Abstract
Higher intakes of cruciferous and allium vegetables are associated with a lower risk of cardiometabolic-related outcomes in observational studies. Whilst acknowledging the many healthy compounds within these vegetables, animal studies indicate that some of these beneficial effects may be partially mediated by S-methyl cysteine sulfoxide (SMCSO), a sulfur-rich, non-protein, amino acid found almost exclusively within cruciferous and alliums. This scoping review explores evidence for SMCSO, its potential roles in human health and possible mechanistic action. After systematically searching several databases (EMBASE, MEDLINE, SCOPUS, CINAHL Plus Full Text, Agricultural Science), we identified 21 original research articles meeting our inclusion criteria. These were limited primarily to animal and in vitro models, with 14/21 (67%) indicating favorable anti-hyperglycemic, anti-hypercholesterolemic, and antioxidant properties. Potential mechanisms included increased bile acid and sterol excretion, altered glucose- and cholesterol-related enzymes, and improved hepatic and pancreatic β-cell function. Raising antioxidant defenses may help mitigate the oxidative damage observed in these pathologies. Anticancer and antibacterial effects were also explored, along with one steroidogenic study. SMCSO is frequently overlooked as a potential mediator to the benefits of sulfur-rich vegetables. More research into the health benefits of SMCSO, especially for cardiometabolic and inflammatory-based pathology, is warranted. Human studies are especially needed.
Introduction
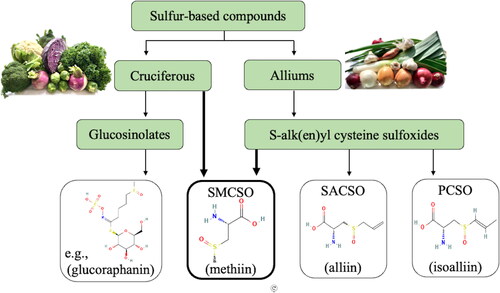
Observational studies suggest that higher consumption of cruciferous and allium vegetables is associated with a lower risk of cardiometabolic disease (Blekkenhorst, Sim, et al. Citation2018; Blekkenhorst et al. Citation2017; Blekkenhorst, Bondonno, et al. Citation2018; Jia et al. Citation2016; Zurbau et al. Citation2020). Cruciferous (e.g., broccoli, cauliflower, cabbage, kale) and alliums (e.g., garlic, onion, leek) contain many vitamins, minerals, fibers, and phytochemicals, and are particularly rich in sulfur-containing compounds: all of which have been linked to health benefits (Hill et al. Citation2022; Mohammed and Qoronfleh Citation2020). In fact, vegetable-derived sulfur-containing compounds are associated with many antimicrobial, anticarcinogenic, lipid-lowering, antioxidant, anti-inflammatory, neuroprotective, hepatoprotective, and cardiometabolic benefits (Petropoulos, Di Gioia, and Ntatsi Citation2017; Ruhee et al. Citation2020). Most of this research has focused on specific sulfur-containing compounds such as glucosinolates (e.g., glucoraphanin, glucoerucin) which are abundant in cruciferous vegetables. Interestingly, all the glucosinolates combined make up approximately 0.1 − 0.6% of the dry weight of cruciferous vegetables, and yet S-methyl cysteine sulfoxide (SMCSO), a non-proteinogenic sulfur-containing cysteine derivative, contributes substantially more at ∼1 - 4% dry weight (Mae, Ohira, and Fujiwara Citation1971; Howard S. Marks et al. Citation1992). Evidence also suggests many of the benefits of allium vegetables are largely due to the presence of sulfur-containing S-alk(en)yl-L-cysteine sulfoxides; the three main ones being alliin (S-allyl-L-cysteine sulfoxide), isoalliin (S-trans-1-propenyl-L-cysteine sulfoxide) and methiin (a.k.a., S-methyl-L-cysteine sulfoxide; SMCSO) (Horníčková et al. Citation2010). Despite SMCSO being one of the main S-alk(en)yl-L-cysteine sulfoxides in alliums and being abundant in cruciferous vegetables, it receives considerably less attention. The interplay of these sulfur-containing glucosinolates and cysteine sulfoxides in cruciferous and alliums is illustrated in .
Figure 1. S-methyl cysteine sulfoxide (SMCSO; a.k.a. methiin) and its interplay between cruciferous and allium vegetables; SACSO S-allyl cysteine sulfoxide; PCSO S-propenyl cysteine sulfoxide. Source of structures provided: https://pubchem.ncbi.nlm.nih.gov/.
Having first been identified in cruciferous and alliums in the 1950s (Morris and Thompson Citation1956; Synge and Wood Citation1956; Yuguri Citation1954), early research into SMCSO centered around the agricultural, livestock, and plant sciences. Crucifers (especially kale) were grown as a low-cost, readily-available source of feed for ruminant livestock animals (e.g., cattle, sheep, goats) (Whittle, Smith, and McIntosh Citation1976). Whilst crucifers were relatively well-tolerated and beneficial, their excess consumption resulted in the development of Heinz-Ehrlich bodies within erythrocytes, leading to hemolysis and even death. It was proposed that SMCSO was the probable cause (Smith, Earl, and Matheson Citation1974; Smith Citation1978). Whilst different ruminants appeared to have varied tolerance levels, intakes of SMCSO above 15 g/100 kg body weight (BW) daily seem sufficient to provoke the hemolytic effect (Smith Citation1978). Subsequent investigations identified that SMCSO can be converted by the ruminant gut microbiota into a highly reactive derivative known as dimethyl disulfide (Smith Citation1978). Dimethyl disulfide has been identified as being responsible for the development of ‘kale poisoning’ (otherwise referred to as ‘kale hemolytic factor’), and although largely restricted to ruminants, has arguably contributed to the paucity of research into SMCSO. Low-SMCSO cruciferous cultivars have since been developed for ruminant feed purposes (Bradshaw Citation2021; Bradshaw and Wilson Citation2012). Potential toxicity concerns (or lethal doses) in other non-ruminant animals have not been established.
Any potential toxicity to humans from SMCSO intake has not been addressed by human research, however, the level of intake from either cruciferous and/or allium vegetables by humans (per BW) would be considerably lower than that of ruminants. Furthermore, raising consumption of these SMCSO-containing vegetables is largely encouraged for their associated health benefits (Abbaoui et al. Citation2018; Alam et al. Citation2023). Evidence suggests that phytochemicals found within these vegetables, beyond glucosinolates, require further investigation, and more specifically that the role of SMCSO in mediating these benefits is needed (Quirante-Moya et al. Citation2020; W.M.B. Edmands et al. Citation2013; Friedrich et al. Citation2022).
The highest known dietary sources of SMCSO include Brussels sprouts (≤420 mg/100g fresh weight [FW]) (Coode-Bate et al. Citation2019), cauliflower (≤285 mg/100g FW) (Kubec and Dadáková Citation2008), Chinese chives (≤413 mg/100g FW) (Yabuki et al. Citation2010), and rakkyo (≤245 mg/100g FW) (Yamazaki et al. Citation2010), whilst also found in broccoli, kale, cabbage, garlic, onion and leek (Hill et al. Citation2022). Briefly, SMCSO levels in vegetables are influenced by plant genetics, cultivar, growing and environmental factors (e.g., exposure to pathogens, temperature, time of harvest, availability of nutrients, storage conditions) (Hill et al. Citation2022; W.M.B. Edmands et al. Citation2013). Whilst stored intact within plant vacuoles (i.e., inactive), SMCSO is released and becomes biologically active upon tissue maceration (i.e., chewing, or slicing) and the subsequent exposure to separately stored cysteine sulfoxide lyases within the plant tissue (W.M.B. Edmands et al. Citation2013). A bacterial-mediated conversion can also occur within the human colonic microbiota (W.M.B. Edmands et al. Citation2013).
As such, SMCSO has been identified as a validated and accurate urinary biomarker to indicate dietary cruciferous intake in humans (Sivapalan et al. Citation2019; W.M. Edmands et al. Citation2011). Recent human studies are beginning to measure and report SMCSO as part of the metabolomic footprint of healthy eating patterns (Chan et al. Citation2022; Li et al. Citation2017; Rafiq et al. Citation2021). As such, an improved understanding of SMCSO and its possible benefits and mechanisms of action that may mediate human physiology is warranted. To date, there have been no systematic or scoping reviews reporting on the health effects of SMCSO. Yet, a scoping review is intended to broadly map literature in an emergent field, determine the extent, range, and nature of current available literature, and aid the development of future research (Peters, Godfrey, et al. Citation2020). It was therefore identified as an ideal process for this topic.
The primary aim of this scoping review was to identify the evidence available for the potential of SMCSO intake to benefit human health and identify the possible mechanisms responsible. In exploring this literature, we also unearthed the extent and nature of previous research and identified gaps for future research.
Methods
Our methodology followed the framework outlined by the Joanna Briggs Institute for scoping reviews (Peters, Marnie, et al. Citation2020; Aromataris and Munn Citation2020) and was reported in accordance with the PRISMA-ScR (Preferred Reporting Items for Systematic Review and Meta-analyses extension for Scoping Reviews) checklist (Tricco et al. Citation2018), provided in Supplementary Table 1.
Research questions
What evidence exists for the potential of SMCSO to bene fit human health?
What are the mechanisms of action that may explain the potential benefits of SMCSO on human health?
Inclusion and exclusion criteria
Our inclusion criteria were broadly developed around three guiding principles: population, concept, and context. [1] Our population included both humans and non-ruminant animals. Being aware of the lack of human studies, it was important to be broad in our definition of population to avoid missing key articles that could offer insights into mechanisms transferable to human health. [2] Our concept was also kept broad. As our overarching phenomenon of interest was the potential of SMCSO to benefit human health, we considered all articles that suggested SMCSO-related health outcomes that could be potentially transferable to human health. Lastly, [3] it was equally important to be broad in context, and as such, no specific setting nor limitation upon context was set. Therefore, articles were considered relevant if they addressed one or more aspects of our research questions, and could be from preclinical (e.g., in vitro, in vivo, animal models) and clinical (e.g., intervention, observational) study designs. They did, however, need to be original research, to prevent duplication of evidence. Our inclusion and exclusion criteria are outlined in Supplementary Table 2.
Search strategy
Our search strategy utilized the expertise and guidance of a discipline-specific librarian. After initially defining our overarching purpose, we broadly became familiar with the literature, being mindful of keywords, definitions, and terms commonly used. It was apparent that synonyms were used interchangeably for SMCSO, and as such, we ensured these were identified prior to finalizing our search strategy. The original search was on 22 September 2020, with an updated search conducted on 30 September 2022. Subject and citation databases searched included EMBASE, MEDLINE, SCOPUS, Agricultural Science collection (including Agricola and TOXLINE), and CINAHL Plus Full Text.
Keywords and index terms were adapted across databases as necessary and always applied across full text whenever possible to avoid missing relevant articles. Search terms used for SMCSO included the following: S-methyl cysteine sulfoxide; S-methyl-L-cysteine sulfoxide; methylcysteine; methyl cysteine; SMCSO; methiin; S-methyl cysteine-sulfoxide; MCSO and S-alk(en)yl-L-cysteine sulfoxide/s, along with an extensive list of health-related outcomes (Supplemental Table 3). Upon initial agreement of our search and screening strategy, 25 randomly selected titles and abstracts were pilot tested for inter-rater reliability between two study authors (CRH and LCB) for our inclusion/exclusion criteria and data extraction process. The total number of publications was recorded, imported, and merged into an Endnote X9 reference library (Thomas Reuters). After the removal of duplicates, articles were imported into Covidence, a web-based literature screening software for the selection process (Covidence, Veritas Health Innovation, Melbourne, Australia) (Veritas Health Innovation Citation2023).
Screening
The screening was a multi-stage process, performed independently by four authors (CRH, AHL, LM, and LCB). Firstly, titles and/or abstracts that included any variation or derivatives of our keyword, or were a food known to contain our keyword (or acronym) and had any relevance to health or mentioned any potential mechanism of action were included at this stage. If the reviewer was unsure or suspected these criteria could be found within the full text, the article progressed for full-text screening, as it was presumed our keywords would be within the full-text, and thus may be relevant. Full-text articles were subsequently assessed for eligibility against our criteria by two authors (CRH and AHL), and any conflicts were resolved by discussion and consensus between these authors in addition to a mediating author as necessary (LCB). A manual citation search of reference lists was further scanned to identify additional sources.
Data extraction
Data were extracted from eligible articles into an Excel spreadsheet (Microsoft Corporation, USA). This extraction spreadsheet followed extraction framework guidelines recommended by the Joanna Briggs Institute (Peters, Godfrey, et al. Citation2020) and was performed by CRH, with guidance and cross-checking by LCB for accuracy. Data extracted included author/s, year of publication, citation details, purpose statement, and overall aim, along with specific health outcomes, design, population, method/dose/duration, outcomes, proposed mechanisms, funding, and any other information deemed relevant. This extraction process enabled our results to be analyzed descriptively (frequencies and percentages) and presented in both a narrative and tabular manner, to meet our intentions for this scoping review. We have provided our data extraction instrument in an Excel file, Supplementary Table 4, whilst excluded studies and their primary reasons for exclusion, are given in Supplementary Table 5. Due to the typically broad nature of varied sources compiled within a scoping review (Peters, Marnie, et al. Citation2020), comparisons of both strength and risk of bias are difficult. Therefore, we did not assess the strength of the evidence extracted nor the risk of bias across the articles extracted, as this is not required of a scoping review (Peters, Marnie, et al. Citation2020), and was beyond the intention of this project.
Results
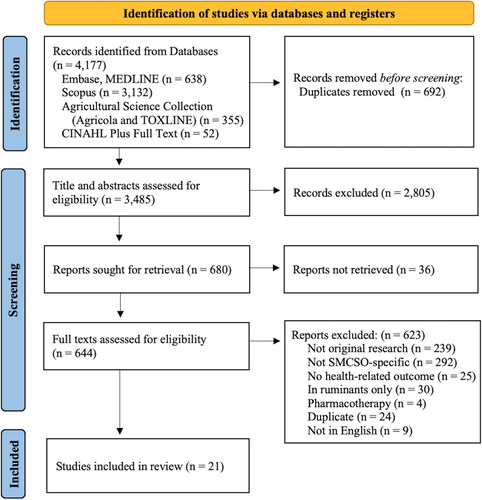
Our search strategy identified 4,177 articles. After removing all duplicates, 3,485 articles were imported into Covidence for title and abstract screening, and a total of 644 articles were screened for full-text eligibility. After removing 623 articles not meeting our criteria, we identified and included a total of 21 original research articles exploring SMCSO intake and health-related outcomes. A PRISMA flow diagram reporting how many articles were identified, screened, and included is illustrated in .
The 21 original research articles originated from Japan (n = 8) (Higuchi, Tateshita, and Nishimura Citation2003; Itokawa et al. Citation1973; Komatsu, Miura, and Yagasaki Citation1998; Kubodera et al. Citation1973; Nakayama et al. Citation2020; Tanaka, Shimada, and Nagaoka Citation2014; Yoshinari, Shiojima, and Igarashi Citation2012; Fujiwara et al. Citation1972), India (n = 5) (Kumari and Augusti Citation1995, Citation2002, Citation2007; Kumari, Mathew, and Augusti Citation1995; Sheela, Kumud, and Augusti Citation1995), United Kingdom (n = 2) (Coode-Bate et al. Citation2019; Traka et al. Citation2019), Brazil (n = 2) (de Castro et al. Citation2021; de Lemos et al. Citation2021), Germany (n = 1) (Sendl et al. Citation1992), Korea (n = 1) (Kook, Kim, and Choi Citation2009), Romania (n = 1) (Bagiu, Vlaicu, and Butnariu Citation2012), and the United States of America (n = 1) (H. S. Marks, Anderson, and Stoewsand Citation1993). The majority were experimental studies conducted in rats (n = 10) (de Castro et al. Citation2021; de Lemos et al. Citation2021; Fujiwara et al. Citation1972; Itokawa et al. Citation1973; Komatsu, Miura, and Yagasaki Citation1998; Kumari and Augusti Citation1995, Citation2002, Citation2007; Kumari, Mathew, and Augusti Citation1995; Sheela, Kumud, and Augusti Citation1995), mice (n = 1) (H. S. Marks, Anderson, and Stoewsand Citation1993), or both rats and mice (n = 1) (Kubodera et al. Citation1973). One meta-analysis was conducted using experimental studies conducted in rats (n = 1) (Kook, Kim, and Choi Citation2009). Others were in vitro studies (n = 6) (Bagiu, Vlaicu, and Butnariu Citation2012; Higuchi, Tateshita, and Nishimura Citation2003; Nakayama et al. Citation2020; Sendl et al. Citation1992; Tanaka, Shimada, and Nagaoka Citation2014; Yoshinari, Shiojima, and Igarashi Citation2012), and the remaining involved humans (n = 2) (Coode-Bate et al. Citation2019; Traka et al. Citation2019). Studies were from 1972 – 2021, with just over half (52%) published in the last two decades. Characteristics of each study are included in (animals), (in vitro), and (humans).
Table 1. Characteristics of experimental non-ruminant animal models (and one meta-analysis) exploring the effects of SMCSO on health-related outcomes (n = 13).
Table 2. Characteristics of in-vitro studies exploring the effects of SMCSO on health-related outcomes or mechanisms of action (n = 6).
Table 3. Characteristics of experimental human studies exploring the effects of SMCSO on health-related outcomes or mechanisms of action (n = 2).
Briefly, most studies explored the effect of SMCSO on lipid and/or glucose levels, demonstrating favorable effects (Komatsu, Miura, and Yagasaki Citation1998; Kumari and Augusti Citation1995, Citation2002, Citation2007; Kumari, Mathew, and Augusti Citation1995; Itokawa et al. Citation1973; Fujiwara et al. Citation1972; de Lemos et al. Citation2021; de Castro et al. Citation2021; Sheela, Kumud, and Augusti Citation1995; Kook, Kim, and Choi Citation2009; Yoshinari, Shiojima, and Igarashi Citation2012; Sendl et al. Citation1992; Tanaka, Shimada, and Nagaoka Citation2014). Others explored the effects of SMCSO as an antioxidant against hypercholesterolemic-induced damage (de Lemos et al. Citation2021; Kumari and Augusti Citation2002), lipid peroxidation (Higuchi, Tateshita, and Nishimura Citation2003), irradiation-induced damage (Kubodera et al. Citation1973), and as an anti-carcinogenic agent (H. S. Marks, Anderson, and Stoewsand Citation1993). The anti-obesogenic (Yoshinari, Shiojima, and Igarashi Citation2012), anti-microbial (Bagiu, Vlaicu, and Butnariu Citation2012), and steroidogenic potential of SMCSO (Nakayama et al. Citation2020) were also studied. The two experimental human studies included dietary interventions with the first study administering cruciferous soup to men with prostate cancer for 4 wk prior to prostate surgery (Coode-Bate et al. Citation2019). The second study gave a weekly cruciferous soup to men undergoing active surveillance for prostate cancer for 12 months prior to prostate surgery (Traka et al. Citation2019). We also found one meta-analysis which set out to collate studies exploring the effects of garlic and onion in diabetic rats (Kook, Kim, and Choi Citation2009). This meta-analysis, however, included just two studies, which we have already captured within this review (Kumari, Mathew, and Augusti Citation1995; Sheela, Kumud, and Augusti Citation1995). Details for each of these studies are outlined below.
SMCSO as an anti-hypercholesterolemic agent
Administering SMCSO to rats with diet-induced hypercholesterolemia has been shown to reduce total cholesterol (by ∼18 - 33%) (Fujiwara et al. Citation1972; Itokawa et al. Citation1973; Komatsu, Miura, and Yagasaki Citation1998; Kumari and Augusti Citation2007; Kumari, Mathew, and Augusti Citation1995), low-density lipoprotein (LDL) (by ∼26%) (Kumari, Mathew, and Augusti Citation1995), and very-low-density lipoprotein (by 35 - 67%) (de Lemos et al. Citation2021; Komatsu, Miura, and Yagasaki Citation1998; Kumari, Mathew, and Augusti Citation1995). Dosages used were between 180 - 364 mg of SMCSO/kg/BW/day administered predominantly via oral gavage over 14 - 60 days. The most frequently used SMCSO dose has been 200 mg/kg/BW/day (de Castro et al. Citation2021; de Lemos et al. Citation2021; Kumari and Augusti Citation1995, Citation2002, Citation2007; Kumari, Mathew, and Augusti Citation1995; Sheela, Kumud, and Augusti Citation1995). Whilst most studies were conducted between ∼45 - 60 days, the lipid-lowering effects of SMCSO were evident after just 2 wk. For example, hypercholesterolemic rats were given either ∼182 mg or 364 mg/kg/BW/day of SMCSO supplemented into their diets, and reported between 21 − 44% reduction in total plasma cholesterol, when compared to control rats (Fujiwara et al. Citation1972; Itokawa et al. Citation1973). When administered at 200 mg/kg/BW, SMCSO also lowered triglycerides in diabetic rats by 65% after 30 days (de Lemos et al. Citation2021), and by 26% in hypercholesterolemic rats after 45 days (Kumari and Augusti Citation2007), when compared to either their diabetic- or hypercholesterolemic-control rats. In contrast, a non-hypercholesterolemic, non-diabetic, hepatoma-induced rat model found a notable increase in hepatic triglycerides (42%) after doses of 250 mg/kg/BW/day via gavage (Komatsu, Miura, and Yagasaki Citation1998). Reductions in liver cholesterol (between 10 - 18%) and liver lipids (between 11 - 33%) have also been observed using oral gavage doses between 182 - 364 mg/kg/BW/day, over 14 − 45 days (Fujiwara et al. Citation1972; Itokawa et al. Citation1973; Kumari, Mathew, and Augusti Citation1995).
Three studies reported that SMCSO increases fecal bile acids in hepatoma (Komatsu, Miura, and Yagasaki Citation1998), diabetic (Kumari, Mathew, and Augusti Citation1995), and hypercholesterolemic rats (Kumari and Augusti Citation2007) when compared to suppressed levels found in controls. Komatsu, Miura, and Yagasaki (Citation1998) gave doses of 250 mg/kg/BW/day over 14 days (% increase not reported), whilst Kumari, Mathew, and Augusti (Citation1995) and Kumari and Augusti (Citation2007) both administered 200 mg/kg/BW/day for 45 days, increasing fecal bile acids by 18 and 25%, respectively. Similarly, increases were observed for fecal sterols (between 15 - 37%) (Kumari and Augusti Citation2007; Kumari, Mathew, and Augusti Citation1995). There is evidence of SMCSO significantly increasing cholesterol 7α-hydroxylase activity (a.k.a., CYP7A1) (Komatsu, Miura, and Yagasaki Citation1998), altering lipogenic enzymes (such as malic enzyme and lipoprotein lipase), and raising the rate-limiting enzyme hydroxymethylglutaryl coenzyme A (HMG CoA) reductase (Kumari and Augusti Citation2007; Kumari, Mathew, and Augusti Citation1995). Each of these are possible contributory mechanisms behind the cholesterol-lowering effect of this compound. However, whilst Sendl et al. (Citation1992) reported an in vitro cholesterol-lowering effect in hypercholesterolemic rat livers exposed to SMCSO, the reduction was insignificant. Furthermore, another in vitro study found exposing human hepatoblastoma cells to SMCSO reduced cholesterol 7α-hydroxylase expression and enhanced HMG CoA reductase levels, further suggestive of a regulatory role of cholesterol-related genes (Tanaka, Shimada, and Nagaoka Citation2014).
SMCSO as an anti-diabetic agent
Administering 200 mg/kg/BW/day of SMCSO via oral gavage to alloxan-induced diabetic rats has been shown to lower blood glucose levels (between ∼19 - 25%) after 30 - 60 days (Kumari and Augusti Citation1995; Kumari, Mathew, and Augusti Citation1995; de Castro et al. Citation2021; de Lemos et al. Citation2021; Kumari and Augusti Citation2002; Sheela, Kumud, and Augusti Citation1995). The same doses have also improved glucose tolerance (Kumari and Augusti Citation1995) and reduced postprandial glucose levels (Sheela, Kumud, and Augusti Citation1995). There is evidence that SMCSO (at 200 mg/kg/BW/day, via oral gavage over 30 − 45 days) acts as an insulin secretagogue, enhances hepatic glycogen stores (by ∼16 - 20%) (de Lemos et al. Citation2021; Kumari and Augusti Citation2007; Sheela, Kumud, and Augusti Citation1995), normalizes glucose-6-phosphatase and hexokinase levels (Kumari and Augusti Citation2007; Kumari, Mathew, and Augusti Citation1995). Furthermore, this aforementioned dose of SMCSO has attenuated the histological changes within pancreatic tissue during the progression of diabetes (de Lemos et al. Citation2021). These anti-diabetic effects have been compared against diabetic medications (i.e., glibenclamide and insulin) indicating favorable results, albeit not as pronounced. For example, diabetic rats given either glibenclamide or insulin reported blood glucose reductions (of 42% and 48%, respectively), whilst diabetic rats given oral gavage of SMCSO (at 200 mg/kg/BW/day) had a 24% reduction (Kumari and Augusti Citation1995). The glibenclamide-administered diabetic rats reported greater serum insulin (81% increase) than the SMCSO-administered rats (50% increase) when compared to the diabetic control rats (Kumari and Augusti Citation1995). This indicates SMCSO acts as an insulin secretagogue, thereby offering anti-diabetic potential.
SMCSO as an antioxidant and anti-inflammatory agent
SMCSO has also been investigated for its role as an antioxidant, suggesting anti-inflammatory potential. SMCSO was found to be a stronger, and more superior antioxidant in a study comparing the glucose-lowering effect of SMCSO against traditional anti-diabetic agents (e.g., glibenclamide and insulin) (Kumari and Augusti Citation2002). For example, diabetic rats administered 200 mg/kg/BW/day of SMCSO (for 60 days, via oral gavage) had lowered malondialdehyde, hydroperoxides, conjugated dienes, superoxide dismutase, and catalase levels, over those diabetic rats treated with either glibenclamide or insulin. In fact, malondialdehyde was lowered by 12%, 8%, and 21% in SMCSO-, glibenclamide- and insulin-treated groups, respectively; hydroperoxides by 34%, 23%, and 31%, respectively; and conjugated dienes by 12%, 8%, and 10%, respectively (Kumari and Augusti Citation2002). SMCSO administered at 200 mg/kg/BW/day was shown to raise hepatic superoxide dismutase and catalase in diabetic rats, when administered over 30 - 60 days, via oral gavage (de Lemos et al. Citation2021; Kumari and Augusti Citation2002). Both rats and mice were also protected against the deleterious effects of radioactivity (Kubodera et al. Citation1973). Firstly, rats given 400 mg/kg/BW via intraperitoneal injection pre- and post-exposure to X-irradiation, had an amelioration in plasma kininogen levels. Within the same study, an intraperitoneal injection of either a one-off 10 or 20 mg dose of SMCSO given 10 min prior to a lethal X-irradiation dose had significantly enhanced survival time (mean survival 17.2 − 17.4 days, compared to 7.6 days in the control mice group (Kubodera et al. Citation1973). An in vitro study reported that copper-induced human LDL oxidized cells (and subsequent lipid peroxidation) were attenuated after exposure to varying concentrations of SMCSO (Higuchi, Tateshita, and Nishimura Citation2003).
A reversal in histological changes has been observed in streptozotocin-induced diabetic rats (de Castro et al. Citation2021; de Lemos et al. Citation2021). Diabetic rats administered SMCSO at 200 mg/kg/BW/day for 30 days, via oral gavage, were found to have restored liver, pancreatic islet (de Lemos et al. Citation2021), and duodenal morphology (de Castro et al. Citation2021), as well as significantly reduced nuclear factor-ĸβ staining in their duodenal tissue (de Castro et al. Citation2021), when compared to their control diabetic groups. The SMCSO-administered rats had no significant change in interleukin (IL)-6 levels, nor did they attenuate an increase in IL-1β, although there was a significant increase in IL-10 levels indicating an anti-inflammatory effect of SMCSO (de Lemos et al. Citation2021). The authors postulated that by improving antioxidant capacity with SMCSO, oxidative damage was reduced, and concomitant lower inflammation (i.e., IL-10) may have led to improved insulin sensitivity, and reduced hyperglycemia seen in both studies (de Castro et al. Citation2021). Importantly, these two studies (de Castro et al. Citation2021; de Lemos et al. Citation2021) reported using (R)-2amino-3-(methylmercapto) propionic acid (molecular weight 135.18 g/mol) (a.k.a., S-methyl cysteine; SMC) within their methodology, which is the oxidized form of SMCSO (molecular weight 151.19 g/mol). Whilst SMC is also present in cruciferous vegetables and may offer similarities to SMCSO, its mechanism is likely to be related, but not identical. To our knowledge, only one study has directly compared SMC and SMCSO, with both compounds administered to diet-induced hypercholesterolemic rats over two weeks (1973). This study reported that SMC was not as effective in either lowering hepatic lipid accumulation nor plasma cholesterol when compared to hypercholesterolemic rats receiving SMCSO (Itokawa et al. Citation1973). The SMCSO-administered rats had a 33% reduction in plasma cholesterol (whilst the SMC-administered rats had 16%, reported as not statistically significant) (Itokawa et al. Citation1973). It has been suggested that sulfoxide-containing amino acids (e.g., SMCSO) offer more anti-hypercholesterolemic advantages than sulfur-containing amino acids (e.g., SMC). More comparative studies on these two compounds are required.
SMCSO and other roles: weight, anti-microbial, anti-cancer
To date, there has been little exploration of SMCSO specifically as an anti-obesity agent, and results are inconsistent. Firstly, this compound does not seem to alter food consumption (Komatsu, Miura, and Yagasaki Citation1998; Kumari and Augusti Citation2002), and whilst favorable body weight maintenance has been reported in diabetic rats (Kumari and Augusti Citation2002), the effect upon body weight changes is very mixed and mostly non-significant across studies (de Castro et al. Citation2021; Itokawa et al. Citation1973; Komatsu, Miura, and Yagasaki Citation1998; Kumari and Augusti Citation1995; Kumari, Mathew, and Augusti Citation1995). However, one study did report a significant (∼11%) weight reduction in hypercholesterolemic (i.e., non-diabetic) rats suggesting a different mechanism may be involved in hypercholesterolemic conditions (Kumari and Augusti Citation2007). We identified one in vitro study that suggested SMCSO inhibits oil drop formation and subsequent accumulation within adipocytes, which may be a possible mechanism (Yoshinari, Shiojima, and Igarashi Citation2012).
Whilst there are suggestions of SMCSO being possibly anti-microbial (W.M. Edmands et al. Citation2011), the evidence found was limited. We found only one in vitro study reporting anti-fungal effects against strains of Candida albicans (Bagiu, Vlaicu, and Butnariu Citation2012). However, it appears that SMCSO derivatives that are produced during the enzyme-induced hydrolysis of SMCSO (e.g., methyl methanethioisulfinate, methyl methanethiolsulfinate [MMTSO], methanesulfinic acid, methanethiol, dimethyl disulfide, pyruvate, and ammonia), may indeed offer a range of anti-microbial potential (W.M.B. Edmands et al. Citation2013). Furthermore, SMCSO, along with the derivative MMTSO, has been suggested to be responsible for reduced genotoxicity in mice, in one of our included papers (H. S. Marks, Anderson, and Stoewsand Citation1993). Marks, Anderson, and Stoewsand (Citation1993) administered weanling mice with varying sulfur compounds (including 2 doses of either 0.5 mmol SMCSO or 0.5 mmol MMTSO, via gavage) both before and 72 h after benzo[1] pyrene-induced genotoxicity. Levels of micronucleated polychromatic erythrocytes in the bone marrow of each mouse (an indicator of carcinogenesis) were reduced by 31% in the SMCSO group (and by 33% in the MMTSO group), compared to controls (H. S. Marks, Anderson, and Stoewsand Citation1993). However, we did not intentionally search for SMCSO derivatives within our search criteria and thus, more research may be available on specific downstream derivatives.
SMCSO in human studies
We identified only two studies exploring the health effect of SMCSO in humans (Coode-Bate et al. Citation2019; Traka et al. Citation2019). Both studies used food (i.e., broccoli) rather than isolating SMCSO as the intervention. Thus, some of the observed effects may be partly explained by other co-existing compounds found in those foods.
Using a double-blinded, three-arm parallel dietary intervention, men undergoing active surveillance for prostate cancer (n = 49) were randomized to receive one of three broccoli soups containing low-, medium- and high-glucoraphanin, for 12 months (Traka et al. Citation2019). Interestingly, the most significant inverse correlation observed was between dietary SMCSO intake averaged over the 12 months and the World Health Organization’s prostate cancer grading level of participants (r = − 0.34, p < 0.05).
The second human study explored the activity (and metabolism) of SMCSO after consuming a glucoraphanin-rich broccoli soup in both men and women (Coode-Bate et al. Citation2019). Men scheduled for a transurethral prostate biopsy (n = 18) were randomly assigned to consume either their habitual diet or their habitual diet plus broccoli soup (containing 227.9 mg/SMCSO/three times/week) for 4 wk pre-surgery (Coode-Bate et al. Citation2019). The researchers reported the accumulation of SMCSO within the prostate and peri-prostatic tissues, more so, in men administered the broccoli soup over the 4 wk. This SMCSO accumulation was also evident in one man within the control group. These results led the researchers to administer a single dose of this same broccoli soup as part of a crossover study with multiple urinary samples collected to further determine the metabolism of SMCSO in another 10 healthy men and women (Coode-Bate et al. Citation2019). In summary, they found that plasma SMCSO was ∼1000-fold higher than plasma glucoraphanin, and remained detectable in human urine 2 wk after the last day of consumption, suggesting not only accumulation but possible biological activities (Coode-Bate et al. Citation2019).
Discussion
To our knowledge, this is the first scoping review to systematically search the literature for evidence on the effects of SMCSO on health-related outcomes, and the possible mechanisms responsible. We have identified that whilst SMCSO is acknowledged as one of the major sulfur-containing compounds within cruciferous and allium vegetables, it has failed to acquire the research attention exploring its role in human health. Of the many articles and citations identified during our screening process, these have emerged from a total of just 21 original research studies. During our screening process, we also separately sub-categorized 182 publications (88 reviews, 17 book chapters, 4 conference abstracts, 4 ethno-surveys, and 69 experiments) mentioning SMCSO within their text. For example, of the 69 experiments, 10 were investigating a food known to contain SMCSO (suggesting SMCSO as a possible mediator), whilst another 34 mentioned SMCSO purely as background context. We found 72 articles discussing the health benefits of food (e.g., garlic and onion), citing the original SMCSO research articles we found, yet not acknowledging SMCSO as a possible mediator for these benefits within their text. During our search, we identified just one review article that focused solely on SMCSO; providing a broad overview of its background, synthesis within plants, its history with ruminants, mammalian and microbial metabolism, and health outcomes (e.g., anti-carcinogenic, anti-diabetic, and cardiovascular) (W.M.B. Edmands et al. Citation2013).
In surmising the articles we found, it appears that the glucose- and lipid-lowering effects of SMCSO in non-ruminant animals may be a result of several possible regulatory mechanisms; [1] raised excretion of bile acid and sterols, [2] impact upon glucose- and cholesterol-regulating enzymes (e.g., glucose-6-phosphatase, HMG-CoA reductase, cholesterol 7α-hydroxylase), [3] normalized insulin-secreting pancreatic cells and/or insulin secretagogue activity, and/or [4] overall improved antioxidant capacity. However, with limited studies on SMCSO, there may be other unexplored effects not yet known.
The liver is critical to the regulation of glucose, lipid, and energy metabolism. It absorbs dietary lipids and cholesterol, directing acetyl-CoA from either glucose or free fatty acids into either lipogenesis (fatty acids) or cholesterol for transport via lipoproteins throughout the body for energy metabolism (Chiang et al. Citation2020). The hepatic catabolism of cholesterol into bile acids is recognized as a key pathway in cholesterol metabolism (Chiang et al. Citation2020; Trauner et al. Citation2010). The ability of SMCSO to raise levels of fecal bile acids may have accelerated the removal of dietary lipids and cholesterol from circulation. Furthermore, fecal bile acids increase in response to increasing hepatic cholesterol 7α-hydroxylase, also observed by Komatsu, Miura, and Yagasaki (Citation1998); the first rate-limiting enzyme for the bile acid synthesis pathway (Chiang et al. Citation2020). Bile acids and sterols are heavily involved in glucose homeostasis, playing an important role as signaling molecules for hepatic gluconeogenesis, glycogen synthesis, and insulin sensitivity (Trauner et al. Citation2010).
It also appears that SMCSO may affect cholesterol- and glucose-regulating enzymes. Malic enzyme, for example, is important for the oxidative carboxylation of malate into pyruvate, CO2, and nicotinamide adenine dinucleotide phosphate (NADPH), with NADPH being an important co-factor needed for lipid synthesis (Simmen, Alhallak, and Simmen Citation2020). One study reported that SMCSO reduced the production of malic enzyme (2007), which in turn likely reduced NADPH availability, subsequently contributing to the cholesterol-lowering effects. Kumari, Matthew, and Augusti (Citation1995) suggest the oxidation of NADPH may also have an insulin-sparing effect, supported by recent research showing the stimulating effect of NADPH, upon pancreatic β-cells to synthesize insulin (Plecitá-Hlavatá et al. Citation2020). Having reduced liver hexokinase concurrently with elevated glucose-6-phosphatase (as occurs in untreated diabetes) negatively impacts glycogen storage and utilization. This becomes exacerbated in hyperglycemic conditions and these changes were ameliorated by the addition of SMCSO (Kumari and Augusti Citation1995). Furthermore, HMG-CoA reductase is a rate-limiting enzyme critical to the production of cholesterol, which in addition to cholesterol 7α-hydroxylase appears positively impacted by SMCSO. Whilst insulin influences HMG-CoA reductase levels (and cholesterol synthesis), hepatic lipogenesis is also influenced by the pancreatic islets and their insulin production. The studies included in this review suggest that SMCSO does indeed impact hepatic changes, intestinal HMG-CoA reductase, hexokinase, glucose-6-phosphatase, and overall lipolytic mechanisms, although additional supportive research to confirm these mechanisms is still required (Kumari and Augusti Citation2007; Kumari, Mathew, and Augusti Citation1995).
There appears to be good evidence that SMCSO is involved in anti-inflammatory pathways, whether that be through the dampening of superoxide dismutase, catalase, hydroperoxides, or malondialdehyde, raising IL-10, and/or ameliorating tissue damage bought on during the chronic disease process (de Lemos et al. Citation2021; Kumari and Augusti Citation2002). It is known that excess oxidative damage and subsequent inflammation are central to chronic disease development (Ferrucci and Fabbri Citation2018; Soysal et al. Citation2020). IL-6 plays a central role in regulating inflammatory responses and can be either pro- and/or anti-inflammatory depending on the stage of response (Kany, Vollrath, and Relja Citation2019). IL-1β is a transcription factor of IL-6 and is often secreted by the pancreatic alpha cells in diabetic models (Kany, Vollrath, and Relja Citation2019). de Lemos et al. (Citation2021) found that SMCSO had no effect on IL-6 levels, and was unable to dampen IL-1β, although it did significantly raise IL-10. As IL-10 is known to be a strong cytokine inhibitor, its increase results in many downstream anti-inflammatory and immune-mediating effects (Kany, Vollrath, and Relja Citation2019). By raising oxidative defenses, and subsequently reducing the inflammatory burden on tissues (i.e., hepatic, pancreatic, gastro-intestinal, etc), these antioxidant effects may be behind SMCSO’s glucose- and lipid-lowering qualities.
Lastly, it has been suggested that the number of sulfur atoms could be partly responsible for the antioxidant effect of sulfoxides, in that SMCSO has an additional sulfur atom compared to its non-sulfoxide (i.e., SMC) equivalent (Higuchi, Tateshita, and Nishimura Citation2003). As previously discussed, administering SMC to hypercholesterolemic rats did not provide the same lipid-lowering effect as SMCSO (Itokawa et al. Citation1973), plausibly partly mediated by greater antioxidant capacity.
Limitations and future directions
The evidence presented by this scoping review suggests that SMCSO administered to rats produces favorable glucose- and lipid-lowering results, however, whether these effects are translatable to human health remains to be seen. There were several limitations that need to be addressed in future studies.
Firstly, whilst humans consume a variety of cruciferous (and allium) vegetables, the doses administered in these aforementioned rats studies (i.e., ∼200 mg/kg/BW/day), is the human SMCSO equivalence of 32.4 mg/kg (or ∼2269 mg/daily for a 70 kg human), using body surface area calculations from animal to humans (Reagan-Shaw, Nihal, and Ahmad Citation2008). Considering that commonly consumed cruciferous (e.g., broccoli, cabbage, cauliflower, Brussels sprouts) contain ∼96 - 318 mg/100g of SMCSO per fresh, raw vegetable (currently unpublished data), this would equate to a human intake of an estimated ∼8 - 23 serves (∼612 - 1745 grams/day) of cruciferous daily. Therefore, dosages used in these studies are likely not achievable by human dietary intake. As such, future studies need to explore whether similar and/or lower doses have the same potential to benefit humans. When considering the greater proportion of SMCSO (i.e., 1 – 4% of dry weight), compared to glucosinolates (0.1 − 0.6% of dry weight) found in cruciferous vegetables (Mae, Ohira, and Fujiwara Citation1971; H. S. Marks, Anderson, and Stoewsand Citation1993), the human intake of SMCSO (gram/gram) therefore, would be far greater than glucosinolates (gram/gram). Despite glucosinolates being ingested in far smaller quantities than SMCSO, their therapeutic benefits have been studied extensively (Costa-Pérez et al. Citation2023). It seems unlikely that SMCSO, or any other compound present in significant amounts, is a bystander devoid of biological activity, and therefore their potential contribution and/or metabolic effects needs to be better understood.
It has been reported that SMCSO appears to accumulate within human prostatic and peri-prostatic tissue and remain in the body for considerably more time than other well-known and well-researched sulfur-containing compounds (Coode-Bate et al. Citation2019). In addition, Traka et al. (Citation2019) have recently reported an inverse association between dietary intake of SMCSO and the severity of prostate cancer grading after 12 months, implying a link deserving more research attention. It is not known if SMCSO accumulates elsewhere in the human body after consuming these vegetables, nor do we have a complete picture of its metabolic pathways in the human body.
Like the glucosinolates and other cysteine sulfoxides, tissue maceration (i.e., slicing or chewing) of these vegetables leads to cysteine sulfoxide lyases (both within the plant tissue and the human gut microbiota) to release downstream SMCSO derivatives (e.g., S-methyl methanethiosulfinate, S-methyl methanethiosulfonate), pyruvate, ammonia, and sulfate (W.M.B. Edmands et al. Citation2013; Kellingray et al. Citation2021; Narbad and Rossiter Citation2018). Whilst SMCSO is absorbed in the gut, a human radioactivity study has identified urine as the major route of excretion (Waring et al. Citation2003). Two 200 mg doses of SMCSO (≥ 16 wk apart) were administered to four healthy men, with over half (60.2%) being excreted in the urine within the initial 24-h, and almost all (96.3%) fully recovered after two weeks (Waring et al. Citation2003). Future metabolic studies at different doses are needed to explore this further. Human omics-based research (especially untargeted metabolomics) would be advantageous in exploring these biological mechanisms. Further well-designed non-ruminant studies should incorporate a non-targeted metabolic approach to enable identification of specific metabolites that could be of interest in human studies.
Furthermore, as suggested by our previous paper (Hill et al. Citation2022, 16), clinical trials “involving sulfur-rich vegetables … must, as a minimum, ensure they measure and report the chemical analysis of their vegetable intake, to infer true meaning to their clinical results, particularly for determining which metabolites might be responsible”. With recent improvements in the detection and identification of SMCSO in human samples, and the knowledge that urinary SMCSO is a biomarker of cruciferous intake (Sivapalan et al. Citation2019), human trials are now in the position to measure adherence to cruciferous vegetable intake and the contribution of SMCSO to their outcomes. The development of a food composition database compiling SMCSO in edible plants (i.e., vegetables) would further facilitate the estimation of dietary intake.
Conclusion
Higher consumption of SMCSO-rich cruciferous and allium vegetables is associated with a lower risk of cardiometabolic diseases. This scoping review has identified that SMCSO exerts favorable anti-diabetic and anti-hypercholesterolemic effects, as well as anti-cancer and anti-inflammatory benefits in non-ruminant animals. Whether the anti-diabetes effects are entirely due to the insulin-secretagogue action of SMCSO, or if the cholesterol-lowering effects are due to raised fecal bile acids or altered expression of cholesterol-related genes remains to be seen. Whilst understanding the mechanisms behind how SMCSO positively impacts cholesterol and glucose levels remains elusive, the antioxidant nature of this compound may be a contributing factor. Most importantly, whether these effects and/or mechanisms translate to humans remains to be explored. To date, the reported results are limited, and restricted to only a few studies, therefore the collective evidence of any mechanism is difficult to ascertain. From only 2 human studies, we know that SMCSO remains detectable 2 wk after ingestion, accumulate in periprostatic tissue and intake is inversely associated with prostate grading. Further research is needed to understand the metabolic fate and biological mechanisms of SMCSO in humans. In addition, a food database can expand the knowledge of SMCSO content within commonly consumed foods, clinical studies on cruciferous vegetables must report on the vegetable matrix used, whilst -omics-based research can explore the metabolic fate and activity of SMCSO after its consumption. It appears likely that SMCSO has potential for human health, however, until further studies are conducted, we are unable to fully determine its role in mediating some of the health benefits associated with consuming cruciferous and allium vegetables.
Authorship
The author’s responsibilities were as follows: CRH and LCB designed the research; CRH conducted the search with guidance from LCB; CRH, AHL, LM, and LCB completed article screening; CRH extracted data with guidance from LCB; CRH analyzed the data and wrote the initial draft with guidance and editing from LCB; All authors critically appraised, edited and agreed to the final published version of this manuscript.
Supplemental Material
Download Zip (165.8 KB)Acknowledgments
The authors acknowledge the assistance of Ms Pam Thornton and Ms Amy Cairns, School of Medical and Health Science librarians at Edith Cowan University, who assisted in developing our search strategy and review criteria.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abbaoui, B., C. R. Lucas, K. M. Riedl, S. K. Clinton, and A. Mortazavi. 2018. Cruciferous vegetables, isothiocyanates, and bladder cancer prevention. Molecular Nutrition & Food Research 62 (18):e1800079. doi: 10.1002/mnfr.201800079.
- Alam, A., A. A. Arif Jahan, M. S. Bari, L. Khandokar, M. H. Mahmud, M. Junaid, M. S. Chowdhury, M. F. Khan, V. Seidel, and M. A. Haque. 2023. Allium vegetables: Traditional uses, phytoconstituents, and beneficial effects in inflammation and cancer. Critical Reviews in Food Science and Nutrition 63 (23):6580–614. doi: 10.1080/10408398.2022.2036094.
- Aromataris, E., and Z. Munn. 2020. JBI Manual for Evidence Synthesis: JBI. https://synthesismanual.jbi.global.
- Bagiu, R. V., B. Vlaicu, and M. Butnariu. 2012. Chemical composition and in vitro antifungal activity screening of the Allium ursinum L. (Liliaceae). International Journal of Molecular Sciences 13 (2):1426–36. doi: 10.3390/ijms13021426.
- Blekkenhorst, L. C., C. P. Bondonno, J. R. Lewis, A. Devine, K. Zhu, W. H. Lim, R. J. Woodman, L. J. Beilin, R. L. Prince, and J. M. Hodgson. 2017. Cruciferous and allium vegetable intakes are inversely associated with 15-year atherosclerotic vascular disease deaths in older adult women. Journal of the American Heart Association 6 (10):1–15. doi: 10.1161/jaha.117.006558.
- Blekkenhorst, L. C., C. P. Bondonno, J. R. Lewis, R. J. Woodman, A. Devine, N. P. Bondonno, W. H. Lim, K. Zhu, L. J. Beilin, P. L. Thompson, et al. 2018. Cruciferous and total vegetable intakes are inversely associated with subclinical atherosclerosis in older adult women. Journal of the American Heart Association 7 (8):e008391. doi: 10.1161/jaha.117.008391.
- Blekkenhorst, L. C., M. Sim, C. P. Bondonno, N. P. Bondonno, N. C. Ward, R. L. Prince, A. Devine, J. R. Lewis, and J. M. Hodgson. 2018. Cardiovascular health benefits of specific vegetable types: A narrative review. Nutrients 10 (5):595. doi: 10.3390/nu10050595.
- Bradshaw, J. E. 2021. Population improvement and synthetic cultivar production in forage kale (Brassica oleracea L.). Euphytica 217 (7):150. doi: 10.1007/s10681-021-02880-2.
- Bradshaw, J. E., and R. N. Wilson. 2012. Kale population improvement and cultivar production. Euphytica 184 (2):275–88. doi: 10.1007/s10681-011-0612-x.
- Chan, Q., G. M. Wren, C. E. Lau, T. M. D. Ebbels, R. Gibson, R. L. Loo, G. S. Aljuraiban, J. M. Posma, A. R. Dyer, L. M. Steffen, et al. 2022. Blood pressure interactions with the DASH dietary pattern, sodium, and potassium: The International Study of Macro-/Micronutrients and Blood Pressure (INTERMAP). The American Journal of Clinical Nutrition 116 (1):216–29. doi: 10.1093/ajcn/nqac067.
- Chiang, J. Y. L., J. M. Ferrell, Y. Wu, and S. Boehme. 2020. Bile acid and cholesterol metabolism in atherosclerotic cardiovascular disease and therapy. Cardiology Plus 5 (4):159–70. doi: 10.4103/2470-7511.305419.
- Coode-Bate, J., T. Sivapalan, A. Melchini, S. Saha, P. W. Needs, J. R. Dainty, J. B. Maicha, G. Beasy, M. H. Traka, R. D. Mills, et al. 2019. Accumulation of dietary S-methyl cysteine sulfoxide in human prostate tissue. Molecular Nutrition & Food Research 63 (20):e1900461. doi: 10.1002/mnfr.201900461.
- Costa-Pérez, A., V. Núñez-Gómez, N. Baenas, G. Di Pede, M. Achour, C. Manach, P. Mena, D. Del Rio, C. García-Viguera, D. A. Moreno, et al. 2023. Systematic review on the metabolic interest of glucosinolates and their bioactive derivatives for human health. Nutrients 15 (6):1424. doi: 10.3390/nu15061424.
- de Castro, V. M., K. C. de Paula Medeiros, L. I. C. de Lemos, L. de Fátima Campos Pedrosa, F. V. L. Ladd, T. G. de Carvalho, R. F. de Araújo Júnior, B. J. Abreu, and N. B. da Silva Farias. 2021. S-methyl cysteine sulfoxide ameliorates duodenal morphological alterations in streptozotocin-induced diabetic rats. Tissue & Cell 69:101483. doi: 10.1016/j.tice.2020.101483.
- de Lemos, L. I. C., M. A. Medeiros, J. Lima, T. O. Teixeira, C. A. Figueiredo, N. B. S. Farias, F. S. Silva, B. J. Abreu, K. C. P. Medeiros, and L. F. C. Pedrosa. 2021. S-methyl cysteine sulfoxide mitigates histopathological damage, alleviate oxidative stress and promotes immunomodulation in diabetic rats. Journal of Complementary & Integrative Medicine 18 (4):719–25. doi: 10.1515/jcim-2020-0220.
- Edmands, W. M., O. P. Beckonert, C. Stella, A. Campbell, B. G. Lake, J. C. Lindon, E. Holmes, and N. J. Gooderham. 2011. Identification of human urinary biomarkers of cruciferous vegetable consumption by metabonomic profiling. Journal of Proteome Research 10 (10):4513–21. doi: 10.1021/pr200326k.
- Edmands, W., M. B. Nigel, J. Gooderham, E. Holmes, and S. C. Mitchell. 2013. S-Methyl-l-cysteine sulphoxide: The Cinderella phytochemical? Toxicology Research 2 (1):11–22. doi: 10.1039/C2TX20030A.
- Ferrucci, L., and E. Fabbri. 2018. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nature Reviews. Cardiology 15 (9):505–22. doi: 10.1038/s41569-018-0064-2.
- Friedrich, K., N. S. Wermter, L. Andernach, K. Witzel, and F. S. Hanschen. 2022. Formation of volatile sulfur compounds and S-methyl-l-cysteine sulfoxide in Brassica oleracea vegetables. Food Chemistry 383:132544. doi: 10.1016/j.foodchem.2022.132544.
- Fujiwara, M., Y. Itokawa, H. Uchino, and K. Inoue. 1972. Anti-hypercholesterolemic effect of a sulfur containing amino acid, S-methyl-L-cysteine sulfoxide, isolated from cabbage. Experientia 28 (3):254–5. doi: 10.1007/bf01928671.
- Higuchi, O., K. Tateshita, and H. Nishimura. 2003. Antioxidative activity of sulfur-containing compounds in Allium species for human low-density lipoprotein (LDL) oxidation in vitro. Journal of Agricultural and Food Chemistry 51 (24):7208–14. doi: 10.1021/jf034294u.
- Hill, C. R., A. Shafaei, L. Balmer, J. R. Lewis, J. M. Hodgson, A. H. Millar, and L. C. Blekkenhorst. 2022. Sulfur compounds: From plants to humans and their role in chronic disease prevention. Critical Reviews in Food Science and Nutrition:1–23. doi: 10.1080/10408398.2022.2057915.
- Horníčková, J., R. Kubec, K. Cejpek, J. Velíšek, J. Ovesná, and H. Stavělíková. 2010. Profiles of S-alk(en)ylcysteine sulfoxides in various garlic genotypes. Czech Journal of Food Sciences 28 (4):298–308. doi: 10.17221/135/2010-CJFS.
- Itokawa, Y., K. Inoue, S. Sasagawa, and M. Fujiwara. 1973. Effect of S-methylcysteine sulfoxide, S-allylcysteine sulfoxide and related sulfur-containing amino acids on lipid metabolism of experimental hypercholesterolemic rats. The Journal of Nutrition 103 (1):88–92. doi: 10.1093/jn/103.1.88.
- Jia, X., L. Zhong, Y. Song, Y. Hu, G. Wang, and S. Sun. 2016. Consumption of citrus and cruciferous vegetables with incident type 2 diabetes mellitus based on a meta-analysis of prospective study. Primary Care Diabetes 10 (4):272–80. doi: 10.1016/j.pcd.2015.12.004.
- Kany, S., J. T. Vollrath, and B. Relja. 2019. Cytokines in inflammatory disease. International Journal of Molecular Sciences 20 (23):6008. doi: 10.3390/ijms20236008.
- Kellingray, L., G. Le Gall, J. F. Doleman, A. Narbad, and R. F. Mithen. 2021. Effects of in vitro metabolism of a broccoli leachate, glucosinolates and S-methylcysteine sulphoxide on the human faecal microbiome. European Journal of Nutrition 60 (4):2141–54. doi: 10.1007/s00394-020-02405-y.
- Komatsu, W., Y. Miura, and K. Yagasaki. 1998. Suppression of hypercholesterolemia in hepatoma-bearing rats by cabbage extract and its component, S-methyl-L-cysteine sulfoxide. Lipids 33 (5):499–503. doi: 10.1007/s11745-998-0233-7.
- Kook, S., G. Kim, and K. Choi. 2009. The antidiabetic effect of onion and garlic in experimental diabetic rats: meta-analysis. Journal of Medicinal Food 12 (3):552–60. doi: 10.1089/jmf.2008.1071.
- Kubec, R., and E. Dadáková. 2008. Quantitative determination of S-alk(en)ylcysteine-S-oxides by micellar electrokinetic capillary chromatography. Journal of Chromatography. A 1212 (1–2):154–7. doi: 10.1016/j.chroma.2008.10.024.
- Kubodera, A., M. Shikita, S. Akaboshi, and S. Turufuji. 1973. Effects of potato kallikrein inhibitor and S-methyl-L-cysteine sulfoxide on plasma and liver kininogen levels of x-irradiated rats and effectiveness of these materials as radioprotectors. Journal of Radiation Research 14 (2):126–35. doi: 10.1269/jrr.14.126.
- Kumari, K., and K. T. Augusti. 1995. Antidiabetic effects of S-methylcysteine sulphoxide on alloxan diabetes. Planta Medica 61 (1):72–4. doi: 10.1055/s-2006-958004.
- Kumari, K., and K. T. Augusti. 2002. Antidiabetic and antioxidant effects of S-methyl cysteine sulfoxide isolated from onions (Allium cepa Linn) as compared to standard drugs in alloxan diabetic rats. Indian Journal of Experimental Biology 40 (9):1005–9. https://pubmed.ncbi.nlm.nih.gov/12587728/.
- Kumari, K., and K. T. Augusti. 2007. Lipid lowering effect of S-methyl cysteine sulfoxide from Allium cepa Linn in high cholesterol diet fed rats. Journal of Ethnopharmacology 109 (3):367–71. doi: 10.1016/j.jep.2006.07.045.
- Kumari, K., B. C. Mathew, and K. T. Augusti. 1995. Antidiabetic and hypolipidemic effects of S-methyl cysteine sulfoxide isolated from Allium cepa Linn. Indian Journal of Biochemistry & Biophysics 32 (1):49–54. https://pubmed.ncbi.nlm.nih.gov/7665195/.
- Li, K. J., E. C. Borresen, N. Jenkins-Puccetti, G. Luckasen, and E. P. Ryan. 2017. Navy bean and rice bran intake alters the plasma metabolome of children at risk for Cardiovascular Disease. Frontiers in Nutrition 4:71. doi: 10.3389/fnut.2017.00071.
- Mae, T., K. Ohira, and A. Fujiwara. 1971. Fate of (+) S-methyl-l-cysteine sulfoxide in Chinese cabbage, Brassica pekinensis RUPR. Plant and Cell Physiology 12 (1):1–11. doi: 10.1093/oxfordjournals.pcp.a074591.
- Marks, H. S., J. A. Anderson, and G. S. Stoewsand. 1993. Effect of S-methyl cysteine sulphoxide and its metabolite methyl methane thiosulphinate, both occurring naturally in Brassica vegetables, on mouse genotoxicity. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 31 (7):491–5. doi: 10.1016/0278-6915(93)90108-b.
- Marks, H. S., J. A. Hilson, H. C. Leichtweis, and G. S. Stoewsand. 1992. S-Methylcysteine sulfoxide in Brassica vegetables and formation of methyl methanethiosulfinate from Brussels sprouts. Journal of Agricultural and Food Chemistry 40 (11):2098–101. doi: 10.1021/jf00023a012.
- Mohammed, S. G., and M. W. Qoronfleh. 2020. Vegetables. Advances in Neurobiology 24:225–77. doi: 10.1007/978-3-030-30402-7_9.
- Morris, C. J., and J. F. Thompson. 1956. The identification of (+) S-methyl-l-cysteine sulfoxide in plants. Journal of the American Chemical Society 78 (8):1605–8. doi: 10.1021/ja01589a028.
- Nakayama, Y., H. J. Ho, M. Yamagishi, H. Ikemoto, M. Komai, and H. Shirakawa. 2020. Cysteine sulfoxides enhance steroid hormone production via activation of the protein kinase A pathway in ttestis-derived I-10 tumor cells. Molecules (Basel, Switzerland) 25 (20):4694. doi: 10.3390/molecules25204694.
- Narbad, A., and J. T. Rossiter. 2018. Gut glucosinolate metabolism and isothiocyanate production. Molecular Nutrition & Food Research 62 (18):e1700991. doi: 10.1002/mnfr.201700991.
- Peters, M. D. J., C. M. Godfrey, P. McInerney, Z. Munn, A. C. Tricco, and H. Khali. 2020. Chapter 11: Scoping reviews (2020 version). In JBI manual for evidence synthesis, eds. E. Aromataris and Z. Munn. JBI. https://synthesismanual.jbi.global. doi: 10.46658/JBIMES-20-12.
- Peters, M. D. J., C. Marnie, A. C. Tricco, D. Pollock, Z. Munn, L. Alexander, P. McInerney, C. M. Godfrey, and H. Khalil. 2020. Updated methodological guidance for the conduct of scoping reviews. JBI Evidence Synthesis 18 (10):2119–26. doi: 10.11124/jbies-20-00167.
- Petropoulos, S., F. Di Gioia, and G. Ntatsi. 2017. Vegetable organosulfur compounds and their health promoting effects. Current Pharmaceutical Design 23 (19):2850–75. doi: 10.2174/1381612823666170111100531.
- Plecitá-Hlavatá, L., M. Jabůrek, B. Holendová, J. Tauber, V. Pavluch, Z. Berková, M. Cahová, K. Schröder, R. P. Brandes, D. Siemen, et al. 2020. Glucose-stimulated insulin secretion fundamentally requires H(2)O(2) signaling by NADPH oxidase 4. Diabetes 69 (7):1341–54. doi: 10.2337/db19-1130.
- Quirante-Moya, S., P. García-Ibañez, F. Quirante-Moya, D. Villaño, and D. A. Moreno. 2020. The role of Brassica bioactives on human health: Are we studying it the right way? Molecules (Basel, Switzerland) 25 (7):1591. doi: 10.3390/molecules25071591.
- Rafiq, T., S. M. Azab, K. K. Teo, L. Thabane, S. S. Anand, K. M. Morrison, R. J. de Souza, and P. Britz-McKibbin. 2021. Nutritional metabolomics and the classification of dietary biomarker candidates: A critical review. Advances in Nutrition (Bethesda, MD) 12 (6):2333–57. doi: 10.1093/advances/nmab054.
- Reagan-Shaw, S., M. Nihal, and N. Ahmad. 2008. Dose translation from animal to human studies revisited. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 22 (3):659–61. doi: 10.1096/fj.07-9574LSF.
- Ruhee, R. T., L. Arwyn Roberts, S. Ma, and K. Suzuki. 2020. Organosulfur compounds: A review of their anti-inflammatory effects in human health. Frontiers in Nutrition 7:64. doi: 10.3389/fnut.2020.00064.
- Sendl, A., M. Schliack, R. Löser, F. Stanislaus, and H. Wagner. 1992. Inhibition of cholesterol synthesis in vitro by extracts and isolated compounds prepared from garlic and wild garlic. Atherosclerosis 94 (1):79–85. doi: 10.1016/0021-9150(92)90190-R.
- Sheela, C. G., K. Kumud, and K. T. Augusti. 1995. Anti-diabetic effects of onion and garlic sulfoxide amino acids in rats. Planta Medica 61 (4):356–7. doi: 10.1055/s-2006-958099.
- Simmen, F. A., I. Alhallak, and R. C. M. Simmen. 2020. Malic enzyme 1 (ME1) in the biology of cancer: It is not just intermediary metabolism. Journal of Molecular Endocrinology 65 (4):R77–R90. doi: 10.1530/JME-20-0176.
- Sivapalan, T., A. Melchini, J. Coode-Bate, P. W. Needs, R. F. Mithen, and S. Saha. 2019. An LC-MS/MS method to measure S-methyl-l-cysteine and S-methyl-l-cysteine sulfoxide in human specimens using isotope labelled internal standards. Molecules (Basel, Switzerland) 24 (13):2427. doi: 10.3390/molecules24132427.
- Smith, R. H. 1978. S-methylcysteine sulphoxide, the brassica anaemia factor (a valuable dietary factor for man?). Veterinary Science Communications 2 (1):47–61. doi: 10.1007/BF02291432.
- Smith, R. H., C. R. Earl, and N. A. Matheson. 1974. The probable role of S-methylcysteine sulphoxide in kale, poisoning in ruminants. Biochemical Society Transactions 2 (1):101–4. doi: 10.1042/bst0020101.
- Soysal, P., F. Arik, L. Smith, S. E. Jackson, and A. T. Isik. 2020. Inflammation, frailty and cardiovascular disease. Advances in Experimental Medicine and Biology 1216:55–64. doi: 10.1007/978-3-030-33330-0_7.
- Synge, R. L. M., and J. C. Wood. 1956. (+)-(S-methyl- L-cysteine S-oxide) in cabbage. The Biochemical Journal 64 (2):252–9. doi: 10.1042/bj0640252.
- Tanaka, Y., M. Shimada, and S. Nagaoka. 2014. L-Cysteine-induced up-regulation of the low-density lipoprotein receptor is mediated via a transforming growth factor-alpha signalling pathway. Biochemical and Biophysical Research Communications 444 (3):401–5. doi: 10.1016/j.bbrc.2014.01.095.
- Traka, M. H., A. Melchini, J. Coode-Bate, O. Al Kadhi, S. Saha, M. Defernez, P. Troncoso-Rey, H. Kibblewhite, C. M. O'Neill, F. Bernuzzi, et al. 2019. Transcriptional changes in prostate of men on active surveillance after a 12-mo glucoraphanin-rich broccoli intervention—results from the Effect of Sulforaphane on prostate CAncer PrEvention (ESCAPE) randomized controlled trial. The American Journal of Clinical Nutrition 109 (4):1133–44. doi: 10.1093/ajcn/nqz012.
- Trauner, M., T. Claudel, P. Fickert, T. Moustafa, and M. Wagner. 2010. Bile acids as regulators of hepatic lipid and glucose metabolism. Digestive Diseases (Basel, Switzerland) 28 (1):220–4. doi: 10.1159/000282091.
- Tricco, A. C., E. Lillie, W. Zarin, K. K. O'Brien, H. Colquhoun, D. Levac, D. Moher, M. D. J. Peters, T. Horsley, L. Weeks, et al. 2018. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Annals of Internal Medicine 169 (7):467–73. doi: 10.7326/m18-0850.
- Veritas Health Innovation. 2023. Covidence Systematic Review Software, Covidence 2.0 edition. Accessed January 3, 2023. https://www.covidence.org/.
- Waring, R. H., R. M. Harris, G. B. Steventon, and S. C. Mitchell. 2003. Degradation to sulphate of S-methyl-L-cysteine sulphoxide and S-carboxymethyl-L-cysteine sulphoxide in man. Drug Metabolism and Drug Interactions 19 (4):241–55. doi: 10.1515/dmdi.2003.19.4.241.
- Whittle, P. J., R. H. Smith, and A. McIntosh. 1976. Estimation of S-methylcysteine sulphoxide (kale anaemia factor) and its distribution among Brassica forage and root crops. Journal of the Science of Food and Agriculture 27 (7):633–42. doi: 10.1002/jsfa.2740270708.
- Yabuki, Y., Y. Mukaida, Y. Saito, K. Oshima, T. Takahashi, E. Muroi, K. Hashimoto, and Y. Uda. 2010. Characterisation of volatile sulphur-containing compounds generated in crushed leaves of Chinese chive (Allium tuberosum Rottler). Food Chemistry 120 (2):343–8. doi: 10.1016/j.foodchem.2009.11.028.
- Yamazaki, Y., K. Iwasaki, M. Mikami, and A. Yagihashi. 2010. Distribution of eleven flavor precursors, S-alk(en)yl-l-cysteine derivatives, in seven Allium vegetables. Food Science and Technology Research 17 (1):55–62. doi: 10.3136/fstr.17.55.
- Yoshinari, O., Y. Shiojima, and K. Igarashi. 2012. Anti-obesity effects of onion extract in Zucker diabetic fatty rats. Nutrients 4 (10):1518–26. doi: 10.3390/nu4101518.
- Yuguri, S. 1954. Studies on vitamin B1 related compounds. LIX reaction between thiamine and ingredients of Allium genus plants. (V). On sulfur-containing ingredients of Allium genus plants. The Pharmaceutical Society of Japan 74:519–24. https://www.jstage.jst.go.jp/article/yakushi1947/74/5/74_5_519/_pdf.
- Zurbau, A., F. Au-Yeung, S. Blanco Mejia, T. A. Khan, V. Vuksan, E. Jovanovski, L. A. Leiter, C. W. C. Kendall, D. J. A. Jenkins, and J. L. Sievenpiper. 2020. Relation of different fruit and vegetable sources with incident cardiovascular outcomes: A systematic review and meta-analysis of prospective cohort studies. Journal of the American Heart Association 9 (19):e017728. doi: 10.1161/jaha.120.017728.