Abstract
Probiotics are not only a food supplement, but they have shown great potential in their nutritional, health and therapeutic effects. To maximize the beneficial effects of probiotics, it is commonly achieved by adding prebiotics. Prebiotics primarily comprise indigestible carbohydrates, specific peptides, proteins, and lipids, with oligosaccharides being the most extensively studied prebiotics. However, these rapidly fermenting oligosaccharides have many drawbacks and can cause diarrhea and flatulence in the body. Hence, the exploration of new prebiotic is of great interest. Besides oligosaccharides, protein hydrolysates have been demonstrated to enhance the expression of beneficial properties of probiotics. Consequently, this paper outlines the mechanism underlying the action of protein hydrolysates on probiotics, as well as the advantageous impacts of proteins hydrolysates derived from various food sources on probiotics. In addition, this paper also reviews the currently reported biological activities of protein hydrolysates. The aim is a theoretical basis for the development and implementation of novel prebiotics.
In 2001, the Food and Agriculture Organization of the United Nations (FAO) and the WHO defined probiotics as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (Hill et al. Citation2014). Not only is it a food supplement, but it is increasingly valued for its nutritional, health and therapeutic effects (Yadav et al. Citation2022). Recently, probiotics have been found to be effective in treating diseases such as dermatitis, asthma, gout, diabetes, hepatic encephalopathy and gastrointestinal infections (Idrees et al. Citation2022; Liu, Walsh, and Sheehan Citation2019). In order to derive health advantages from probiotic products, it is crucial to determine the existence of an adequate number of viable bacteria in the food before consumption, as well as the successful delivery of probiotics to the colon subsequent to consumption. Research indicates that a probiotic product must contain at least 106-107 CFU/g of live bacteria to exhibit efficacy (Tripathi and Giri Citation2014). However, during processing, transport, storage, marketing, and consumption, the number of bacteria that survive rapidly decreases. Various factors, including heat, oxygen, water activity, pH, enzymes, bile salts, and other stress conditions, have the potential to influence strain activity (Tripathi and Giri Citation2014; Champagne et al. Citation2011). Adding growth promoting factors, known as prebiotics, to probiotics is a successful strategy for improving its survival rate. International Scientific Association for Probiotics and Prebiotics (ISAPP) defines prebiotics as “a substrate that was selectively utilized by host microorganisms conferring a health benefit” (Gibson et al. Citation2017). It is believed that prebiotics function to improve immunity, resist pathogens, influence metabolism, increase mineral absorption, and enhance health (Peredo-Lovillo, Romero-Luna, and Jiménez-Fernández Citation2020). Based on the established definition of prebiotics, any form of sustenance that successfully reaches the colon, including indigestible carbohydrates, specific peptides, proteins, and particular lipids, possesses the potential to be regarded as candidate for prebiotic (Gibson et al. Citation2017; Zhang et al. Citation2020). Widely used prebiotics include xylo-oligosaccharides, galacto-oligosaccharides, lactulose as well as inulin and its fructose-oligosaccharide derivatives (You et al. Citation2022), but these rapidly fermenting oligosaccharides have many drawbacks, causing diarrhea and flatulence in human body. Hence, the identification of new prebiotic types was currently of great interest to researchers. It has recently been shown that protein hydrolysates can also promote the growth of probiotics (Zhang et al. Citation2020; Zhang et al. Citation2022). So, this paper outlines the mechanism underlying the action of protein hydrolysates on probiotics and the beneficial effects of proteins from different food sources on probiotics. Meanwhile, biological activities of currently reported protein hydrolysates are reviewed in this paper.
1. Mechanism of action of protein hydrolysates on probiotics
The interaction between protein hydrolysates and probiotics has been studied in recent years, but no consistent conclusion has been reached. There is some evidence that protein hydrolysates offer free amino acids, low molecular weight peptides, and growth factors, which potentially contribute to bacterial growth and resistance (Ummadi and Curic-Bawden Citation2008). Other researchers have suggested that protein hydrolysates possess the potential to function as stimulators of polypeptide transport systems, thereby promoting the expression of a variety of beneficial traits (Doeven, Kok, and Poolman Citation2005).
1.1. Providing amino acids, oligopeptides and growth factors for probiotics
Amino acids have many important roles in the growth and metabolism of strains, including regulation of intracellular pH, energy supply, regulation of redox balance, and anti-stress (De Angelis et al. Citation2016). The amino acid metabolism of lactic acid bacteria (LAB) includes mainly aspartic acid metabolism, glutamic acid metabolism, methionine metabolism, alanine and proline metabolism, arginine metabolism, and glycine metabolism. The growth of LAB is limited by the availability of free amino acids and/or peptides due to the auxotrophic effects of multiple amino acids (De Angelis et al. Citation2016). LAB can obtain energy (e.g., ATP) from free amino acids during the stationary phase or under environmental stress (e.g., starvation, acid) (Wu et al. Citation2011; Suokko et al. Citation2008). Furthermore, enhanced amounts of enzymes involved in the catabolism of free amino acids, such as cystathionine beta-lyase/cystathionine gamma-synthase and succinate dehydrogenase, facilitates the synthesis of lactate, acetate and ATP via the pyruvate pathway (De Angelis et al. Citation2016). Indeed, one study showed that protein hydrolysates obtained by enzymatic digestion and spray drying contained high levels of free amino acids, among which threonine and leucine, supporting the growth of many LAB such as Lactobacillus plantarum (L. plantarum), Lactobacillus acidophilus (L. acidophilus), and Lactobacillus reuteri (L. reuteri) (Solval et al. Citation2019). The impact of incorporating amino acids such as L-proline, L-glutamic acid, L-glycine, L-serine, and L-isoleucine into the medium on the growth of LAB has been reported (Jingjing et al. Citation2023). Jingjing et al. (Citation2023) reported that L-glutamic acid improved the lyophilization survival of four strains of L. plantarum. This improvement was attributed to the modulation of the bacterial cell membrane and cell wall structure. Furthermore, the internal mechanism was also explored to include two main pathways. Initially, the rapid growth of the strain and subsequent acid production caused a swift decrease in pH within the culture environment. The acidic environment subsequently induced the overexpression of the dnaK gene and secG gene, leading to increased levels of DnaK protein. Consequently, the DnaK protein demonstrated an augmented capacity for binding to the cell membrane, consequently reinforcing its structural integrity. Additionally, the overexpression of the murL, murD, and vanY genes resulted in enhanced peptidoglycan biosynthesis and reinforced cellular wall integrity. Chen et al. (Citation2012) observed a notable stimulatory impact of glutamic acid and lysine on the growth of Streptococcus thermophilus (S. thermophilus). Furthermore, the concentrations of these amino acids were positively correlated with the number of viable bacteria. Stuart, Chou, and Weimer (Citation1999) showed that arginine can act as a precursor for the metabolic synthesis of certain growth-essential nutrients in strains, and that arginine can contribute to the increase in bacterial mass through the metabolic energy production (ADI pathway).
Several researchers have undertaken surveillance of the ingestion of peptides and amino acids by LAB. Moreover, their investigations have substantiated that the proliferation of LAB is contingent upon oligopeptides serving as a nitrogenous substrate (Juillard et al. Citation1995). Oligopeptides have also been shown to be a major source of nitrogen for LAB (Zhao et al. Citation2013). Ding and Li (Citation2021) observed that the utilization of walnut oligopeptides resulted in the augmentation of bacterial growth and reproduction. This enhancement was attributed to the modulation of the bacterial quorum sensing system, which regulated the secretion of biofilm and extracellular polymeric substances. Consequently, the bacteria exhibited an improved capacity to withstand unfavorable environmental conditions. According to Zhang et al. (Citation2021), soy protein hydrolysates enhance Lactobacillus rhamnosus (L. rhamnosus) growth and short-chain fatty acid synthesis. When co-cultured with E. coli, these hydrolysates also diminished E. coli’s competitive ability and enhanced L. rhamnosus’ resistance. Oligopeptides exhibit enhanced nutritional value in comparison to free amino acids due to their capacity to facilitate the transport of multiple amino acids into the cell by consuming a single ATP molecule. The direct transportation of peptides (consisting of 2-3 units) into the cell offers the advantage of reducing the metabolic energy required for amino acid uptake (Juillard et al. Citation1995). In addition, the bioefficacy of particular protein hydrolysates in culture media as a nitrogen-containing substrate depends on a number of factors, including the composition of amino acids, the amino acid form (which was limited to L-amino acids or L-stereoisomers for cellular biomass assimilation), and the form of peptides (encompassing oligopeptides, dipeptides, tripeptides, or free amino acids) (Ummadi and Curic-Bawden Citation2008).
Interestingly, researchers observed that the inclusion of protein hydrolysates derived from poultry bone and meat in the culture resulted in a noteworthy enhancement in the growth promotion of LAB, surpassing the efficacy of two widely used commercial hydrolysates. Additionally, the activity of aminopeptidases PepC, PepN, PepL, and PepX exhibited a notable increase (Meli et al. Citation2014). Leu-Pro was also shown to regulate Lactobacillus helveticus (L. helveticus) protease activity by acting as an effector. In addition, it has been reported that peptide p (CHWPR) acts as a transcription factor, augmenting the expression of c-myc and interleukin (IL)-6 genes. This phenomenon may subsequently activate the intestinal defense mechanisms in Bifidobacterium (Mitsuma et al. Citation2008).
1.2. As promoter of polypeptide transport systems
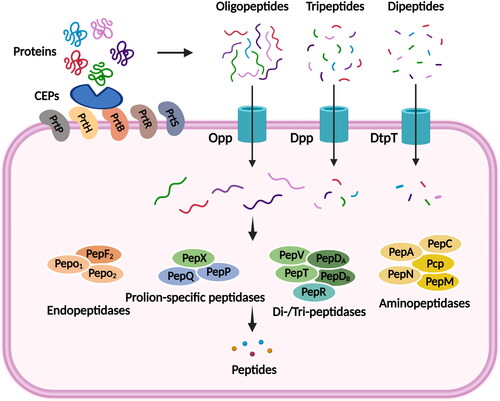
LAB cannot directly use exogenous protein and inorganic nitrogen sources during their growth and metabolism. In order to meet their nutritional requirements for growth, they must degrade exogenous proteins or peptides through a proteolysis system. The proteolysis system of LAB consists of a cell wall-associated protease (CEPs), several membrane-based peptide transport systems (DtpT, Dpp and Opp), and a series of intracellular peptidases (Picon, García-Casado, and Nuñez Citation2010). The utilization of exogenous proteins by the strain begins with the degradation of the exogenous proteins into dipeptides, tripeptides and oligopeptides by cell wall proteases. Subsequently, the corresponding peptides are then transferred into the cell via a specific peptide transport system. Ultimately, the transferred peptides were again degraded to form briefer peptides and amino acids under the synergistic action of multiple peptidase enzymes in the cell, thus meeting the nutritional requirements of the strain. Comparative genomic analyses showed significant differences in the components of the proteolysis system of LAB at the strain level (Liu et al. Citation2010). This strain specificity explains the high phenotypic diversity of proteolytic activity and the resulting release of bioactive peptides, and also makes it necessary to analyze in depth the characteristics of each strain selected for functional food applications.
illustrates the protein hydrolysis system of LAB. The use of exogenous proteins by strains begins with the degradation of proteins by CEPs, which was the serine protease of the Bacillus subtilis (B. subtilis) protease family (Sinezen Citation1999). Current research on CEPs has focused more on lactic acid bacteria grown in milk, with the most widely studied being Lactococcus lactis (L. lactis). Five distinct enzymes were cloned and identified from LAB, namely PrtP from L. lactis and Lactobacillus paracasei (L. paracasei) (Holck and Naes Citation1992), PrtH from L. lactis (Pederson et al. Citation1999), PrtR from L. rhamnosus (Pastar et al. Citation2003), PrtS from S. thermophilus (Fernandez-Espla and Rul Citation1999) and PrtB from Lactobacillus bulgaricus (L. bulgaricus) (Gilbert et al. Citation1996). The second stage of protein utilization was the transport of dipeptides, tripeptides and oligopeptides into the cell via different peptide transport systems. Biochemical analysis has shown that Lactobacillus has at least three functional peptide transport systems, DtpT, Dpp and Opp (Kunji et al. Citation1996). The Opp and dtpT genes were initially cloned and induced, leading to the identification of the corresponding transport proteins (Opp and DtpT) responsible for facilitating the absorption of oligopeptides and di/tripeptides, respectively (Doeven, Kok, and Poolman Citation2005). The Opp system is a member of the ABC transporter protein superfamily, comprising five distinct proteins: OppA, OppB, OppC, OppD, and OppF. OppA functions as an oligopeptide-binding protein, while OppB and OppC serve as integral membrane proteins. OppD and OppF, on the other hand, act as nucleotide-binding proteins (Zhang et al. Citation2020). Dpp systems also is members of the ABC (ATP-binding cassette) transporter family, and is composed of two (DppA and DppP) oligopeptide binding proteins and a membrane-bound complex formed by two hydrophobic integral membrane proteins and two membrane associated proteins that carry the ATP (adenosine 5′-triphosphate) binding cassette motif (Doeven, Kok, and Poolman Citation2005). OppA transports peptides ranging in size from 4 to 35 amino acids (Detmers et al. Citation2000). DppA is specific for di- and tripeptides, whereas DppP transports peptides up to at least 9 residues in length (Lamarque et al. Citation2004). DtpT functioned as a secondary transporter belonging to the PTR family of peptide transporters (Picon, García-Casado, and Nuñez Citation2010). The regulation of peptide transport is achieved through the action of the multipotent transcriptional repressor CodY, which detects the intracellular pool of branched amino acids (Guédon, Serror, et al. Citation2001). A variety of intracellular peptidases with different specificities, such as endonucleases, aminopeptidases and proline-specific peptidases, hydrolyze the peptide fragments that were transported into the cell into free amino acids for strain growth, the third step in protein utilization. Currently, more than a dozen peptidases have been purified and identified from different LAB. The main characteristic of endopeptidases such as PepF and PepO was that they could not hydrolyze intact casein, but could hydrolyze the peptide bonds within casein-derived peptides, resulting in smaller peptides. Studies have shown that PepF, besides cleaving oligopeptides of 7-17 residues in length, was also important for the renewal of proteins in L. lactis in the absence of nitrogen (Christensson et al. Citation2002; Sridhar et al. Citation2005). Additionally, the broadly specific metallopeptidases PepN and cysteine peptidase PepC can also act on oligopeptides. The primary attribute of these enzymes lies in their hydrolysis from the N-terminal end of the peptide chain, whereby the specificity of their action is contingent upon the length of the chain and the composition of the N-terminal amino acid residues (Christensen et al. Citation1999). The resulting short chain peptides such as dipeptides and tripeptides were further hydrolyzed to free amino acids by the dipeptidases PepV and PepD, the tripeptidases PepT and some aminopeptidases (Klein et al. Citation1995). Other peptidases with more substrate specificity (Fernandez-Espla and Rul Citation1999; Christensen et al. Citation1999; Kunji et al. Citation1996): as PepP prefers to hydrolyze intermediate proline-containing tripeptides, PepQ exhibits enzymatic activity in the cleavage of dipeptides containing proline at the second position, while PepR and PepI demonstrate a greater affinity for dipeptides with proline at the second position.
Tynkkynen et al. (Citation1993) showed that the Lactobacillus MG1363 utilized the DtpT and Dpp systems to take up short peptides (2-3 residues) and the Opp system to take up oligopeptides as a means of promoting bacterial growth. Detmers et al. (Citation1998) conducted a study to investigate the selectivity of peptide uptake through the oligopeptide transport system of LAB. They utilized casein-derived peptides and observed that Opp was capable of transporting peptides consisting of up to 18 residues. Furthermore, they discovered that the affinity of nine- and ten-peptides exhibited a 2-4 fold alteration when the carboxyl terminus of the amino acid was modified. Picon, García-Casado, and Nuñez (Citation2010) researched the peptide transport capacity characterized by the growth of 24 wild Lactobacillus strains containing several peptides of essential amino acids and found that most wild LAB strains (18 out of 24) contained Opp and Dpp oligopeptide transport systems, a fact that may demonstrate the advantage of using amino acids as substrates for LAB growth. The inclusion of casein hydrolysate in sourdough facilitated the proliferation of Lactobacillus sanfranciscensis (L. sanfranciscensis) strain LTH 2581, a strain devoid of proteinase activity. During the process of sourdough fermentation, the expression of Opp, DtpT, PepT, PepC, PepR, and PepN was observed. The introduction of the peptide led to a decrease in the transcription of both dtpT and opp-pepN in L. sanfranciscensis cells that were in the exponential growth phase. Therefore, the observed increase in amino nitrogen content in dough caused by L. sanfranciscensis can be attributed to peptide hydrolysis rather than proteolysis. The proteins identified in L. sanfranciscensis exhibited a significant resemblance to the corresponding proteins found in L. lactis (Guédon, Renault, et al. Citation2001). However, it was observed that the expression of Opp was significantly diminished in both organisms upon the introduction of peptides, while the expression of PepT was reduced by a factor of two to five. Furthermore, DtpT exhibited regulation in response to the availability of peptides in L. sanfranciscensis, but not in L. lactis. Therefore, the uptake characteristics of probiotics for peptides in the medium under different protein hydrolysate culture conditions remain to be studied.
2. Beneficial impact of protein hydrolysates from diverse sources on probiotics
Protein sources, namely casein, meat, gelatin, and soya, exhibit disparities in both their overall amino acid composition and protein structure. Consequently, protein hydrolysates derived from diverse sources elicit distinct impacts on different strains, with certain hydrolysates promoting organismal growth and others instigating specific amino acid metabolic pathways. It is imperative to conduct a comprehensive investigation into the impacts of diverse protein hydrolysates on LAB. provides a concise overview of the hydrolysis parameters employed for different protein hydrolysate sources, as well as the advantageous outcomes observed on probiotics.
Table 1. Effect of protein hydrolysates on probiotics.
2.1. Casein
Casein is the main protein component of milk, accounting for approximately 80% of the total protein content, making it the most studied peptide supplement in microbiology (Kunz and Lönnerdal Citation1990). Many casein hydrolysis contribute significantly to the proliferation, viability and production of LAB and synthesis of extracellular polysaccharides in LAB, such as Lactobacillus delbrueckii (L. delbrueckii), S. thermophilus, L. plantarum, L. sanfranciscensis, Lactobacillus brevis (L. brevis), and were also able to promote growth of Bifidobacteria and enhance proteolytic activity of L. acidophilus and L. helveticus (Dysin et al. Citation2023). Furthermore, the enhanced efficacy of the papain-produced hydrolysate, in comparison to casein hydrolyzed by alternative enzymes, can likely be attributed to the greater susceptibility of smaller peptide fragments (less than 3 kDa) to bacterial absorption (Dysin et al. Citation2023).
The stimulation of LAB growth is significantly influenced by the molecular weight distribution of peptides obtained from casein hydrolysates, irrespective of the amino acid composition (St-Gelais et al. Citation1993). Arakawa et al. (Citation2015) showed suboptimal growth of all three strains of Lactobacillus gasseri (L. gasseri) cultured in a medium without a nitrogen source. However, the addition of a casein-derived peptide mixture promoted growth. In contrast, the addition of casein or casein-derived amino acid mixtures had scant effect. These results suggest that L. gasseri requires peptides for growth, rather than proteins or free amino acids. Some research groups have also reported that the growth of Lactobacillus acidophilus (L. acidophilus), L. rhamnosus and L. plantarum strains could be promoted by supplementing the medium with pepsinolytic casein and whey protein (Kafley et al. Citation2010; Robitaille and Champagne Citation2014). Previous studies have demonstrated that the growth of Lactobacillus acidophilus (L. acidophilus) was enhanced by casein fragments that have undergone digestion by proteases other than pepsin (Saxena, Mital, and Garg Citation1994; Masuda et al. Citation2003). Casein hydrolysate has the potential to stimulate the proliferation of Bifidobacterium, with fractions smaller than 1 kDa demonstrating a notable enhancement in the growth of Bifidobacterium infants, Bifidobacterium breve, and Bifidobacterium longum (Proulx et al. Citation1994). Poch and Bezkorovainy (Citation1991) employed B. bifidum and B. longum strains, isolated from infant fecal samples, to assess the impact of the casein constituent of milk on the growth-promoting efficacy of Bifidobacteria. The study indicate that the digestion of casein with trypsin resulted in the promotion of Bifidobacteria growth when compared to TPY medium. And, к-casein was identified as the most effective growth promoter. Previous research has demonstrated that hydrophilic amino acid residues found within casein hydrolysates, such as His, Lys, Glu, and Ser, play a role in facilitating bacterial growth, but their growth stimulating mechanisms remain to be investigated (Zhang et al. Citation2011). Furthermore, Xue et al. (Citation2023) reported that most of the peptides in casein gastrointestinal hydrolysates were able to be transported in an intact form through a monolayer of cell membranes to perform immunologically active functions. However, there were also studies indicated that the supplementation of peptide fractions with varying molecular weights did not yield any statistically significant impact on the growth of L. delbrueckii, L. acidophilus, S. thermophilus and L. helveticus (Gandhi and Shah Citation2014). Nevertheless, in the presence of macropeptides (410 kDa), all bacterial strains exhibited elevated levels of proteolytic activity in the medium, resulting in increased production of angiotensin-converting enzyme (ACE)-inhibiting peptides (Gandhi and Shah Citation2014). This implies that the proteolytic systems exhibit variations among different bacterial species, as well as within different strains of the same species (de Giori et al. Citation1985).
Casein glycomacropeptide (GMP) is a 7 kDa sugar phosphate polypeptide fragment produced by the hydrolysis of κ-casein by pepsin. GMP is an amphiphilic glycopeptide containing sialic acid and N-acetyl-galactosamine (Farrell et al. Citation2004). The enhancement of the growth and metabolic activity of Bifidobacteria by GMP has been found (Cicvárek et al. Citation2010). Janer, Pelaez, and Requena (Citation2004) described the growth-promoting activity of GMP on LAB at a concentration of 2%. Also, GMP hydrolysis products (fractions less than 1 kDa obtained by protease hydrolysis) were reported to have a significant growth-promoting effect on Bifidobacterium (Fukudome et al. Citation2021). Tian et al. (Citation2015) found that the GMP hydrolysate produced by the hydrolysis of papain had a significant growth promoting effect on Bacillus thermophilus (p < 0.05). Meanwhile, it showed that the growth-enhancing properties of GHP may be linked to its heightened concentrations of Glu, Leu, and Ala, while exhibiting no discernible relationship with sialic acid content.
2.2. Whey protein
Whey protein, a by-product of casein coagulation in the dairy industry, accounts for about 15-20% of total bovine milk protein and belongs to the globular protein family, which mainly consists of β-lactoglobulin (50-60%) and α-lactoglobulin (about 20%) and is an excellent source of food-grade protein (Patel Citation2015; Sun, Wang, and Guo Citation2018). The peptides released through enzymatic digestion confer numerous biological functions to whey protein in vivo, including advantageous effects on the nervous, endocrine, immune, cardiovascular, and digestive systems (Fenelon et al. Citation2019). Whey protein hydrolysate, known for its abundance of essential amino acids and other nutrients (Dullius, Goettert, and de Souza Citation2018), has emerged as a prominent hypoallergenic dairy product in the commercial market (Chatterton et al. Citation2006). Notably, whey protein-derived peptides have been found to exhibit advantageous effects on the intestinal microbiota, functioning as prebiotics (Peled and Livney Citation2021). One study showed that whey protein-derived peptides not only exhibited antibacterial effects on pathogenic bacteria such as E. coli, B. subtilis and Staphylococcus aureus, but also promoted the growth of probiotics such as B. longum ATCC 15, 707 (Chatterton et al. Citation2006). Furthermore, whey protein-derived peptides were associated with improved oxidative stress and maintained intestinal barriers, such as a commercial whey protein-derived product HILMAR™ 8350 (Liu et al. Citation2021).
During in vitro digestion, whey protein demonstrated complete protein hydrolysis and exhibited the highest ratio of EAA to NEAA when compared to various common protein sources, including collagen, maize alcoholic protein, sorghum flour, wheat bran grains, peanuts, black beans, and pigeon peas (Sousa et al. Citation2020). Studies have shown that whey protein hydrolysates could promote proliferation and protection of probiotics (Zhang et al. Citation2020; Krunić and Rakin Citation2022; Wang et al. Citation2022). Potential effects of whey protein hydrolysate on infant intestinal microbiota were assessed by static digestion models and fecal culture fermentation simulating colonic fermentation, and it was found that whey protein hydrolysate significantly increased the relative abundance of Proteobacteria, Bacteroides and Streptococcus, particularly promoting the growth of Lactobacillus acidophilus (L. acidophilus) NCFM, and increased the production of short-chain fatty acids (Feng et al. Citation2022). Through the utilization of cellular measurements and metabolite analysis of L. acidophilus and B. bifidum, it was determined that whey peptides exhibit a stimulatory effect on the proliferation of probiotic microorganisms. Additionally, the findings were corroborated through the examination of an obese male Wistar rat model, which further confirmed the ability of whey peptides to stimulate the proliferation of intestinal probiotics (Yu et al. Citation2016). It indicated that whey protein hydrolysate exerts a beneficial influence on the composition and function of the intestinal microbiota. It has been shown that whey protein hydrolysate into the culture medium has been demonstrated to exert a favorable impact on the synthesis of extracellular polysaccharides and the mitigation of antigenicity in probiotics, which was an efficacious strategy for augmenting the proliferation rate of probiotic microorganisms (Zisu and Shah Citation2003). For example, supplementation of whey protein hydrolysate in infant formula provided a positive boost to the growth of Bifidobacterium L80 (Wang et al. Citation2021). Krunić and Rakin (Citation2022) reported that whey protein hydrolysate as a component of the probiotic encapsulation matrix promoted the growth of probiotics. Moreover, the carrier supplemented with hydrolysate exhibited superior encapsulation efficiency, spherical factor, and antioxidant capacity both pre- and post-fermentation in comparison to the carrier containing non-hydrolyzed proteins. Wang et al. (Citation2022) conducted an assessment on the efficacy of whey protein hydrolysate as a lyoprotectant in preserving the cell viability of Bifidobacterium animalis ssp. lactis Probio-M8 throughout the freeze-drying process and subsequent storage. The results revealed that the utilization of whey protein hydrolysate led to enhanced cell membrane integrity, decreased levels of reactive oxygen species and malondialdehyde production, thereby offering protective benefits to probiotics during freeze drying and storage. Whey protein hydrolysates were also found to have potential as probiotic protectors during spray or freeze-drying processes, and probiotics show greater sustained viability after gastrointestinal digestion (Xie et al. Citation2023).
2.3. Soybean protein
Soybean protein is rich in nutrients, balanced in amino acids and has functional properties such as antioxidant, blood pressure reduction, easy digestion and absorption, cholesterol reduction, which is widely recognized in academic circles for its natural functionality and exceptional nutritional composition (Li, Li, Zhang, He, et al. Citation2021; Nishinari et al. Citation2014). Based on immunological techniques, soybean protein can be classified as soybean globulin, β-conglycinin, γ-conglycinin, and α-conglycinin. Additionally, the protein can be further classified into four components, namely 2S, 7S, 11S, and 15S, based on the sedimentation rate observed during super centrifugation. Notably, the 7S globulin and 11S globulin represent the predominant constituents, constituting approximately 70% of the total protein content (Chen et al. Citation2014). The effects of soy protein on reduced blood lipids (Ascencio et al. Citation2004) and cholesterol (Butteiger et al. Citation2016) and improved immunity (Liu et al. Citation2018) have been extensively studied. Soybean peptides, as hydrolysates of soybean protein, have the same nutritional characteristics as soybean protein and are widely used in foods, pharmaceuticals and nutraceuticals (Darmawan, Bringe, and de Mejia Citation2010).
Several studies have demonstrated that soybean peptides possess the capacity to enhance the proliferation of probiotics (Zhang et al. Citation2020). Zhao et al. (Citation2013) effectively acquired soybean protein hydrolysates that are appropriate for uptake by Bacillus thermophilus, featuring fragment lengths spanning from 2 to 8 amino acid residues. The fragments stimulated the proliferation of bacteria. Zhang, Zhang, Liu, et al. (Citation2021) and Zhang et al. (Citation2022) conducted a simulation of gastrointestinal digestion on soybean isolates and soybean peptides, followed by the addition of resulting hydrolysates to a medium. The researchers observed that this intervention led to enhanced growth and production of short-chain fatty acids in L. rhamnosus and L. reuteri LR08. Subsequently, microscopic observation and iTRAQ-based proteomics and bioinformatics analysis of L. rhamnosus treated with soybean isolate and soybean peptide hydrolysate screened for differentially abundant proteins (DAPs) affecting growth and morphologic changes (Zhang, Zhang, Xia, et al. Citation2021). In another study, the growth and metabolism of B. animalis subsp. animalis JCM 1190 were found to be enhanced by soybean protein and soybean peptide hydrolysates. Additionally, the study demonstrated that the observed phenomenon can be ascribed to the heightened abundance of digested soybean peptides, thereby significantly increasing the number of viable cells and the levels of lactic acid and acetic acid in the culture. This augmentation was achieved through the regulation of glycine, serine, and threonine metabolism, as well as pyruvate metabolism, the tricarboxylic acid cycle, glycolipid metabolism, and various other metabolic pathways (Li, Li, Zhang, He, et al. Citation2021). Sun et al. (Citation2021) showed that the addition of soy protein hydrolysate had a positive effect on the proliferation and metabolism of S. thermophilus. The cell density and survival number of S. thermophilus were significantly enhanced and contained high levels of organic acids and H2O2. Li, Li, Zhang, Zhang, et al. (Citation2021) found that soybean protein-derived peptides with molecular weights between 150 and 1000 Da significantly increased the abundance and uniformity of the gut microbiota and had a significant effect on the proliferation of Lactobacillus and Phascolarctobacterium. Similar to casein hydrolysate, the molecular weight of soybean protein hydrolysate plays an important role in the growth of probiotics and may be related to the peptide transport mechanism (Hsieh, Yang, and Iannotti Citation1999). Zhao and Zhang (Citation2004) proposed that the incorporation of soybean peptides into the diet of laying hens resulted in a noteworthy enhancement in the proliferation of gut LAB, exhibiting a tenfold rise in comparison to the control group. Additionally, soybean peptides exhibited a substantial inhibitory effect on the growth of E. coli.
2.4. Other sources of protein hydrolysate
Many studies have shown that other sources of protein besides the ones mentioned earlier can also promote the growth of probiotics. Protein hydrolysates derived from poultry bones and feather by-products were extremely potent promoters of probiotics (Lazzi et al. Citation2013). Meli et al. (Citation2013) achieved the extraction of peptide hydrolysates from poultry by-products utilizing keratin protein as the foundation. These peptides have the potential to enhance the growth and aminopeptidase functionality of various Lactobacillus and Bifidobacterium species. Studies have shown that the addition of egg white hydrolysate also had a proliferative effect on the growth of LAB (Solval et al. Citation2019). Quinoa protein or its hydrolysate was able to alleviate gut microbiota dysbiosis in mice with colorectal cancer by reducing the abundance of pathogenic bacteria and increasing the abundance of probiotics (Fan et al. Citation2023). Yang et al. (Citation2014) showed that a tensin A-magainin heteropeptide promoted the growth of Bifidobacteria and Lactobacillus and inhibited the proliferation of E. coli in the mouse gut.
3. Biological activity of protein hydrolysates
Numerous studies have documented that protein hydrolysates (peptides) obtained from various food proteins exhibit diverse bioactivities, encompassing antioxidant, antimicrobial, immunomodulatory, anticancer, and mineral binding properties (Chalamaiah, Yu, and Wu Citation2018).
3.1. Antioxidant
A specific amino acid composition was found to be associated with antioxidative properties of protein hydrolysates. Notably, the initial identification of peptides with antioxidant activity revealed the presence of acid peptides. Within the acidic amino acids, certain metal ions (Fe2+ and Cu+) were observed to interact with the carboxyl residues of side chains, resulting in oxidative passivation. This interaction effectively diminished the occurrence of free radical chain reactions, thereby serving as an antioxidative mechanism (Saiga, Tanabe, and Nishimura Citation2003). Using equal amounts of alkaline protease and complex protease, Damgaard, Lametsch, and Otte (Citation2015) hydrolyzed porcine colon, bovine lung, and pancreatic samples for 2 h. The results indicated that the lung hydrolysates exhibited a notable presence of Glu and His residues, thereby demonstrating a strong iron chelating capacity. Additionally, the pancreatic hydrolysates displayed a potential correlation between their antioxidative ability and the elevated levels of Phe and Val, which were observed to inhibit lipid oxidation within the linoleic acid emulsion system. The colon hydrolysates were found to possess a significant quantity of Tyr residues, which facilitated the release of free radicals via direct electron transfer. The presence of proton donors, specifically within amino acid residues like Tyr, plays a vital role in the generation of antioxidative peptides. These peptides rely on hydrogen atoms to donate electrons, effectively counteracting the harmful effects of free radicals. Consequently, the resonance stabilization of the indole radical and phenoxy radical occurs, hindering or ceasing the radical chain reaction (Wang et al. Citation2008).
3.2. Antimicrobial
Antimicrobial peptides exhibit structural diversity, yet possess certain shared characteristics such as helical peptide structure, small size, cationic properties, and a significant presence of hydrophobic amino acids. The amphiphilic α-helix structure, pronounced hydrophobic moment, and net positive charge of the antibacterial peptide BCp12, which has been recently identified from buffalo casein hydrolysates, are noteworthy (Zhao et al. Citation2020). This peptide exhibits notable efficacy against bacterial pathogens and displays remarkable stability in the presence of salts and temperatures as high as 121 °C (Zhao et al. Citation2020). Collagen peptides derived from collagen hydrolysates of Atlantic mackerel by-products were found to possess inhibitory effects against Staphylococcus aureus. These peptides exhibited a secondary structure primarily composed of β-sheet and β-turn motifs (Ennaas et al. Citation2016). In a similar vein, Catiau et al. (Citation2011) conducted a study on the structural characteristics of antimicrobial peptides found in bovine hemoglobin. Their findings indicated that antimicrobial activity was notably high in peptides featuring short-chain, positively charged, non-hydrophobic residues, or a limited number of peptides lacking secondary structure elements. Notably, it has been shown that casein hydrolysates with a molecular weight of 5 kDa have a proliferative effect on probiotics (L. plantarum, L. reuteri and B. thermophilus), whereas they showed an inhibitory effect on pathogenic bacteria (Salmonella typhimurium) (Venardou et al. Citation2021). However, the mechanism of this promotive or antibacterial effect on various bacteria remains to be investigated.
3.3. Immunomodulatory
Proteins are hydrolyzed by proteases to produce immunologically active peptides. The precise mechanism underlying the immunomodulatory impact of food-derived peptides remains incompletely understood. However, the prevailing belief is that immunomodulation primarily occurs through the activation of macrophages, stimulation of phagocytosis, elevation of leukocyte count, enhanced production of immune modulators such as cytokines, nitric oxide, and immunoglobulins, stimulation of natural killer cells, promotion of splenocyte activity, activation of CD4+, CD8+, CD11b+, and CD56+ cells, triggering of transcription factor nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) dependent pathways, and inhibition of pro-inflammatory mediators. (Chalamaiah, Yu, and Wu Citation2018). Hou et al. (Citation2012) obtained three immunomodulatory peptides, AsnGly-Met-Thr-Tyr, Asn-Gly-Leu-Ala-Pro, and Trp-Thr, from the trypsin hydrolysate of Alaskan pollock frames, which showed high lymphoproliferative activity. Pan et al. (Citation2013) conducted enzymatic digestion using papain and trypsin to obtain a whole milk hydrolysate. Their findings indicated that the milk protein hydrolysate exhibited immunomodulatory effects in mice, as evidenced by an increase in spleen and thymus weight, as well as enhanced phagocytic activity of macrophages. In their study, Javier et al. (Citation2014) demonstrated a variety of ex vivo immune responses induced by β-lactoglobulin tryptic-digested fractions. These responses included an increased secretion of IFN-γ from T cells and an elevated production of TNF-α from monocytes.
3.4. Anticancer
Certain peptides or protein hydrolysates derived from dietary proteins containing specific amino acids (e.g., Pro, Leu, Gly, Ala, Lys, Arg, Ser, Glu, Thr, Tyr) have been reported to be important in terms of anticancer activity (Chi et al. Citation2015; Hung et al. Citation2014; Vital et al. Citation2014; Wang and Zhang Citation2017). Kannan et al. (Citation2010) purified and isolated the anti-cancer pentapeptide Glu-Gln-Arg-Pro-Arg from rice bran. The pentapeptide demonstrated its efficacy in inhibiting cancer cells at a concentration of 600-700 µg/mL. Notably, the growth inhibition rates for colon cancer cells (Caco-2, HCT-116) and breast cancer cells (MCF-7, MDA-MB-231) reached 84%, and liver cancer cells (HepG-2) exhibited an inhibition rate of 80%. A separate investigation revealed that the antitumor tripeptide trp-thrp-pro (408.2 Da), derived from rapeseed through enzymatic digestion and fermentation, demonstrated noteworthy anticancer attributes against HepG2 cells. This was achieved through the upregulation of p53 and Bax, while concurrently downregulating Bcl2 (Wang et al. Citation2016).
3.5. Mineral binding
Metal binding capacity of peptides is determined by their amino acid sequence, as determined by their parent proteins (Guo et al. Citation2014). Additionally, the mineral binding capacity of a peptide is influenced by various factors including peptide structure, spatial effects, and molecular mass. In their investigation on the impact of duck egg protein peptides on iron bioavailability in an iron-deficiency anemia model, Li et al. (Citation2019) found that specific amino acid residues, including Glu, Asp, Lys, His, Ser, and Cys, potentially contribute significantly to the chelation of the peptides with iron. Additionally, Pro residues, while not directly involved in Fe coordination, play a vital role in facilitating peptide folding and promoting the formation of a ring structure with the Fe atom at its center (Eckert et al. Citation2016). The Ca-binding capacity of casein phosphopeptides (CPPs), derived from phosphorylated casein peptides released through enzymatic hydrolysis of αs1, αs2, and β-casein, has been extensively investigated (Miquel, Gomez, et al. Citation2005). One notable characteristic of CPPs is the inclusion of the SpSpSpEE cluster, which is a considerably polar acidic sequence comprised of three phosphoseryl groups followed by two Glu residues. Minerals such as Ca, Fe, and Zn were bound to this cluster, which plays an essential role in mineral absorption (Miquel, Alegria, et al. Citation2005; Miquel et al. Citation2006).
4. Prospects
This review aims to provide a comprehensive overview of the existing literature regarding the impacts and mechanisms of protein hydrolysates on probiotics, as well as to summaries the biological activities of protein hydrolysates reported. Although in this paper we summarize the effects of a number of different sources of protein hydrolysates on probiotics, fewer protein species have been reported. However, the diverse specificities of probiotics result in varying capacities to utilize proteins (Day et al. Citation2019). Hence, it is imperative to actively investigate the potential impacts and underlying mechanisms of protein hydrolysates derived from diverse protein-rich food sources on probiotics in the future. There is evidence that edible mushrooms are high in protein, generally containing more than 20% (dry weight) of protein, with individual edible mushrooms containing up to 35% (dry weight) or more, and contain diverse range of biologically active functional proteins such as immunomodulatory, antioxidant, and anti-cardiovascular diseases (Chen et al. Citation2017; Maseko et al. Citation2013; Zhang, Roytrakul, and Sutheerawattananonda Citation2017; Lau et al. Citation2014), a new class of alternative high-quality food protein ingredients with great potential (Stephan et al. Citation2018; Bach et al. Citation2017). The effect of edible mushroom protein hydrolysates on probiotics has not yet been reported. Therefore, the beneficial properties of edible mushroom protein hydrolysates as prebiotic effectors on probiotics could be explored in the future.
Furthermore, the correlation between the molecular conformation and activity of bioactive peptides remains largely unexplored. As advancements in bioengineering techniques and separation technologies continue to emerge, a more comprehensive understanding of the structure-property relationships of bioactive peptides is anticipated. Liquid chromatography − electrospray ionization mass spectrometry (LC-ESI-MS/MS) and matrix assisted laser desorption/ionization time-of-flight/time-off light (MALDI-TOF/TOF) mass spectrometry are among the potent methodologies employed for the characterization and quantification of peptide morphology (Tang et al. Citation2023). The utilization of these analytical techniques will significantly enhance the process of identifying novel functional peptides.
Authors’ contributions
Ping-Ping Gao (PP G) and Jun-Fang Lin (JF L) conceived and designed the study. PP G and Han-Qing Liu (HQ L) conducted systematic search, screened articles and read the full texts for eligibility. PP G and Zhi-Wei Ye (ZW Y) extracted data from original studies. PP G and JF L performed risk of bias assessments. PPG performed the analyses. Yuan Zou (Y Z), Qian-Wang Zheng (QW Z), and Tao Wei (T W) contributed to the interpretation of the results and wrote the first draft of the manuscript. JF L and Li-Qiong (LQ G) contributed to the interpretation of the results and critically revised the manuscript. All authors acknowledge full responsibility for the analyses and interpretation of the report. All authors have read and approved the final manuscript. JF L is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arakawa, K., K. Matsunaga, S. Takihiro, A. Moritoki, S. Ryuto, Y. Kawai, T. Masuda, and T. Miyamoto. 2015. Lactobacillus gasseri requires peptides, not proteins or free amino acids, for growth in milk. Journal of Dairy Science 98 (3):1593–1603. doi:10.3168/jds.2014-8860.
- Ascencio, C., N. Torres, F. Isoard-Acosta, F. J. Gómez-Pérez, R. Hernández-Pando, and A. R. Tovar. 2004. Soy protein affects serum insulin and hepatic SREBP-1 mRNA and reduces fatty liver in rats. The Journal of Nutrition 134 (3):522–529. doi:10.1093/jn/134.3.522.
- Bach, F., C. V. Helm, M. B. Bellettini, G. M. Maciel, and C. W. I. Haminiuk. 2017. Edible mushrooms: A potential source of essential amino acids, glucans and minerals. International Journal of Food Science & Technology 52 (11):2382–2392. doi:10.1111/ijfs.13522.
- Butteiger, D. N., A. A. Hibberd, N. J. McGraw, N. Napawan, J. M. Hall-Porter, and E. S. Krul. 2016. Soy protein compared with milk protein in a western diet increases gut microbial diversity and reduces serum lipids in golden Syrian hamsters. The Journal of Nutrition 146 (4):697–705. doi:10.3945/jn.115.224196.
- Catiau, L., J. Traisnel, V. Delval-Dubois, N. E. Chihib, D. Guillochon, and N. Nedjar-Arroume. 2011. Minimal antimicrobial peptidic sequence from hemoglobin alpha-chain: KYR. Peptides 32 (4):633–638. doi:10.1016/j.peptides.2010.12.016.
- Chalamaiah, M., W. L. Yu, and J. P. Wu. 2018. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chemistry 245:205–222. doi:10.1016/j.foodchem.2017.10.087.
- Champagne, C. P., R. P. Ross, M. Saarela, K. F. Hansen, and D. Charalampopoulos. 2011. Recommendations for the viability assessment of probiotics as concentrated cultures and in food matrices. International Journal of Food Microbiology 149 (3):185–193. doi:10.1016/j.ijfoodmicro.2011.07.005.
- Chatterton, D. E. W., G. Smithers, P. Roupas, and A. Brodkorb. 2006. Bioactivity of β-lactoglobulin and α-lactalbumin – Technological implications for processing. International Dairy Journal 16 (11):1229–1240. doi:10.1016/j.idairyj.2006.06.001.
- Chen, D. L., C. Q. Zheng, J. Yang, J. Li, J. Su, Y. Z. Xie, and G. X. Lai. 2017. Immunomodulatory activities of a fungal protein extracted from Hericium erinaceus through regulating the gut microbiota. Frontiers in Immunology 8:666. doi:10.3389/fimmu.2017.00666.
- Chen, H., C. N. Li, G. W. Shu, and C. F. Wang. 2012. Screening of nitrogen sources in the medium for Streptococcus thermophilus using Plackett-Burman design. Advanced Materials Research 531:532–535. doi:10.4028/www.scientific.net/AMR.531.532.
- Chen, J. S., J. Wang, P. X. Song, and X. Ma. 2014. Determination of glycinin in soybean and soybean products using a sandwich enzyme-linked immunosorbent assay. Food Chemistry 162:27–33. doi:10.1016/j.foodchem.2014.04.065.
- Chi, C., F. Hu, B. Wang, T. Li, and G. Ding. 2015. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. Journal of Functional Foods 15:301–313. doi:10.1016/j.jff.2015.03.045.
- Christensen, J. E., E. G. Dudley, J. A. Pederson, and J. L. Steele. 1999. Peptidases and amino acid catabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 76 (1/4):217–246. doi:10.1023/A:1002001919720.
- Christensson, C., H. Bratt, L. J. Collins, T. Coolbear, R. Holland, M. W. Lubbers, P. W. O’Toole, and J. R. Reid. 2002. Cloning and expression of an oligopeptidase, PepO, with novel specificity from Lactobacillus rhamnosus HN001 (DR20). Applied and Environmental Microbiology 68 (1):254–262. doi:10.1128/AEM.68.1.254-262.2002.
- Cicvárek, J., L. Čurda, O. Elich, E. Dvořáková, and M. Dvořák. 2010. Effect of caseinomacropeptide concentrate addition on the growth of Bifidobacteria. Czech Journal of Food Sciences 28 (6):485–494. doi:10.17221/269/2009-CJFS.
- Damgaard, T., R. Lametsch, and J. Otte. 2015. Antioxidant capacity of hydrolyzed animal by-products and relation to amino acid composition and peptide size distribution. Journal of Food Science and Technology 52 (10):6511–6519. doi:10.1007/s13197-015-1745-z.
- Darmawan, R., N. A. Bringe, and E. G. de Mejia. 2010. Antioxidant capacity of alcalase hydrolysates and protein profiles of two conventional and seven low glycinin soybean cultivars. Plant Foods for Human Nutrition (Dordrecht, Netherlands) 65 (3):233–240. doi:10.1007/s11130-010-0185-1.
- Day, R. L., A. J. Harper, R. M. Woods, O. G. Davies, and L. M. Heaney. 2019. Probiotics: Current landscape and future horizons. Future Science OA 5 (4):FSO391. doi:10.4155/fsoa-2019-0004.
- De Angelis, M., M. Calasso, N. Cavallo, R. Di Cagno, and M. Gobbetti. 2016. Functional proteomics within the genus Lactobacillus. Proteomics 16 (6):946–962. doi:10.1002/pmic.201500117.
- de Giori, G. S., G. F. de Valdez, A. P. de Ruiz Holgado, and G. Oliver. 1985. Effect of pH and temperature on the proteolytic activity of lactic acid bacteria. Journal of Dairy Science 68 (9):2160–2164. doi:10.3168/jds.S0022-0302(85)81085-7.
- Detmers, F. J. M., F. C. Lanfermeijer, R. Abele, R. W. Jack, R. Tampe, W. N. Konings, and B. Poolman. 2000. Combinatorial peptide libraries reveal the ligand binding mechanism of the oligopeptide binding protein OppA of Lactococcus lactis. Proceedings of the National Academy of Sciences of the United States of America 97 (23):12487–12492. doi:10.1073/pnas.220308797.
- Detmers, F. J., E. R. Kunji, F. C. Lanfermeijer, B. Poolman, and W. N. Konings. 1998. Kinetics and specificity of peptide uptake by the oligopeptide transport system of Lactococcus lactis. Biochemistry 37 (47):16671–16679. doi:10.1021/bi981712t.
- Ding, T., and Y. Li. 2021. Beneficial effect and mechanism of walnut oligopeptide on Lactobacillus plantarum Z7. Food Science & Nutrition 9 (2):672–681. doi:10.1002/fsn3.2029.
- Doeven, M. K., J. Kok, and B. Poolman. 2005. Specificity and selectivity determinants of peptide transport in Lactococcus lactis and other microorganisms. Molecular Microbiology 57 (3):640–649. doi:10.1111/j.1365-2958.2005.04698.x.
- Dullius, A., M. I. Goettert, and C. F. V. de Souza. 2018. Whey protein hydrolysates as a source of bioactive peptides for functional foods-Biotechnological facilitation of industrial scale-up. Journal of Functional Foods 42:58–74. doi:10.1016/j.jff.2017.12.063.
- Dysin, A. P., A. R. Egorov, A. A. Godzishevskaya, A. A. Kirichuk, A. G. Tskhovrebov, and A. S. Kritchenkov. 2023. Biologically active supplements affecting producer microorganisms in food biotechnology: A review. Molecules (Basel, Switzerland) 28 (3):1413. doi:10.3390/molecules28031413.
- Eckert, E., L. Lu, L. D. Unsworth, L. Chen, J. Xie, and R. Xu. 2016. Biophysical and in vitro absorption studies of iron chelating peptide from barley proteins. Journal of Functional Foods 25:291–301. doi:10.1016/j.jff.2016.06.011.
- Ennaas, N., R. Hammami, A. Gomaa, F. Bédard, E. ́. Biron, M. Subirade, L. Beaulieu, and I. Fliss. 2016. Collagencin, an antibacterial peptide from fish collagen: Activity, structure and interaction dynamics with membrane. Biochemical and Biophysical Research Communications 473 (2):642–647. doi:10.1016/j.bbrc.2016.03.121.
- Fadda, S., Y. Sanz, G. Vignolo, M. C. Aristoy, G. Oliver, and F. Toldrá. 1999. Characterization of muscle sarcoplasmic and myofibrillar protein hydrolysis caused by Lactobacillus plantarum. Applied and Environmental Microbiology 65 (8):3540–3546. doi:10.1089/oli.1.1999.9.359.
- Fan, X., H. M. Guo, C. Teng, X. S. Yang, P. Y. Qin, A. Richel, L. Z. Zhang, C. Blecker, and G. X. Ren. 2023. Supplementation of quinoa peptides alleviates colorectal cancer and restores gut microbiota in AOM/DSS-treated mice. Food Chemistry 408:135196. doi:10.1016/j.foodchem.2022.135196.
- Farrell, H. M., R. Jimenez-Flores, G. T. Bleck, E. M. Brown, J. E. Butler, L. K. Creamer, C. L. Hicks, C. M. Hollar, K. F. Ng-Kwai-Hang, and H. E. Swaisgood. 2004. Nomenclature of the proteins of cows’ milk-sixth revision. Journal of Dairy Science 87 (6):1641–1674. doi:10.3168/jds.S0022-0302(04)73319-6.
- Fenelon, M. A., R. M. Hickey, A. Buggy, N. McCarthy, and E. G. Murphy. 2019. Whey proteins in infant formula. Whey Proteins 439–494. doi:10.1016/B978-0-12-812124-5.00013-8.
- Feng, C. S., L. Tian, H. Hong, Q. Y. Wang, X. Zhan, Y. K. Luo, and Y. Q. Tan. 2022. In vitro gut fermentation of whey protein hydrolysate: An evaluation of its potential modulation on infant gut microbiome. Nutrients 14 (7):1374. doi:10.3390/nu14071374.
- Fernandez-Espla, M. D., and F. Rul. 1999. PepS from Streptococcus thermophilus - a new member of the aminopeptidase T family of thermophilic bacteria. European Journal of Biochemistry 263 (2):502–510. doi:10.1046/j.1432-1327.1999.00528.x.
- Fukudome, H., T. Yamaguchi, J. Higuchi, A. Ogawa, Y. Taguchi, J. Li, T. Kabuki, K. Ito, and F. Sakai. 2021. Large-scale preparation and glycan characterization of sialylglycopeptide from bovine milk glycomacropeptide and its bifidogenic properties. Journal of Dairy Science 104 (2):1433–1444. doi:10.3168/jds.2019-17865.
- Gandhi, A., and N. P. Shah. 2014. Cell growth and proteolytic activity of Lactobacillus acidophilus, Lactobacillus helveticus, Lactobacillus delbrueckii ssp. bulgaricus, and Streptococcus thermophilus in milk as affected by supplementation with peptide fractions. International Journal of Food Sciences and Nutrition 65 (8):937–941. doi:10.3109/09637486.2014.945154.
- Gibson, G. R., R. Hutkins, M. E. Sanders, S. L. Prescott, R. A. Reimer, S. J. Salminen, K. Scott, C. Stanton, K. S. Swanson, P. D. Cani, et al. 2017. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews. Gastroenterology & Hepatology 14 (8):491–502. doi:10.1038/nrgastro.2017.75.
- Gilbert, C., D. Atlan, B. Blanc, R. Portailer, J. E. Germond, L. Lapierre, and B. Mollet. 1996. A new cell surface proteinase: Sequencing and analysis of the prtB gene from Lactobacillus delbrueckii subsp. bulgaricus. Journal of Bacteriology 178 (11):3059–3065. doi:10.1128/jb.178.11.3059-3065.1996.
- Guédon, E., P. Renault, S. D. Ehrlich, and C. Delorme. 2001. Transcriptional pattern of genes coding for the proteolytic system of Lactococcus lactis and evidence for coordinated regulation of key enzymes by peptide supply. Journal of Bacteriology 183 (12):3614–3622. doi:10.1128/JB.183.12.3614-3622.2001.
- Guédon, E., P. Serror, S. D. Ehrlich, P. Renault, and C. Delorme. 2001. Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in Lactococcus lactis. Molecular Microbiology 40 (5):1227–1239. doi:10.1046/j.1365-2958.2001.02470.x.
- Guo, L., P. A. Harnedy, B. Li, H. Hou, Z. Zhang, X. Zhao, and R. J. FitzGerald. 2014. Food protein-derived chelating peptides: Biofunctional ingredients for dietary mineral bioavailability enhancement. Trends in Food Science & Technology 37 (2):92–105. doi: 10.1016/j.tifs.2014.02.007.
- Hill, C., F. Guarner, G. Reid, G. R. Gibson, D. J. Merenstein, B. Pot, L. Morelli, R. B. Canani, H. J. Flint, S. Salminen, et al. 2014. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews. Gastroenterology & Hepatology 11 (8):506–514. doi:10.1038/nrgastro.2014.66.
- Holck, A., and H. Naes. 1992. Cloning, sequencing and expression of the gene encoding the cell-envelope-associated proteinase from Lactobacillus paracasei subsp. paracasei NCDO151. Journal of General Microbiology 138 (7):1353–1364. doi:10.1099/00221287-138-7-1353.
- Hou, H., Y. Fan, B. F. Li, C. H. Xue, G. L. Yu, Z. H. Zhang, and X. Zhao. 2012. Purification and identification of immunomodulating peptides from enzymatic hydrolysates of Alaska pollock frame. Food Chemistry 134 (2):821–828. doi:10.1016/j.foodchem.2012.02.186.
- Hsieh, C. M., F. C. Yang, and E. L. Iannotti. 1999. The effect of soy protein hydrolyzates on fermentation by Lactobacillus amylovorus. Process Biochemistry 34 (2):173–179. doi:10.1016/S0032-9592(98)00081-8.
- Hung, C. C., Y. H. Yang, P. F. Kuo, and K. C. Hsu. 2014. Protein hydrolysates from tuna cooking juice inhibit cell growth and induce apoptosis of human breast cancer cell line MCF-7. Journal of Functional Foods 11:563–570. doi:10.1016/j.jff.2014.08.015.
- Idrees, M., M. Imran, N. Atiq, R. Zahra, R. Abid, M. Alreshidi, T. Roberts, A. Abdelgadir, M. K. Tipu, A. Farid, et al. 2022. Probiotics, their action modality and the use of multi-omics in metamorphosis of commensal microbiota into target-based probiotics. Frontiers in Nutrition 9:959941. doi:10.3389/fnut.2022.959941.
- Janer, C., C. Pelaez, and T. Requena. 2004. Caseinomacropeptide and whey protein concentrate enhance Bifidobacterium lactis growth in milk. Food Chemistry 86 (2):263–267. doi:10.1016/j.foodchem.2003.09.034.
- Javier, R., F. Ayoa, A. R. Francisco, and S. Ana. 2014. Immunomodulatory activities of whey β-lactoglobulin tryptic-digested fractions. International Dairy Journal 34 (1):65–73. doi:10.1016/j.idairyj.2013.07.004.
- Jingjing, E., Z. Jingya, M. Rongze, C. Zichao, Y. Caiqing, W. Ruixue, Z. Qiaoling, Y. Ying, L. Jing, and W. Junguo. 2023. Study of the internal mechanism of L-glutamate for improving the survival rate of Lactiplantibacillus plantarum LIP-1 after freeze-drying. Innovative Food Science & Emerging Technologies 84:103253. doi:10.1016/j.ifset.2022.103253.
- Juillard, V., D. Le Bars, E. R. Kunji, W. N. Konings, J. C. Gripon, and J. Richard. 1995. Oligopeptides are the main source of nitrogen for Lactococcus lactis during growth in milk. Applied and Environmental Microbiology 61 (8):3024–3030. doi:10.1128/AEM.61.8.3024-3030.1995.
- Kafley, S., W. S. Kim, H. Kumura, and K. Shimazaki. 2010. Growth performance of whey protein hydrolysates in the media on different strains of probiotic bacteria. Milchwissenschaft-Milk Science International 65 (3):245–248.
- Kannan, A., N. S. Hettiarachchy, J. O. Lay, and R. Liyanage. 2010. Human cancer cell proliferation inhibition by a pentapeptide isolated and characterized from rice bran. Peptides 31 (9):1629–1634. doi:10.1016/j.peptides.2010.05.018.
- Klein, J. R., A. Dick, J. Schick, H. T. Matern, B. Henrich, and R. Plapp. 1995. Molecular-cloning and DNA-sequence analysis of pepL, a leucyl aminopeptidase gene from Lactobacillus delbrueckii subsp. Lactis DSM7290. European Journal of Biochemistry 228 (3):570–578. doi:10.1111/j.1432-1033.1995.0570m.x.
- Krunić, T. Ž., and M. B. Rakin. 2022. Enriching alginate matrix used for probiotic encapsulation with whey protein concentrate or its trypsin-derived hydrolysate: Impact on antioxidant capacity and stability of fermented whey-based beverages. Food Chemistry 370:130931. doi:10.1016/j.foodchem.2021.130931.
- Kunji, E. R. S., I. Mierau, A. Hagting, B. Poolman, and W. N. Konings. 1996. The proteolytic systems of lactic acid bacteria. Antonie van Leeuwenhoek 70 (2-4):187–221. doi:10.1007/BF00395933.
- Kunz, C., and B. Lönnerdal. 1990. Human-milk proteins: Analysis of casein and casein subunits by anion-exchange chromatography, gel electrophoresis, and specific staining methods. The American Journal of Clinical Nutrition 51 (1):37–46. doi:10.1093/ajcn/51.1.37.
- Lamarque, M., P. Charbonnel, D. Aubel, J. C. Piard, D. Atlan, and V. Juillard. 2004. A multifunction ABC transporter (Opt) contributes to diversity of peptide uptake specificity within the genus Lactococcus. Journal of Bacteriology 186 (19):6492–6500. doi:10.1128/JB.186.19.6492-6500.2004.
- Lau, C. C., N. Abdullah, A. S. Shuib, and N. Aminudin. 2014. Novel angiotensin-I converting enzyme inhibitory peptides derived from edible mushroom Agaricus bisporus (J. E. Lange) Imbach identified by LC-MS/MS. Food Chemistry 148:396–401. doi:10.1016/j.foodchem.2013.10.053.
- Lazzi, C., F. Meli, F. Lambertini, C. Bottesini, I. Nikolaev, M. Gatti, S. Sforza, O. Koroleva, V. Popov, E. Neviani, et al. 2013. Growth promotion of Bifidobacterium and Lactobacillus species by proteinaceous hydrolysates derived from poultry processing leftovers. International Journal of Food Science & Technology 48 (2):341–349. doi:10.1111/j.1365-2621.2012.03192.x.
- Li, B., H. He, W. Shi, and T. Hou. 2019. Effect of duck egg white peptide-ferrous chelate on iron bioavailability in vivo and structure characterization. Journal of the Science of Food and Agriculture 99 (4):1834–1841. doi:10.1002/jsfa.9377.
- Li, W. H., H. Li, Y. X. Zhang, C. Zhang, J. Zhang, and X. Q. Liu. 2021. Differences in the gut microbiota composition of rats fed with soybean protein and their derived peptides. Journal of Food Science 86 (12):5452–5465. doi:10.1111/1750-3841.15948.
- Li, W. H., H. Li, Y. X. Zhang, L. J. He, C. Zhang, and X. Q. Liu. 2021. Different effects of soybean protein and its derived peptides on the growth and metabolism of Bifidobacterium animalis subsp. animalis JCM 1190. Food & Function 12 (13):5731–5744. doi:10.1039/d1fo00480h.
- Liu, M. M., B. Qi, J. S. Zhan, Y. Y. Chen, and G. Q. Zhao. 2018. Effects of low-abundant soybean protein extracts on the immune function and antioxidant capacity of mice. Journal of China Agricultural University 23 (02):57–63.
- Liu, M., J. R. Bayjanov, B. Renckens, A. Nauta, and R. J. Siezen. 2010. The proteolytic system of lactic acid bacteria revisited: A genomic comparison. BMC Genomics 11 (1):36. doi:10.1186/1471-2164-11-36.
- Liu, R. T., R. F. Walsh, and A. E. Sheehan. 2019. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neuroscience and Biobehavioral Reviews 102:13–23. doi:10.1016/j.neubiorev.2019.03.023.
- Liu, X. R., N. Zhu, Y. T. Hao, X. C. Yu, Z. Li, R. X. Mao, R. Liu, J. W. Kang, J. N. Hu, and Y. Li. 2021. Radioprotective effect of whey hydrolysate peptides against γ-radiation-induced oxidative stress in BALB/c mice. Nutrients 13 (3):816. doi:10.3390/nu13030816.
- Maseko, T., D. L. Callahan, F. R. Dunshea, A. Doronila, S. D. Kolev, and K. Ng. 2013. Chemical characterization and speciation of organic selenium in cultivated selenium-enriched Agaricus bisporus. Food Chemistry 141 (4):3681–3687. doi:10.1016/j.foodchem.2013.06.027.
- Masuda, T., R. Taguchi, T. Kabuki, H. Nakajima, and T. Itoh. 2003. Improvement of the growth of Lactobacillus acidophilus in milk by addition of enzymatically digested casein. Milchwissenschaft-Milk Science International 58:124–127.
- Meli, F., C. Lazzi, E. Neviani, and M. Gatti. 2013. Effect of protein hydrolizates on growth kinetics and aminopeptidase activities of some Bifidobacterium species. Anaerobe 22:130–133. doi:10.1016/j.anaerobe.2013.05.003.
- Meli, F., C. Lazzi, E. Neviani, and M. Gatti. 2014. Effect of protein hydrolysates on growth kinetics and aminopeptidase activities of Lactobacillus. Current Microbiology 68 (1):82–87. doi:10.1007/s00284-013-0445-z.
- Miquel, E., A. Alegria, R. Barbera, and R. Farre. 2005. Speciation analysis of calcium, iron, and zinc in casein phosphopeptide fractions from toddler milk-based formula by anion exchange and reversed phase high-performance liquid chromatography-mass spectrometry/flame atomic-absorption spectroscopy. Analytical and Bioanalytical Chemistry 381 (5):1082–1088. doi:10.1007/s00216-004-3002-6.
- Miquel, E., A. Alegria, R. Barbera, and R. Farre. 2006. Casein phosphopeptides released by simulated gastrointestinal digestion of infant formulas and their potential role in mineral binding. International Dairy Journal 16 (9):992–1000. doi:10.1016/j.idairyj.2005.10.010.
- Miquel, E., J. A. Gomez, A. Alegria, R. Barbera, R. Farre, and I. Recio. 2005. Identification of casein phosphopeptides released after simulated digestion of milk-based infant formulas. Journal of Agricultural and Food Chemistry 53 (9):3426–3433. doi:10.1021/jf0482111.
- Mitsuma, T., H. Odajima, Z. Momiyama, K. Watanabe, M. Masuguchi, T. Sekine, S. Shidara, and S. Hirano. 2008. Enhancement of gene expression by a peptide p(CHWPR) produced by Bifidobacterium lactis BB-12. Microbiology and Immunology 52 (3):144–155. doi:10.1111/j.1348-0421.2008.00022.x.
- Nishinari, K., Y. Fang, S. Guo, and G. O. Phillips. 2014. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocolloids 39:301–318. doi:10.1016/j.foodhyd.2014.01.013.
- Pan, D. D., Z. Wu, J. Liu, X. Y. Cao, and X. Q. Zeng. 2013. Immunomodulatory and hypoallergenic properties of milk protein hydrolysates in ICR mice. Journal of Dairy Science 96 (8):4958–4964. doi:10.3168/jds.2013-6758.
- Pastar, I., I. Tonic, N. Golic, M. Kojic, R. van Kranenburg, M. Kleerebezem, L. Topisirovic, and G. Jovanovic. 2003. Identification and genetic characterization of a novel proteinase, PrtR, from the human isolate Lactobacillus rhamnosus BGT10. Applied and Environmental Microbiology 69 (10):5802–5811. doi:10.1128/AEM.69.10.5802-5811.2003.
- Patel, S. 2015. Functional food relevance of whey protein: A review of recent findings and scopes ahead. Journal of Functional Foods 19:308–319. doi:10.1016/j.jff.2015.09.040.
- Pederson, J. A., G. J. Mileski, B. C. Weimer, and J. L. Steele. 1999. Genetic characterization of a cell envelope-associated proteinase from Lactobacillus helveticus CNRZ32. Journal of Bacteriology 181 (15):4592–4597. doi:10.1128/JB.181.15.4592-4597.1999.
- Peled, S., and Y. D. Livney. 2021. The role of dietary proteins and carbohydrates in gut microbiome composition and activity: A review. Food Hydrocolloids 120:106911. doi:10.1016/j.foodhyd.2021.106911.
- Peredo-Lovillo, A., H. E. Romero-Luna, and M. Jiménez-Fernández. 2020. Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Research International (Ottawa, Ontario) 136:109473. doi:10.1016/j.foodres.2020.109473.
- Picon, A., M. A. García-Casado, and M. Nuñez. 2010. Proteolytic activities, peptide utilization and oligopeptide transport systems of wild Lactococcus lactis strains. International Dairy Journal 20 (3):156–162. doi:10.1016/j.idairyj.2009.10.002.
- Poch, M., and A. Bezkorovainy. 1991. Bovine milk-casein trypsin digest is a growth enhancer for the genus Bifidobacterium. Journal of Agricultural and Food Chemistry 39 (1):73–77. doi:10.1021/jf00001a013.
- Proulx, M., P. Ward, S. F. Gauthier, and D. Roy. 1994. Comparison of bifidobacterial growth-promoting activity of ultrafiltered casein hydrolysate fractions. Le Lait 74 (2):139–152. doi:10.1051/lait:1994212.
- Robitaille, G., and C. P. Champagne. 2014. Growth-promoting effects of pepsin- and trypsin-treated caseinomacropeptide from bovine milk on probiotics. The Journal of Dairy Research 81 (3):319–324. doi:10.1017/S0022029914000247.
- Saiga, A. I., S. Tanabe, and T. Nishimura. 2003. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. Journal of Agricultural and Food Chemistry 51 (12):3661–3667. doi:10.1021/jf021156g.
- Saxena, S. N., B. K. Mital, and S. K. Garg. 1994. Effect of casitone and fructose on the growth of Lactobacillus acidophilus and its survival during storage. International Journal of Food Microbiology 21 (3):271–276. doi:10.1016/0168-1605(94)90034-5.
- Sinezen, R. J. 1999. Multi-domain, cell-envelope proteinases of lactic acid bacteria. Antonie Van Leeuwenhoek International Journal of General and Molecular Microbiology 76 (1-4):139–155. doi:10.1023/A:1002036906922.
- Solval, K. M., A. Chouljenko, A. Chotiko, and S. Sathivel. 2019. Growth kinetics and lactic acid production of Lactobacillus plantarum NRRL B-4496, L. acidophilus NRRL B-4495, and L. reuteri B-14171 in media containing egg white hydrolysates. Lwt 105:393–399. doi:10.1016/j.lwt.2019.01.058.
- Sousa, R., R. Portmann, S. Dubois, I. Recio, and L. Egger. 2020. Protein digestion of different protein sources using the INFOGEST static digestion model. Food Research International (Ottawa, Ont.) 130:108996. doi:10.1016/j.foodres.2020.108996.
- Sridhar, V. R., J. E. Hughes, D. L. Welker, J. R. Broadbent, and J. L. Steele. 2005. Identification of endopeptidase genes from the genomic sequence of Lactobacillus helveticus CNRZ32 and the role of these genes in hydrolysis of model bitter peptides. Applied and Environmental Microbiology 71 (6):3025–3032. doi:10.1128/AEM.71.6.3025-3032.2005.
- Stephan, A., J. Ahlborn, M. Zajul, and H. Zorn. 2018. Edible mushroom mycelia of Pleurotus sapidus as novel protein sources in a vegan boiled sausage analog system: Functionality and sensory tests in comparison to commercial proteins and meat sausages. European Food Research and Technology 244 (5):913–924. doi:10.1007/s00217-017-3012-1.
- St-Gelais, D., D. Roy, S. Haché, M. L. Desjardins, and S. F. Gauthier. 1993. Growth of nonproteolytic Lactococcus lactis in culture medium supplemented with different casein hydrolyzates. Journal of Dairy Science 76 (11):3327–3337. doi:10.3168/jds.S0022-0302(93)77670-5.
- Stuart, M. R., L. S. Chou, and B. C. Weimer. 1999. Influence of carbohydrate starvation and arginine on culturability and amino acid utilization of Lactococcus lactis subsp. lactis. Applied and Environmental Microbiology 65 (2):665–673. doi:10.1177/0146167206298564.
- Sun, X. M., C. N. Wang, and M. R. Guo. 2018. Interactions between whey protein or polymerized whey protein and soybean lecithin in model system. Journal of Dairy Science 101 (11):9680–92. doi:10.3168/jds.2018-14998.
- Sun, Z.-H., M.-J. Yao, X. Bian, Q.-Q. Guo, H.-N. Guan, Y. Yang, B. Wang, Y.-G. Shi, W. Piekoszewski, X.-W. Yang, et al. 2021. The influence of soy protein hydrolysate (SPH) addition to infant formula powder on Streptococcus thermophilus proliferation and metabolism. Food Research International (Ottawa, Ont.) 141:110103. doi:10.1016/j.foodres.2020.110103.
- Suokko, A., M. Poutanen, K. Savijoki, N. Kalkkinen, and P. Varmanen. 2008. ClpL is essential for induction of thermotolerance and is potentially part of the HrcA regulon in Lactobacillus gasseri. Proteomics 8 (5):1029–1041. doi:10.1002/pmic.200700925.
- Tang, T. T., N. Wu, S. S. Tang, N. H. Xiao, Y. Jiang, Y. G. Tu, and M. S. Xu. 2023. Industrial application of protein hydrolysates in food. Journal of Agricultural and Food Chemistry 71 (4):1788–1801. doi:10.1021/acs.jafc.2c06957.
- Tian, Q., T. T. Wang, X. Tang, M. Z. Han, X. J. Leng, and X. Y. Mao. 2015. Developing a potential prebiotic of yogurt: Growth of Bifidobacterium and yogurt cultures with addition of glycomacropeptide hydrolysate. International Journal of Food Science & Technology 50 (1):120–127. doi:10.1111/ijfs.12611.
- Tripathi, M. K., and S. K. Giri. 2014. Probiotic functional foods: Survival of probiotics during processing and storage. Journal of Functional Foods 9:225–241. doi:10.1016/j.jff.2014.04.030.
- Tynkkynen, S., G. Buist, E. Kunji, J. Kok, B. Poolman, G. Venema, and A. Haandrikman. 1993. Genetic and biochemical characterization of the oligopeptide transport system of Lactococcus lactis. Journal of Bacteriology 175 (23):7523–7532. doi:10.1128/JB.175.23.7523-7532.1993.
- Ummadi, M., and M. Curic-Bawden. 2008. Use of protein hydrolysates in industrial starter culture fermentations. Springer: Dordrecht, The Netherlands.
- Venardou, B., J. V. O’Doherty, M. J. McDonnell, A. Mukhopadhya, C. Kiely, M. T. Ryan, and T. Sweeney. 2021. Evaluation of the in vitro effects of the increasing inclusion levels of yeast beta-glucan, a casein hydrolysate and its 5 kDa retentate on selected bacterial populations and strains commonly found in the gastrointestinal tract of pigs. Food & Function 12 (5):2189–2200. doi:10.1039/d0fo02269a.
- Vital, D. A. L., E. G. de Mejia, V. P. Dia, and G. Loarca-Pina. 2014. Peptides in common bean fractions inhibit human colorectal cancer cells. Food Chemistry 157:347–355. doi:10.1016/j.foodchem.2014.02.050.
- Wang, B., Y. Yang, X. Bian, H.-N. Guan, L.-L. Liu, X.-X. Li, Q.-Q. Guo, W. Piekoszewski, F.-L. Chen, N. Wu, et al. 2021. Proliferation of Bifidobacterium L80 under different proportions of milk protein hydrolysate. Microbial Cell Factories 20 (1):213. doi:10.1186/s12934-021-01702-3.
- Wang, H. Q., T. Huang, K. L. Liu, J. Yu, G. Q. Yao, W. Y. Zhang, H. P. Zhang, and T. S. Sun. 2022. Protective effects of whey protein hydrolysate on Bifidobacterium animalis ssp. lactis Probio-M8 during freeze-drying and storage. Journal of Dairy Science 105 (9):7308–7321. doi:10.3168/jds.2021-21546.
- Wang, J. R., D. Teng, Z. G. Tian, H. U. Jian-Cheng, and J. H. Wang. 2008. Preparation and mechanism of functional antioxidant peptides. Natural Product Research & Development 20:49. doi:10.16333/j.1001-6880.2008.02.010.
- Wang, L. F., J. Zhang, Q. Yuan, H. H. Xie, J. Y. Shi, and X. R. Ju. 2016. Separation and purification of an anti-tumor peptide from rapeseed (Brassica campestris L.) and the effect on cell apoptosis. Food & Function 7 (5):2239–2248. doi:10.1039/c6fo00042h.
- Wang, Z. J., and X. W. Zhang. 2017. Isolation and identification of anti-proliferative peptides from Spirulina platensis using three-step hydrolysis. Journal of the Science of Food and Agriculture 97 (3):918–922. doi:10.1002/jsfa.7815.
- Wu, R. N., W. Y. Zhang, T. S. Sun, J. R. Wu, X. Q. Yue, H. Meng, and H. P. Zhang. 2011. Proteomic analysis of responses of a new probiotic bacterium Lactobacillus casei to low acid stress. International Journal of Food Microbiology 147 (3):181–187. doi:10.1016/j.ijfoodmicro.2011.04.003.
- Xie, H. X., Y. Liao, M. W. Woo, H. Xiong, and Q. Zhao. 2023. Whey protein hydrolysates as prebiotic and protective agent regulate growth and survival of Lactobacillus rhamnosus CICC22152 during spray/freeze-drying, storage and gastrointestinal digestion. Journal of the Science of Food and Agriculture 103 (3):1237–1246. doi:10.1002/jsfa.12218.
- Xue, H., J. Han, J. Ma, H. Song, B. He, X. Liu, M. Yi, and L. Zhang. 2023. Identification of immune-active peptides in casein hydrolysates and its transport mechanism on a Caco-2 monolayer. Foods (Basel, Switzerland) 12 (2):373. doi:10.3390/foods12020373.
- Yadav, M. K., I. Kumari, B. Singh, K. K. Sharma, and S. K. Tiwari. 2022. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Applied Microbiology and Biotechnology 106 (2):505–521. doi:10.1007/s00253-021-11646-8.
- Yang, T. T., L. M. Yu, E. Q. Liu, X. J. Chen, M. X. Zhu, and X. Q. Wang. 2014. Effects of cecropin a-magainin hybrid peptide on small intestinal mucosal structure, mucosal immune function and intestinal microflora in mice. Chinese Journal of Animal Nutrition 26:3387–3395.
- You, S., Y. Ma, B. Yan, W. Pei, Q. Wu, C. Ding, and C. Huang. 2022. The promotion mechanism of prebiotics for probiotics: A review. Frontiers in Nutrition 9:1000517. doi:10.3389/fnut.2022.1000517.
- Yu, Y. J., M. Amorim, C. Marques, C. Calhau, and M. Pintado. 2016. Effects of whey peptide extract on the growth of probiotics and gut microbiota. Journal of Functional Foods 21:507–516. doi:10.1016/j.jff.2015.10.035.
- Zhang, C., S. Xia, Y. Zhang, S. Zhu, H. Li, and X. Liu. 2022. Identification of soybean peptides and their effect on the growth and metabolism of Limosilactobacillus reuteri LR08. Food Chemistry 369:130923. doi:10.1016/j.foodchem.2021.130923.
- Zhang, C., Y. X. Zhang, G. R. Liu, W. H. Li, S. Q. Xia, H. Li, and X. Q. Liu. 2021. Effects of soybean protein isolates and peptides on the growth and metabolism of Lactobacillus rhamnosus. Journal of Functional Foods 77:104335. doi:10.1016/j.jff.2020.104335.
- Zhang, C., Y. X. Zhang, H. Li, and X. Q. Liu. 2020. The potential of proteins, hydrolysates and peptides as growth factors for Lactobacillus and Bifidobacterium: Current research and future perspectives. Food & Function 11 (3):1946–1957. doi:10.1039/c9fo02961c.
- Zhang, C., Y. X. Zhang, S. Q. Xia, S. Y. Zhu, W. H. Li, S. M. Aboelenin, M. M. Soliman, X. Li, and X. Liu. 2021. iTRAQ-based proteomic analysis of the differential effects of digested soy peptides and digested soy protein isolates on Lacticaseibacillus rhamnosus. Food Bioscience 43:101296. doi:10.1016/j.fbio.2021.101296.
- Zhang, P., S. Roytrakul, and M. Sutheerawattananonda. 2017. Production and purification glucosamine and angiotensin-I converting enzyme (ACE) inhibitory peptides from mushroom hydrolysates. Journal of Functional Foods 36:72–83. doi:10.1016/j.jff.2017.06.049.
- Zhang, Q. L., J. Y. Ren, H. F. Zhao, M. M. Zhao, J. Y. Xu, and Q. Z. Zhao. 2011. Influence of casein hydrolysates on the growth and lactic acid production of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus. International Journal of Food Science & Technology 46 (5):1014–1020. doi:10.1111/j.1365-2621.2011.02578.x.
- Zhao, F. F., and R. J. Zhang. 2004. Effect of soybean peptide on intestinal flora of laying hens. Feed China 9:17–18.
- Zhao, H. F., F. L. Bai, F. Zhou, P. Walczak, X. N. Jiang, and B. L. Zhang. 2013. Characterization of soybean protein hydrolysates able to promote the proliferation of Streptococcus Thermophilus ST. Journal of Food Science 78 (4):M575–M581. doi:10.1111/1750-3841.12075.
- Zhao, Q., Y. Shi, X. Wang, and A. Huang. 2020. Characterization of a novel antimicrobial peptide from buffalo casein hydrolysate based on live bacteria adsorption. Journal of Dairy Science 103 (12):11116–11128. doi:10.3168/jds.2020-18577.
- Zisu, B., and N. P. Shah. 2003. Effects of pH, temperature, supplementation with whey protein concentrate, and adjunct cultures on the production of exopolysaccharides by Streptococcus thermophilus 1275. Journal of Dairy Science 86 (11):3405–3415. doi:10.3168/jds.S0022-0302(03)73944-7.