreak to evade sunlight exposure (Flaherty et al. Citation2000). A similar finding was reported by Iriarte et al. (Citation2007), in which the efficacy of phage treatment decreased by 80% with an increase in UV light intensity from 0 to 8 mJ/cm2 (Iriarte et al. Citation2007). Therefore, shielding phages from UV irradiation is important to enhance the phage survivability and improve the treatment efficacy as a biocontrol approach in the pre-harvest stage.
2.1.4. Desiccation
Apart from the aforementioned environmental factor, the phage activity of liquid formulation remains stable for one year of storage under refrigerated temperature (Merabishvili et al. Citation2013). Turning phage suspension into powder form is an effective strategy that can maintain phage activity during long-term storage, and it is also convenient for long-trip transportation. Spray drying and freeze drying are mostly involved techniques for producing phage powder. However, the drying process is considered a detrimental condition for phages.
In spray drying, high inlet temperatures (exceeding 100 °C) are usually involved for atomization, ensuring the quality and stability of dried product due to the low residual moisture content (Erbay et al. Citation2015; Luna-Solano et al. Citation2005). The rapid dehydration caused by water evaporation under extremely high temperatures is a detriment to phages because removing water results in conformational changes in DNA (Hegedüs et al. Citation2006). Although a spray-drying process may occur in milliseconds, the thermal energy used to evaporate the liquid part is still intense for phages (Vandenheuvel et al. Citation2013). Furthermore, high shear forces involved in feeding suspension through the nozzle and atomizing the liquid into tiny droplets can also negatively influence the integrity of phage particles, inactivating phages (Vandenheuvel et al. Citation2013). Excipients acting as water-replacing agents, such as sugar and protein, should be used to protect phages from desiccation. For example, trehalose is the most frequently used excipient that provides thermal and dehydration protection by forming hydrogen bonds directly or vitrifying the protein, thus stabilizing the phage’s conformational structure (Vandenheuvel et al. Citation2014). A study shows sugar is the most important excipient for retaining the activity in spray-dried powder (Chang et al. Citation2017). More than 6-log phage reduction after spray drying was observed if sugar was absent, while phages showed less than 1-log reduction when spray dried with lactose or trehalose mixed with leucine as a protectant formulation (Chang et al. Citation2017). Freeze drying involves cooling phage suspension, forming ice crystals of pure water, and sublimating, which may involve two mechanisms influencing phage stability: osmotic shock and dehydration (Shapira and Kohn Citation1974). Both freezing and drying stresses result in protein aggregation, irreversible structural changes, phage destabilization, and inactivation (Ergin Citation2022). A study revealed that reducing the moisture content to lower than 4% was detrimental to phage stability because the non-frozen water adsorbed to the phage tail or head might be removed, leading to significant titer reduction after drying (Puapermpoonsiri, Ford, and Van Der Walle Citation2010). The rapid change in osmotic pressure during the cooling process may cause phage DNA to extrude from the tail or heads to break, especially when the cooling rate is low. Similar to spray drying, stabilizers acting as cryo/lyo-protectant are responsible for increasing phage survivability during the freeze-drying process. Sugars like trehalose protect phages by preventing water crystallization during dehydration-rehydration cycles, and also forming a protein-matrix interface of hydrogen bonds to stabilize phage’s protein capsid (Petsong, Benjakul, and Vongkamjan Citation2021). In order to improve phage stability during lyophilization, three lytic phages were subjected to freeze-drying protecting by six different excipients: glucose, sucrose gelatin, mannitol, polyethylene glycol and sorbitol (Manohar and Ramesh Citation2019). The results showed sucrose, gelatin and their mixture effectively improved phage stability during lyophilization, which maintained the phage viability with less than 1-log reduction after drying (Manohar and Ramesh Citation2019). Thus, drying techniques elongate phage storability but desiccation normally take place with some unfavorable conditions threating phage viability.
2.2. Impacts of multiplicity of infection (MOI) on antimicrobial efficacy
Phage-based biocontrol strategies can be divided into two categories: passive and active approaches, depending on the kinetics of phage replication (Gill Citation2010). For the passive approach, an abundant quantity of phages are added into the system, and the host bacteria will be killed mainly by primary infection or lysis. The antimicrobial action may become overwhelming and speedy as sufficient phages are supplied. In contrast, the active approach relies on phage replication in their host bacteria and requires a relatively lower phage population (Gill Citation2010). In this process, most of the host bacteria will be inactivated by secondary infection, and multiple generations of phages are usually needed. More specifically, the antimicrobial efficacy of the active approach depends on the number of phages being able to spread between susceptible bacterial hosts. Phage mobility is influenced by the physical conditions of the surrounding environment such as viscosity, which could be increased due to the presence of overnumbered host bacteria. From the perspective of microbial inactivation in food applications, rapid and effective inactivation is highly demanded, which suggests that the passive strategy is preferred. To achieve a high infection rate, the attachment rate of phages onto a susceptible host cell plays an important role since this is the first step of phage infection (Shao and Wang Citation2008). The phage adsorption rate to a host cell is governed by two factors: the multiplicity of infection (MOI) and the random distribution of phage adsorption events across bacteria (Gill Citation2010). A low concentration of host bacterial cells can delay phage replication, and a bacterial concentration of 104 CFU/mL is considered the minimum density required for phage replication, which is named as ‘replication threshold’ (Wiggins and Alexander Citation1985). However, Kasman et al. (Citation2002) argued about the concept of replication threshold density of the host and suggested that the phage adsorption rate can be accurately predicted using the Schlesinger mathematical model (Schlesinger Citation1932). In this mode, phage adsorption can be influenced by four factors: the density of host cells, the adsorption constant of the phage, the number of phages, and the time of bacteria-phage interaction. The definition of MOI has been further refined to MOIinput and MOIactual, while the former indicates the simple ratio of input phage to input bacterial cells, and the latter strictly indicates the number of phages calculated to infect bacteria instead of merely added into the system (Kasman et al. Citation2002). Based on the concept of MOIactual, Bigwood, Hudson, and Billington (Citation2009) further stated that by giving a sufficiently high concentration of initial phages (107-108 PFU/mL), the inactivation of bacteria with low density (<104 CFU/mL) in liquid foods is achievable regardless of knowing the concentration of host cells (Bigwood, Hudson, and Billington Citation2009). Phages with the MOI ratio of 104 inactivated a Salmonella liquid culture with more than 6-log reduction after 2-h treatment at 37 °C, while the reduction rate and lytic activities of phages were diminished at MOI of 103 and 102, with 4.5-log and 3-log reduction after 5-h infection, respectively (Bao et al. Citation2015). Apart from liquid culture, the antimicrobial ability of phages can also be enhanced with a high MOI ratio in food samples. Atterbury et al. (Citation2003) inoculated the combination of Campylobacter (102, 104 and 106 CFU/sample) and phages onto sections of chicken skin with MOIs ranging from 0.001 to 100,000. The highest-titer (107 PFU/sample) phage treatment inhibited bacterial growth on all samples regardless of bacterial concentration, reducing it by more than 1 log after 5 days of refrigeration. In contrast, under the same conditions there is no significant reduction in pathogen numbers observed with the lowest phage titer (103 PFU) (Atterbury et al. Citation2003). Therefore, an excessive population of phages is needed to guarantee an efficient biocontrol treatment.
Despite significant progress in exploring various types of phages against foodborne pathogens, including Salmonella (Islam et al. Citation2019), Listeria (Miguéis, Saraiva, and Esteves Citation2017), Shigella (Soffer et al. Citation2017), E. coli O157:H7 (Carter et al. Citation2012) and Campylobacter (Bigwood et al. Citation2008), to achieve a higher MOI value in complex food systems remains challenging. Many studies have demonstrated that the categories of foods lead to different efficacy of phage treatments due to varied MOIactual values. The physical topology of the food is critical for phage mobility and their adsorption to bacteria, affecting antimicrobial efficacy. It has been reported that phages destroy host bacteria with single-hit kinetics, suggesting that although multiple phages can adsorb to a single bacterium, only the first phage is the actual killer (Abedon Citation2011). Therefore, solid foods with large and uneven surface areas are more challenging to treat than liquid foods, where phages can diffuse much faster. Guenther and colleagues added aliquots of A511 phages with the final concentration of 3 x 108 PFU/g or mL as an antimicrobial agent against Listeria monocytogenes that was spiked (103 CFU/g or mL) to a range of RTE foods (Guenther et al. Citation2009). Under the same treatment conditions, the A511 phages were more effective in preventing the growth of bacteria in liquid foods such as chocolate milk and mozzarella cheese brine compared to fresh produce and meat products, with more than 5-log (CFU/g) reduction of L. monocytogenes after 6 days at 6 °C. The higher inactivation efficacy in liquid foods could be attributed to the mobility and accessibility of phages in an aqueous environment, leading to a higher MOIactual value. In addition, the results suggest the treatment efficacy on the surfaces of leafy greens was higher than sliced turkey breast and smoked salmon. This is due to the difference in surface properties between leafy greens and meat surfaces, such as surface roughness and hydrophobicity, which can physically shield bacteria from contact with phages, resulting in relatively lower MOIactual values in certain areas (Guenther et al. Citation2009).
In summary, the proportion of phages that can infect their host bacteria highly depends on several intrinsic factors of food (reviewed in Shannon, Radford, and Balamurugan Citation2020). Although phages possess higher mobility and accessibility in liquid foods, it is challenging and costly to achieve a high quantity of freely dispersed phages in an aqueous environment, which has been a major barrier to applying phages in large-scale applications.
3. Novel strategies to overcome challenges
3.1. Overview of phage delivery systems
Phage-based treatment is one of the most promising alternatives to conventional antibiotic-based approaches (Endersen and Coffey Citation2020). Among most pre-and post-harvest practices, those challenges significantly limit the phage stability and efficacy to inactivate pathogenic microbes. Phages have great potential for use in food and agricultural systems, including animal feeding supplements, preharvest disease control, postharvest sanitation of perishable foods, and antimicrobial coatings on food-contact surfaces. To realize these applications, a biocompatible carrier is needed to stabilize phages under harsh conditions and efficiently deliver them to the target site. An ideal phage delivery system used in food and agriculture systems would possess several unique features to facilitate an enhanced antimicrobial efficacy: (1) being composed of food-grade materials; (2) protecting phages from unfavorable conditions (e.g., acid, high temperature, UV irradiation, and desiccation); (3) localizing high concentration of phages to achieve a high MOI value; and (4) controlling the release of phages for prolonged antimicrobial efficacy. Recently, there has been a growing interest in the design of phage delivery systems (). and present novel methods for enhancing phage stability and delivery, respectively, including encapsulation, embedding, and immobilization.
Table 3. Strategies of phage incorporation that improves stability of phages in harsh encironments
3.1.1. Encapsulation
Encapsulation aims to create micro-sized capsules to entrap phages within a shell. These capsules can be spherical or irregular shapes with a size varying from nano (< 1 µm) to macroscale (> 1000 µm). Based on their structures, these capsules can be categorized into mononuclear, polynuclear, and core-shell structures. Numerous encapsulation techniques have been summarized in a previous review (Choińska-Pulit et al. Citation2015), in which the common methods used to encapsulate phages include extrusion, emulsification, liposomal encapsulation, and so on. Extrusion and emulsification are frequently utilized for encapsulating phages, providing robust protection against harsh conditions. Extrusion operation includes extruding the hydrocolloid solution containing phages (free-fall droplet via syringe needle) into a hardening solution or setting bath containing multivalent cations (usually Ca2+) (Krasaekoopt, Bhandari, and Deeth Citation2003). The hydrocolloid solution usually contains biocompatible materials, such as alginate, chitosan, whey proteins, and so on (Ma et al. Citation2012; Silva Batalha et al. Citation2021; Tang et al. Citation2013). The extrusion technique is highly adjustable. While spherical beads are produced, their size and quantity can vary with operational conditions and polymer concentration (Silva Batalha et al. Citation2021). However, the main disadvantages of extrusion include the difficulty of scaling up, low production rate, and relatively large particle size (reviewed in Choińska-Pulit et al. Citation2015). Similarly, emulsification can also be used to construct microspheres encapsulating phages. The formation of a water-in-oil (W/O) emulsion system involves adding a mixture of phages and hydrogel precursors into a non-miscible phase with vigorous stirring. The droplets formed can be solidified by hardening solutions (Gbassi and Vandamme Citation2012). Emulsification is more complicated than extrusion due to the risk of contamination and potential damage to phages caused by high-shear stress. Strict asepsis is required when adding emulsifiers to stabilize the emulsion. However, it is challenging to sterilize vegetable oils (reviewed in Choińska-Pulit et al. Citation2015). The droplet size of emulsions can be adjusted by changing the shear rate (Vinner, Richards, et al. Citation2019), but high shear force can negatively influence the viability of the phage particles (Vandenheuvel et al. Citation2013). Multiple emulsions called water-in-oil-in-water (W/O/W) with compartmentalized internal structures can be constructed, providing better protection for phages (Richards and Malik Citation2021a). Liposomal encapsulation involves entrapping phages into liposomes, which are sphere-shaped vesicles consisting of phospholipid bilayers (Akbarzadeh et al. Citation2013). The liposomal encapsulated phages can exhibit better performance during oral administration because adherence of liposomes to the intestinal mucosa enhances phage delivery in the GI tract (Colom et al. Citation2015). Other encapsulation techniques, such as spraying drying and freeze drying, produce powder-form encapsulated phages, which are convenient for distribution, and usually have an extended shelf-life in cool and dry storage (Carrigy et al. Citation2020; Puapermpoonsiri, Ford, and Van Der Walle Citation2010). However, these encapsulation types are not within this review’s scope due to the insufficient stability in harsh environments.
3.1.2. Embedding
Embedding refers to loading a mass of phages into a large biopolymer matrix. Typical examples of embedded phages are phage-based films or nanofibers (Alves et al. Citation2019; Korehei and Kadla Citation2013). Phage-based films as novel bioactive packaging materials attract lots of interest in the food and biomedical areas. Phages can be added to the film-forming solutions, which commonly consist of bioactive polymers such as proteins or polysaccharides, and then phage-incorporated films are formed during material manufacturing such as molding and casting (Amarillas et al. Citation2018; Radford et al. Citation2017; Vonasek, Le, and Nitin Citation2014). Electrospinning is a technique that produces nanofibers containing phages, which can be recognized as embedding and encapsulation. Phage-based nanofibrous mats can be further constructed, potentially as antimicrobial materials for food packages or biomedical usage (Korehei and Kadla Citation2013; Korehei and Kadla Citation2014; Sarhan and Azzazy Citation2017). In general, dried nanofibers are formed by ejecting a charged and molten polymer solution under a high-voltage electric field, where phages are added into liquid precursors before operation (Díaz et al. Citation2018). The drawback is that the high voltages and rapid evaporation of solvent may impair the phage survivability (Koo et al. Citation2016). In order to maintain a high phage titer, the coaxial electrospinning process was developed to produce core-shell electrospun fibers where phages were located in the core of the fibers (Korehei and Kadla Citation2014).
3.1.3. Immobilization
Immobilization is the creation of a phage coating on various surfaces via noncovalent or covalent bonds using physical (Patel, Zhou, and Ramasamy Citation2021), chemical (Xu et al. Citation2020), and genetic modification (Kadiri, Günther, and Fischer Citation2019) approaches. Unlike the encapsulation or embedding strategy, the immobilized phages are partially or entirely exposed to the external environment (reviewed in Rosner and Clark Citation2021). The understanding of the bonding mechanism is still limited, but some studies stated that the main driving force for the adsorption of phages could be electrostatic interaction, van der Waals forces, hydrophobic bonding, weak H+ bonding, or covalent bonding through Cys-SH groups (reviewed in O’Connell, Marcoux, and Roupioz Citation2021). In general, at least 96% of phages are tailed (reviewed in Ackermann Citation2003), and those phages have a net dipole moment at their physiological pH. To be more specific, the protein capsid head exhibits a net negative charge, and the tail carries a net positive charge (Anany et al. Citation2011). The dipole moment enables the adhesion of phages to a diversity of substrates via electrostatic bonding. Moreover, phage’s protein capsid heads consist of long chains of amino acid subunits that offer several active sites for chemical conjugations, including endogenous primary amine groups (-NH2) and carboxylic groups (-COOH) (reviewed in O’Connell, Marcoux, and Roupioz Citation2021). For example, phages can covalently bond to silanized solid substrates susceptible to nucleophilic attack by the primary amine of amino acids (Handa et al. Citation2008). In the other study, the amine-terminated substrates can form amide bonds with the carboxylic groups on phages (Shabani et al. Citation2008). The genetic engineering of phages is another method to facilitate phage immobilization, which can be used as an alternative strategy when a chemical-free approach is required. The site-directed mutagenesis occurs by fuzing the target peptides into the phage capsid protein gene via splicing a foreign coding sequence that endows an affinity for given substrates (reviewed in O’Connell, Marcoux, and Roupioz Citation2021). However, genetic modification is only limited to those well-characterized phages, such as M13, T4, and T7, and the issues associated with regulatory compliance limit the use of genetically modified phages in the food industry (Vonasek et al. Citation2017).
Overall, these three novel strategies are currently used to overcome the challenges associated with phage-based antimicrobial treatments in food and agricultural systems. The following sections illustrate how these strategies can be adapted to address specific challenges.
3.2. Enhancing phage stability
3.2.1. Acidic environment
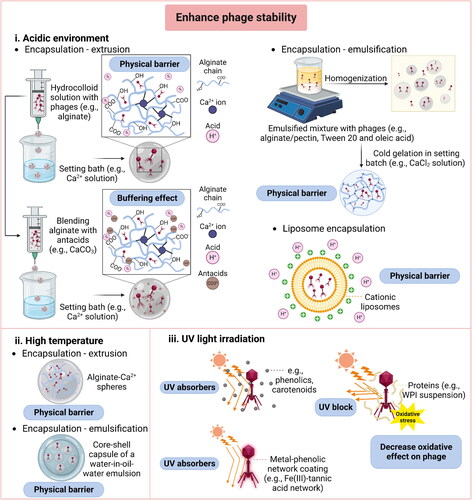
Incorporating phages into protective matrices can reduce acidic denaturation and enhance their survivability under low pH conditions. Embedding and immobilization strategies are rarely involved in resisting acidic denaturation because neither of them can isolate phages entirely from the acidic environment. Encapsulation is an optimal strategy to improve phage’s acid tolerance, which can provide physical protection to insulate phages from the surrounding acidic stress. Within an encapsulation system, the wall materials can act as a boundary to obstruct H+ proton permeation and provide buffer effects.
Various food-grade materials, such as biopolymers and antacids, are commonly used to form a microcapsule for protecting the encapsulated phages from acid denaturation (Colom et al. Citation2015; Tang, Dettmar, and Batchelor Citation2005). Extrusion is the main approach to fabricate phage microcapsules, while the emulsification and liposome encapsulation methods are also feasible (Colom et al. Citation2015; Dini et al. Citation2012; Silva Batalha et al. Citation2021).
Alginate, one of the most studied polymer materials for encapsulating food bioactive ingredients (reviewed in Li, Wei, and Xue Citation2021), has been frequently used to improve phage stability under acidic conditions. This is because the protonation of the carboxylic groups on alginate molecules can neutralize acidity, which occurs when pH is below its pKa value (3.3-3.5) (Silva Batalha et al. Citation2021). In addition, the alginate network (‘egg-box’ matrix) restricts proton diffusion compared to that in an aqueous solution (Tang, Dettmar, and Batchelor Citation2005). For example, alginate-Ca2+ spheres (diameter of 2.5-3 mm) were formed through the extrusion method, containing 106 PFU/g of Salmonella Enteritidis phages (f3aSE). The acid stability test showed that 80.6% of encapsulated phages survived at pH 3 after 1-h incubation, but only 1% of freely dispersed phages were alive (Soto et al. Citation2018). In terms of simulated gastric fluid (SGF) with a pH of 2.5, the smaller alginate-Ca2+ spheres (diameter of ≈ 1.54 mm) formed through extrusion protected phages from acid denaturation. The titer of encapsulated E. coli O157:H7 phages (UFV-AREG1) reduced by 2.39 log (PFU/g) after 120-min incubation, while non-encapsulated phages were completely inactivated after 150 s (Silva Batalha et al. Citation2021).
In addition to pure alginate hydrogel, ionic cross-linking alginate with polycationic polymers or proteins can also provide a barrier property to improve phage stability in an acidic environment, in which the alginate networks can be tailored by composition, sequential structure, and molecular size (Soto et al. Citation2018). Firstly, alginate can form polyelectrolyte complexes by interacting with polycations (Thu et al. Citation1996). The anionic carboxyl groups on alginate can electrostatically bond with polycationic polymers such as chitosan and polyethyleneimine (PEI), which compacts the alginate network and reduces proton permeability (Degroot and Neufeld Citation2001). For example, chitosan-coated alginate microspheres protected Salmonella phages (Felix O1) in SGF (pH 2.4), with phage decreased by about 2 log (PFU/g) after 40 min incubation while there were no freely dispersed phages detected (Ma et al. Citation2008). Another study encapsulated E. coli phages into the chitosan- or PEI-coated alginate beads (Moghtader, Eğri, and Piskin Citation2017). The results demonstrate that the phages in these two types of alginate beads decreased by about 2.2 log and 1 log (PFU/g), respectively, after 40-min incubation in SGF (pH 2.5), while the activity of free phages was below the detection limit (103 PFU/mL) after exposure to the same conditions (Moghtader, Eğri, and Piskin Citation2017). Second, the protective capacity of the alginate network can be promoted by blending whey proteins into the gel systems. The denatured whey protein effectively limits the acid penetration by two mechanisms: (a) at neutral pH, whey protein carrying negative charges can neutralize the acidity upon interacting with protons; and (b) when pH is below the isoelectronic point (pI of whey protein = 5.2) whey proteins carrying positive charges can interact with negatively charged alginate molecules, facilitating the further strengthening of the hydrogel network (Tang et al. Citation2015). The enhanced acid stability of phages using alginate-whey protein microcapsules has been demonstrated in several studies. Only 0.04-log reduction was observed from the encapsulated phages in alginate-whey protein spheres after 120-min incubation in SGF (pH 2.5), while the pure alginate formulation resulted in 2.39-log reduction (Silva Batalha et al. Citation2021). Tang et al. also reported that the alginate-whey protein microspheres (diameter < 300 µm) with much smaller particle size could stabilize Staphylococcus aureus phages in the SGF (pH 2.5), achieving less than 0.5-log PFU/g reduction after 120-min exposure (Tang et al. Citation2015). A further study by Tang et al. (Citation2015) suggested that the large microsphere size offered an extra physical barrier, due to the high polymer concentration that can reduce acid permeation and thus provide better protection for phages.
Apart from enhancing physical barrier properties, the buffering effect of encapsulating matrices can protect phages from acid denaturation, which is usually achieved by blending alginate with antacids, such as calcium carbonate (CaCO3). The solubility of CaCO3 is increased under acidic conditions, causing the release of carbonate ions (CO32-) and subsequent interaction with H+ protons and neutralization of the acidity. Meanwhile, those solubilized calcium ions (Ca2+) can bond with carboxyl groups on the alginate molecule, enhancing the alginate network and improving the protective effect for the encapsulated phages (Ma et al. Citation2012). This has been demonstrated in a previous study, where co-encapsulation of S. aureus phages with CaCO3 into alginate microspheres led to only 0.17-log (PFU/g) reduction after 2-h incubation in SGF (pH 2.5) (Ma et al. Citation2012). Similar results were observed in a more miniature capsule (diameter of ≈ 150 µm) of alginate-CaCO3, in which entrapped Salmonella phages showed no significant reduction after 1-h incubation in SGF (pH 2.8) (Colom et al. Citation2017).
Emulsification and liposome encapsulations are also used to protect phages from acidic environments. For example, the ionic motifs of low methoxylated (LM) pectin-containing poly(galacturonic acid) strands form rigid hydrophobic pockets where phages can be accommodated, and the interactions of phages with protons can be prevented in an aqueous environment (Dini et al. Citation2012). The emulsification of LM pectin with oleic acid showed the effective protection offered to phage against acidity at pH 1.6 with less than 1-log reduction (PFU/bead) after 30-min incubation (Dini et al. Citation2012). In addition, the cationic lipids used in liposome encapsulation can improve the phage viability under acidic conditions (pH 1-3) by acting as a barrier against protons and offering distinguished performance in controlling the release of phages in intestinal digestion (Colom et al. Citation2015).
3.2.2. High temperature
The strategies used to enhance the thermal stability of phages closely resemble those for improving their acid tolerance. A physical barrier that insulates phages within a thermal-stable microenvironment is the typical strategy to protect phages from capsid denaturation and DNA melting when exposed to a high temperature. Encapsulation is a versatile technique which can create a polymeric solid (Soto et al. Citation2018) or semisolid core-shell (Abdelsattar et al. Citation2019) particles for stabilizing phages. Alginate as an ionotropic gelation polymer is frequently selected to encapsulate phages and protect them from high temperatures because alginate hydrogel presents a thermal resistance property (Bennacef et al. Citation2021). For example, Soto et al. (Citation2018) were the first to propose using the alginate-Ca2+ spheres to encapsulate phages via an extrusion process, which improved the thermal stability of Salmonella enteritidis phages. About 50% of encapsulated phages survived after 1-h incubation at 60 °C, while only 10% of non-encapsulated phages were alive (Soto et al. Citation2018). Moreover, the core-shell particle combines the outer shell and inner core features, whose thermal stability can be further modified by combining appropriate materials (Abdelsattar et al. Citation2019; Galogahi et al. Citation2020). A recent study stated that the oil-in-water (O/W) emulsion core could improve phage thermal tolerance by using phase change agents (Richards and Malik Citation2021a). The aqueous solution of E. coli phages was emulsified with miglyol and polyglycerol polyricinoleate in the core and wrapped by an outer shell of Eudragit® polymer S100 and alginate. Such encapsulated phages maintained a final concentration of 105 log (PFU/g) after 120 s of exposure to 95 °C, while the freely dispersed phages were completely inactivated after 15 s (Richards and Malik Citation2021a). A complete phage inactivation within 30 s at 95 °C still occurred if phages were encapsulated in the capsules without the emulsion core, which highlighted the role of O/W emulsion with high thermal capacity, protecting phages in a short period during heating (Richards and Malik Citation2021a).
3.2.3. UV light irradiation
Despite significant efforts to investigate acidic and thermal stable matrices, studies on phage protection against UV light are still very limited. Instead of incorporating phages into well-structured biomaterials via encapsulation, embedding, or immobilization approaches, current studies tend to explore some useful compounds for absorbing UV irradiation or reducing oxidative stress.
Mimicking the natural biopolymer eumelanin, polydopamine (PDA) formed by self-polymerization from dopamine in alkaline conditions can absorb UV irradiation and protect substances from sunburn (Kim et al. Citation2014; Yan et al. Citation2022). For example, a recent study added PDA into the formula of whey protein isolate (WPI)-glycerol. After 4-h UV-A exposure, T7 phages suspended in a solution containing PDA survived well with 0.8-log (PFU/mL) reduction, while the phages suspended in water were inactivated significantly with more than 6-log (PFU/mL) reduction (Huang and Nitin Citation2020). Additionally, some plant-derived materials can act as UV absorbers, especially for phenolic acids (Boo Citation2020; Pacios-Michelena et al. Citation2023). Given their chemical structure, the aromatic ring and carboxylic group have absorption maxima (λmax) in the 200-400 nm range, covering the UV region (Robbins Citation2003). Born et al. (Citation2015) reported that, under the same UV-C irradiation for 5 min, the phage titers in the carrot, peppers or beetroots juice samples were approximately 2 log (PFU/mL) higher than those in SM buffer solution. Phenolic compounds and carotenoids can directly absorb UV irradiation, thus reducing the UV-induced inactivation of phages (Born et al. Citation2015). More recently, natural phenolic compounds extracted from tarbush leaves were added to the encapsulation formulation of alginate-polyvinyl acetate (PVA) or alginate-chitosan to enhance phage stability against UV light (Pacios-Michelena et al. Citation2023). Significant differences in phage stability were observed after adding polyphenols from tarbush leaves. The tarbush contains a high amount of tannic acid, which might be the reason for enhanced phage stability under UV irradiation (De León-Zapata et al. Citation2016). After 20-min UV irradiation, about 4 log (PFU/mL) of phages survived if they were encapsulated with polyphenols, while lower phage titer was observed in the absence of polyphenols and no freely dispersed phage could be detected under the same condition (Pacios-Michelena et al. Citation2023). Additionally, a novel encapsulation strategy of metal-phenolic networks (MPNs) showed significant protective ability for T4 phages under UV-C irradiation (Sun et al. Citation2022). The metal-organic nano shell composed of a natural polyphenol, tannic acid, and Fe (III) encapsulated T7 phages individually, in which tannic acid can effectively absorb the UV light, especially in the UV-C region (Park et al. Citation2014; Sun et al. Citation2022). According to Sun et al. (Citation2022), there was about 3-log (PFU/mL) reduction of encapsulated T4 phages after 20-min UV-C treatment. In contrast, more than 8 log (PFU/mL) of native phages were inactivated under the same conditions (Sun et al. Citation2022).
Apart from UV absorbers, proteins can attenuate the harmful effect of UV-light on phages based on two hypotheses: (a) cloudy protein solution impedes the penetration of UV irradiation; and (b) excessive proteins in the microenvironment might contribute to the majority of oxidation reactions induced by UV light and decrease the oxidative effect on phages (Huang and Nitin Citation2020). For example, skim milk formulation increased phage longevity on tomatoes in the greenhouse, which reduced disease severity on plants by 62% compared to 1% of the nontreated sample (Balogh et al. Citation2003). Another example was reported that soy peptone and casein protected phages from UV-induced damage due to the presence of proteins and peptides (Born et al. Citation2015). After 5-min UV-C irradiation, the loss of phage was reduced by more than 2 logs and about 1 log (PFU/mL) in the present of peptone (50 mg/mL) and casein (10 mg/mL), respectively (Born et al. Citation2015).
In summary, most of the current studies to overcome the UV instability of phages still focus on exploring materials that can absorb UV irradiation or reduce oxidative stress. However, the feasibility of using a phage dispersion system in food and agricultural applications remains challenging. An encapsulation or immobilization system for improving phage stability against UV irradiation is highly demanded in future studies.
3.3. Phage delivery systems
The overwhelming initial concentration of phages promises an effective antimicrobial treatment due to the high probability of phage-bacteria adsorption (Gill Citation2010). The high MOIinput value results in a strong antimicrobial efficacy of phage treatment in liquid (Bai, Jeon, and Ryu Citation2019), food (Bigwood et al. Citation2008; Islam et al. Citation2019), and biofilms (Islam et al. Citation2019). However, applying freely dispersed phages with high concentration is improbable and wasteful, a major concern for large-scale applications. Incorporating phages into or on a matrix is beneficial to increase the local concentration and enhance their antimicrobial efficacy. This section will overview the phage incorporation approaches based on two categories: phage-based films and phage-immobilized particles. In addition, the controlled release function based on these two methods will be emphasized.
3.3.1. Antimicrobial films
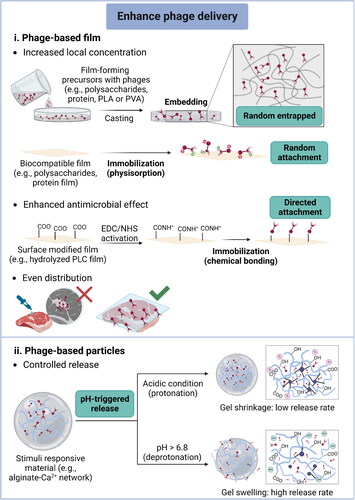
Phages are incorporated into a film matrix by immobilization or embedding method, in which phages are capable of capturing and inactivating target bacteria straight because they are fully or partially exposed to the external environment. Phage encapsulation is not a preferred option for antimicrobial applications. This is because phages are entirely entrapped in a robust matrix, providing impressive protection, but the release of phages usually requires matrix destruction. For phage immobilization, physisorption is a popular method for making phage-based films and preserving antimicrobial activity because it only needs gentle operations and can keep the phage’s original conformation, while covalent bonding between phage and substrate dominates phage-based bio-detectors (reviewed in O’Connell, Marcoux, and Roupioz Citation2021). Physical adsorption has been subdivided into three categories: nonspecific adsorption, protein-ligand interaction, and electrostatic interaction (Vonasek et al. Citation2017). The former is achieved by incubating phages with native substrates, while protein-ligand interaction involves substrate modification with affinity ligands (Vonasek et al. Citation2017). The electrostatic bonding approach requires opposite charges on substrates, which are usually pretreated with polycationic polymers (Vonasek et al. Citation2017). In terms of embedding, phages are located nonspecific by simply mixing phages with precursors and then casting, in which phages can only lie randomly (Vonasek, Le, and Nitin Citation2014).
There are some frequently used polymer materials for creating films, such as polysaccharides (Amarillas et al. Citation2018), proteins (Vonasek, Le, and Nitin Citation2014; Weng et al. Citation2021), poly(lactic acid) (Radford et al. Citation2017), and polyvinyl alcohol (López de Dicastillo et al. Citation2021). Although phages on films cannot survive in a harsh environment due to a lack of robust physical barriers, phages are gathered by biocompatible materials, resulting in an increased local concentration. The increased phage concentration on films can enhance bacterial capturing ability and antimicrobial activity (Choi et al. Citation2021; Weng et al. Citation2021). Phage-functionalized films can also prolongate phage’s stability as well as deliver phages uniformly. For example, T4 phages (10 log PFU/mL) were embedded in WPI/glycerol film and the stability of phages under both ambient (light and 22 °C) and refrigerated (dark and 4 °C) storage conditions was improved. After five-week storage, phages-based WPI/glycerol film that was stored under ambient conditions showed no significant loss in phage activity, and the phages activity decreased under refrigerated conditions only exhibited between week 3 and week 5, with about 1-log reduction. The WPI/glycerol film matrix enhanced the stability of phages, because the WPI films act as oxygen permeation barrier limiting oxidative damage, and glycerol and WPI protein can stabilize viral capsid upon drying of film (Vonasek, Le, and Nitin Citation2014). The uniformly distributed phages on the films also enhanced their antimicrobial efficacy. Phage-based WPI/glycerol film displayed about 5-log reduction (CFU/mL) after 24-h incubation compared with the negative control group (Vonasek, Le, and Nitin Citation2014). Based on these key benefits, the phage-functionalized films have raised attention as sustainable antimicrobial packaging materials to preserve food products in the food industry, which particularly benefit fresh produce and meat products (Alves et al. Citation2020; Alves et al. Citation2019; Anany et al. Citation2011; Cui, Yuan, and Lin Citation2017; Sezer, Tayyarcan, and Boyaci Citation2022). A dip-coating method was reported to create a phage-based coating on the surfaces of food products such as cucumbers, apples, and tomatoes, as well as food contact surfaces such as glass (Vonasek et al. Citation2018). Adding a crosslinker into the film-forming matrix can further increase the loading capacity by limiting the mobility of phages (Alves et al. Citation2019). In particular, WPI coating loaded about 7.2 log (PFU/cm2) of phages on the apple surface compared to the coating of phage suspension with 0.9 log (PFU/cm2) (Vonasek et al. Citation2018). Moreover, there is a significant difference between physisorption and chemical bonding. The latter fixes phages by generating irreversible and robust interaction that can endure shear stress and changes in surroundings, while the simply physisorbed phages have a high risk of detaching (reviewed in Hosseinidoust, Olsson, and Tufenkji Citation2014). In a recent study, an antibacterial film was developed by covalently immobilizing T4 phages onto an alkaline-modified polycaprolactone (PCL) film, using 1-ethyl-3(3-dimethylaminopropyl) carbodiimide (EDC)/N-hydroxy succinimide (NHS)-mediated ligand conjugation (Choi et al. Citation2021). The results in this study demonstrate that the chemically immobilized phages had higher antimicrobial efficacy, with the density of covalently bonded T4 phages being three times higher than that of physically adsorbed films. The chemically functionalized films significantly decreased the number of E. coli in bacterial suspension and on raw beef samples, achieving approximately 3-log and 1.5-log (CFU/mL) reduction, respectively. In comparison, the physically adsorbed films only resulted in less than 1-log (CFU/mL) reduction in both systems (Choi et al. Citation2021). These studies highlight the potential of using phage-based antimicrobial films in the food system, but more consideration of the incorporation approaches is needed to achieve higher antimicrobial efficacy.
3.3.2. Antimicrobial particles
Phages can bind onto the particle surface through immobilization, otherwise, they can be incorporated into particles through encapsulation. Generally, phages have a high affinity to binding their host due to their specificity. The phage-based particles have been recognized as a promising alternative to antibodies in biosensing applications. Moreover, immobilized phages allow easy separation and recycling, overcoming issues from the presence of exogenous phages (Tolba et al. Citation2010). The immobilization can be achieved through both covalent and non-covalent bonding. The oriented (i.e., tail-upward orientation) immobilization is favorable for maintaining the phage’s properties because the tail component of phages is responsible for bacterial receptor recognition (Cuervo et al. Citation2019). Modifying the substrate surface or genetically engineering phages are usually involved in achieving more robust immobilization, which can increase the phage density on particles and enhance the efficiency of capturing their host bacteria (Sun, Brovko, and Griffiths Citation2001; Tawil et al. Citation2013). For example, phages were immobilized onto a gold surface through several strategies, including physisorption and covalent chemistries after treating the surface with glutaraldehyde, L-cysteine combined with 11-mercaptoundecanoic acid, or EDC/NHS (Niyomdecha et al. Citation2018; Tawil et al. Citation2013). Physisorption resulted in low surface coverage and showed the lowest antimicrobial activity with a final bacterial concentration of 7.1 ± 0.9 × 1010 CFU/mL compared with the control value of 7.9 ± 0.9 × 1010 CFU/mL. In contrast, modifying the gold surface with L-cysteine increased the surface density of phages and showed a two-fold improvement on antimicrobial activity (Tawil et al. Citation2013). Another study also demonstrated that phages maintained their infectivity after being immobilized onto modified cationic silica particles through electrostatically-facilitated physisorption (Cademartiri et al. Citation2010).
Although immobilized phages can maintain infectivity, encapsulation is the dominant strategy for making phage-functionalized antimicrobial particles due to their sturdy structure. The preceding part of this review has emphasized encapsulation to improve phage stability in various harsh conditions. Delivering encapsulated phages inside spherical beads is normally done for antimicrobials in oral administration because the formed outer shell/matrix can effectively protect phages passing through the GI tract (Colom et al. Citation2015; Lorenzo-Rebenaque et al. Citation2021). Apart from increasing phage stability, encapsulation through moderate methods results in high phage viability in matrices. Among various encapsulation methods, extrusion is the most frequently used method, whereas alginate is the most commonly used material. Several studies have demonstrated that high encapsulation efficiency (> 90%) can be achieved after encapsulating phages into alginate-based beads by extrusion (Colom et al. Citation2017; Ergin et al. Citation2021; Silva Batalha et al. Citation2021; Tang et al. Citation2013; Tang et al. Citation2015). Thus, encapsulation is a useful and promising approach to concentrate phages in antimicrobial particles, which facilitates the delivery of phages to the target site.
3.3.3. Control release
Controlled release is designed to release the incorporated phages in a predictable pattern to maintain antimicrobial efficacy over an extended time. In particular, releasing phages from a polymeric matrix in response to external stimuli allows target delivery of phages to a site of interest, improving their antimicrobial efficiency. Amongst phage-based antimicrobial applications, pH-triggered controlled release has been widely studied, and modulated by the swelling-dissolution-erosion mechanism (Kikuchi et al. Citation1999; Lima et al. Citation2018). Ionic cross-linked alginate hydrogels have been recognized as an excellent wall material for encapsulating phages because of their naturally occurring pH responsiveness (Moghtader, Eğri, and Piskin Citation2017). The alginate network can tightly pack the phages in a matrix under acidic conditions, where the gel shrinkage causes a decrease in pore size. As the pH increases to neutral (close to pH 6.8), the dissociation of carboxyl groups in alginate molecules occurs due to ion exchanges between the monovalent ions (such as K+ and Na+) and complexed Ca2+ ions in the medium (Kikuchi et al. Citation1999), resulting in gel swelling and disintegration, subsequently causing a slow release of phages. In addition to alginate, a series of commercial polymers named Eudragit®, consisting of various polymethacrylate-based copolymers, have been used as pH-triggered release systems (Vinner, Rezaie-Yazdi, et al. Citation2019; Vinner, Richards, et al. Citation2019; Vinner et al. Citation2017).
Several studies have investigated phage survivability and antimicrobial activity in an in vivo animal model of infection and evidenced that phage delivery systems with a controlled release property can potentially be applied for delivering phages through farm animals’ GI tract (Gomez-Garcia et al. Citation2021; Jaiswal et al. Citation2013; Lorenzo-Rebenaque et al. Citation2021; Sheng et al. Citation2006; Wall et al. Citation2010). Orally delivering phages to inactivate human pathogens should be more deliberate, so few in vivo studies in humans have been conducted. However, a study investigated the stability of lactococcal phage P008 in a dynamic human gastrointestinal model (TIM-1 system) and displayed the importance of encapsulating phages for GI tract delivery (Samtlebe et al. Citation2018). In terms of controlled release in GI tract, carriers can protect encapsulated phages from extremely acidic conditions (i.e., in the stomach) and release phages in the small intestine because of increased pH conditions. For instance, the alginate-Ca2+ matrix protected phages in simulated gastric fluid (SGF, around pH 2.5), then the encapsulated phages were sustained released in simulated intestinal fluid (SIF, around pH 6.8), available examples including E. coli phages (Abdelsattar et al. Citation2019; Moghtader, Eğri, and Piskin Citation2017; Silva Batalha et al. Citation2021), Salmonella phages (Ergin et al. Citation2021; Ma et al. Citation2008) and Staphylococcus phages (Ma et al. Citation2012). Eudragit® polymers are synthetic polymers that can be used as enteric coatings, and the type of Eudragit® polymers depends on the ratio of copolymers of methacrylate and ethyl acrylate as ester components with methacrylic acid. Therefore, a more precise controlled release of encapsulated phages can be achieved because this type of material’s dissolution pH is controlled by the carboxylic group’s content (Thakral, Thakral, and Majumdar Citation2013). For example, three Eudragit® polymers (L-55, L100, and S100) were used to encapsulate T3 phages, and they possess different release profiles under specific pH conditions: polymer L-55 matrix degrades and releases phages at pH 5.5 and above, while polymer L100 matrix degrades at pH 6. In addition, the polymer S100 matrix could deliver encapsulated phages in the ileum and colon where the pH value is around 7 (Richards and Malik Citation2021b).
Apart from the success of in vitro studies, the controlled release of phages has also been demonstrated in several in vivo studies under more complicated and dynamic conditions. Targeted delivery and sufficient retention of phages in the GI tract are critical for enhanced inactivation of foodborne pathogens, which usually colonize in the small intestine and cecum in livestock. In general, the release behavior can be influenced by polymeric network compositions and concentrations (Moghtader, Eğri, and Piskin Citation2017; Silva Batalha et al. Citation2021; Tang et al. Citation2013). Polymers with hydrophilic functional groups (-COOH) are recognized as mucoadhesive agents that can form hydrogen bonds with intestinal mucus glycoproteins (Chickering and Mathiowitz Citation1995). Alginate with carboxyl ends is a potential mucoadhesive agent which has been used in numerous in vivo studies. For example, Colom et al. (Citation2017) investigated the survivability of phages in the broiler’s GI tract after encapsulating by the alginate-CaCO3 matrix. Based on Salmonella concentrations in the cecum, they observed that 95.2% of encapsulated phages were detected 48 h after oral feeding, and 71.4% remained after 72 h. In contrast, only 38.1% and 9.5% of non-encapsulated phages were detectable after 48 and 72 h, respectively (Colom et al. Citation2017). Additionally, cationic lipids used in liposome encapsulation of phages extended the retention time in the GI tract due to the adherence of liposomes to the intestinal mucosa (Colom et al. Citation2015). The antimicrobial efficacy of phages evaluated in animal models is still inappreciable, although many encouraging effects have been observed during in vitro studies (Stanford et al. Citation2010; Wagenaar et al. Citation2005). Beyond pH conditions, retention time, and delivery location, numerous challenges existing in the GI tract limit the phage efficacy during oral administration such as immune clearance (Donati et al. Citation2021; Hodyra-Stefaniak et al. Citation2015). Thus, developing a more sophisticated delivery system would be critical for using phages as an alternative to antibiotics in livestock feed supplements.
4. Conclusion and outlook
This review highlights the environmental and practical challenges of using phages as antimicrobial agents in food and agricultural systems, such as low pH, improper temperature, UV irradiation, and complex food matrices. This review also overviews the state-of-the-art strategies for improving the stability of phages and enhancing their antimicrobial efficacy. Phage-based treatment has demonstrated significant potential to enhance the inactivation of foodborne pathogens in the whole food supply chain. Although a range of phage products have been commercialized, the stability and antimicrobial efficacy of applied phages in food and agricultural systems are unsatisfactory. Phage incorporation, including encapsulation, immobilization or embedding techniques, is an effective strategy to stabilize phages against harsh conditions, increase localized concentration, and facilitate high treatment efficacy. Apart from these benefits, other properties such as controlled release and stimuli-responsive release provide an advance for enhancing the delivery efficiency of phages to the site of interest. However, further investigations are required to translate these concepts for large-scale applications in food and agricultural systems. Although numerous encouraging exemplifications exist for utilizing phages as antimicrobials or preservatives in food on a laboratory scale, the antimicrobial efficacy is unsettled and food-dependent, which impedes industrial-scale applications. The investigation of protecting phages against sunlight remains largely unexplored, which becomes a crucial barrier to utilizing phages for plant disease control. Oral administration of phages for livestock is attractive for treating GI tract infections as an alternative to antibiotics. However, bio-based carriers and optimum feeding operations are required for enhanced delivery efficiency. Together, both improving phage stability and enhancing phage delivery can significantly overcome the challenges and reduce the risk of foodborne diseases, bringing food safety to a new era and developing sustainable food and agricultural industry.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Abdelsattar, A. S., F. Abdelrahman, A. Dawoud, I. F. Connerton, and A. El-Shibiny. 2019. Encapsulation of E. coli phage ZCEC5 in chitosan–alginate beads as a delivery system in phage therapy. AMB Express 9 (1):98. doi:10.1186/s13568-019-0810-9.
- Abedon, S. 2011. Phage therapy pharmacology: Calculating phage dosing. Advances in applied microbiology 77:1–40. doi:10.1016/B978-0-12-387044-5.00001-7.
- Abhisingha, M., J. Dumnil, and C. Pitaksutheepong. 2020. Efficiency of phage cocktail to reduce Salmonella Typhimurium on chicken meat during low temperature storage. LWT 129:109580. doi:10.1016/j.lwt.2020.109580.
- Ackermann, H. W. 2001. Frequency of morphological phage descriptions in the year 2000. Archives of Virology 146 (5):843–57. doi:10.1007/s007050170120.
- Ackermann, H.-W. 2003. Bacteriophage observations and evolution. Research in Microbiology 154 (4):245–51. doi:10.1016/s0923-2508(03)00067-6.
- Ackermann, H.-W. 2009. Phage classification and characterization. In Methods in molecular biology, 127–40. New York, NY: Humana Press. doi:10.1007/978-1-60327-164-6_13.
- AgriPhage™. n.d. Product info. Accessed March 16, 2023. https://www.agriphage.com/product-info/.
- Ahmadi, H., D. Radford, A. M. Kropinski, L.-T. Lim, and S. Balamurugan. 2017. Thermal-stability and reconstitution ability of Listeria phages P100 and A511. Frontiers in Microbiology 8:2375. doi:10.3389/fmicb.2017.02375.
- Akbarzadeh, A., R. Rezaei-Sadabady, S. Davaran, S. W. Joo, N. Zarghami, Y. Hanifehpour, M. Samiei, M. Kouhi, and K. Nejati-Koshki. 2013. Liposome: Classification, preparation, and applications. Nanoscale Research Letters 8 (1):102. doi:10.1186/1556-276X-8-102.
- Alves, D., A. Marques, C. Milho, M. J. Costa, L. M. Pastrana, M. A. Cerqueira, and S. M. Sillankorva. 2019. Bacteriophage ϕIBB-PF7A loaded on sodium alginate-based films to prevent microbial meat spoilage. International Journal of Food Microbiology 291:121–7. doi:10.1016/j.ijfoodmicro.2018.11.026.
- Alves, D., M. A. Cerqueira, L. M. Pastrana, and S. Sillankorva. 2020. Entrapment of a phage cocktail and cinnamaldehyde on sodium alginate emulsion-based films to fight food contamination by Escherichia coli and Salmonella Enteritidis. Food Research International (Ottawa, Ont.) 128:108791. doi:10.1016/j.foodres.2019.108791.
- Amarillas, L., L. Lightbourn‐Rojas, A. K. Angulo‐Gaxiola, J. Basilio Heredia, A. González‐Robles, and J. León‐Félix. 2018. The antibacterial effect of chitosan‐based edible coating incorporated with a lytic bacteriophage against Escherichia coli O157:H7 on the surface of tomatoes. Journal of Food Safety 38 (6):e12571. doi:10.1111/jfs.12571.
- Anany, H., W. Chen, R. Pelton, and M. W. Griffiths. 2011. Biocontrol of Listeria monocytogenes and Escherichia coli O157:H7 in meat by using phages immobilized on modified cellulose membranes. Applied and Environmental Microbiology 77 (18):6379–87. doi:10.1128/AEM.05493-11.
- APS Biocontrol. n.d. Products. Accessed March 16, 2023. https://www.apsbiocontrol.com/technology.
- ARM & HAMMER™. n.d. Finalyse SAL. Accessed March 16, 2023. https://ahfoodchain.com/en/segments/food-production/products/finalyse-sal.
- Atterbury, R. J., M. A. P. V. Bergen, F. Ortiz, M. A. Lovell, J. A. Harris, A. D. Boer, J. A. Wagenaar, V. M. Allen, and P. A. Barrow. 2007. Bacteriophage therapy to reduce Salmonella colonization of broiler chickens. Applied and Environmental Microbiology 73 (14):4543–9. doi:10.1128/AEM.00049-07.
- Atterbury, R. J., P. L. Connerton, C. E. R. Dodd, C. E. D. Rees, and I. F. Connerton. 2003. Application of host-specific bacteriophages to the surface of chicken skin leads to a reduction in recovery of Campylobacter jejuni. Applied and Environmental Microbiology 69 (10):6302–6. doi:10.1128/AEM.69.10.6302-6306.2003.
- Bai, J., B. Jeon, and S. Ryu. 2019. Effective inhibition of Salmonella Typhimurium in fresh produce by a phage cocktail targeting multiple host receptors. Food Microbiology 77:52–60. doi:10.1016/j.fm.2018.08.011.
- Balogh, B., J. B. Jones, M. T. Momol, S. M. Olson, A. Obradovic, P. King, and L. E. Jackson. 2003. Improved efficacy of newly formulated bacteriophages for management of bacterial spot on tomato. Plant Disease 87 (8):949–54. doi:10.1094/pdis.2003.87.8.949.
- Bao, H., P. Zhang, H. Zhang, Y. Zhou, L. Zhang, and R. Wang. 2015. Bio-control of Salmonella Enteritidis in foods using bacteriophages. Viruses 7 (8):4836–53. doi:10.3390/v7082847.
- Bardina, C., D. A. Spricigo, P. Cortés, and M. Llagostera. 2012. Significance of the bacteriophage treatment schedule in reducing Salmonella colonization of poultry. Applied and Environmental Microbiology 78 (18):6600–7. doi:10.1128/AEM.01257-12.
- Bennacef, C., S. Desobry-Banon, L. Probst, and S. Desobry. 2021. Advances on alginate use for spherification to encapsulate biomolecules. Food Hydrocolloids. 118:106782. doi:10.1016/j.foodhyd.2021.106782.
- Bigwood, T., J. A. Hudson, and C. Billington. 2009. Influence of host and bacteriophage concentrations on the inactivation of food-borne pathogenic bacteria by two phages. FEMS Microbiology Letters 291 (1):59–64. doi:10.1111/j.1574-6968.2008.01435.x.
- Bigwood, T., J. A. Hudson, C. Billington, G. V. Carey-Smith, and J. A. Heinemann. 2008. Phage inactivation of foodborne pathogens on cooked and raw meat. Food Microbiology 25 (2):400–6. doi:10.1016/j.fm.2007.11.003.
- Blaustein, A. R., and C. Searle. 2013. Ultraviolet radiation. In Encyclopedia of biodiversity, 296–303. Cambridge, MA: Elsevier. doi:10.1016/b978-0-12-384719-5.00147-7.
- Bonnain, C., M. Breitbart, and K. N. Buck. 2016. The ferrojan horse hypothesis: Iron-virus interactions in the ocean. Frontiers in Marine Science 3:1–11. doi:10.3389/fmars.2016.00082.
- Boo, Y. C. 2020. Emerging strategies to protect the skin from ultraviolet rays using plant-derived materials. Antioxidants 9 (7):637. https://link.gale.com/apps/doc/A641063408/AONE?u=learn&sid=bookmark-AONE&xid=4e5aba6c. doi:10.3390/antiox9070637.
- Borin, J. M., S. Avrani, J. E. Barrick, K. L. Petrie, and J. R. Meyer. 2021. Coevolutionary phage training leads to greater bacterial suppression and delays the evolution of phage resistance. Proceedings of the National Academy of Sciences 118 (23):e2104592118. doi:10.1073/pnas.2104592118.
- Born, Y., L. Bosshard, B. Duffy, M. J. Loessner, and L. Fieseler. 2015. Protection of Erwinia amylovora bacteriophage Y2 from UV-induced damage by natural compounds. Bacteriophage, 5 (4):e1074330. doi:10.1080/21597081.2015.1074330.
- Brimrose. n.d. Prevention & treatment. Accessed March 16, 2023. https://www.brimrosetechnology.com/prevention-treatment.
- Brovko, L. Y., H. Anany, and M. W. Griffiths. 2012. Bacteriophages for detection and control of bacterial pathogens in food and food-processing environment. In Advances in food and nutrition research, ed. J. Henry, vol. 67, 241–88. Cambridge, MA: Academic Press. doi:10.1016/B978-0-12-394598-3.00006-X.
- Bueno, E., P. García, B. Martínez, and A. Rodríguez. 2012. Phage inactivation of Staphylococcus aureus in fresh and hard-type cheeses. International Journal of Food Microbiology 158 (1):23–7. doi:10.1016/j.ijfoodmicro.2012.06.012.
- Burrell, C. J., C. R. Howard, and F. A. Murphy. 2017. Virion structure and composition. In Fenner and white’s medical virology, C. J. Burrell, C. R. Howard, & F. A. Murphy eds., 5th ed., 27–37. Cambridge, MA: Academic Press. doi:10.1016/B978-0-12-375156-0.00003-5.
- Cademartiri, R., H. Anany, I. Gross, R. Bhayani, M. Griffiths, and M. A. Brook. 2010. Immobilization of bacteriophages on modified silica particles. Biomaterials 31 (7):1904–10. doi:10.1016/j.biomaterials.2009.11.029.
- Campbell, A. 2003. The future of bacteriophage biology. Nature Reviews. Genetics 4 (6):471–7. doi:10.1038/nrg1089.
- Carrigy, N. B., L. Liang, H. Wang, S. Kariuki, T. E. Nagel, I. F. Connerton, and R. Vehring. 2020. Trileucine and pullulan improve anti-campylobacter bacteriophage stability in engineered spray-dried microparticles. Annals of Biomedical Engineering 48 (4):1169–80. doi:10.1007/s10439-019-02435-6.
- Carter, C. D., A. Parks, T. Abuladze, M. Li, J. Woolston, J. Magnone, A. Senecal, A. M. Kropinski, and A. Sulakvelidze. 2012. Bacteriophage cocktail significantly reduces Escherichia coli O157. Bacteriophage, 2 (3):178–85. doi:10.4161/bact.22825.
- Chang, R. Y., J. Wong, A. Mathai, S. Morales, E. Kutter, W. Britton, J. Li, and H.-K. Chan. 2017. Production of highly stable spray dried phage formulations for treatment of Pseudomonas aeruginosa lung infection. European Journal of Pharmaceutics and Biopharmaceutics : Official Journal of Arbeitsgemeinschaft Fur Pharmazeutische Verfahrenstechnik e.V 121:1–13. doi:10.1016/j.ejpb.2017.09.002.
- CheilJedang. n.d. Biotector. Accessed March 16, 2023. https://www.cjbio.net/kr/products/biotector.do.
- Chen, Y., E. Sun, J. Song, Y. Tong, and B. Wu. 2018. Three Salmonella enterica serovar Enteritidis bacteriophages from the Siphoviridae family are promising candidates for phage therapy. Canadian Journal of Microbiology 64 (11):865–75. doi:10.1139/cjm-2017-0740.
- Chickering, D. E., and E. Mathiowitz. 1995. Bioadhesive microspheres: I. A novel electrobalance-based method to study adhesive interactions between individual microspheres and intestinal mucosa. Journal of Controlled Release 34 (3):251–62. doi:10.1016/0168-3659(95)00011-v.
- Choi, I., D. S. Yoo, Y. Chang, S. Y. Kim, and J. Han. 2021. Polycaprolactone film functionalized with bacteriophage T4 promotes antibacterial activity of food packaging toward Escherichia coli. Food Chemistry 346:128883. doi:10.1016/j.foodchem.2020.128883.
- Choińska-Pulit, A., P. Mituła, P. Śliwka, W. Łaba, and A. Skaradzińska. 2015. Bacteriophage encapsulation: Trends and potential applications. Trends in Food Science & Technology 45 (2):212–21. doi:10.1016/j.tifs.2015.07.001.
- Clavijo, V., D. Baquero, S. Hernandez, J. C. Farfan, J. Arias, A. Arévalo, P. Donado-Godoy, and M. Vives-Flores. 2019. Phage cocktail SalmoFREE® reduces Salmonella on a commercial broiler farm. Poultry Science 98 (10):5054–63. doi:10.3382/ps/pez251.
- Cole, L. 2005. Xanthomonas Campestris pv. Vesicatoria and Pseudomonas Syringae pv. tomato specific bacteriophages; exemption from the requirement of a tolerance (Document No. 05-24540). Environmental Protection Agency. https://www.federalregister.gov/documents/2005/12/28/05-24540/xanthomonas-campestris-pv-vesicatoria-and-pseudomonas-syringae-pv-tomato-specific-bacteriophages
- Colom, J., M. Cano-Sarabia, J. Otero, J. Aríñez-Soriano, P. Cortés, D. Maspoch, and M. Llagostera. 2017. Microencapsulation with alginate/CaCO3: A strategy for improved phage therapy. Scientific Reports 7 (1):41441. doi:10.1038/srep41441.
- Colom, J., M. Cano-Sarabia, J. Otero, P. Cortés, D. Maspoch, and M. Llagostera. 2015. Liposome-encapsulated bacteriophages for enhanced oral phage therapy against Salmonella spp. Applied and Environmental Microbiology 81 (14):4841–9. doi:10.1128/aem.00812-15.
- Cong, C., B. Wei, H. Cui, X. Li, Y. Yuan, L. Wang, S. Li, and Y. Xu. 2021. Isolation, characterization and comparison of lytic Epseptimavirus phages targeting Salmonella. Food Research International (Ottawa, Ont.) 147:110480. doi:10.1016/j.foodres.2021.110480.
- Cuervo, A., M. Fàbrega-Ferrer, C. Machón, J. J. Conesa, F. J. Fernández, R. Pérez-Luque, M. Pérez-Ruiz, J. Pous, M. C. Vega, J. L. Carrascosa, et al. 2019. Structures of T7 bacteriophage portal and tail suggest a viral DNA retention and ejection mechanism. Nature Communications 10 (1):3746. doi:10.1038/s41467-019-11705-9.
- Cui, H., L. Yuan, and L. Lin. 2017. Novel chitosan film embedded with liposome-encapsulated phage for biocontrol of Escherichia coli O157:H7 in beef. Carbohydrate Polymers 177:156–64. doi:10.1016/j.carbpol.2017.08.137.
- Dąbrowska, K. 2019. Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Medicinal Research Reviews 39 (5):2000–25. doi:10.1002/med.21572.
- De León-Zapata, M. A., L. Pastrana-Castro, M. L. Rua-Rodríguez, O. B. Alvarez-Pérez, R. Rodríguez-Herrera, and C. N. Aguilar. 2016. Experimental protocol for the recovery and evaluation of bioactive compounds of tarbush against postharvest fruit fungi. Food Chemistry 198:62–7. doi:10.1016/j.foodchem.2015.11.034.
- Degroot, A. R., and R. J. Neufeld. 2001. Encapsulation of urease in alginate beads and protection from α-chymotrypsin with chitosan membranes. Enzyme and Microbial Technology 29 (6-7):321–7. doi:10.1016/s0141-0229(01)00393-3.
- Díaz, A., L. J. Del Valle, N. Rodrigo, M. T. Casas, G. Chumburidze, R. Katsarava, and J. Puiggalí. 2018. Antimicrobial activity of poly(ester urea) electrospun fibers loaded with bacteriophages. Fibers 6 (2):33. https://www.mdpi.com/2079-6439/6/2/33. doi:10.3390/fib6020033.
- Dini, C., G. A. Islan, P. J. De Urraza, and G. R. Castro. 2012. Novel biopolymer matrices for microencapsulation of phages: Enhanced protection against acidity and protease activity. Macromolecular Bioscience 12 (9):1200–8. doi:10.1002/mabi.201200109.
- Donati, V. L., I. Dalsgaard, K. Sundell, D. Castillo, M. Er-Rafik, J. Clark, T. Wiklund, M. Middelboe, and L. Madsen. 2021. Phage-mediated control of Flavobacterium psychrophilum in aquaculture: In vivo experiments to compare delivery methods. Frontiers in Microbiology 12:628309. doi:10.3389/fmicb.2021.628309.
- Egyeki, M., G. Turóczy, Z. Majer, K. Tóth, A. Fekete, P. Maillard, and G. Csík. 2003. Photosensitized inactivation of T7 phage as surrogate of non-enveloped DNA viruses: Efficiency and mechanism of action. Biochimica et Biophysica Acta 1624 (1-3):115–24. doi:10.1016/j.bbagen.2003.10.003.
- El-Shibiny, A., A. Scott, A. Timms, Y. Metawea, P. Connerton, and I. Connerton. 2009. Application of a group II Campylobacter bacteriophage to reduce strains of Campylobacter jejuni and Campylobacter coli colonizing broiler chickens. Journal of Food Protection 72 (4):733–40. doi:10.4315/0362-028x-72.4.733.
- Endersen, L., and A. Coffey. 2020. The use of bacteriophages for food safety. Current Opinion in Food Science 36:1–8. doi:10.1016/j.cofs.2020.10.006.
- Erbay, Z., N. Koca, F. Kaymak-Ertekin, and M. Ucuncu. 2015. Optimization of spray drying process in cheese powder production. Food and Bioproducts Processing 93:156–65. doi:10.1016/j.fbp.2013.12.008.
- Ergin, F. 2022. Effect of freeze drying, spray drying and electrospraying on the morphological, thermal, and structural properties of powders containing phage Felix O1 and activity of phage Felix O1 during storage. Powder Technology 404:117516. doi:10.1016/j.powtec.2022.117516.
- Ergin, F., Z. Atamer, E. M. Comak Göcer, M. Demir, J. Hinrichs, and A. Kucukcetin. 2021. Optimization of Salmonella bacteriophage microencapsulation in alginate-caseinate formulation using vibrational nozzle technique. Food Hydrocolloids. 113:106456. doi:10.1016/j.foodhyd.2020.106456.
- Fekete, A., G. Rontó, L. A. Feigin, V. V. Tikhonychev, and K. Módos. 1982. Temperature dependent structural changes of intraphage T7 DNA. Biophysics of Structure and Mechanism 9 (1):1–9. doi:10.1007/bf00536011.
- Fellows, P. 2017. Pasteurisation. In Food processing technology, 563–80. Amsterdam, NL: Elsevier. doi:10.1016/b978-0-08-100522-4.00011-0.
- Ferguson, S., C. Roberts, E. Handy, and M. Sharma. 2013. Lytic bacteriophages reduce Escherichia coli O157. Bacteriophage, 3 (1):e24323. doi:10.4161/bact.24323.
- Flaherty, J. E., G. C. Somodi, J. B. Jones, B. K. Harbaugh, and L. E. Jackson. 2000. Control of bacterial spot on tomato in the greenhouse and field with H-mutant bacteriophages. HortScience 35 (5):882–4. doi:10.21273/HORTSCI.35.5.882.
- Galogahi, F. M., Y. Zhu, H. An, and N.-T. Nguyen. 2020. Core-shell microparticles: Generation approaches and applications. Journal of Science: Advanced Materials and Devices, 5 (4):417–35. doi:10.1016/j.jsamd.2020.09.001.
- Gbassi, G. K., and T. Vandamme. 2012. Probiotic encapsulation technology: From microencapsulation to release into the gut. Pharmaceutics 4 (1):149–63. doi:10.3390/pharmaceutics4010149.
- Gill, J. J. 2010. Practical and theoretical considerations for the use of bacteriophages in food systems. In Bacteriophages in the Control of Food‐ and Waterborne Pathogens, eds P. M. Sabour and M. W. Griffiths, 217–35. Amsterdam, Netherlands. doi:10.1128/9781555816629.ch11.
- Gomez-Garcia, J., A. Chavez-Carbajal, N. Segundo-Arizmendi, M. G. Baron-Pichardo, S. E. Mendoza-Elvira, E. Hernandez-Baltazar, A. P. Hynes, and O. Torres-Angeles. 2021. Efficacy of Salmonella bacteriophage S1 delivered and released by alginate beads in a chicken model of infection. Viruses 13 (10):1932. doi:10.3390/v13101932.
- González-Menéndez, E., L. Fernández, D. Gutiérrez, D. Pando, B. Martínez, A. Rodríguez, and P. García. 2018. Strategies to encapsulate the Staphylococcus aureus bacteriophage phiIPLA-RODI. Viruses 10 (9):495. doi:10.3390/v10090495.
- Guan, J., A. Oromí-Bosch, S. D. Mendoza, S. Karambelkar, J. D. Berry, and J. Bondy-Denomy. 2022. Bacteriophage genome engineering with CRISPR–Cas13a. Nature Microbiology 7 (12):1956–66. doi:10.1038/s41564-022-01243-4.
- Guenther, S., D. Huwyler, S. Richard, and M. J. Loessner. 2009. Virulent bacteriophage for efficient biocontrol of Listeria monocytogenes in ready-to-eat foods. Applied and Environmental Microbiology 75 (1):93–100. doi:10.1128/AEM.01711-08.
- Guo, Y., J. Li, M. S. Islam, T. Yan, Y. Zhou, L. Liang, I. F. Connerton, K. Deng, and J. Li. 2021. Application of a novel phage vB_SalS-LPSTLL for the biological control of Salmonella in foods. Food Research International (Ottawa, Ont.) 147:110492. doi:10.1016/j.foodres.2021.110492.
- Hammerl, J. A., A. Barac, P. Erben, J. Fuhrmann, A. Gadicherla, F. Kumsteller, A. Lauckner, F. Müller, and S. Hertwig. 2021. Properties of two broad host range phages of Yersinia enterocolitica isolated from wild animals. International Journal of Molecular Sciences 22 (21):11381. doi:10.3390/ijms222111381.
- Handa, H., S. Gurczynski, M. P. Jackson, G. Auner, J. Walker, and G. Mao. 2008. Recognition of Salmonella typhimurium by immobilized phage P22 monolayers. Surface Science 602 (7):1392–400. doi:10.1016/j.susc.2008.01.036.
- Hankin, E. H. 1896. L’action bactericide des eaux de la Jumna et du Gange sur le vibrion du cholera. Ann Inst Pasteur 10 (11):511–23.
- Hegedüs, M., G. Kovács, K. Módos, G. Rontó, H. Lammer, C. Panitz, and A. Fekete. 2006. Exposure of phage T7 to simulated space environment: The effect of vacuum and UV-C radiation. Journal of Photochemistry and Photobiology. B, Biology 82 (2):94–104. doi:10.1016/j.jphotobiol.2005.09.002.
- Hodyra-Stefaniak, K., P. Miernikiewicz, J. Drapała, M. Drab, E. Jończyk-Matysiak, D. Lecion, Z. Kaźmierczak, W. Beta, J. Majewska, M. Harhala, et al. 2015. Mammalian host-versus-phage immune response determines phage fate in vivo. Scientific Reports 5 (1):14802. doi:10.1038/srep14802.
- Hosseinidoust, Z., A. L. J. Olsson, and N. Tufenkji. 2014. Going viral: Designing bioactive surfaces with bacteriophage. Colloids and Surfaces. B, Biointerfaces 124:2–16. doi:10.1016/j.colsurfb.2014.05.036.
- Hu, Y. O. O., L. W. Hugerth, C. Bengtsson, A. Alisjahbana, M. Seifert, A. Kamal, Å. Sjöling, T. Midtvedt, E. Norin, J. Du, et al. 2018. Bacteriophages synergize with the gut microbial community to combat Salmonella. mSystems 3 (5):e00119-00118. doi:10.1128/mSystems.00119-18.
- Huang, C., J. Shi, W. Ma, Z. Li, J. Wang, J. Li, and X. Wang. 2018. Isolation, characterization, and application of a novel specific Salmonella bacteriophage in different food matrices. Food Research International (Ottawa, Ont.) 111:631–41. doi:10.1016/j.foodres.2018.05.071.
- Huang, K., and N. Nitin. 2019. Edible bacteriophage based antimicrobial coating on fish feed for enhanced treatment of bacterial infections in aquaculture industry. Aquaculture 502:18–25. doi:10.1016/j.aquaculture.2018.12.026.
- Huang, K., and N. Nitin. 2020. Food-grade microscale dispersion enhances UV stability and antimicrobial activity of a model bacteriophage (T7) for reducing bacterial contamination (Escherichia coli) on the plant surface. Journal of Agricultural and Food Chemistry 68 (39):10920–7. doi:10.1021/acs.jafc.0c02795.
- Hudson, J. A., T. Bigwood, A. Premaratne, C. Billington, B. Horn, and L. McIntyre. 2010. Potential to use ultraviolet-treated bacteriophages to control foodborne pathogens. Foodborne Pathogens and Disease 7 (6):687–93. doi:10.1089/fpd.2009.0453.
- Intralytix. n.d. 2023. ListShield™. Accessed August 24, 2023. https://www.intralytix.com/product/1.
- Intralytix. n.d. Bacteriaophage products. Accessed March 16, 2023. http://www.intralytix.com/index.php?page=prod.
- Iriarte, F. B., B. Balogh, M. T. Momol, L. M. Smith, M. Wilson, and J. B. Jones. 2007. Factors affecting survival of bacteriophage on tomato leaf surfaces. Applied and Environmental Microbiology 73 (6):1704–11. doi:10.1128/AEM.02118-06.
- Islam, M. S., Y. Zhou, L. Liang, I. Nime, K. Liu, T. Yan, X. Wang, and J. Li. 2019. Application of a phage cocktail for control of Salmonella in foods and reducing biofilms. Viruses 11 (9):841. doi:10.3390/v11090841.
- Jaiswal, A., H. Koley, A. Ghosh, A. Palit, and B. Sarkar. 2013. Efficacy of cocktail phage therapy in treating Vibrio cholerae infection in rabbit model. Microbes and Infection 15 (2):152–6. doi:10.1016/j.micinf.2012.11.002.
- Jancheva, M., and T. Böttcher. 2021. A metabolite of Pseudomonas triggers prophage-selective lysogenic to lytic conversion in Staphylococcus aureus. Journal of the American Chemical Society 143 (22):8344–51. doi:10.1021/jacs.1c01275.
- Jarvis, N. A., C. A. O’Bryan, T. M. Dawoud, S. H. Park, Y. M. Kwon, P. G. Crandall, and S. C. Ricke. 2016. An overview of Salmonella thermal destruction during food processing and preparation. Food Control. 68:280–90. doi:10.1016/j.foodcont.2016.04.006.
- Jurczak-Kurek, A., T. Gąsior, B. Nejman-Faleńczyk, S. Bloch, A. Dydecka, G. Topka, A. Necel, M. Jakubowska-Deredas, M. Narajczyk, M. Richert, et al. 2016. Biodiversity of bacteriophages: Morphological and biological properties of a large group of phages isolated from urban sewage. Scientific Reports 6 (1):34338. doi:10.1038/srep34338.
- Kadiri, A.-C., R. Günther, and B. R. Fischer, (2019). Genetically modified M13 bacteriophage nanonets for enzyme catalysis and recovery. Catalysts, 9(9), 723. doi:10.3390/catal9090723.
- Kafri, Y., D. Mukamel, and L. Peliti. 2000. Why is the DNA denaturation transition first order? Physical Review Letters 85 (23):4988–91. doi:10.1103/physrevlett.85.4988.
- Kasman, L. M., A. Kasman, C. Westwater, J. Dolan, M. G. Schmidt, and J. S. Norris. 2002. Overcoming the phage replication threshold: A mathematical model with implications for phage therapy. Journal of Virology 76 (11):5557–64. doi:10.1128/JVI.76.11.5557-5564.2002.
- Kikuchi, A., M. Kawabuchi, A. Watanabe, M. Sugihara, Y. Sakurai, and T. Okano. 1999. Effect of Ca2+-alginate gel dissolution on release of dextran with different molecular weights. Journal of Controlled Release 58 (1):21–8. doi:10.1016/s0168-3659(98)00141-2.
- Kim, B. J., T. Park, H. C. Moon, S.-Y. Park, D. Hong, E. H. Ko, J. Y. Kim, J. W. Hong, S. W. Han, Y.-G. Kim, et al. 2014. Cytoprotective alginate/polydopamine core/shell microcapsules in microbial encapsulation. Angewandte Chemie (International ed. in English) 53 (52):14443–6. doi:10.1002/anie.201408454.
- Kim, C., R. Alrefaei, M. Bushlaibi, E. Ndegwa, P. Kaseloo, and C. Wynn. 2019. Influence of growth temperature on thermal tolerance of leading foodborne pathogens. Food Science & Nutrition 7 (12):4027–36. doi:10.1002/fsn3.1268.
- Kim, S. H., S. Kim, S. G. Chun, M-s Park, J. H. Park, and B-k Lee. 2008. Phage types and pulsed-field gel electrophoresis patterns of Salmonella enterica serovar Enteritidis isolated from humans and chickens. Journal of Microbiology (Seoul, Korea) 46 (2):209–13. doi:10.1007/s12275-007-0197-1.
- Kim, S., S.-H. Kim, M. Rahman, and J. Kim. 2018. Characterization of a Salmonella Enteritidis bacteriophage showing broad lytic activity against Gram-negative enteric bacteria. Journal of Microbiology (Seoul, Korea) 56 (12):917–25. doi:10.1007/s12275-018-8310-1.
- Kolenda, C., Medina, M., Bonhomme, M., Laumay, F., Roussel-Gaillard, T., Martins-Simoes, P., Tristan, A., Pirot, F., Ferry, T., Laurent, F., & Santella, B. (2022). Phage therapy against Staphylococcus aureus: Selection and optimization of production protocols of novel broad-spectrum silviavirus phages. Pharmaceutics, 14(9), 1885. doi:10.3390/pharmaceutics14091885.
- Koo, C. K., K. Senecal, A. Senecal, and S. R. Nugen. 2016. Dehydration of bacteriophages in electrospun nanofibers: Effect of excipients in polymeric solutions. Nanotechnology 27 (48):485102. doi:10.1088/0957-4484/27/48/485102.
- Korehei, R., and J. F. Kadla. 2014. Encapsulation of T4 bacteriophage in electrospun poly(ethylene oxide)/cellulose diacetate fibers. Carbohydrate Polymers 100:150–7. doi:10.1016/j.carbpol.2013.03.079.
- Korehei, R., and J. Kadla. 2013. Incorporation of T4 bacteriophage in electrospun fibres. Journal of Applied Microbiology 114 (5):1425–34. doi:10.1111/jam.12158.
- Krasaekoopt, W., B. Bhandari, and H. Deeth. 2003. Evaluation of encapsulation techniques of probiotics for yoghurt. International Dairy Journal, 13 (1):3–13. doi:10.1016/s0958-6946(02)00155-3.
- Kutter, E., B. Guttman, and K. Carlson. 1994. The transition from host to phage metabolism after T4 infection. Molecular Biology of Bacteriophage 4:343–6.
- Lepock, J. R. 2004. Role of nuclear protein denaturation and aggregation in thermal radiosensitization. International Journal of Hyperthermia 20 (2):115–30. doi:10.1080/02656730310001637334.
- Li, D., Z. Wei, and C. Xue. 2021. Alginate-based delivery systems for food bioactive ingredients: An overview of recent advances and future trends. Comprehensive Reviews in Food Science and Food Safety 20 (6):5345–69. doi:10.1111/1541-4337.12840.
- Lima, D. S., E. T. Tenório-Neto, M. K. Lima-Tenório, M. R. Guilherme, D. B. Scariot, C. V. Nakamura, E. C. Muniz, and A. F. Rubira. 2018. pH-responsive alginate-based hydrogels for protein delivery. Journal of Molecular Liquids 262:29–36. doi:10.1016/j.molliq.2018.04.002.
- Liu, A., Y. Liu, L. Peng, X. Cai, L. Shen, M. Duan, Y. Ning, S. Liu, C. Li, Y. Liu, et al. 2020. Characterization of the narrow-spectrum bacteriophage LSE7621 towards Salmonella Enteritidis and its biocontrol potential on lettuce and tofu. LWT 118:108791. doi:10.1016/j.lwt.2019.108791.
- López de Dicastillo, C., L. Settier-Ramírez, R. Gavara, P. Hernández-Muñoz, and G. López Carballo. 2021. Development of biodegradable films loaded with phages with antilisterial properties. Polymers 13 (3):327. https://www.mdpi.com/2073-4360/13/3/327. doi:10.3390/polym13030327.
- Lorenzo-Rebenaque, L., D. J. Malik, P. Catalá-Gregori, C. Marin, and S. Sevilla-Navarro. 2021. In Vitro and In Vivo gastrointestinal survival of non-encapsulated and microencapsulated Salmonella bacteriophages: Implications for bacteriophage therapy in poultry. Pharmaceuticals (Basel, Switzerland) 14 (5):434. doi:10.3390/ph14050434.
- Lu, T. K., and J. J. Collins. 2007. Dispersing biofilms with engineered enzymatic bacteriophage. Proceedings of the National Academy of Sciences of the United States of America 104 (27):11197–202. doi:10.1073/pnas.0704624104.
- Luna-Solano, G., M. A. Salgado-Cervantes, G. C. Rodríguez-Jimenes, and M. A. García-Alvarado. 2005. Optimization of brewer’s yeast spray drying process. Journal of Food Engineering 68 (1):9–18. doi:10.1016/j.jfoodeng.2004.05.019.
- Ma, Y., J. C. Pacan, Q. Wang, P. M. Sabour, X. Huang, and Y. Xu. 2012. Enhanced alginate microspheres as means of oral delivery of bacteriophage for reducing Staphylococcus aureus intestinal carriage. Food Hydrocolloids. 26 (2):434–40. doi:10.1016/j.foodhyd.2010.11.017.
- Ma, Y., J. C. Pacan, Q. Wang, Y. Xu, X. Huang, A. Korenevsky, and P. M. Sabour. 2008. Microencapsulation of bacteriophage Felix O1 into chitosan-alginate microspheres for oral delivery. Applied and Environmental Microbiology 74 (15):4799–805. doi:10.1128/AEM.00246-08.
- Ma, Y.-H., G. S. Islam, Y. Wu, P. M. Sabour, J. R. Chambers, Q. Wang, S. X. Y. Wu, and M. W. Griffiths. 2016. Temporal distribution of encapsulated bacteriophages during passage through the chick gastrointestinal tract. Poultry Science 95 (12):2911–20. doi:10.3382/ps/pew260.
- Manohar, P., and N. Ramesh. 2019. Improved lyophilization conditions for long-term storage of bacteriophages. Scientific Reports 9 (1):15242. doi:10.1038/s41598-019-51742-4.
- Masuma, R., S. Kashima, M. Kurasaki, and T. Okuno. 2013. Effects of UV wavelength on cell damages caused by UV irradiation in PC12 cells. Journal of Photochemistry and Photobiology. B, Biology 125:202–8. doi:10.1016/j.jphotobiol.2013.06.003.
- Merabishvili, M., C. Vervaet, J.-P. Pirnay, D. De Vos, G. Verbeken, J. Mast, N. Chanishvili, and M. Vaneechoutte. 2013. Stability of Staphylococcus aureus phage ISP after freeze-Drying (Lyophilization). PLos One, 8 (7):e68797. doi:10.1371/journal.pone.0068797.
- Miguéis, S., C. Saraiva, and A. Esteves. 2017. Efficacy of LISTEX P100 at different concentrations for reduction of Listeria monocytogenes inoculated in sashimi. Journal of Food Protection 80 (12):2094–8. doi:10.4315/0362-028x.jfp-17-098.
- Moghtader, F., S. Eğri, and E. Piskin. 2017. Phages in modified alginate beads. Artificial Cells, Nanomedicine, and Biotechnology 45 (2):357–63. doi:10.3109/21691401.2016.1153485.
- Moye, Z., J. Woolston, and A. Sulakvelidze. 2018. Bacteriophage applications for food production and processing. Viruses 10 (4):205. doi:10.3390/v10040205.
- Nanduri, V., S. Balasubramanian, S. Sista, V. J. Vodyanoy, and A. L. Simonian. 2007. Highly sensitive phage-based biosensor for the detection of β-galactosidase. Analytica Chimica Acta 589 (2):166–72. doi:10.1016/j.aca.2007.02.071.
- Niyomdecha, S., W. Limbut, A. Numnuam, P. Kanatharana, R. Charlermroj, N. Karoonuthaisiri, and P. Thavarungkul. 2018. Phage-based capacitive biosensor for Salmonella detection. Talanta 188:658–64. doi:10.1016/j.talanta.2018.06.033.
- O’Connell, L., P. R. Marcoux, and Y. Roupioz. 2021. Strategies for Surface Immobilization of Whole Bacteriophages: A Review. ACS Biomaterials Science & Engineering 7 (6):1987–2014. doi:10.1021/acsbiomaterials.1c00013.
- Oliveira, M., I. Viñas, P. Colàs, M. Anguera, J. Usall, and M. Abadias. 2014. Effectiveness of a bacteriophage in reducing Listeria monocytogenes on fresh-cut fruits and fruit juices. Food Microbiology 38:137–42. doi:10.1016/j.fm.2013.08.018.
- Orlova, E. V. 2012. Bacteriophages and their structural organisation. In Bacteriophages, ed. I. Kurtböke. InTech. doi:10.5772/34642.
- Pacios-Michelena, S., R. Rodríguez-Herrera, G. Rincón-Enríquez, R. Ramos-González, A. C. Flores-Gallegos, M. L. Chávez-González, J. E. de la Peña González, and A. Ilina. 2023. Effect of encapsulation and natural polyphenolic compounds on bacteriophage stability and activity on Escherichia coli in Lactuca sativa L. var. longifolia. Journal of Food Safety, 43 (2):e13000. doi:10.1111/jfs.13000.
- Park, J. H., K. Kim, J. Lee, J. Y. Choi, D. Hong, S. H. Yang, F. Caruso, Y. Lee, and I. S. Choi. 2014. A cytoprotective and degradable metal–polyphenol nanoshell for single-cell encapsulation. Angewandte Chemie (International ed. in English) 53 (46):12420–5. doi:10.1002/anie.201405905.
- Patel, D., Y. Zhou, and R. P. Ramasamy. 2021. A bacteriophage-based electrochemical biosensor for detection of methicillin-resistant Staphylococcus aureus. Journal of the Electrochemical Society 168 (5):057523. doi:10.1149/1945-7111/abef85.
- Pereira, C. S., M. T. N. d Almeida, M. L. Festivo, R. G. Costa, E. M. F. d Reis, and D. d P. Rodrigues. 2007. Salmonella hadar phage types isolated from different sources of foodchain in Brazil. Brazilian Journal of Microbiology 38 (4):620–3. doi:10.1590/S1517-83822007000400008.
- Pereira, C., C. Moreirinha, M. Lewicka, P. Almeida, C. Clemente, A. Cunha, I. Delgadillo, J. L. Romalde, M. L. Nunes, and A. Almeida. 2016. Bacteriophages with potential to inactivate Salmonella Typhimurium: Use of single phage suspensions and phage cocktails. Virus Research 220:179–92. doi:10.1016/j.virusres.2016.04.020.
- Petsong, K., S. Benjakul, and K. Vongkamjan. 2021. Optimization of wall material for phage encapsulation via freeze-drying and antimicrobial efficacy of microencapsulated phage against Salmonella. Journal of Food Science and Technology 58 (5):1937–46. doi:10.1007/s13197-020-04705-x.
- PhageGuard. n.d. Approvals. Accessed August 24, 2023. https://phageguard.com/approvals.
- PhageGuard. n.d. Home. Accessed March 16, 2023. https://phageguard.com/.
- Price, R. E., M. Longtin, S. Conley-Payton, J. A. Osborne, S. D. Johanningsmeier, D. Bitzer, and F. Breidt. 2020. Modeling buffer capacity and pH in acid and acidified foods. Journal of Food Science 85 (4):918–25. doi:10.1111/1750-3841.15091.
- Puapermpoonsiri, U., S. J. Ford, and C. F. Van Der Walle. 2010. Stabilization of bacteriophage during freeze drying. International Journal of Pharmaceutics 389 (1-2):168–75. doi:10.1016/j.ijpharm.2010.01.034.
- Qiu, X. 2012. Heat induced capsid disassembly and DNA release of bacteriophage λ. Plos One, 7 (7):e39793. doi:10.1371/journal.pone.0039793.
- Radford, D., B. Guild, P. Strange, R. Ahmed, L.-T. Lim, and S. Balamurugan. 2017. Characterization of antimicrobial properties of Salmonella phage Felix O1 and Listeria phage A511 embedded in xanthan coatings on Poly(lactic acid) films. Food Microbiology 66:117–28. doi:10.1016/j.fm.2017.04.015.
- Rakhuba, D. V., E. I. Kolomiets, E. S. Dey, and G. I. Novik. 2010. Bacteriophage receptors, mechanisms of phage adsorption and penetration into host cell. Polish Journal of Microbiology 59 (3):145–55. doi:10.33073/pjm-2010-023.
- Ramirez, K., C. Cazarez-Montoya, H. S. Lopez-Moreno, and N. Castro-Del Campo. 2018. Bacteriophage cocktail for biocontrol of Escherichia coli O157:H7: Stability and potential allergenicity study. PloS One 13 (5):e0195023. doi:10.1371/journal.pone.0195023.
- Ravanat, J.-L., T. Douki, and J. Cadet. 2001. Direct and indirect effects of UV radiation on DNA and its components. Journal of Photochemistry and Photobiology. B, Biology 63 (1-3):88–102. doi:10.1016/s1011-1344(01)00206-8.
- Richards, K., and D. J. Malik. 2021a. Bacteriophage encapsulation in pH-responsive core-shell capsules as an animal feed additive. Viruses 13 (6):1131. doi:10.3390/v13061131.
- Richards, K., and D. J. Malik. 2021b. Microencapsulation of bacteriophages using membrane emulsification in different pH-triggered controlled release formulations for oral administration. Pharmaceuticals (Basel, Switzerland) 14 (5):424. doi:10.3390/ph14050424.
- Robbins, R. J. 2003. Phenolic acids in foods: An overview of analytical methodology. Journal of Agricultural and Food Chemistry 51 (10):2866–87. doi:10.1021/jf026182t.
- Rodriguez, R. A., S. Bounty, S. Beck, C. Chan, C. Mcguire, and K. G. Linden. 2014. Photoreactivation of bacteriophages after UV disinfection: Role of genome structure and impacts of UV source. Water Research 55:143–9. doi:10.1016/j.watres.2014.01.065.
- Rodriguez-Mozaz, S., S. Chamorro, E. Marti, B. Huerta, M. Gros, A. Sànchez-Melsió, C. M. Borrego, D. Barceló, and J. L. Balcázar. 2015. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Research 69:234–42. doi:10.1016/j.watres.2014.11.021.
- Rosner, D., and J. Clark. 2021. Formulations for bacteriophage therapy and the potential uses of immobilization. Pharmaceuticals (Basel, Switzerland) 14 (4):359. doi:10.3390/ph14040359.
- Saltmarsh, M. 2020. Food additives and why they are used. In Saltmarsh’s essential guide to food additives, 1–9. London, UK: The Royal Society of Chemistry. doi:10.1039/9781839161063-00001.
- Samtlebe, M., F. Ergin, N. Wagner, H. Neve, A. Küçükçetin, C. M. A. P. Franz, K. J. Heller, J. Hinrichs, and Z. Atamer. 2016. Carrier systems for bacteriophages to supplement food systems: Encapsulation and controlled release to modulate the human gut microbiota. LWT - Food Science and Technology 68:334–40. doi:10.1016/j.lwt.2015.12.039.
- Samtlebe, M., S. Denis, S. Chalancon, Z. Atamer, N. Wagner, H. Neve, C. Franz, H. Schmidt, S. Blanquet-Diot, and J. Hinrichs. 2018. Bacteriophages as modulator for the human gut microbiota: Release from dairy food systems and survival in a dynamic human gastrointestinal model. LWT 91:235–41. doi:10.1016/j.lwt.2018.01.033.
- Sarhan, W. A., and H. M. Azzazy. 2017. Apitherapeutics and phage-loaded nanofibers as wound dressings with enhanced wound healing and antibacterial activity. Nanomedicine (London, England) 12 (17):2055–67. doi:10.2217/nnm-2017-0151.
- Schlesinger, M. 1932. Adsorption of bacteriophages to homologous bacteria. II. Quantitative investigation of adsorption velocity and saturation. Estimation of the particle size of the bacteriophage. Immunitaetsforschung 114:149–60.
- Seeley, N. D., and S. B. Primrose. 1980. The effect of temperature on the ecology of aquatic bacteriophages. Journal of General Virology 46 (1):87–95. doi:10.1099/0022-1317-46-1-87.
- Sezer, B., E. K. Tayyarcan, and I. H. Boyaci. 2022. The use of bacteriophage-based edible coatings for the biocontrol of Salmonella in strawberries. Food Control. 135:108812. doi:10.1016/j.foodcont.2022.108812.
- Shabani, A., M. Zourob, B. Allain, C. A. Marquette, M. F. Lawrence, and R. Mandeville. 2008. Bacteriophage-modified microarrays for the direct impedimetric detection of bacteria. Analytical Chemistry 80 (24):9475–82. doi:10.1021/ac801607w.
- Shahin, K., M. Barazandeh, L. Zhang, A. Hedayatkhah, T. He, H. Bao, M. Mansoorianfar, M. Pang, H. Wang, R. Wei, et al. 2021. Biodiversity of new lytic bacteriophages infecting Shigella spp. in freshwater environment [Original Research]. Frontiers in Microbiology 12:619323. doi:10.3389/fmicb.2021.619323.
- Shannon, R., D. R. Radford, and S. Balamurugan. 2020. Impacts of food matrix on bacteriophage and endolysin antimicrobial efficacy and performance. Critical Reviews in Food Science and Nutrition 60 (10):1631–40. doi:10.1080/10408398.2019.1584874.
- Shao, Y., and I.-N. Wang. 2008. Bacteriophage adsorption rate and optimal lysis time. Genetics 180 (1):471–82. doi:10.1534/genetics.108.090100.
- Shapira, A., and A. Kohn. 1974. The effects of freeze-drying on bacteriophage T4. Cryobiology 11 (5):452–64. doi:10.1016/0011-2240(74)90113-8.
- Sharp, D. G., A. E. Hook, A. R. Taylor, D. Beard, and J. W. Beard. 1946. Sedimentation characters and pH stability of the T2 bacteriophage of Escherichia coli. The Journal of Biological Chemistry 165 (1):259–70. doi:10.1016/s0021-9258(17)41228-2.
- Sheng, H., H. J. Knecht, I. T. Kudva, and C. J. Hovde. 2006. Application of bacteriophages to control intestinal Escherichia coli O157:H7 levels in ruminants. Applied and Environmental Microbiology 72 (8):5359–66. doi:10.1128/AEM.00099-06.
- Shi, X., A. Namvar, M. Kostrzynska, R. Hora, and K. Warriner. 2007. Persistence and growth of different Salmonella Serovars on pre- and postharvest tomatoes. Journal of Food Protection 70 (12):2725–31. doi:10.4315/0362-028x-70.12.2725.
- Sillankorva, S. M., H. Oliveira, and J. Azeredo. 2012. Bacteriophages and their role in food safety. International Journal of Microbiology 2012:863945. doi:10.1155/2012/863945.
- Silva Batalha, L., M. T. Pardini Gontijo, A. Vianna Novaes De Carvalho Teixeira, D. Meireles Gouvêa Boggione, M. E. Soto Lopez, M. Renon Eller, and R. C. Santos Mendonça. 2021. Encapsulation in alginate-polymers improves stability and allows controlled release of the UFV-AREG1 bacteriophage. Food Research International (Ottawa, Ont.) 139:109947. doi:10.1016/j.foodres.2020.109947.
- Slieman, T. A., and W. L. Nicholson. 2000. Artificial and solar UV radiation induces strand breaks and cyclobutane pyrimidine dimers in Bacillus subtilis spore DNA. Applied and Environmental Microbiology 66 (1):199–205. doi:10.1128/AEM.66.1.199-205.2000.
- Smith, G. P. 1985. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science (New York, N.Y.) 228 (4705):1315–7. doi:10.1126/science.4001944.
- Soffer, N., J. Woolston, M. Li, C. Das, and A. Sulakvelidze. 2017. Bacteriophage preparation lytic for Shigella significantly reduces Shigella sonnei contamination in various foods. PloS One 12 (3):e0175256. doi:10.1371/journal.pone.0175256.
- Soto, M. J., J. Retamales, H. Palza, and R. Bastías. 2018. Encapsulation of specific Salmonella Enteritidis phage f3αSE on alginate-spheres as a method for protection and dosification. Electronic Journal of Biotechnology 31:57–60. doi:10.1016/j.ejbt.2017.11.006.
- Stanford, K., T. A. Mcallister, Y. D. Niu, T. P. Stephens, A. Mazzocco, T. E. Waddell, and R. P. Johnson. 2010. Oral delivery systems for encapsulated bacteriophages targeted at Escherichia coli O157:H7 in feedlot cattle. Journal of Food Protection 73 (7):1304–12. doi:10.4315/0362-028x-73.7.1304.
- Sun, W., J. Xu, B. Liu, Y.-D. Zhao, L. Yu, and W. Chen. 2022. Controlled release of metal phenolic network protected phage for treating bacterial infection. Nanotechnology 33 (16):165102. doi:10.1088/1361-6528/ac4aa7.
- Sun, W., L. Brovko, and M. Griffiths. 2001. Use of bioluminescent Salmonella for assessing the efficiency of constructed phage-based biosorbent. Journal of Industrial Microbiology & Biotechnology 27 (2):126–8. doi:10.1038/sj.jim.7000198.
- Takahashi, R., S. Bowatte, K. Taki, Y. Ohashi, S. Asakawa, and M. Kimura. 2011. High frequency of phage-infected bacterial cells in a rice field soil in Japan. Soil Science and Plant Nutrition 57 (1):35–9. doi:10.1080/00380768.2010.550864.
- Tanaka, C., K. Yamada, H. Takeuchi, Y. Inokuchi, A. Kashiwagi, T. Toba, and J. Björkroth. 2018. A lytic bacteriophage for controlling Pseudomonas lactis in raw cow’s milk. Applied and Environmental Microbiology 84 (18):e00111-00118. doi:10.1128/AEM.00111-18.
- Tang, M., P. Dettmar, and H. Batchelor. 2005. Bioadhesive oesophageal bandages: Protection against acid and pepsin injury. International Journal of Pharmaceutics 292 (1-2):169–77. doi:10.1016/j.ijpharm.2004.11.039.
- Tang, Z., X. Huang, P. M. Sabour, J. R. Chambers, and Q. Wang. 2015. Preparation and characterization of dry powder bacteriophage K for intestinal delivery through oral administration. LWT - Food Science and Technology 60 (1):263–70. doi:10.1016/j.lwt.2014.08.012.
- Tang, Z., X. Huang, S. Baxi, J. R. Chambers, P. M. Sabour, and Q. Wang. 2013. Whey protein improves survival and release characteristics of bacteriophage Felix O1 encapsulated in alginate microspheres. Food Research International 52 (2):460–6. doi:10.1016/j.foodres.2012.12.037.
- Tawil, N., E. Sacher, R. Mandeville, and M. Meunier. 2013. Strategies for the immobilization of bacteriophages on gold surfaces monitored by surface plasmon resonance and surface morphology. The Journal of Physical Chemistry C 117 (13):6686–91. doi:10.1021/jp400565m.
- Thakral, S., N. K. Thakral, and D. K. Majumdar. 2013. Eudragit®: A technology evaluation. Expert Opinion on Drug Delivery 10 (1):131–49. doi:10.1517/17425247.2013.736962.
- Thu, B., P. Bruheim, T. Espevik, O. Smidsrød, P. Soon-Shiong, and G. Skjåk-Braek. 1996. Alginate polycation microcapsules: I. Interaction between alginate and polycation. Biomaterials 17 (10):1031–40. doi:10.1016/0142-9612(96)84680-1.
- Thung, T. Y., J. M. Krishanthi Jayarukshi Kumari Premarathne, W. San Chang, Y. Y. Loo, Y. Z. Chin, C. H. Kuan, C. W. Tan, D. F. Basri, C. W. Jasimah Wan Mohamed Radzi, and S. Radu. 2017. Use of a lytic bacteriophage to control Salmonella Enteritidis in retail food. LWT 78:222–5. doi:10.1016/j.lwt.2016.12.044.
- Tokman, J. I., D. J. Kent, M. Wiedmann, and T. Denes. 2016. Temperature significantly affects the plaquing and adsorption efficiencies of Listeria phages [Original Research]. Frontiers in Microbiology 7:631. doi:10.3389/fmicb.2016.00631.
- Tolba, M., O. Minikh, L. Y. Brovko, S. Evoy, and M. W. Griffiths. 2010. Oriented immobilization of bacteriophages for biosensor applications. Applied and Environmental Microbiology 76 (2):528–35. doi:10.1128/AEM.02294-09.
- Torres-Acosta, M. A., V. Clavijo, C. Vaglio, A. F. González-Barrios, M. J. Vives-Flórez, and M. Rito-Palomares. 2019. Economic evaluation of the development of a phage therapy product for the control of Salmonella in poultry. Biotechnology Progress 35 (5):e2852. doi:10.1002/btpr.2852.
- Tóth, K., J. Bolard, G. Rontó, and D. Aslanian. 1984. UV-Induced small structural changes in the T7 bacteriophage studied by melting methods. Biophysics of Structure and Mechanism 10 (4):229–39. doi:10.1007/bf00535551.
- Towse, A., C. K. Hoyle, J. Goodall, M. Hirsch, J. Mestre-Ferrandiz, and J. H. Rex. 2017. Time for a change in how new antibiotics are reimbursed: Development of an insurance framework for funding new antibiotics based on a policy of risk mitigation. Health Policy (Amsterdam, Netherlands) 121 (10):1025–30. doi:10.1016/j.healthpol.2017.07.011.
- Tucker, G. A. 1993. Introduction. In Biochemistry of fruit ripening, eds. G. B. Seymour, J. E. Taylor, & G. A. Tucker, 1–51. Dordrecht, NL: Springer Netherlands. doi:10.1007/978-94-011-1584-1_1.
- Vandenheuvel, D., A. Singh, K. Vandersteegen, J. Klumpp, R. Lavigne, and G. Van den Mooter. 2013. Feasibility of spray drying bacteriophages into respirable powders to combat pulmonary bacterial infections. European Journal of Pharmaceutics and Biopharmaceutics : official Journal of Arbeitsgemeinschaft Fur Pharmazeutische Verfahrenstechnik e.V 84 (3):578–82. doi:10.1016/j.ejpb.2012.12.022.
- Vandenheuvel, D., J. Meeus, R. Lavigne, and G. Van den Mooter. 2014. Instability of bacteriophages in spray-dried trehalose powders is caused by crystallization of the matrix. International Journal of Pharmaceutics 472 (1-2):202–5. doi:10.1016/j.ijpharm.2014.06.026.
- Vinner, G. K., G. T. Vladisavljevic, M. R. J. Clokie, and D. J. Malik. 2017. Microencapsulation of Clostridium difficile specific bacteriophages using microfluidic glass capillary devices for colon delivery using pH triggered release. PloS One 12 (10):e0186239. doi:10.1371/journal.pone.0186239.
- Vinner, G. K., K. Richards, M. Leppanen, A. P. Sagona, and D. J. Malik. 2019. Microencapsulation of enteric bacteriophages in a pH-responsive solid oral dosage formulation using a scalable membrane emulsification process. Pharmaceutics 11 (9):475. doi:10.3390/pharmaceutics11090475.
- Vinner, G. K., Z. Rezaie-Yazdi, M. Leppanen, A. G. F. Stapley, M. C. Leaper, and D. J. Malik. 2019. Microencapsulation of Salmonella-specific bacteriophage Felix O1 using spray-drying in a pH-responsive formulation and direct compression tableting of powders into a solid oral dosage form. Pharmaceuticals (Basel, Switzerland) 12 (1):43. doi:10.3390/ph12010043.
- Vonasek, E. L., A. H. Choi, J. Sanchez, and N. Nitin. 2018. Incorporating phage therapy into WPI dip coatings for applications on fresh whole and cut fruit and vegetable surfaces. Journal of Food Science 83 (7):1871–9. doi:10.1111/1750-3841.14188.
- Vonasek, E., P. Le, and N. Nitin. 2014. Encapsulation of bacteriophages in whey protein films for extended storage and release. Food Hydrocolloids. 37:7–13. doi:10.1016/j.foodhyd.2013.09.017.
- Vonasek, E., P. Lu, Y.-L. Hsieh, and N. Nitin. 2017. Bacteriophages immobilized on electrospun cellulose microfibers by non-specific adsorption, protein–ligand binding, and electrostatic interactions. Cellulose 24 (10):4581–9. doi:10.1007/s10570-017-1442-3.
- Wagenaar, J. A., M. A. P. V. Bergen, M. A. Mueller, T. M. Wassenaar, and R. M. Carlton. 2005. Phage therapy reduces Campylobacter jejuni colonization in broilers. Veterinary Microbiology 109 (3-4):275–83. doi:10.1016/j.vetmic.2005.06.002.
- Wall, S. K., J. Zhang, M. H. Rostagno, and P. D. Ebner. 2010. Phage therapy to reduce preprocessing Salmonella infections in market-weight swine. Applied and Environmental Microbiology 76 (1):48–53. doi:10.1128/AEM.00785-09.
- Weng, S., A. López, S. Sáez-Orviz, I. Marcet, P. García, M. Rendueles, and M. Díaz. 2021. Effectiveness of bacteriophages incorporated in gelatine films against Staphylococcus aureus. Food Control. 121:107666. doi:10.1016/j.foodcont.2020.107666.
- Wiggins, B. A., and M. Alexander. 1985. Minimum bacterial density for bacteriophage replication: Implications for significance of bacteriophages in natural ecosystems. Applied and Environmental Microbiology 49 (1):19–23. doi:10.1128/aem.49.1.19-23.1985.
- Xu, J., C. Zhao, Y. Chau, and Y.-K. Lee. 2020. The synergy of chemical immobilization and electrical orientation of T4 bacteriophage on a micro electrochemical sensor for low-level viable bacteria detection via Differential Pulse Voltammetry. Biosensors & Bioelectronics 151:111914. doi:10.1016/j.bios.2019.111914.
- Yan, G., H. Wang, J. Lv, C. Li, and B. Zhang. 2022. Surface modification of nucleopolyhedrovirus with polydopamine to improve its properties. Pest Management Science 78 (2):456–66. doi:10.1002/ps.6640.
- Yanagida, M., Y. Suzuki, and T. Toda. 1984. Molecular organization of the head of bacteriophage Teven: Underlying design principles. Advances in Biophysics 17:97–146. doi:10.1016/0065-227x(84)90026-1.
- Ye, J., M. Kostrzynska, K. Dunfield, and K. Warriner. 2009. Evaluation of a biocontrol preparation consisting of Enterobacter asburiae JX1 and a lytic bacteriophage cocktail to suppress the growth of Salmonella Javiana associated with tomatoes. Journal of Food Protection 72 (11):2284–92. doi:10.4315/0362-028x-72.11.2284.
- Yin, H., J. Li, H. Huang, Y. Wang, X. Qian, J. Ren, F. Xue, J. Dai, and F. Tang. 2021. Microencapsulated phages show prolonged stability in gastrointestinal environments and high therapeutic efficiency to treat Escherichia coli O157:H7 infection. Veterinary Research 52 (1):118. doi:10.1186/s13567-021-00991-1.
- Yin, Y., P. E. Ni, D. Liu, S. Yang, A. Almeida, Q. Guo, Z. Zhang, L. Deng, and D. Wang. 2019. Bacteriophage potential against Vibrio parahaemolyticus biofilms. Food Control. 98:156–63. doi:10.1016/j.foodcont.2018.11.034.
- Yuan, Y. Y., L. L. Wang, X. Y. Li, D. M. Tan, C. Cong, and Y. P. Xu. 2019. Efficacy of a phage cocktail in controlling phage resistance development in multidrug resistant Acinetobacter baumannii. Virus Research 272:8, Article 197734. doi:10.1016/j.virusres.2019.197734.
- Zaburlin, D., A. Quiberoni, and D. Mercanti. 2017. Changes in environmental conditions modify infection kinetics of dairy phages. Food and Environmental Virology 9 (3):270–6. doi:10.1007/s12560-017-9296-2.
- Zhang, H., Z. Yang, Y. Zhou, H. Bao, R. Wang, T. Li, M. Pang, L. Sun, and X. Zhou. 2018. Application of a phage in decontaminating Vibrio parahaemolyticus in oysters. International Journal of Food Microbiology 275:24–31. doi:10.1016/j.ijfoodmicro.2018.03.027.
- Zhou, H.-X., and X. Pang. 2018. Electrostatic interactions in protein structure, folding, binding, and condensation. Chemical Reviews 118 (4):1691–741. doi:10.1021/acs.chemrev.7b00305.