Abstract
Fermentation is one of the most effective methods of food preservation. Since ancient times, food has been fermented using lactic acid bacteria (LAB). Fermented milk is a very intricate fermentation ecosystem, and the microbial metabolism of fermented milk largely determines its metabolic properties. The two most frequently used dairy starter strains are Streptococcus thermophilus (S. thermophilus) and Lactobacillus delbrueckii subsp. bulgaricus (L. bulgaricus). To enhance both the culture growth rate and the flavor and quality of the fermented milk, it has long been customary to combine S. thermophilus and L. bulgaricus in milk fermentation due to their mutually beneficial and symbiotic relationship. On the one hand, the symbiotic relationship is reflected by the nutrient co-dependence of the two microbes at the metabolic level. On the other hand, more complex interaction mechanisms, such as quorum sensing between cells, are involved. This review summarizes the application of LAB in fermented dairy products and discusses the symbiotic mechanisms and interactions of milk LAB starter strains from the perspective of nutrient supply and intra- and interspecific quorum sensing. This review provides updated information and knowledge on microbial interactions in a fermented milk ecosystem.
HIGHLIGHTS
The symbiotic relationship between Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus is reviewed.
Their nutrient co-dependence is discussed.
The role of quorum sensing in their interaction is discussed for the first time.
This review is of interest to colleagues interested in exploiting LAB starter cultures.
1. Introduction
Fermented foods are an important part of long-standing traditional food cultures around the world. (Wang et al. Citation2023). Fermented foods are generally defined as foods produced through controlled microbial growth and enzymatic conversion of major and minor food components (Marco et al. Citation2017). They provide and preserve nutrients and sensory qualities (flavors, aromas, and textures), enriching the human diet. Fermentation improves the sensory properties, shelf life, and nutritional value of foods while reducing toxic and anti-nutritional food factors. Humans, microbial fermentation, and traditional fermented foods have always had a close relationship (Kabak and Dobson Citation2011; Tamang et al. Citation2020; Xu et al. Citation2021; Yang, Fan, and Xu Citation2020). More importantly, fermented foods promote human health primarily through the fermentative activities of the associated food microbes (Marco et al. Citation2021). Consumers are becoming more health conscious and aware of the benefits of fermented dairy products, which is driving demand for fermented dairy products (Sakandar and Zhang Citation2021). Major known health-promoting mechanisms of fermented foods, including improving the nutritional value of raw food materials, synthesizing active compounds, modulating the gut microbiota, and boosting host immunity, are summarized in .
Figure 1. Beneficial effects of fermented foods on human health promotion. The beneficial effects of fermented foods on human health are observed when food ingredients, fermentation products, and live microorganisms are consumed and enter the gastrointestinal tract. These components, along with the resident gut microflora, are transformed into bioactive substances, such as peptides, bacteriocins, amino acids, conjugated linoleic acids, short-chain fatty acids (SCFAs), and other organic acids. Furthermore, microorganisms and their byproducts related to fermentation have the potential to interact with the natural gut microflora, epithelial cells in the intestines, and the immune system of the host.
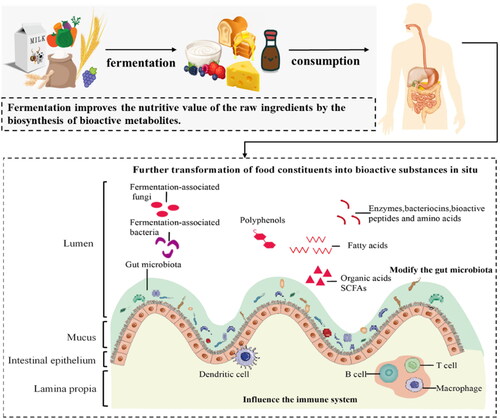
Fermented milk products are widely produced and consumed across the globe. For thousands of years, milk fermentation has been one of the oldest and most practical techniques utilized to extend the shelf-life of milk (Sakandar and Zhang Citation2022). Back-slopping is a traditional production method of naturally fermented foods. In this method, some material from a previous fermentation is inoculated into the fresh substrate, preserving the natural microbiota that imparts the properties to the new batch of the fermented product (Zannini et al. Citation2016). Some traditional starter cultures have started to be exploited and used in the large-scale industrial production of fermented foods in the last century (Macori and Cotter Citation2018).
A starter culture comprises living microorganisms intentionally used to initiate fermentation to modify the chemical composition and sensory qualities of the substrate, leading to more standardized products. Such cultures include one or more species of lactic acid bacteria (LAB) fermenting together as a co-culture or mixed culture (Hill et al. Citation2017; Medina-Pradas et al. Citation2017). Inoculating microbial starter cultures during fermentation initiates biochemical reactions in the raw materials, thereby optimizing the traditional fermentation and production process. Lactic acid bacteria are phylogenetically located in the Clostridia branch of Gram-positive bacteria. They are non-sporing cocci, coccobacilli or rods, and aero-tolerant anaerobes, with a molar DNA base composition of less than 50% G ± C (George et al. Citation2018; Pasolli et al. Citation2020). They are among the most widely studied microorganisms worldwide. Given the important role that LABs in different biotechnological processes, it is not surprising that they have received much attention from the scientific community for decades (De Filippis, Pasolli, and Ercolini Citation2020).
Lactic acid bacteria are the main starter cultures used for producing fermented dairy products and have great application market potential and value. Fermented milk is an intricate fermentation ecosystem that regulates microbial fermentative metabolism and nutrient interactions (Gesudu et al. Citation2016; Yu et al. Citation2018). In this ecosystem, microorganisms interact directly through physical contact, quorum sensing (QS), symbiosis, parasitism, predation, and inhibition, while extracellular metabolites mediate indirect interactions through commensalism, neutralism, amensalism, mutualism, and competition (Wang et al. Citation2023). Streptococcus thermophilus (S. thermophilus) and Lactobacillus delbrueckii subsp. bulgaricus (L. bulgaricus) are the most commonly used starter cultures to manufacture fermented dairy products. The interaction between L. bulgaricus and S. thermophilus directly affects the quality of the fermented milk. Thousands of years ago, humans began to use fermentation to sour milk (McAuliffe Citation2018). For thousands of years, S. thermophilus and L. bulgaricus have been used in combination to ferment yogurt; and these two mutually beneficial symbiotic bacteria could support each other to improve the growth rate, taste, and quality of fermented milk (Iyer et al. Citation2010). This review discusses their mutualistic interaction and mechanism during milk fermentation from multiple perspectives.
2. Lactic acid bacteria and fermented milk
2.1. Definition and biochemical metabolic characteristics of LAB
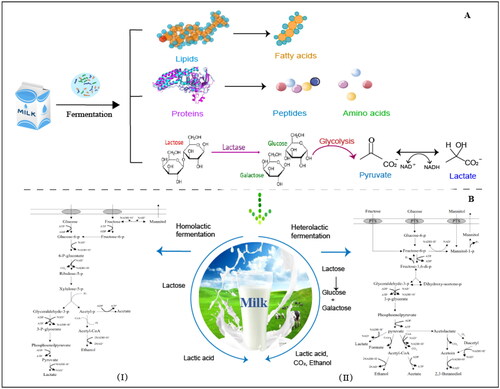
Although humans have used LAB for producing naturally fermented dairy products and other fermented foods for thousands of years, there is still a limited understanding of LAB (Sakandar and Zhang Citation2022). The typical LAB are Gram-positive, nonspore-forming, nonmotile, catalase-negative, devoid of cytochromes, anaerobic to aerotolerant cocci or rods, which are acid-resistant and produce lactic acid as the main end product of carbohydrate fermentation (Teneva-Angelova et al. Citation2018). Lactic acid was first discovered in 1780 by C.W. Scheele in sour milk, which was later used in its industrial production (Castillo Martinez et al. Citation2013). Through the progress of lactic acid fermentation, microorganisms break down numerous nutrients, including proteins, lipids, and carbohydrates, into simpler and absorbable forms, such as peptides, free amino acids, free fatty acids, and lactic acid (; Sharma et al. Citation2023; Fernandez, Picard-Deland, and Anne Citation2019). Therefore, the available nutrients in fermented dairy products are higher than in non-fermented food due to the greater protein solubility, release of micronutrients, and restrictive amino acids (Sharma et al. Citation2020).
Figure 2. Milk fermentation by LAB through homolactic and heterolactic fermentation pathways. A. Degradation of main milk nutrient components by lactic acid bacteria during fermentation is a critical process in the production of various dairy products. These pathways result in the degradation of protein, fat, and carbohydrates in milk, leading to the development of unique flavors, textures, and other desired characteristics. B. Lactic acid bacteria utilize homolactic or heterolactic fermentation pathways (designated I and II, respectively) to break down lactose into lactic acid with and without other by-products.
In 2020, a taxonomic revision of the LAB categorized more than 300 species in seven genera and two families into a single family, the Lactobacillaceae, with 31 genera, comprising 23 new genera to encompass microorganisms previously grouped as Lactobacillus species (Qiao et al. Citation2022). A merger of Lactobacillaceae and Leuconostocaceae into Lactobacillaceae as one family has been recommended. Furthermore, the genus Lactobacillus has been reclassified into 25 genera (Zheng et al. Citation2020). Currently, 12 LAB genera are utilized in the production of fermented foods (). The primary LAB found in starter cultures for producing fermented dairy products primarily comprise Streptococcus, Lactobacillus, Bifidobacterium, Lactococcus lactis, and Leuconostoc species (Chen et al. Citation2017; Karaman and Ozcan Citation2021; Macori and Cotter Citation2018; Makarova et al. Citation2006).
Table 1. Lactic acid bacteria used in fermented food production.
Lactic acid bacteria are divided into homotypic and heterotypic fermentation according to the types of homofermentative end products (Salim-ur-Rehman, Paterson, and Piggott Citation2006). During LAB fermentation, sugars are converted to lactic acid (Bintsis Citation2018). Homolactic fermentation refers to the fermentation pathway that only produces two molecules of lactic acid by fermenting one molecule of glucose, using the glycolytic pathway (also known as the Embden-Meyerhof-Parnans pathway, EMP pathway; ) (Gänzle Citation2015). Lactobacillus delbrueckii, S. thermophilus, and Lactococcus lactis are the most common edible LAB fermented by homolactic acid (Macori and Cotter Citation2018; Mokoena Citation2017). Heterolactic fermentation refers to the fermentation pathway in which glucose produces not only lactic acid but also ethanol, acetic acid, formic acid, and carbon dioxide after fermentation () (Gänzle Citation2015). Leuconostoc mesenteroides and L. bulgaricus are representative LAB fermented by the heterolactic acid fermentation pathway (Macori and Cotter Citation2018). Other LAB are considered to be “facultatively” heterofermentative, meaning that they produce carbon dioxide and other products under certain conditions or from specific substrates (Cousin et al. Citation2012; Hutkins Citation2006).
2.2. Fermented milk as a carrier of LAB starter
Dairy and milk consumption are frequently included as important elements in a healthy and balanced diet (Pereira Citation2014). There is a long history of human production and consumption of fermented dairy products worldwide (Akdeniz and Akalın Citation2019). The historical development of fermented milk starters is closely related to that of fermented milk, passing through two stages: empirical starter and selective starter (Shrivastava and Ananthanarayan Citation2015; Tamime Citation2002). The practice of introducing old fermented yogurt, or “back-slopping,” into fresh milk for fermentation has been utilized by humans for centuries, unbeknownst to them, in fermenting various dairy foods (McAuliffe Citation2018; Steensels et al. Citation2019). Technological advancements have allowed for the isolation and cultivation of pure LAB to produce desired fermentation traits, such as acid, fragrance, and aroma from various raw materials.
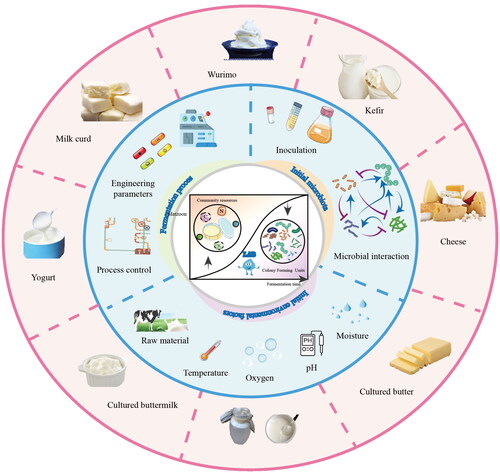
The choice of starter cultures is one of the most important factors determining the success and outcome of fermentation. In the process, fermented milk is produced as a curd-like product due to the fermentative activities of LAB. The milk matrix acts as a carrier, safeguarding the vitality of LAB during gastrointestinal transit and enabling them to contribute to host health upon ingestion (De Filippis, Pasolli, and Ercolini Citation2020). Under appropriate conditions, dairy starter cultures can produce lactic acid, peptides, vitamins, extracellular polysaccharides, and other substances through metabolism. Lactic acid bacteria convert lactose in milk into lactic acid, and protein is degraded into polypeptides or amino acids. Such a process produces organic acids, alcohols, esters, ketones, and other flavor components, endowing fermented milk with specific flavors and rich nutrients. The fermentation process of naturally fermented milk is a dynamic ecological succession of the complex community of milk-associated microbiota that transforms the raw materials. This is mainly driven by the LAB community under specific fermentation conditions (). Factors that control the fermentation process include the inoculum size, starter culture interaction with the raw milk and the endogenous microbiota, time of fermentation, oxygen, pH, temperature, and so on. By adjusting these parameters, the microecological succession in the milk fermentation process can be finely tuned to yield a variety of fermented milk products including wurimo, kefir, cheese, cultured butter, matzoon, cultured buttermilk, yogurt, milk curd, and others (Wang et al. Citation2023).
Figure 3. Driving factors of the dynamic succession of lactic acid bacteria in fermented dairy products and typical fermented dairy products. The inner ring shows various driving factors that control the dynamic succession of lactic acid bacteria in fermented dairy products. These driving factors include the initial microbial inoculum, its interaction with the endogenous microbiota, and environmental factors like raw materials, temperature, oxygen content, pH, and moisture. By tuning these variables in the fermentation process, a variety of fermented dairy products can be produced. Some of the representative fermented dairy products are shown in the outer ring.
2.3. Interaction and symbiosis of LAB starter in fermented milk
The interaction of commensal microorganisms in nature is indispensable to global carbon and nitrogen cycling and has been used for food fermentation and preservation for thousands of years (Sieuwerts et al. Citation2008). Interactions among microorganisms during fermentation can result in beneficial, neutral, or detrimental effects on the overall community. These interactions include mutualism (in which the microorganisms interact to their mutual benefit), favoritism symbiosis (in which one microorganism has negative effects on the other without inhibiting its growth), competition (in which the microorganisms compete for energy and nutrients), and parasitism (in which one microbe benefits at the expense of the other, and the suffering microbe provides nutrients to the benefiting microbe while its growth is inhibited). These interactions are important drivers for the assembly and evolution of microbial communities in the fermented food ecosystem. Moreover, they contribute to determining the safety and quality of the final product to some extent (Ivey, Massel, and Phister Citation2013; Sieuwerts et al. Citation2008; Smid and Lacroix Citation2013; Winters et al. Citation2019). However, classical theories alone are insufficient in explaining the intricate relationships and interdependence among microorganisms within complex networks (Frey-Klett et al. Citation2011; Ponomarova et al. Citation2017; Wang, Xu, et al. Citation2016; Wolfe and Dutton Citation2015). Most known natural and industrial food fermentation processes are driven by either simple or complex microbial communities. Microbial interaction is an important factor in maintaining the stable coexistence of microbial communities (Wang et al. Citation2023). Positive interactions between associated microbes are crucial in achieving improved fermentation processes and substrate conversion in fermented food production. The mechanisms behind positive interactions have garnered considerable research attention in recent decades (Canon et al. Citation2020). A thorough comprehension of the interaction mechanisms is necessary to enhance the utilization of multi-starters and natural fermentation in the food production process (Albergaria and Arneborg Citation2016).
Microbial communities are not simple aggregates of individual microorganisms, but rather complex network ecosystems formed by the interactions among microbial species, other organisms, and their environments through a variety of means, including resource competition, growth inhibition, cross-feeding, QS, and horizontal gene transfer (Settachaimongkon et al. Citation2014; Sieuwerts Citation2016). The interaction of microflora is driven by more than just exchanging electron donors and substance metabolism pathways. In fact, over 98% of the microorganisms are auxotrophic and require specific exogenous nutrients for growth, including amino acids, vitamins, and other nutrients (Zengler and Zaramela Citation2018). Differences in auxotrophs have a significant impact on the interaction between microorganisms in the microbial community, especially in terms of their ability to acquire and assimilate nutrients despite having similar energy requirements (Mu et al. Citation2022).
3. Effects of metabolites on the interaction between L. bulgaricus and S. thermophilus
3.1. Interdependence of LAB at the metabolic level
Food fermentation often depends on mutualistic interaction. The most notable example of this interaction is the symbiotic relationship between fermented milk and its leavening agents, specifically S. thermophilus and L. bulgaricus (Ge et al. Citation2023). The mutualism of the fermented milk system is mainly reflected in the respective metabolism of the two bacteria, utilizing metabolites released by each other to meet their own metabolic needs. Such interaction is also known as cross-feeding, which refers to the utilization and catabolism of metabolites (including carbon source, nitrogen source, amino acid, vitamin, nucleotide, electron donor, electron receptor, and other growth factors) secreted by one bacterium by another bacterium. Such metabolite transfer and consumption are widespread in microbial communities to improve the survival of microorganisms in a resource-poor environment, shaping the composition, structure, and evolution of microbial communities (Mu et al. Citation2022). The metabolic interaction between S. thermophilus and L. bulgaricus in milk fermentation is intricate and extensively researched (Sieuwerts Citation2016). The two bacteria can grow together through mutual exchange and utilization of metabolites and growth-promoting factors generated during their metabolism. These interactions depend on the release of chemical molecules into the environment, or direct contact between bacterial cells. Despite intense competition for resources, microbial communities exhibit metabolic interdependence among populations within the community, even across different habitats (Zelezniak et al. Citation2015). Since each strain has unique metabolic potential, the presence and intensity of interactions between L. bulgaricus and S. thermophilus depend on the specific combination of strains. In milk, these strains are symbiotic and mutually beneficial (). Typically, yogurt fermentation involves two exponential growth phases separated by a transition phase of slower growth () (Sieuwerts Citation2016).
Figure 4. The symbiosis between Streptococcus (S.) thermophilus and Lactobacillus (L.) delbrueckii subsp. bulgaricus in milk fermentation. A. Metabolite-level interactions between and nutrient codependency of S. thermophilus (red) and L. bulgaricus (green) during the milk fermentation process 
 positive effect of the component;
positive effect of the component;  negative effect;
negative effect;  neutral or yet-to-be-confirmed effect. B. Schematic diagram showing the different bacterial growth phases during milk fermentation. The growth of S. thermophilus and L. bulgaricus are shown by the red and green curves, respectively. AA: amino acid; EPS: exopolysaccharide; LCFA: long-chain fatty acid.
neutral or yet-to-be-confirmed effect. B. Schematic diagram showing the different bacterial growth phases during milk fermentation. The growth of S. thermophilus and L. bulgaricus are shown by the red and green curves, respectively. AA: amino acid; EPS: exopolysaccharide; LCFA: long-chain fatty acid.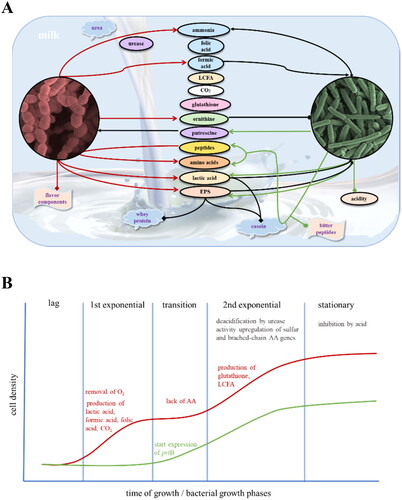
3.2. Interaction between L. bulgaricus and S. thermophilus in the first growth phase
During the initial phase of co-culture growth, S. thermophilus grows more rapidly than L. bulgaricus due to its higher tolerance to neutral pH and higher efficiency in absorbing amino acids and trace elements compared with L. bulgaricus (Sieuwerts Citation2016). During this stage, S. thermophilus is capable of producing formic and folic acids while growing. In contrast, the growth of L. bulgaricus is limited in a milk environment due to its inability to independently produce formic and folic acids. However, when the two bacteria are mixed during fermentation, S. thermophilus provides formic acid to L. bulgaricus through the use of pyruvic acid in the EMP pathway under anoxic conditions (Lecomte et al. Citation2016). At the same time, S. thermophilus can provide L. bulgaricus with the necessary folic acid for growth and compensate for its lack of folic acid due to the incomplete folate biosynthesis pathway in L. bulgaricus (Sybesma et al. Citation2003; Van de Guchte et al. Citation2006; Wegkamp et al. Citation2004). Furthermore, formic acid and folic acid can serve as a precursor and a cofactor, respectively, enabling purine biosynthesis in L. bulgaricus and thereby providing purine substrates for S. thermophilus (Sybesma et al. Citation2003; Vinderola, Mocchiutti, and Reinheimer Citation2002). In addition, S. thermophilus hydrolyzes lactose in milk with β-galactosidase (LacZ) to produce glucose and galactose (Cui et al. Citation2016; Tarrah et al. Citation2018; Wu, Cheung, and Shah Citation2015). The glucose produced then undergoes glycolysis to generate lactic acid (Iskandar et al. Citation2019; Vaillancourt et al. Citation2008). Moreover, S. thermophilus consumes oxygen and produces carbon dioxide, facilitating the anaerobic growth of L. bulgaricus (Sasaki et al. Citation2014).
During the transition stage of milk fermentation, the growth of S. thermophilus is restricted due to the limited content of free amino acids, especially sulfur and branched-chain amino acids (Sieuwerts Citation2016). The nitrogen sources required for the growth of S. thermophilus and L. bulgaricus are mainly small molecular peptides and amino acids. To fulfill the requirements of cell growth, casein is first degraded into peptides and amino acids, serving as the primary nitrogen source (Liu et al. Citation2016). At this stage, the growth of L. bulgaricus and the expression of its extracellular protease gene prtB are initiated, and the extracellular protease prtB of L. bulgaricus can degrade casein to produce amino acids (such as methionine, histidine, and proline) and peptides (Garault et al. Citation2000; Zhao, Qu, and Cui Citation2015). These amino acids and peptides facilitate the second stage of exponential growth of S. thermophilus, which is also beneficial to the exponential growth of L. bulgaricus (Sieuwerts et al. Citation2010). However, the protease of S. thermophilus, prtS, has a weak ability to degrade casein and cannot directly obtain enough amino acids needed for growth from the milk environment. The hydrolysis of proteins by L. bulgaricus supplies an ample quantity of amino acids by up-regulating the amiABCDE operon, which is responsible for oligopeptide transport to support the growth of S. thermophilus. In addition, S. thermophilus can also synthesize three branched-chain amino acids that L. bulgaricus cannot synthesize (Ng et al. Citation2003). Consequently, the amino acid requirements of both bacteria are met under symbiotic conditions, facilitating fermentation.
3.3. Interaction between L. bulgaricus and S. thermophilus in the second growth phase
In the second exponential stage, genes participating in the production of long-chain fatty acids are usually up-regulated in S. thermophilus. Since L. bulgaricus lacks some biological pathways needed for the de novo synthesis of long-chain fatty acids (Partanen, Marttinen, and Alatossava Citation2001), S. thermophilus can provide long-chain fatty acids to L. bulgaricus (Sieuwerts et al. Citation2008). Glutathione (GSH) is an antioxidant. Streptococcus thermophilus can absorb exogenous GSH and synthesize GSH through its glutathione synthetase, gshF, which can protect the bacteria from stressors like oxygen and acids (Cui et al. Citation2021). At the same time, S. thermophilus exports GSH from the cell and provides it to the non-GSH synthesizing L. bulgaricus, enhancing its growth and ability to resist acid stress (Wang et al. Citation2015; Wang, Xu, et al. Citation2016). In addition, the biosynthesis of extracellular polysaccharides is upregulated due to the increased availability of casein-derived nitrogen (Zhang et al. Citation2014).
Urease is a multi-subunit urea amide hydrolase, accounting for 0.9% of the estimated core genome of S. thermophilus (Rasmussen et al. Citation2008). Among all LAB involved in dairy fermentation, only S. thermophilus exhibits urease activity (Mora et al. Citation2005; Yamauchi et al. Citation2019). The urease activity of S. thermophilus has a metabolic relationship with the biosynthesis pathways involved in the metabolism of aspartic acid, glutamine, arginine, and carbon dioxide (Arioli et al. Citation2007). During the process, the release of ammonia raises the pH levels both inside and outside the cells, facilitating bacterial acid production and growth. Urease is the key growth limiting factor whether S. thermophilus is cultured alone or with L. bulgaricus. Urease promotes lactose utilization by S. thermophilus and the production of lactic acid and acetaldehyde (Mora, Arioli, and Compagno Citation2013; Scala et al. Citation2019; Yu et al. Citation2020). At the same time, the urease activity of S. thermophilus facilitates the homolactic acid fermentation of L. bulgaricus (Arioli et al. Citation2017). The urease activity of S. thermophilus would affect the acidification of L. bulgaricus by regulating the carbon dioxide content, thereby promoting acidification, yogurt fermentation, and reducing production costs (Yamauchi et al. Citation2019).
The interaction between Lactobacillus bulgaricus and S. thermophilus in milk co-fermentation is well-studied at the metabolic level. Recent investigations into the role of probiotics in milk fermentation ecosystems have broadened our understanding of microbial interactions. Vinderola, Mocchiutti, and Reinheimer (Citation2002) studied the growth kinetics of co-culturing 48 LAB strains, including S. thermophilus, L. bulgaricus, Lactobacillus acidophilus, Lacticaseibacillus paracasei, and Bifidobacterium. They identified four modes of growth interactions between the basic starters and probiotics: growth stimulation, growth inhibition, growth delay, and no impact (Vinderola, Mocchiutti, and Reinheimer Citation2002). Although the primary metabolic characteristics of fermented milk are formed by the fermentation of L. bulgaricus and S. thermophilus (Sieuwerts et al. Citation2010), the metabolic and protein hydrolysis activities of probiotics also affect the quality of fermented milk, including its sensory characteristics, nutritional value, and probiotic function (Li et al. Citation2019; Yu et al. Citation2018). A study compared the volatile and nonvolatile metabolomic characteristics of fermented milk produced singly by Bifidobacterium animalis subsp. lactis Probio-M8 or co-fermented together with Lacticaseibacillus paracasei Zhang. Analyses by LC-MS and GC-MS revealed that co-fermentation with Lacticaseibacillus paracasei Zhang had a greater impact on the volatile and nonvolatile milk metabolomes compared with single fermentation with Bifidobacterium animalis subsp. lactis Probio-M8, particularly enhancing the volatile metabolites of aroma. Thus, the combined use of these two strains in the fermentation exerted an additive effect on the metabolomics changes (Wang et al. Citation2021). Peng et al. (Citation2022) studied the effects of the combined application of Lacticaseibacillus paracasei Zhang and Bifidobacterium animalis subsp. lactis V9 in milk fermentation on the bacterial growth and milk metabolomics changes during the fermentation and storage processes. The findings indicated that the co-cultivation of these two strains promoted the growth of Bifidobacterium animalis subsp. lactis V9, leading to an increase in both volatile and nonvolatile metabolites and short-chain fatty acids during storage (Peng et al. Citation2022).
In future research, it would be worthwhile to investigate the metabolomics effects of using different probiotic strains in the fermentation process, the interactions between traditional starter cultures and probiotics, and the contribution of probiotic implementation in improving the techno-functional properties of the product.
4. The role of QS in the interaction among LAB
4.1. Overview of quorum sensing and signal molecules
Microbes are ubiquitous on Earth, living in mixed or single-species communities (Weiland-Bräuer Citation2021). The quality of fermented food is highly dependent on the involved microorganisms their metabolic activities and interactions (Johansen and Jespersen Citation2017). In addition to metabolic-level interactions, researchers are investigating more intricate interaction mechanisms, such as cell-to-cell communication, in such ecosystems. Quorum sensing is a molecular signaling mechanism, through which microorganisms use cell communication to control population density and communicate within or between species through secreted signal molecules to coordinate gene expression and group behavior (Linciano et al. Citation2020; Mukherjee and Bassler Citation2019; Papenfort and Bassler Citation2016). It is a precise cell density-dependent microbial communication mechanism that acts via a QS molecule-mediated adaptive switch for gene transcription (Bai and Rai Citation2011; Sieuwerts et al. Citation2008; Smid and Lacroix Citation2013). In recent years, QS has also been found to involve in the regulation of many important physiological functions and features of LAB, such as bacterial biofilm formation (Liu et al. Citation2018), resistance to environmental pressures (Zhang Citation2022), autolysis (Sun Citation2010), and0 acid tolerance (Moslehi-Jenabian, Gori, and Jespersen Citation2009), biosyntheses of exopolysaccharides, antimicrobial peptides, and extracellular enzymes (Pezzulo et al. Citation2012; Wang Citation2022; Wasfi et al. Citation2018), adhesion to intestinal epidermal cells (Buck, Azcarate-Peril, and Klaenhammer Citation2009), and intestinal colonization (Medellin-Peña and Griffiths Citation2009).
During the QS process, bacteria secrete specific signal molecules (i.e., the language for microbial communication) into the environment while growing. As the microbial cell density increases, the concentration of these signaling molecules also rises. When the concentration of signal molecules reaches a threshold, these signal molecules combine with transcription factors to form a complex that triggers the expression of downstream genes, leading to the desired physiological effects and regulation (Waters and Bassler Citation2005). Quorum sensing is an important mechanism for interaction between bacterial cells and plays a critical regulatory role in bacterial population control and specific physiological functions (De Kievit and Iglewski Citation2000).
A recurring theme for QS has emerged after years of study. Briefly, LuxI homologs catalyze acylated homoserine lactone synthesis, while the luxR counterparts exhibit specificity for their cognate acylated homoserine lactone (). When it reaches the threshold concentration, it specifically interacts with the luxR transcriptional regulator, leading to the expression of the luxICDABE operon and bioluminescence. In a complex ecosystem, bacterial communication can be viewed from a broad perspective of inter- or intramicrobial population communication, which includes three modes of QS: self-talk, where a species communicates within its own population; crosstalk, where different species communicate using common AI; and eavesdropping, where species that are unable to produce AI “listen” () (Coquant et al. Citation2021; Whiteley, Diggle, and Greenberg Citation2017).
Figure 5. Schematic diagrams showing the mechanisms of quorum sensing (QS) and autolysis of Lactobacillus (L.) bulgaricus. A. Illustration of QS bioluminescence mechanism. (a) a general QS mechanism – LuxI produces N-acyl-homoserine lactones (AHL, represented by yellow spheres). (b) QS signaling relies on the release of automatic inducers (AIs) into the environment for three modes of communication between bacteria, including self-talk, crosstalk, and eavesdropping. B. Common QS systems regulate three types of gene expression. (a) Quorum sensing in Gram-negative bacteria consists of LuxI and LuxR proteins. (b) The most common signal molecules in the QS system of Gram-positive bacteria are autoinducing peptides (AIPs). (c) LuxS/AI-2 is a quorum sensing system that can be used for interspecific information exchange. It is mediated by the signal molecule, furanosyl borate dister (AI-2), facilitating communications between Gram-negative and Gram-positive bacteria. C. Mechanism of autolysis in L. bulgaricus.
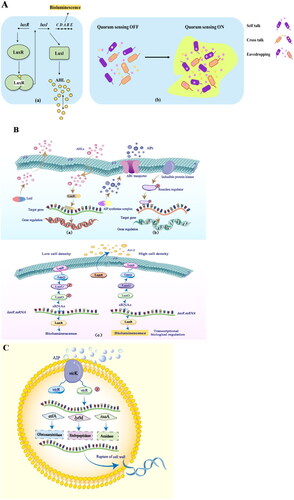
There are two categories of information exchange in LAB: intra- and interspecific. Notably, the AI-2 signaling molecule is different from all other QS signals in that it can achieve interspecific communication among bacteria, making it the “common language” for bacterial communication (Pereira, Thompson, and Xavier Citation2013). This common QS system regulates the expression of three types of genes, as shown in . Currently, QS system is mainly divided into three categories according to the bacterial signal molecules, including N-acyl-homoserine lactones in Gram-negative bacteria (Coquant, Grill, and Seksik Citation2020), autoinducing peptide in Gram-positive bacteria (Prazdnova et al. Citation2022), and furanosyl borate dister, AI-2, in both Gram-positive and Gram-negative bacteria (Pereira, Thompson, and Xavier Citation2013). Among them, the action mechanism of signal molecule N-acyl homoserine lactones existing in gram-negative bacteria is shown in . LuxI protein is an enzyme that regulates the synthesis of signal molecules, including N-acyl-homoserine lactones (AHLs). LuxR protein is a cytoplasmic signal molecule receptor and a DNA binding transcription activation element responsible for binding AHLs. Acyl-homoserine lactones are released into the environment through the cell membrane following synthesis. The level of AHLs increases with the number of bacteria. As AHL concentrations reach a certain threshold, these molecules will form AHL-LuxR complexes with the N-terminus of the receptor protein, LuxR, in the bacterial membrane, thus activating the expression specific downstream genes. There are also some other QS signaling molecules, such as quinolone in Pseudomonas (Dubern and Diggle Citation2008), the diffusion signaling factor (Majdura et al. Citation2023; Deng et al. Citation2011), γ-butyrolactone (Shi et al. Citation2022; Takano Citation2006), 2-amino acetophenone (Kesarwani et al. Citation2011), and bradyoxetin (Loh et al. Citation2002).
AI-2 is a furanone compound, which is a symmetrical double-pentacyclic-structure furanose boratediester, a by-product of the methionine cycle-methyl cycle (Zhang et al. Citation2020). AI-2 is a signal molecule that exists in both Gram-positive bacteria and Gram-negative bacteria. The specific mechanism of action is shown in . The synthesis of AI-2 is catalyzed by the luxS gene, which is essential for producing AI-2 for intercellular signal transduction (Tannock et al. Citation2005). The AI-2/luxS QS system plays a crucial role in the information exchange of many bacteria (Chen et al. Citation2002; Keller and Surette Citation2006). At present, the genomes of over 70 known bacterial species contain homologous sequences of luxS and the biosynthetic pathways of AI-2 (Schauder et al. Citation2001).
AI-1, a small autoinducing signaling peptide, is a crucial signaling molecule for intraspecific information exchange. It is derived from the two-component phosphokinase protein and is secreted after post-translational processing. However, unlike AHLs, it cannot pass through the cell membrane by diffusion, but relying on certain transport systems for membrane translocation, such as the ATP-binding-cassette. The growth of LAB constantly synthesizes and excretes AI-1, which is recognized by its specific receptors when accumulating to a certain level. Autoinducing peptide is recognized by the histidine kinase protein of the two-component system (TCS), and it binds to and interacts with the corresponding protein to induce the transcription of QS regulatory genes and other downstream genes, forming a dynamic communication system (Sturme et al. Citation2002; Waters and Bassler Citation2005). The molecular synthesis pathway of typical autoinducing peptides is shown in . Bacterial TCS are widely found signal transduction pathways that facilitate environmental adaptation; they are mainly composed of a detection membrane-reactive histidine protein kinase and a response regulator protein (Groisman Citation2016). These two proteins transmit signals through phosphorylation and dephosphorylation (Stock, Robinson, and Goudreau Citation2000).
4.2. The role of QS in the autolysis of LAB
Although numerous studies have been conducted on the QS of LAB, there is insufficient reporting on the effects of QS on the interactions between LAB during the milk fermentation process. Notably, autolysis is an interesting feature of LAB that can influence the outcome of the milk fermentation process and the microbial interactions in the milk fermentation ecosystem. Further research has found that QS systems play an active role in regulating bacterial interactions within co-cultures, as demonstrated by significant changes in the protein-level expression of luxS during the co-cultivation of different LAB strains (Di Cagno et al. Citation2009).
4.2.1. Autolysis of LAB affects the milk fermentation process
Autolysis of LAB is a frequent phenomenon, in which spontaneous cellular lysis occurs to maintain a favorable environment (Blaya, Barzideh, and LaPointe Citation2018; Lazzi et al. Citation2016). Autolytic enzymes causing bacterial autolysis hydrolyze the peptidoglycan network structure of cell walls under certain conditions. The expression of these enzymes leads to the dissolution and disappearance of bacterial murein, releasing intracellular metabolites into the surrounding environment (Gasson Citation1996). Autolysis is a vital and natural physiological process in cellular growth (Buist, Venema, and Kok Citation1998). During milk fermentation, autolysis is a mechanism for LAB to prevent excessive cell density in the fermentation medium. As cells lyse, intracellular enzymes, such as proteases and lipases, will be released into the fermentation medium, which will decompose the inherent components of protein and fat in milk, shaping the texture, flavor, physical and chemical properties, and shelf life of fermented dairy products (Cibik and Chapot-Chartier Citation2000; Ortakci et al. Citation2015). Accelerating the mechanical autolysis of LAB can improve the quality of fermented dairy products (Pang et al. Citation2017). Rapid autolysis of mixed strain starters endows dairy products with unique sensory properties, reducing the need to add flavors and spices. At the same time, rapid autolysis can effectively control the adverse effects of acidification on the quality of yogurt after shelf life, improving its acceptance by consumers (Sun Citation2010). Accelerating autolysis also reduces the detrimental effects of acidification on the fermented milk quality during shelf life, improving consumer acceptance rates. In cheese fermentation, rapid autolysis of LAB shortens the ripening period and reduces the production cost, and the post-autolysis release of proteases and lipases enhances the flavor of the cheese products (Pang et al. Citation2014). However, excessively rapid autolysis of starter bacteria can result in a decline in viable bacteria, insufficient acid production, poor curd, and excessive lactose residue (Pillidge et al. Citation2002). Despite this, autolysis of starter strains is generally considered a desirable property in terms of improving the quality of fermented dairy products. Therefore, the mechanism and regulation of autolysis in LAB have attracted much attention.
4.2.2. Quorum sensing regulates the autolysis of LAB
Bacteria typically sense and respond to environmental changes via a TCS (Cui et al. Citation2012). Genome sequencing and gene function analysis found that LAB genomes contain multiple TCS that are responsible for various functions, such as bacteriocin synthesis, acid resistance, bile tolerance, and bacterial adhesion (Borland et al. 2015; Marx, Meiers, and Brückner Citation2014; Guo and Sun Citation2017). The TCS in QS of LAB can regulate the autolysis in L. bulgaricus BAA-365 by secreting signal molecules to the surrounding environment during growth (Pang et al. Citation2017, Citation2020). When the concentration of the signal molecule in the surrounding environment reaches or exceeds the threshold, the cell wall-located histidine protein kinase, vicK, will initiate protein phosphorylation of vicR. Phosphorylated vicR binds to RNA polymerases and promotes the gene expression of lytM, ssaA, and atlA, accelerating cell wall rupture (). It was shown that the QS activity of L. bulgaricus regulates its autolysis (Pang et al. Citation2017). Another study analyzed the autolysis of L. bulgaricus LJJ at different growth stages under different treatment conditions and found that the bacteria were highly susceptible to autolysis during its logarithmic growth phase, and the susceptibility was further increased by elevating ambient temperature (4 ∼ 50 °C) and prolonging the incubation time. Additionally, the cellular lysozyme activity and thus the autolysis are influenced by environmental pH, temperature, incubation time, the concentrations of sodium dodecyl sulfate and Triton X-100, and the bacterial growth stage (Cui et al. Citation2013). Another studied bacterium is S. thermophilus strain DN-001065, which shows extensive autolysis in the later stage of growth in milk (Husson-Kao et al. Citation1999). The autolysis of S. thermophilus is triggered by unfavorable environmental conditions, such as lactose deprivation and the addition of salt or organic solvents (Husson-Kao et al. Citation2000)
4.2.3. Autolysis of LAB affects the microbial interaction in the milk fermentation ecosystem
Autolysis of Lactobacillus bulgaricus is related to the drop of environmental acidity (Chen et al. Citation2021). The study conducted by Xu (Citation2017) investigated the role of two key genes of the acid adaptation-related TCS, hpk1 and rr1, in the bacterial symbiosis between Lactobacillus bulgaricus CH3 and s Streptococcus thermophilus strain by inactivating these two genes. It was found that the growth ability, acetaldehyde production ability, and viscosity production ability of the double gene-inactivated mutant were lower than that of the wild-type CH3 strain (Xu Citation2017). The findings highlight the active involvement of TCS in the interaction between L. bulgaricus and S. thermophilus. Moreover, it was found that L. bulgaricus and S. thermophilus strains with a higher rate of autolysis have shorter coagulation times and a better acid production capacity in fermentation experiments compared with those with a lower autolysis rate (Sun Citation2010). However, excessive autolysis of LAB would substantially decrease the extent of post-acidification of fermented milk, as high autolytic strains are prone to lyse in the later stages of fermentation. The damaged cell wall and membrane of autolytic cells reduce environmental acidity, curbing bacterial growth during fermentation. Additionally, the lower number of viable cells restricts lactose metabolism and acid production, resulting in a considerable reduction of post-acidification of fermented milk. Thus, a high autolytic rate of LAB is desirable for fermentation.
The autolysis of two most commonly used dairy starters, L. bulgaricus and S. thermophilus, will certainly affect the microbial interaction in the fermented milk ecosystem, and the autolysis characteristics of LAB starters are of practical importance in improving the quality of fermented dairy products. Lactic acid bacteria regulate their autolysis through the TCS in QS. Moderate autolysis will positively affect the fermentation characteristics of LAB starter strains, and the release of intracellular constituents during autolysis will benefit the microbial interaction in milk fermentation, although the complex mechanism requires further exploration. The autolysis of LAB is strain-specific and is influenced by different fermentation conditions. Therefore, selecting autolytic strains and optimizing fermentation conditions would enhance the properties of fermented dairy products.
4.3. The role of QS in the acid tolerance of LAB
4.3.1. Acid tolerance of LAB
Lactic acid bacteria need to survive different environmental conditions, such as different temperatures, acid levels, and salt concentrations (Rao et al. Citation2004). Acid stress is the most important factor affecting the growth and metabolism of LAB. The acid stress of LAB in the process of growth and metabolism mainly comes from two aspects, i.e., its acid production and the external environment from food processing and storage and the gastrointestinal tract after being ingested (Gu Citation2017). The acidic metabolites, such as lactic acid and acetic acid, produced by LAB in the process of milk fermentations have dual effects. On the one hand, they may inhibit the growth and reproduction of other microorganisms, resulting in better storage properties of fermented milk. Moreover, they may react with aldehydes and ketones to produce aromatic compounds, enhancing the flavor of fermented milk (Cogan et al. Citation2007). On the other hand, excessive acid accumulation during fermentation inhibits bacterial growth due to the elevated intracellular proton levels and the decreased intracellular pH, affecting the transmembrane δ pH, consuming the proton driving force in the bacteria, and deteriorating their proliferation ability and biological activity (Lorca and de Valdez Citation1999). Therefore, the acid adaptability and tolerance capacities of LAB, especially those used as fermentation starter strains, are crucial (Wang, Cui, and Qu Citation2018).
4.3.2. Quorum sensing regulates the acid tolerance of LAB
The QS system can regulate the acid tolerance of LAB and influence the interaction of starter strains in milk. The ability of LAB to survive in an acidic environment is very important for the stability and quality of fermented milk. Lactic acid bacteria use luxS/AI-2 to respond to environmental stimuli and regulate their growth and metabolism (Park et al. Citation2016). Under the conditions of acid shock, high temperature, and hunger stress, the AI-2 activity of LAB increases, while a decline in AI-2 activity is observed under conditions of low temperature and high osmotic pressure stress (Gu et al. Citation2018). At the same time, acid shock, low temperature, high osmotic pressure, and nutritional stress induce the over-expression of the luxS gene (Yeo et al. Citation2015). Under acid stress, bacteria can detect environmental changes and release AI-2 to regulate gene expression. This mechanism aids bacterial adaptation to the fluctuating environment (Gu Citation2017).
A previous study used quantitative polymerase chain reaction to show that the gene expression of a TCS (JN675228/JN675229) of the L. bulgaricus CH3 strain was up-regulated with the acid adaptation ability (Cui et al. Citation2012). To confirm the causal relationship, gene knockout mutants of JN675228 and JN675229 were constructed. The growth ability of the two mutants was substantially lower than the wild type, and the mutant lacking the JN675229 gene had reduced acid adaptability. These outcomes indicate that JN675228 and JN675229, particularly the latter, play a critical role in the acid adaptability of this strain. To investigate the signal transduction mechanism of JN675228/JN675229 in the acid adaptation in L. bulgaricus, differential protein expression among the two mutants and wild-type strain was screened using two-dimensional gel electrophoresis, transcriptional analysis, and time-of-flight mass spectrometry under natural fermentation and acid adaptation conditions (Wang, Cui, and Qu Citation2018). The results suggested that the TCS (comprising JN675228 and JN675229) regulates the acid adaptability of L. bulgaricus in many ways, including regulating proton pump-related proteins, classical stress shock proteins, carbohydrate metabolism, nucleotide biosynthesis, DNA repair transcription and translation, peptide transport and degradation, and cell wall biosynthesis.
Thermophilic bacteria can use lsrB and rbsB genes for AI-2 signal transduction, accomplishing biofilm formation and environmental stress adaptation (Kaur, Capalash, and Sharma Citation2018). The number of TCS in S. thermophilus varies between strains, usually ranging from six to eight (Hols et al. Citation2005). While S. thermophilus Sfi39 exhibits excellent fermentation proficiency, it possesses poor stress resilience (Lemoine et al. Citation1997). This bacterial strain is sensitive to acid and oxygen in the logarithmic growth period (Zotta et al. Citation2008), and its rr01 gene is involved in heat and acid tolerance (Zotta et al. Citation2009). A study isolated, identified, and analyzed the TCS of 22 strains of S. thermophilus from traditional yogurt samples in Inner Mongolia. The results showed that all 22 strains of S. thermophilus had eight pairs of greatly variable TCS, and their stress response corresponded to the unique TCS gene sequences. Therefore, it was speculated that the strain characteristics are linked to the differences in TCS (Hu et al. Citation2018). The LMD-9 genome contains six complete TCS, each with a sensor kinase and response regulator (RR; TCS 2, 4, 5, 6, 7, and 9), and two orphan RRs (RR01 and 08) with truncated sensor kinase. The RRs in the TCS were systematically inactivated, and subsequent experimentation using a fermented milk model revealed that among all the RRs tested, only RR05 was essential for the growth of S. thermophilus LMD-9 (Thevenard et al. Citation2011).
The AI-2 activity of Lactobacillus acidophilus NCFM (L. acidophilus NCFM) and Lacticaseibacillus rhamnosus GG (L. rhamnosus GG) significantly increased with pH reduction under acid stress (Moslehi-Jenabian, Gori, and Jespersen Citation2009). In addition, the transcriptional changes in the luxS gene of L. acidophilus NCFM and L. rhamnosus GG were consistent with the AI-2 activity, suggesting that the acid tolerance of this strain could be associated with the expression of luxS and other related genes. Moreover, the TCS mutant of L. acidophilus NCFM was more sensitive to acid than the wild-type, with an upregulation of expression of more than 80 genes, including luxS, under acid stress, indicating TCS and the related genes like luxS play a role in adapting to an acidic environment (Azcarate-Peril et al. Citation2005). The Limosilactobacillus fermentum 2-1 utilizes a similar acid stress coping mechanism (Gu Citation2017). In Lacticaseibacillus casei 12 A, it was found that the nicotinamide adenine dinucleotide dehydrogenase, the adenosine triphosphate binding protein (pstB), and the histidine protein kinase in the TCS can work together to increase the acid tolerance (Overbeck et al. Citation2017). This could have a significant impact on how bacteria survive in the gastrointestinal tract and their interactions with the local gut microbial community. During the logarithmic phase, the acid-producing ability of Limosilactobacillus fermentum 332 was found to be directly proportional to the activity of signal molecule AI-2 (Qiao et al. Citation2022). Furthermore, adding exogenous AI-2 significantly enhanced the activity of LAB and exopolysaccharide production (Wang Citation2022).
4.3.3. Acid tolerance of LAB affects the microbial interaction in the milk fermentation ecosystem
Quorum sensing systems, particularly the luxS/AI-2 system, play an important role in the interactions among LAB by aiding in environmental stress tolerance. AI-2 has been demonstrated to improve the acid resistance of LAB and promote the metabolism of S. thermophilus, thus enhancing its interaction with L. bulgaricus (Meng, Zhao, and Lu Citation2022).
Glutathione is an important signaling molecule in QS. It is a tripeptide compound capable of scavenging free radicals and enhancing cell stress resistance (Wang et al. Citation2019). Bacterial GSH levels are regulated by QS in response to changes in cell density and the cellular redox state (Zhou et al. Citation2019). It is interesting to note that GSH synthesized by S. thermophilus not only improves its acid resistance but also the vitality of L. bulgaricus, enhancing the growth of both starter cultures and the performance of milk fermentation (Wang, Xu, et al. Citation2016; Xue et al. Citation2023). Thus, the QS activity based on GSH is beneficial to the mutual interaction of the starter cultures during milk fermentation.
The acid tolerance of LAB is an adaptive response to environmental changes. These research findings suggest that LAB can increase their acid tolerance under acid stress by using a luxS/AI-2 signaling system or a TCS, enabling the bacteria to survive in an acidic environment. The QS of L. bulgaricus, S. thermophilus, or other LAB promotes their growth, metabolism, and interactions in fermented milk. The acid tolerance of LAB is undoubtedly regulated by QS, but its precise mechanism needs to be investigated in the future. Currently, the study of this aspect remains largely unexplored.
5. Concluding remarks and future perspectives
One of the earliest efficient food preservation techniques is fermentation, which has a long history and is ingrained in global food culture. People have used LAB to produce naturally fermented dairy products and other fermented foods for thousands of years. These bacteria are widely distributed in nature and have rich biodiversity, making them an essential biological resource with practical applications in human everyday life. Dairy fermentation ecosystems comprise complex microbial communities, affecting microbial proliferation, metabolic activity, and interactions to determine the quality of fermented dairy products. The most frequently employed starter species for producing fermented dairy products, such as yogurt, are S. thermophilus and L. bulgaricus. These microbes interact with each other in the fermentation process through symbiosis at the nutrient and metabolomics levels. In the process of microbial interaction, the role of QS through TCS, especially the luxS/AI-2 signal system, is crucial in governing microbial population and dynamics and regulating some growth characteristics of LAB, such as autolysis and acid stress tolerance. The regulation of autolysis and acid tolerance by QS in fermented food starter cultures is beneficial to their growth, metabolism, and interactions during fermentation, ultimately enhancing the functional and sensory quality of the final products. This article reviews the effects and mechanisms of nutrient assimilation and intra- and interspecific QS on the interaction of LAB during milk fermentation.
The food fermentation process constitutes a micro-ecosystem co-fermented by multiple microorganisms, encompassing intricate and dynamic microbial interactions. The stability, safety, flavor, and nutritional value of the final product are reliant on the microbial fermentation process and interactions. High-throughput DNA sequencing, RNA-seq, functional genomics, and metabolomics technologies have been widely used in exploring the microbiology behind a variety of traditional fermentation processes in recent years. These technologies can be used to track the changes in the taxonomic and functional microbiome. Furthermore, the fermented food industry has gradually evolved from a small, family-based workshop production mode to a highly integrated industrial factory production scale, in light of the rapid development and innovation of modern biotechnology. The ability to characterize and trace the microbiome associated with traditional fermented foods is made possible by implementing the omics tools. Bioinformatics, machine learning, and other cutting-edge techniques can predict microbial community evolution and metabolic outcomes, shedding light on the fermentation process. By bringing together biotechnology advancements, the nutritional value and safety of fermented foods can also be improved. In the future, a deeper understanding of the intricate microbial interactive mechanism can be explored with the aid of more sophisticated technological tools.
Author contributions
Shujuan Yang Writing – Original Draft. Mei Bai Conceptualization. Lai-Yu Kwok: Writing – Reviewing and Editing. Zhi Zhong Writing – Reviewing and Editing. Zhihong Sun Conceptualization, Project administration.
Competing interests
The authors have no conflicts of interest to declare. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Akdeniz, V., and A. S. Akalın. 2019. New approach for yoghurt and ice cream production: High-intensity ultrasound. Trends in Food Science & Technology 86:392–8. doi: 10.1016/j.tifs.2019.02.046.
- Albergaria, H., and N. Arneborg. 2016. Dominance of Saccharomyces cerevisiae in alcoholic fermentation processes: Role of physiological fitness and microbial interactions. Applied Microbiology and Biotechnology 100 (5):2035–46. doi: 10.1007/s00253-015-7255-0.
- Arioli, S., C. Monnet, S. Guglielmetti, C. Parini, I. De Noni, J. Hogenboom, P. M. Halami, and D. Mora. 2007. Aspartate biosynthesis is essential for the growth of Streptococcus thermophilus in milk, and aspartate availability modulates the level of urease activity. Applied and Environmental Microbiology 73 (18):5789–96. doi: 10.1128/AEM.00533-07.
- Arioli, S., G. Della Scala, M. C. Remagni, M. Stuknyte, S. Colombo, S. Guglielmetti, I. De Noni, E. Ragg, and D. Mora. 2017. Streptococcus thermophilus urease activity boosts Lactobacillus delbrueckii subsp. bulgaricus homolactic fermentation. International Journal of Food Microbiology 247:55–64. doi: 10.1016/j.ijfoodmicro.2016.01.006.
- Azcarate-Peril, M. A., O. McAuliffe, E. Altermann, S. Lick, W. M. Russell, and T. R. Klaenhammer. 2005. Microarray analysis of a two-component regulatory system involved in acid resistance and proteolytic activity in Lactobacillus acidophilus. Applied and Environmental Microbiology 71 (10):5794–804. doi: 10.1128/AEM.71.10.5794-5804.2005.
- Bai, A. J., and V. R. Rai. 2011. Bacterial Quorum Sensing and Food Industry. Comprehensive Reviews in Food Science and Food Safety 10 (3):183–93. doi: 10.1111/j.1541-4337.2011.00150.x.
- Bintsis, T. 2018. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiology 4 (4):665–84. doi: 10.3934/microbiol.2018.4.665.
- Blaya, J., Z. Barzideh, and G. LaPointe. 2018. Symposium review: Interaction of starter cultures and nonstarter lactic acid bacteria in the cheese environment. Journal of Dairy Science 101 (4):3611–29. doi: 10.3168/jds.2017-13345.
- Borland, S.,A. Oudart,C. Prigent-Combaret,C. Prigent-Combaret, andF. Wisniewski-Dyé. 2015. Genome-wide survey of two-component signal transduction systems in the plant growth-promoting bacterium Azospirillum. BMC Genomics. 16, 833. doi: 10.1186/s12864-015-1962-x.
- Buck, B. L., M. A. Azcarate-Peril, and T. R. Klaenhammer. 2009. Role of autoinducer-2 on the adhesion ability of Lactobacillus acidophilus. Journal of Applied Microbiology 107 (1):269–79. doi: 10.1111/j.1365-2672.2009.04204.x.
- Buist, G., G. Venema, and J. Kok. 1998. Autolysis of Lactococcus lactis is influenced by proteolysis. Journal of Bacteriology 180 (22):5947–53. doi: 10.1128/JB.180.22.5947-5953.1998.
- Canon, F., T. Nidelet, E. Guédon, A. Thierry, and V. Gagnaire. 2020. Understanding the mechanisms of positive microbial interactions that benefit lactic acid bacteria co-cultures. Frontiers in Microbiology 11:2088. doi: 10.3389/fmicb.2020.02088.
- Castillo Martinez, F. A., E. M. Balciunas, J. M. Salgado, J. M. Domínguez González, A. Converti, and R. P. d S. Oliveira. 2013. Lactic acid properties, applications and production: A review. Trends in Food Science & Technology 30 (1):70–83. doi: 10.1016/j.tifs.2012.11.007.
- Chen, C., S. Zhao, G. Hao, H. Yu, H. Tian, and G. Zhao. 2017. Role of lactic acid bacteria on the yogurt flavour: A review. International Journal of Food Properties 20 (sup1):S316–S330. doi: 10.1080/10942912.2017.1295988.
- Chen, S., Y. Wu, H. Niu, J. Sun, X. Han, and L. Zhang. 2021. Imbalance between peptidoglycan synthases and hydrolases regulated lysis of Lactobacillus bulgaricus in batch culture. Archives of Microbiology 203 (7):4571–8. doi: 10.1007/s00203-021-02433-0.
- Chen, X., S. Schauder, N. Potier, A. Van Dorsselaer, I. Pelczer, B. L. Bassler, and F. M. Hughson. 2002. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415 (6871):545–9. doi: 10.1038/415545a.
- Cibik, R., and M. P. Chapot-Chartier. 2000. Autolysis of dairy leuconostocs and detection of peptidoglycan hydrolases by renaturing SDS-PAGE. Journal of Applied Microbiology 89 (5):862–9. doi: 10.1046/j.1365-2672.2000.01191.x.
- Cogan, T. M., T. P. Beresford, J. Steele, J. Broadbent, N. P. Shah, and Z. Ustunol. 2007. Invited review: Advances in starter cultures and cultured foods. Journal of Dairy Science 90 (9):4005–21. doi: 10.3168/jds.2006-765.
- Coquant, G., D. Aguanno, S. Pham, N. Grellier, S. Thenet, V. Carrière, J.-P. Grill, and P. Seksik. 2021. Gossip in the gut: Quorum sensing, a new player in the host-microbiota interactions. World Journal of Gastroenterology 27 (42):7247–70. doi: 10.3748/wjg.v27.i42.7247.
- Coquant, G., J.-P. Grill, and P. Seksik. 2020. Impact of N-Acyl-homoserine lactones, quorum sensing molecules, on gut immunity. Frontiers in Immunology 11:1827. doi: 10.3389/fimmu.2020.01827.
- Cousin, F. J., S. Louesdon, M.-B. Maillard, S. Parayre, H. Falentin, S.-M. Deutsch, G. Boudry, and G. Jan. 2012. The first dairy product exclusively fermented by Propionibacterium freudenreichii: A new vector to study probiotic potentialities in vivo. Food Microbiology 32 (1):135–46. doi: 10.1016/j.fm.2012.05.003.
- Cui, M., L. Liu, S. Zhang, X. H. Li, and J. Lu. 2013. Analysis of factors influencing the autolysis of Lactobacillus delbrueckii subsp. bulgaricus LJJ. Food and Fermentation Industry 39 (7):7. doi: 10.13995/j.cnki.11-1802/ts.2013.07.026.
- Cui, X., Y. Sun, K. Wang, Z. Wang, Y. Liang, Z. Xu, and T. Wang. 2021. Research progress on symbiotic relationship between Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus. Food Research and Development 42 (6):184–9. doi: 10.12161/j.issn.1005-6521.2021.06.030.
- Cui, Y., T. Xu, X. Qu, T. Hu, X. Jiang, and C. Zhao. 2016. New Insights into Various Production Characteristics of Streptococcus thermophilus Strains. International Journal of Molecular Sciences 17 (10):1701. doi: 10.3390/ijms17101701.
- Cui, Y., W. Liu, X. Qu, Z. Chen, X. Zhang, T. Liu, and L. Zhang. 2012. A two component system is involved in acid adaptation of Lactobacillus delbrueckii subsp. bulgaricus. Microbiological Research 167 (5):253–61. doi: 10.1016/j.micres.2011.11.003.
- De Filippis, F., E. Pasolli, and D. Ercolini. 2020. The food-gut axis: Lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiology Reviews 44 (4):454–89. doi: 10.1093/femsre/fuaa015.
- De Kievit, T. R., and B. H. Iglewski. 2000. Bacterial quorum sensing in pathogenic relationships. Infection and Immunity 68 (9):4839–49. doi: 10.1128/IAI.68.9.4839-4849.2000.
- Deng, Y., J. Wu, F. Tao, and L.-H. Zhang. 2011. Listening to a new language: DSF-based quorum sensing in Gram-negative bacteria. Chemical Reviews 111 (1):160–73. doi: 10.1021/cr100354f.
- Di Cagno, R., M. De Angelis, R. Coda, F. Minervini, and M. Gobbetti. 2009. Molecular adaptation of sourdough Lactobacillus plantarum DC400 under co-cultivation with other lactobacilli. Research in Microbiology 160 (5):358–66. doi: 10.1016/j.resmic.2009.04.006.
- Dubern, J.-F., and S. P. Diggle. 2008. Quorum sensing by 2-alkyl-4-quinolones in Pseudomonas aeruginosa and other bacterial species. Molecular bioSystems 4 (9):882–8. doi: 10.1039/b803796p.
- Fernandez, A. M., É. Picard-Deland, and M. Anne. 2019. Yogurt: Roles in Nutrition and Impacts on Health. Chapter 1 Yogurt Composition, 1st ed. 3–10. Boca Raton: CRC Press.doi: 10.1201/b21826.
- Frey-Klett, P., P. Burlinson, A. Deveau, M. Barret, M. Tarkka, and A. Sarniguet. 2011. Bacterial-fungal interactions: Hyphens between agricultural, clinical, environmental, and food microbiologists. Microbiology and Molecular Biology Reviews: MMBR 75 (4):583–609. doi: 10.1128/MMBR.00020-11.
- Gänzle, M. G. 2015. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Current Opinion in Food Science 2:106–17. doi: 10.1016/j.cofs.2015.03.001.
- Garault, P., C. Letort, V. Juillard, and V. Monnet. 2000. Branched-chain amino acid biosynthesis is essential for optimal growth of Streptococcus thermophilus in milk. Applied and Environmental Microbiology 66 (12):5128–33. doi: 10.1128/AEM.66.12.5128-5133.2000.
- Gasson, M. J. 1996. Lytic systems in lactic acid bacteria and their bacteriophages. Antonie Van Leeuwenhoek 70 (2–4):147–59. doi: 10.1007/BF00395931.
- Ge, Y., X. Yu, X. Zhao, C. Liu, T. Li, S. Mu, L. Zhang, Z. Chen, Z. Zhang, Z. Song, et al. 2023. Fermentation characteristics and post-acidification of yogurt by Streptococcus thermophilus CICC 6038 and Lactobacillus delbrueckii ssp. Bulgaricus CICC 6047 at optimal inoculum ratio. Journal of Dairy Science S0022-0302(23):00577-5. doi: 10.3168/jds.2023-23817.
- George, F., C. Daniel, M. Thomas, E. Singer, A. Guilbaud, F. J. Tessier, A.-M. Revol-Junelles, F. Borges, and B. Foligné. 2018. Occurrence and dynamism of lactic acid bacteria in distinct ecological niches: A multifaceted functional health perspective. Frontiers in Microbiology 9:2899. doi: 10.3389/fmicb.2018.02899.
- Gesudu, Q., Y. Zheng, X. Xi, Q. C. Hou, H. Xu, W. Huang, H. Zhang, B. Menghe, and W. Liu. 2016. Investigating bacterial population structure and dynamics in traditional koumiss from Inner Mongolia using single molecule real-time sequencing. Journal of Dairy Science 99 (10):7852–63. doi: 10.3168/jds.2016-11167.
- Groisman, E. A. 2016. Feedback control of two-component regulatory systems. Annual Review of Microbiology 70 (1):103–24. doi: 10.1146/annurev-micro-102215-095331.
- Gu, Y. 2017. The effects of environmental stresses and yeast on LuxS/AI-2 quorum sensing system of lactic acid bacteria., PHD thesis., Inner Mongolia Agricultural University.
- Gu, Y., B. Li, J. Tian, R. Wu, and Y. He. 2018. The response of LuxS/AI-2 quorum sensing in Lactobacillus fermentum 2-1 to changes in environmental growth conditions. Annals of Microbiology 68 (5):287–94. doi: 10.1007/s13213-018-1337-z.
- Guo, X. P., and Y. C. Sun. 2017. New Insights into the non-orthodox two component rcs ohosphorelay system. Frontiers in Microbiology 8:2014. doi: 10.3389/fmicb.2017.02014.
- Hill, D., I. Sugrue, E. Arendt, C. Hill, C. Stanton, and R. P. Ross. 2017. Recent advances in microbial fermentation for dairy and health. F1000Research 6:751. doi: 10.12688/f1000research.10896.1.
- Hols, P., F. Hancy, L. Fontaine, B. Grossiord, D. Prozzi, N. Leblond-Bourget, B. Decaris, A. Bolotin, C. Delorme, S. Dusko Ehrlich, et al. 2005. New insights in the molecular biology and physiology of Streptococcus thermophilus revealed by comparative genomics. FEMS Microbiology Reviews 29 (3):435–63. doi: 10.1016/j.femsre.2005.04.008.
- Hu, T., Y. Zhang, Y. Cui, C. Zhao, X. Jiang, X. Zhu, Y. Wang, and X. Qu. 2018. Technological properties assessment and two component systems distribution of Streptococcus thermophilus strains isolated from fermented milk. Archives of Microbiology 200 (4):567–80. doi: 10.1007/s00203-017-1468-9.
- Husson-Kao, C., J. Mengaud, J.-C. Gripon, L. Benbadis, and M.-P. Chapot-Chartier. 2000. Characterization of Streptococcus thermophilus strains that undergo lysis under unfavourable environmental conditions. International Journal of Food Microbiology 55 (1–3):209–13. doi: 10.1016/S0168-1605(00)00166-5.
- Husson-Kao, Clara, Jérôme, Mengaud, Jean-Claude, Gripon, Laurent Benbadis, and Marie-Pierre Chapot-Chartier. (1999). The autolysis of Streptococcus thermophilus DN-001065 is triggered by several food-grade environmental signals. International Dairy Journal, 9(10), 715–723. doi: 10.1016/S0958-6946(99)00145-4.
- Hutkins, R. W. (ed.) 2006. Microbiology and technology of fermented foods Chapter 2 Microorganisms and Metabolism. 22–36. Oxford: Blackwell Publishing. doi: 10.1002/9780470277515.
- Iskandar, C. F., C. Cailliez-Grimal, F. Borges, and A.-M. Revol-Junelles. 2019. Review of lactose and galactose metabolism in Lactic Acid Bacteria dedicated to expert genomic annotation. Trends in Food Science & Technology 88:121–32. doi: 10.1016/j.tifs.2019.03.020.
- Ivey, M., M. Massel, and T. G. Phister. 2013. Microbial interactions in food fermentations. Annual Review of Food Science and Technology 4 (1):141–62. doi: 10.1146/annurev-food-022811-101219.
- Iyer, R., S. K. Tomar, T. Uma Maheswari, and R. Singh. 2010. Streptococcus thermophilus strains: Multifunctional lactic acid bacteria. International Dairy Journal 20 (3):133–41. doi: 10.1016/j.idairyj.2009.10.005.
- Johansen, P., and L. Jespersen. 2017. Impact of quorum sensing on the quality of fermented foods. Current Opinion in Food Science 13:16–25. doi: 10.1016/j.cofs.2017.01.001.
- Kabak, B., and A. D. W. Dobson. 2011. An introduction to the traditional fermented foods and beverages of Turkey. Critical Reviews in Food Science and Nutrition 51 (3):248–60. doi: 10.1080/10408390903569640.
- Karaman, S., and T. Ozcan. 2021. Determination of gelation properties and bio-therapeutic potential of black carrot fibre-enriched functional yoghurt produced using pectin and gum arabic as prebiotic. International Journal of Dairy Technology 74 (3):505–17. doi: 10.1111/1471-0307.12776.
- Kaur, A., N. Capalash, and P. Sharma. 2018. Quorum sensing in thermophiles: Prevalence of autoinducer-2 system. BMC Microbiology 18 (1):62. doi: 10.1186/s12866-018-1204-x.
- Keller, L., and M. G. Surette. 2006. Communication in bacteria: An ecological and evolutionary perspective. Nature Reviews. Microbiology 4 (4):249–58. doi: 10.1038/nrmicro1383.
- Kesarwani, M., R. Hazan, J. He, Y.-A. Que, Y. Apidianakis, B. Lesic, G. Xiao, V. Dekimpe, S. Milot, E. Deziel, et al. 2011. A quorum sensing regulated small volatile molecule reduces acute virulence and promotes chronic infection phenotypes. PLoS Pathogens 7 (8):e1002192. doi: 10.1371/journal.ppat.1002192.
- Lazzi, C., M. Povolo, F. Locci, V. Bernini, E. Neviani, and M. Gatti. 2016. Can the development and autolysis of lactic acid bacteria influence the cheese volatile fraction? The case of Grana Padano. International Journal of Food Microbiology 233:20–8. doi: 10.1016/j.ijfoodmicro.2016.06.009.
- Lecomte, X., V. Gagnaire, S. Lortal, A. Dary, and M. Genay. 2016. Streptococcus thermophilus, an emerging and promising tool for heterologous expression: Advantages and future trends. Food Microbiology 53 (Pt A):2–9. doi: 10.1016/j.fm.2015.05.003.
- Lemoine, J., F. Chirat, J. M. Wieruszeski, G. Strecker, N. Favre, and J. R. Neeser. 1997. Structural characterization of the exocellular polysaccharides produced by Streptococcus thermophilus SFi39 and SFi12. Applied and Environmental Microbiology 63 (9):3512–8. doi: 10.1128/aem.63.9.3512-3518.1997.
- Li, S., S. Tang, Q. He, J. Hu, and J. Zheng. 2019. Changes in Proteolysis in Fermented Milk Produced by Streptococcus thermophilus in Co-Culture with Lactobacillus plantarum or Bifidobacterium animalis subsp. Lactis during refrigerated storage. Molecules (Basel, Switzerland) 24 (20):3699. doi: 10.3390/molecules24203699.
- Linciano, P., V. Cavalloro, E. Martino, J. Kirchmair, R. Listro, D. Rossi, and S. Collina. 2020. Tackling antimicrobial resistance with small molecules targeting LsrK: Challenges and opportunities. Journal of Medicinal Chemistry 63 (24):15243–57. doi: 10.1021/acs.jmedchem.0c01282.
- Liu, E., H. Zheng, T. Shi, L. Ye, T. Konno, M. Oda, H. Shen, and Z.-S. Ji. 2016. Relationship between Lactobacillus bulgaricus and Streptococcus thermophilus under whey conditions: Focus on amino acid formation. International Dairy Journal 56:141–50. doi: 10.1016/j.idairyj.2016.01.019.
- Liu, L., R. Wu, J. Zhang, and P. Li. 2018. Overexpression of luxS promotes stress resistance and biofilm formation of Lactobacillus paraplantarum L-ZS9 by regulating the expression of multiple genes. Frontiers in Microbiology 9:2628. doi: 10.3389/fmicb.2018.02628.
- Loh, J., R. W. Carlson, W. S. York, and G. Stacey. 2002. Bradyoxetin, a unique chemical signal involved in symbiotic gene regulation. Proceedings of the National Academy of Sciences of the United States of America 99 (22):14446–51. doi: 10.1073/pnas.222336799.
- Lorca, G. L., & G. F. de Valdez. (1999). The effect of suboptimal growth temperature and growth phase on resistance of Lactobacillus acidophilus to environmental stress. Cryobiology, 39(2), 144–149. doi: 10.1006/cryo.1999.2193.
- Macori, G., and P. D. Cotter. 2018. Novel insights into the microbiology of fermented dairy foods. Current Opinion in Biotechnology 49:172–8. doi: 10.1016/j.copbio.2017.09.002.
- Majdura, J., U. Jankiewicz, A. Gałązka, and S. Orzechowski. 2023. The role of quorum sensing molecules in bacterial-plant interactions. Metabolites 13 (1):114. doi: 10.3390/metabo13010114.
- Makarova, K., A. Slesarev, Y. Wolf, A. Sorokin, B. Mirkin, E. Koonin, A. Pavlov, N. Pavlova, V. Karamychev, N. Polouchine, et al. 2006. Comparative genomics of the lactic acid bacteria. Proceedings of the National Academy of Sciences of the United States of America 103 (42):15611–6. doi: 10.1073/pnas.0607117103.
- Marco, M. L., D. Heeney, S. Binda, C. J. Cifelli, P. D. Cotter, B. Foligné, M. Gänzle, R. Kort, G. Pasin, A. Pihlanto, et al. 2017. Health benefits of fermented foods: Microbiota and beyond. Current Opinion in Biotechnology 44:94–102. doi: 10.1016/j.copbio.2016.11.010.
- Marco, M. L., M. E. Sanders, M. Gänzle, M. C. Arrieta, P. D. Cotter, L. De Vuyst, C. Hill, W. Holzapfel, S. Lebeer, D. Merenstein, et al. 2021. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on fermented foods. Nature Reviews. Gastroenterology & Hepatology 18 (3):196–208. doi: 10.1038/s41575-020-00390-5.
- Marx, P., M. Meiers, and R. Brückner. 2014. Activity of the response regulator CiaR in mutants of Streptococcus pneumoniae R6 altered in acetyl phosphate production. Frontiers in Microbiology 5:772. doi: 10.3389/fmicb.2014.00772.
- McAuliffe, O. 2018. Symposium review: Lactococcus lactis from nondairy sources: Their genetic and metabolic diversity and potential applications in cheese1. Journal of Dairy Science 101 (4):3597–610. doi: 10.3168/jds.2017-13331.
- Medellin-Peña, M. J., and M. W. Griffiths. 2009. Effect of molecules secreted by Lactobacillus acidophilus strain La-5 on Escherichia coli O157:H7 colonization. Applied and Environmental Microbiology 75 (4):1165–72. doi: 10.1128/AEM.01651-08.
- Medina-Pradas, E., I. M. Pérez-Díaz, A. Garrido-Fernández, and F. N. Arroyo-López. 2017. The Microbiological Quality of Food. Chapter 9—Review of vegetable fermentations with particular emphasis on processing modifications, microbial ecology, and spoilage, ed. A. Bevilacqua, M. R. Corbo, & M. Sinigaglia, 211–236. New York: Woodhead Publishing. doi: 10.1016/B978-0-08-100502-6.00012-1.
- Meng, F., M. Zhao, and Z. Lu. 2022. The LuxS/AI-2 system regulates the probiotic activities of lactic acid bacteria. Trends in Food Science & Technology 127:272–9. doi: 10.1016/j.tifs.2022.05.014.
- Mokoena, M. P. 2017. Lactic acid bacteria and their bacteriocins: Classification, biosynthesis and applications against uropathogens: A mini-review. Molecules (Basel, Switzerland) 22 (8):1255. doi: 10.3390/molecules22081255.
- Mora, D., C. Monnet, C. Parini, S. Guglielmetti, A. Mariani, P. Pintus, F. Molinari, D. Daffonchio, and P. L. Manachini. 2005. Urease biogenesis in Streptococcus thermophilus. Research in Microbiology 156 (9):897–903. doi: 10.1016/j.resmic.2005.04.005.
- Mora, D., S. Arioli, and C. Compagno. 2013. Food environments select microorganisms based on selfish energetic behavior. Frontiers in Microbiology 4:348. doi: 10.3389/fmicb.2013.00348.
- Moslehi-Jenabian, S., K. Gori, and L. Jespersen. 2009. AI-2 signalling is induced by acidic shock in probiotic strains of Lactobacillus spp. International Journal of Food Microbiology 135 (3):295–302. doi: 10.1016/j.ijfoodmicro.2009.08.011.
- Mu, T., L. Rong, J. Wu, and R. Li. 2022. Advances in microbial interaction in fermented foods community ecosystem: Focus on cross-feeding. Food and Fermentation Industries, 49 (18):1–10. doi: 10.13995/j.cnki.11-1802/ts.033879.
- Mukherjee, S., and B. L. Bassler. 2019. Bacterial quorum sensing in complex and dynamically changing environments. Nature Reviews. Microbiology 17 (6):371–82. doi: 10.1038/s41579-019-0186-5.
- Ng, W.-L., K. M. Kazmierczak, G. T. Robertson, R. Gilmour, and M. E. Winkler. 2003. Transcriptional regulation and signature patterns revealed by microarray analyses of Streptococcus pneumoniae R6 challenged with sublethal concentrations of translation inhibitors. Journal of Bacteriology 185 (1):359–70. doi: 10.1128/JB.185.1.359-370.2003.
- Ortakci, F., J. R. Broadbent, C. J. Oberg, and D. J. McMahon. 2015. Late blowing of Cheddar cheese induced by accelerated ripening and ribose and galactose supplementation in presence of a novel obligatory heterofermentative nonstarter Lactobacillus wasatchensis. Journal of Dairy Science 98 (11):7460–72. doi: 10.3168/jds.2015-9468.
- Overbeck, T. J., D. L. Welker, J. E. Hughes, J. L. Steele, and J. R. Broadbent. 2017. Transient MutS-based hypermutation system for adaptive evolution of Lactobacillus casei to low pH. Applied and Environmental Microbiology 83 (20):e01120-17. doi: 10.1128/AEM.01120-17.
- Pang, X., Q. Zhu, J. Lu, S. Zhang, L. Liu, L. Yang, W. L, and J. Lv. 2020. Progress in quorum sensing system of lactic acid bacteria. Chinese Journal of Bioprocess Engineering 18 (02):141–9. doi: 10.3969/j.issn.1672-3678.2020.02.002
- Pang, X., S. Zhang, J. Lu, L. Liu, C. Ma, Y. Yang, P. Ti, W. Gao, and J. Lv. 2017. Identification and functional validation of autolysis-associated genes in Lactobacillus bulgaricus ATCC BAA-365. Frontiers in Microbiology 8:1367. doi: 10.3389/fmicb.2017.01367.
- Pang, X.-Y., W.-M. Cui, L. Liu, S.-W. Zhang, and J.-P. Lv. 2014. Gene knockout and overexpression analysis revealed the role of N-acetylmuramidase in autolysis of Lactobacillus delbrueckii subsp. Bulgaricus ljj-6. PLoS One 9 (8):e104829. doi: 10.1371/journal.pone.0104829.
- Papenfort, K., and B. L. Bassler. 2016. Quorum sensing signal-response systems in Gram-negative bacteria. Nature Reviews. Microbiology 14 (9):576–88. doi: 10.1038/nrmicro.2016.89.
- Park, H., H. Shin, K. Lee, and W. Holzapfel. 2016. Autoinducer-2 properties of kimchi are associated with lactic acid bacteria involved in its fermentation. International Journal of Food Microbiology 225:38–42. doi: 10.1016/j.ijfoodmicro.2016.03.007.
- Partanen, L., N. Marttinen, and T. Alatossava. 2001. Fats and fatty acids as growth factors for Lactobacillus delbrueckii. Systematic and Applied Microbiology 24 (4):500–6. doi: 10.1078/0723-2020-00078.
- Pasolli, E., F. De Filippis, I. E. Mauriello, F. Cumbo, A. M. Walsh, J. Leech, P. D. Cotter, N. Segata, and D. Ercolini. 2020. Large-scale genome-wide analysis links lactic acid bacteria from food with the gut microbiome. Nature Communications 11 (1):2610. doi: 10.1038/s41467-020-16438-8.
- Peng, C., G. Yao, Y. Sun, S. Guo, J. Wang, X. Mu, Z. Sun, and H. Zhang. 2022. Comparative effects of the single and binary probiotics of Lacticaseibacillus casei Zhang and Bifidobacterium lactis V9 on the growth and metabolomic profiles in yogurts. Food Research International (Ottawa, Ont.) 152:110603. doi: 10.1016/j.foodres.2021.110603.
- Pereira, C. S., J. A. Thompson, and K. B. Xavier. 2013. AI-2-mediated signalling in bacteria. FEMS Microbiology Reviews 37 (2):156–81. doi: 10.1111/j.1574-6976.2012.00345.x.
- Pereira, P. C. 2014. Milk nutritional composition and its role in human health. Nutrition (Burbank, Los Angeles County, Calif.) 30 (6):619–27. doi: 10.1016/j.nut.2013.10.011.
- Pezzulo, A. A., E. E. Hornick, M. V. Rector, M. Estin, A. C. Reisetter, P. J. Taft, S. C. Butcher, A. B. Carter, J. R. Manak, D. A. Stoltz, et al. 2012. Expression of human paraoxonase 1 decreases superoxide levels and alters bacterial colonization in the gut of Drosophila melanogaster. PLoS One 7 (8):e43777. doi: 10.1371/journal.pone.0043777.
- Pillidge, C. J., P. S. V. S. Rallabhandi, X.-Z. Tong, P. K. Gopal, P. C. Farley, and P. A. Sullivan. 2002. Autolysis of Lactococcus lactis. International Dairy Journal 12 (2-3):133–40. doi: 10.1016/S0958-6946(01)00135-2.
- Ponomarova, O., N. Gabrielli, D. C. Sévin, M. Mülleder, K. Zirngibl, K. Bulyha, S. Andrejev, E. Kafkia, A. Typas, U. Sauer, et al. 2017. Yeast creates a niche for symbiotic lactic acid bacteria through nitrogen overflow. Cell Systems 5 (4):345–57.e6. doi: 10.1016/j.cels.2017.09.002.
- Prazdnova, E. V., A. V. Gorovtsov, N. G. Vasilchenko, M. P. Kulikov, V. N. Statsenko, A. A. Bogdanova, A. G. Refeld, Y. A. Brislavskiy, V. A. Chistyakov, and M. L. Chikindas. 2022. Quorum-Sensing inhibition by gram-positive bacteria. Microorganisms 10 (2):350. doi: 10.3390/microorganisms10020350.
- Qiao, N., S. Wittouck, P. Mattarelli, J. Zheng, S. Lebeer, G. E. Felis, and M. G. Gänzle. 2022. After the storm-perspectives on the taxonomy of Lactobacillaceae. JDS Communications 3 (3):222–7. doi: 10.3168/jdsc.2021-0183.
- Qiao, R. 2022. Study on the relationship between signal molecule AI-2 and fermentation characteristics of lactic acid bacteriain meat products. Master’s thesis, Inner Mongolia Agricultural University. doi: 10.27229/d.cnki.gnmnu.2022.000759.
- Rao, M. S., J. Pintado, W. F. Stevens, and J. P. Guyot. 2004. Kinetic growth parameters of different amylolytic and non-amylolytic Lactobacillus strains under various salt and pH conditions. Bioresource Technology 94 (3):331–7. doi: 10.1016/j.biortech.2003.11.028.
- Rasmussen, T. B., M. Danielsen, O. Valina, C. Garrigues, E. Johansen, and M. B. Pedersen. 2008. Streptococcus thermophilus core genome: Comparative genome hybridization study of 47 strains. Applied and Environmental Microbiology 74 (15):4703–10. doi: 10.1128/AEM.00132-08.
- Sakandar, H. A., and H. Zhang. 2021. Trends in probiotic(s)-fermented milks and their in vivo functionality: A review. Trends in Food Science & Technology 110:55–65. doi: 10.1016/j.tifs.2021.01.054.
- Sakandar, H. A., and H. Zhang. 2022. Curious case of the history of fermented milk: Tangible evidence. Science Bulletin 67 (16):1625–7. doi: 10.1016/j.scib.2022.07.013.
- Salim-ur-Rehman, Alistair Paterson, and John R. Piggott. (2006). Flavour in sourdough breads: A review. Trends in Food Science & Technology 17(10):557–66. doi: 10.1016/j.tifs.2006.03.006.
- Sasaki, Y., H. Horiuchi, H. Kawashima, T. Mukai, and Y. Yamamoto. 2014. NADH Oxidase of Streptococcus thermophilus 1131 is required for the effective yogurt fermentation with Lactobacillus delbrueckii subsp. Bulgaricus 2038. Bioscience of Microbiota, Food and Health 33 (1):31–40. doi: 10.12938/bmfh.33.31.
- Scala, G. D., F. Volontè, G. Ricci, M. B. Pedersen, S. Arioli, and D. Mora. 2019. Development of a milk-based medium for the selection of urease-defective mutants of Streptococcus thermophilus. International Journal of Food Microbiology 308:108304. doi: 10.1016/j.ijfoodmicro.2019.108304.
- Schauder, S., K. Shokat, M. G. Surette, and B. L. Bassler. 2001. The LuxS family of bacterial autoinducers: Biosynthesis of a novel quorum-sensing signal molecule. Molecular Microbiology 41 (2):463–76. doi: 10.1046/j.1365-2958.2001.02532.x.
- Settachaimongkon, S., Nout, M. J. R. Fernandes, E. C. A. Hettinga, K. A. Vervoort, J. J. M. Hooijdonk, A. C. M.van, Zwietering, M. H.Smid, E. J. Valenberg, H. J. F. van, et al. 2014. Influence of different proteolytic strains of Streptococcus thermophilus in co-culture with Lactobacillus delbrueckii subsp. Bulgaricus on the metabolite profile of set-yoghurt. International Journal of Food Microbiology 177:29–36. doi: 10.1016/j.ijfoodmicro.2014.02.008.
- Sharma, H., F. Ozogul, E. Bartkiene, and J. M. Rocha. 2023. Impact of lactic acid bacteria and their metabolites on the techno-functional properties and health benefits of fermented dairy products. Critical Reviews in Food Science and Nutrition 63 (21):4819–41. doi: 10.1080/10408398.2021.2007844.
- Sharma, R., P. Garg, P. Kumar, S. K. Bhatia, and S. Kulshrestha. 2020. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 6 (4):106. doi: 10.3390/fermentation6040106.
- Shi, R., Q. Luo, Y. Liu, G. Meng, W. Chen, and C. Wang. 2022. Effect of γ-butyrolactone, a quorum sensing molecule, on morphology and secondary metabolism in Monascus. LWT 172:114225. doi: 10.1016/j.lwt.2022.114225.
- Shrivastava, N., and L. Ananthanarayan. 2015. Use of the backslopping method for accelerated and nutritionally enriched idli fermentation. Journal of the Science of Food and Agriculture 95 (10):2081–7. doi: 10.1002/jsfa.6923.
- Sieuwerts, S. 2016. Microbial interactions in the yoghurt consortium: current status and product implications. SOJ Microbiology & Infectious Diseases 4 (2):01–5. doi: 10.15226/sojmid/4/2/00150.
- Sieuwerts, S., D. Molenaar, S. A. F. T. van Hijum, M. Beerthuyzen, M. J. A. Stevens, P. W. M. Janssen, C. J. Ingham, F. A. M. de Bok, W. M. de Vos, and J. E. T. van Hylckama Vlieg. 2010. Mixed-culture transcriptome analysis reveals the molecular basis of mixed-culture growth in Streptococcus thermophilus and Lactobacillus bulgaricus. Applied and Environmental Microbiology 76 (23):7775–84. doi: 10.1128/AEM.01122-10.
- Sieuwerts, S., F. A. M. de Bok, J. Hugenholtz, and J. E. T. van Hylckama Vlieg. 2008. Unraveling microbial interactions in food fermentations: From classical to genomics approaches. Applied and Environmental Microbiology 74 (16):4997–5007. doi: 10.1128/AEM.00113-08.
- Smid, E. J., and C. Lacroix. 2013. Microbe-microbe interactions in mixed culture food fermentations. Current Opinion in Biotechnology 24 (2):148–54. doi: 10.1016/j.copbio.2012.11.007.
- Steensels, J., B. Gallone, K. Voordeckers, and K. J. Verstrepen. 2019. Domestication of industrial microbes. Current Biology: CB 29 (10):R381–R393. doi: 10.1016/j.cub.2019.04.025.
- Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annual Review of Biochemistry 69 (1):183–215. doi: 10.1146/annurev.biochem.69.1.183.
- Sturme, M. H. J., M. Kleerebezem, J. Nakayama, A. D. L. Akkermans, E. E. Vaugha, and W. M. de Vos. 2002. Cell to cell communication by autoinducing peptides in gram-positive bacteria. Antonie Van Leeuwenhoek 81 (1-4):233–43. doi: 10.1023/a:1020522919555.
- Sun, J. 2010. Studies of autolysis properties and mechanism of lactobic acid bacteria starters. PhD thesis., Chinese Academy of Agricultural Sciences. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CDFD0911&filename=2010171370.nh.
- Sybesma, W., M. Starrenburg, L. Tijsseling, M. H. N. Hoefnagel, and J. Hugenholtz. 2003. Effects of cultivation conditions on folate production by lactic acid bacteria. Applied and Environmental Microbiology 69 (8):4542–8. doi: 10.1128/AEM.69.8.4542-4548.2003.
- Takano, E. 2006. Gamma-butyrolactones: Streptomyces signalling molecules regulating antibiotic production and differentiation. Current Opinion in Microbiology 9 (3):287–94. doi: 10.1016/j.mib.2006.04.003.
- Tamang, J. P., P. D. Cotter, A. Endo, N. S. Han, R. Kort, S. Q. Liu, B. Mayo, N. Westerik, and R. Hutkins. 2020. Fermented foods in a global age: East meets West. Comprehensive Reviews in Food Science and Food Safety 19 (1):184–217. doi: 10.1111/1541-4337.12520.
- Tamime, A. Y. 2002. Fermented milks: A historical food with modern applications–a review. European Journal of Clinical Nutrition 56 Suppl 4:S2–S15. doi: 10.1038/sj.ejcn.1601657.
- Tannock, G. W., S. Ghazally, J. Walter, D. Loach, H. Brooks, G. Cook, M. Surette, C. Simmers, P. Bremer, F. Dal Bello, et al. 2005. Ecological behavior of Lactobacillus reuteri 100-23 is affected by mutation of the luxS gene. Applied and Environmental Microbiology 71 (12):8419–25. doi: 10.1128/AEM.71.12.8419-8425.2005.
- Tarrah, A., L. Treu, S. Giaretta, V. Duarte, V. Corich, and A. Giacomini. 2018. Differences in carbohydrates utilization and antibiotic resistance between Streptococcus macedonicus and Streptococcus thermophilus strains isolated from dairy products in Italy. Current Microbiology 75 (10):1334–44. doi: 10.1007/s00284-018-1528-7.
- Teneva-Angelova, T., I. Hristova, A. Pavlov, and D. Beshkova. 2018. Advances in Biotechnology for Food Industry. Chapter 4—Lactic acid bacteria—From nature through food to health, ed. A. M. Holban & A. M. Grumezescu, 91–133. New York: Academic Press. doi: 10.1016/B978-0-12-811443-8.00004-9.
- Thevenard, B., N. Rasoava, P. Fourcassié, V. Monnet, P. Boyaval, and F. Rul. 2011. Characterization of Streptococcus thermophilus two-component systems: In silico analysis, functional analysis and expression of response regulator genes in pure or mixed culture with its yogurt partner, Lactobacillus delbrueckii subsp. bulgaricus. International Journal of Food Microbiology 151 (2):171–81. doi: 10.1016/j.ijfoodmicro.2011.08.019.
- Vaillancourt, K., N. Bédard, C. Bart, M. Tessier, G. Robitaille, N. Turgeon, M. Frenette, S. Moineau, and C. Vadeboncoeur. 2008. Role of galK and galM in galactose metabolism by Streptococcus thermophilus. Applied and Environmental Microbiology 74 (4):1264–7. doi: 10.1128/AEM.01585-07.
- Van de Guchte, M., S. Penaud, C. Grimaldi, V. Barbe, K. Bryson, P. Nicolas, C. Robert, S. Oztas, S. Mangenot, A. Couloux, et al. 2006. The complete genome sequence of Lactobacillus bulgaricus reveals extensive and ongoing reductive evolution. Proceedings of the National Academy of Sciences of the United States of America 103 (24):9274–9. doi: 10.1073/pnas.0603024103.
- Vinderola, C. G., P. Mocchiutti, and J. A. Reinheimer. 2002. Interactions among lactic acid starter and probiotic bacteria used for fermented dairy products. Journal of Dairy Science 85 (4):721–9. doi: 10.3168/jds.S0022-0302(02)74129-5.
- Wang, C., Y. Cui, and X. Qu. 2018. Identification of proteins regulated by acid adaptation related two component system HPK1/RR1 in Lactobacillus delbrueckii subsp. Bulgaricus. Archives of Microbiology 200 (9):1381–93. doi: 10.1007/s00203-018-1552-9.
- Wang, J., H. Sun, S. Guo, Y. Sun, L.-Y. Kwok, H. Zhang, and C. Peng. 2021. Comparison of the effects of single probiotic strains Lactobacillus casei Zhang and Bifidobacterium animalis ssp. Lactis Probio-M8 and their combination on volatile and nonvolatile metabolomic profiles of yogurt. Journal of Dairy Science 104 (7):7509–21. doi: 10.3168/jds.2020-20099.
- Wang, T., W. Lu, S. Lu, and J. Kong. 2015. Protective role of glutathione against oxidative stress in Streptococcus thermophilus. International Dairy Journal 45:41–7. doi: 10.1016/j.idairyj.2015.01.015.
- Wang, T., Z. Xu, S. Lu, M. Xin, and J. Kong. 2016. Effects of glutathione on acid stress resistance and symbiosis between Streptococcus thermophilus and Lactobacillus delbrueckii subsp. Bulgaricus. International Dairy Journal 61:22–8. doi: 10.1016/j.idairyj.2016.03.012.
- Wang, Y. 2022. Study on the relationship between signal molecule AI-2 and exopolysaccharides production by lactic acid bacteria. Master’s thesis., Inner Mongolia Agricultural University. doi: 10.27229/d.cnki.gnmnu.2022.000179.
- Wang, Y., C. Zhang, F. Liu, Z. Jin, and X. Xia. 2023. Ecological succession and functional characteristics of lactic acid bacteria in traditional fermented foods. Critical Reviews in Food Science and Nutrition 63 (22):5841–55. doi: 10.1080/10408398.2021.2025035.
- Wang, Y., H. Li, T. Li, X. Du, X. Zhang, T. Guo, and J. Kong. 2019. Glutathione biosynthesis is essential for antioxidant and anti-inflammatory effects of Streptococcus thermophilus. International Dairy Journal 89:31–6. doi: 10.1016/j.idairyj.2018.08.012.
- Wang, Z. M., Z. M. Lu, J. S. Shi, and Z. H. Xu. 2016. Exploring flavour-producing core microbiota in multispecies solid-state fermentation of traditional Chinese vinegar. Scientific Reports 6 (1):26818. doi: 10.1038/srep26818.
- Wasfi, R., O. A. Abd El-Rahman, M. M. Zafer, and H. M. Ashour. 2018. Probiotic Lactobacillus sp. Inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. Journal of Cellular and Molecular Medicine 22 (3):1972–83. doi: 10.1111/jcmm.13496.
- Waters, C. M., and B. L. Bassler. 2005. Quorum sensing: Cell-to-cell communication in bacteria. Annual Review of Cell and Developmental Biology 21 (1):319–46. doi: 10.1146/annurev.cellbio.21.012704.131001.
- Wegkamp, A., M. Starrenburg, W. M. de Vos, J. Hugenholtz, and W. Sybesma. 2004. Transformation of folate-consuming Lactobacillus gasseri into a folate producer. Applied and Environmental Microbiology 70 (5):3146–8. doi: 10.1128/AEM.70.5.3146-3148.2004.
- Weiland-Bräuer, N. 2021. Friends or foes-microbial interactions in nature. Biology 10 (6):496. doi: 10.3390/biology10060496.
- Whiteley, M., S. P. Diggle, and E. P. Greenberg. 2017. Progress in and promise of bacterial quorum sensing research. Nature 551 (7680):313–20. doi: 10.1038/nature24624.
- Winters, M., D. Panayotides, M. Bayrak, G. Rémont, C. G. Viejo, D. Liu, B. Le, Y. Liu, J. Luo, P. Zhang, et al. 2019. Defined co-cultures of yeast and bacteria modify the aroma, crumb and sensory properties of bread. Journal of Applied Microbiology 127 (3):778–93. doi: 10.1111/jam.14349.
- Wolfe, B. E., and R. J. Dutton. 2015. Fermented foods as experimentally tractable microbial ecosystems. Cell 161 (1):49–55. doi: 10.1016/j.cell.2015.02.034.
- Wu, Q., C. K. W. Cheung, and N. P. Shah. 2015. Towards galactose accumulation in dairy foods fermented by conventional starter cultures: Challenges and strategies. Trends in Food Science & Technology 41 (1):24–36. doi: 10.1016/j.tifs.2014.08.010.
- Xu, T. 2017. Preliminary study on the role of two-component system in the protocooperation relationship of fermented yoghurt strains. Master’s thesis., Harbin Institute of Technology. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C475KOm_zrgu4lQARvep2SAk-6BvX81hrs37AaEFpExs0Cr4COSHHX-CutwaIcxoHuAxKtsRfRr3D6kTYZw-dz4_&uniplatform=NZKPT.
- Xu, Y., J. Zang, J. M. Regenstein, and W. Xia. 2021. Technological roles of microorganisms in fish fermentation: A review. Critical Reviews in Food Science and Nutrition 61 (6):1000–12. doi: 10.1080/10408398.2020.1750342.
- Xue, Z. P., Cu, X. Xu, K. Peng, J. H. Liu, H. R. Zhao, R. T. Wang, Z. Wang, T. Xu, and Z. S. 2023. The effect of glutathione biosynthesis of Streptococcus thermophilus ST-1 on cocultured Lactobacillus delbrueckii ssp. Bulgaricus ATCC11842. Journal of Dairy Science 106 (2):884–96. doi: 10.3168/jds.2022-22123.
- Yamauchi, R., E. Maguin, H. Horiuchi, M. Hosokawa, and Y. Sasaki. 2019. The critical role of urease in yogurt fermentation with various combinations of Streptococcus thermophilus and Lactobacillus delbrueckii ssp. Bulgaricus. Journal of Dairy Science 102 (2):1033–43. doi: 10.3168/jds.2018-15192.
- Yang, L., W. Fan, and Y. Xu. 2020. Metaproteomics insights into traditional fermented foods and beverages. Comprehensive Reviews in Food Science and Food Safety 19 (5):2506–29. doi: 10.1111/1541-4337.12601.
- Yeo, S., H. Park, Y. Ji, S. Park, J. Yang, J. Lee, J. M. Mathara, H. Shin, and W. Holzapfel. 2015. Influence of gastrointestinal stress on autoinducer-2 activity of two Lactobacillus species. FEMS Microbiology Ecology 91 (7):fiv065. doi: 10.1093/femsec/fiv065.
- Yu, J., L. Mo, L. Pan, C. Yao, D. Ren, X. An, T. Tsogtgerel, H. Zhang, and W. Liu. 2018. Bacterial microbiota and metabolic character of traditional sour cream and butter in Buryatia, Russia. Frontiers in Microbiology 9:2496. doi: 10.3389/fmicb.2018.02496.
- Yu, P., N. Li, M. Geng, Z. Liu, X. Liu, H. Zhang, J. Zhao, H. Zhang, and W. Chen. 2020. Short communication: Lactose utilization of Streptococcus thermophilus and correlations with β-galactosidase and urease. Journal of Dairy Science 103 (1):166–71. doi: 10.3168/jds.2019-17009.
- Zannini, E., D. M. Waters, A. Coffey, and E. K. Arendt. 2016. Production, properties, and industrial food application of lactic acid bacteria-derived exopolysaccharides. Applied Microbiology and Biotechnology 100 (3):1121–35. doi: 10.1007/s00253-015-7172-2.
- Zelezniak, A., S. Andrejev, O. Ponomarova, D. R. Mende, P. Bork, and K. R. Patil. 2015. Metabolic dependencies drive species co-occurrence in diverse microbial communities. Proceedings of the National Academy of Sciences of the United States of America 112 (20):6449–54. doi: 10.1073/pnas.1421834112.
- Zengler, K., and L. S. Zaramela. 2018. The social network of microorganisms—How auxotrophies shape complex communities. Nature Reviews. Microbiology 16 (6):383–90. doi: 10.1038/s41579-018-0004-5.
- Zhang, L., S. Li, X. Liu, Z. Wang, M. Jiang, R. Wang, L. Xie, Q. Liu, X. Xie, D. Shang, et al. 2020. Sensing of autoinducer-2 by functionally distinct receptors in prokaryotes. Nature Communications 11 (1):1. doi: 10.1038/s41467-020-19243-5.
- Zhang, Q., B. Yang, M. M. Brashears, Z. Yu, M. Zhao, N. Liu, and Y. Li. 2014. Influence of casein hydrolysates on exopolysaccharide synthesis by Streptococcus thermophilus and Lactobacillus delbrueckii ssp. Bulgaricus. Journal of the Science of Food and Agriculture 94 (7):1366–72. doi: 10.1002/jsfa.6420.
- Zhang, Y. 2022. Study on mechanism of biofilm formation and stress resistence by lactic acid bacteria based on luxS/AI-2 quorum sensing system. PHD thesis., Inner Mongolia Agricultural University. doi: 10.27229/d.cnki.gnmnu.2022.000030.
- Zhao, C., X. Qu, Y. Cui, and T. X. 2015. Review on protocooperation between Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus. Journal of Dairy Science and Technology 38 (4):21–4. doi: 10.7506/rykxyjs1671-5187-201504006.
- Zheng, J., S. Wittouck, E. Salvetti, C. M. A. P. Franz, H. M. B. Harris, P. Mattarelli, P. W. O’Toole, B. Pot, P. Vandamme, J. Walter, et al. 2020. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. International Journal of Systematic and Evolutionary Microbiology 70 (4):2782–858. doi: 10.1099/ijsem.0.004107.
- Zhou, H., M. Wang, N. E. Smalley, M. Kostylev, A. L. Schaefer, E. P. Greenberg, A. A. Dandekar, and F. Xu. 2019. Modulation of pseudomonas aeruginosa quorum sensing by glutathione. Journal of Bacteriology 201 (9):e00685-18. doi: 10.1128/JB.00685-18.
- Zotta, T., A. Ricciardi, F. Ciocia, R. Rossano, and E. Parente. 2008. Diversity of stress responses in dairy thermophilic streptococci. International Journal of Food Microbiology 124 (1):34–42. doi: 10.1016/j.ijfoodmicro.2008.02.024.
- Zotta, T., K. Asterinou, R. Rossano, A. Ricciardi, M. Varcamonti, and E. Parente. 2009. Effect of inactivation of stress response regulators on the growth and survival of Streptococcus thermophilus Sfi39. International Journal of Food Microbiology 129 (3):211–20. doi: 10.1016/j.ijfoodmicro.2008.11.024.
