Abstract
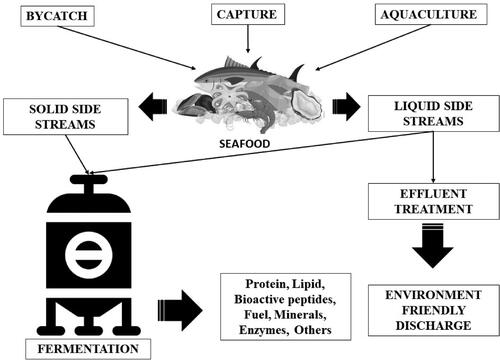
Fermentation technology is a biorefining tool that has been used in various industrial processes to recover valuable nutrients from different side streams. One promising application of this technique is in the reclamation of nutritional components from seafood side streams. Seafood processing generates significant amounts of waste, including heads, shells, and other side streams. These side streams contain high quantities of valued nutritional components that can be extracted using fermentation technology. The fermentation technology engages the application of microorganisms to convert the side stream into valuable products like biofuels, enzymes, and animal feed. Natural polymers such as chitin and chitosan have various purposes in the food, medicinal, and agricultural industry. Another example is the fish protein hydrolysates (FPH) from seafood side streams. FPHs are protein-rich powders which could be used in animal nutrition and nutraceutical industry. The resulting hydrolysate is further filtered and dried resulting in a FPH powder. Fermentation technology holds great possibility in the recovery of valuable nutrients from seafood side streams. The process can help reduce waste and generate new value-added products from what would otherwise be considered a waste product. With further research and development, fermentation technology can become a key tool in the biorefining industry.
Introduction
The fast increase in the world’s population, which is anticipated to reach more than 9 billion people by 2050, will cause a major increase in the need for food (Karimi et al. Citation2021). Conversely, when individuals get more affluent, there is an increased demand for premium food and other products. Nevertheless, food resources, particularly captured, will be limited (Välimaa et al. Citation2019). The aquatic ecology, which is very diverse, provides around 20% of global food (Sharma et al. Citation2020). Additionally, it contains millions of species containing nutrients and bioactive compounds that are little explored but could have high added value for food, pharmaceuticals, and cosmetics (Ucak et al. Citation2021). By 2030, 183 million tons of seafood are anticipated to be needed for human sustenance (Sharma et al. Citation2020). The seafood industry produces significant amounts of processing discards, such as heads, viscera, scales, and bones, which make up approximately 40–60% of the weight of raw materials (Singh et al. Citation2022). Consequently, seafood generally has a lower usable mass and produces more side streams (Sharma et al. Citation2020). Proper disposal of such products is still a major issue since their discharge might result in significant environmental risks that adversely affect the aquaculture and fishing sectors (Marti-Quijal et al. Citation2020; Ucak et al. Citation2021). According to research, by-catch and discards from seafood processing are abundant in a variety of biologically and nutritionally significant protein components, and lipids with high amounts of poly unsaturated fatty acids (PUFA), minerals, gelatin, collagen, and carotenoids (Singh et al. Citation2022; Ucak et al. Citation2021; Välimaa et al. Citation2019). These side streams should be given a second chance in order to prevent pollution and improve the fishing and aquaculture industries, in addition to supplying many vital nutritional and functional components (Marti-Quijal et al. Citation2020). The presence of bioactive and health-promoting components makes it possible to develop applications for healthy foods, nutrients, and special feeds in addition to technologies other than food, such as cosmetics (Singh et al. Citation2022; Välimaa et al. Citation2019). Consumers are adopting a health-conscious lifestyle, which supports this trend. It is also possible to create a positive image for new products derived from the by-product due to increased consumer awareness of environmental issues (Välimaa et al. Citation2019). Furthermore, the European Union (EU) Commission proposed the Green Deal in 2020, promoting a circular economy as a social goal and an innovative development model (Donzella et al. Citation2022; Leong et al. Citation2021). Sustainable product design, consumer empowerment, and rotation in production processes are all discussed in this central document. Another idea is to develop an industry for secondary raw materials. These are side streams that are often thrown away as trash but may instead be reused as raw materials. Identifying alternatives to supply chains has the dual goal of reducing side streams and environmental problems (Allegretti et al. Citation2022). As a result, functional foods, nutrient supplements, pharmaceuticals, and cosmetics can be developed at a low cost. Furthermore, using side streams and low-value seafood efficiently and rationally can help improve sustainable and environmentally friendly production (González-Camejo et al. Citation2023; Ucak et al. Citation2021). Current economic dynamics require more sustainable and renewable technologies to maximize resource recovery from side streams. Recent years have seen the development of several new processing techniques for improving nutritional quality and microbial safety while additionally increasing food physicochemical properties by reducing process intensity, decreasing energy usage, reducing side streams loads, and enhancing process productivity and production. Although these advantages are undeniable, in some instances, the cost of using innovative technologies and the dependence on them pose obstacles. Under these circumstances, microbial fermentation or enzymatic hydrolysis are frequently used as bioconversion procedures (Singh et al. Citation2022). Green techniques are anticipated to displace traditional technologies for extracting resources from food side streams in the future due to their simplicity of use and environmental safety (Venugopal and Sasidharan Citation2022). In recent years, “green chemistry” has grown in popularity, which relies on creating and improving green extracting processes that use microorganisms and enzymes to recover bioactive substances side streams from seafood (Singh et al. Citation2022). Biorefining based on green chemistry is secure, effective, and ecologically responsible since it doesn’t compromise the extracted chemicals’ inherent qualities (Venugopal and Sasidharan Citation2022). By-products of the seafood industry can be valorized through biorefining to increase their value as biomaterials and energy sources (Singh et al. Citation2022). Green technologies can demonstrate promising performance for the recovery of bioactive compounds when assessed with established considering the efficiency, energy efficiency, and cost effectiveness (Venugopal and Sasidharan Citation2022). Chemical extraction, which uses strong acids and alkalis at high temperatures, is widely used. These conditions require more energy and are associated with disadvantages such as being time-consuming, having higher treatment costs, requiring a large volume of water (to neutralize and wash acids and alkalis), and having worse properties (physical and chemical) of the obtained product. Recent developments in the seafood processing industry have introduced biotechnology processes, such as bio catalysis and fermentation, that could be applied to recover valuable components (Singh et al. Citation2022; Venugopal and Sasidharan Citation2022). The food, energy, pharmaceutical, and side stream treatment industries are all paying attention to fermentation since it has less effect on the environment and lower operating costs than traditional chemical processes (Chai et al. Citation2022). Therefore, integrating biological processing facilities and implementing innovation-based exploitation methods can maximize resource recovery by creating a bioeconomy (Singh et al. Citation2022). Incorporating green chemistry into industries related to the ocean can lead to a more profitable, sustainable, and conscious ocean economy (Venugopal and Sasidharan Citation2022). This study investigates the significance of fermentation processes used in biorefining to recover high-value resources involving protein, lipids, and bioactive peptides, as well as different fermentation strategies used to transform seafood by-products.
Significance of fermentation as a resource recovery tool
Preventive measures can reduce the production of inedible food side streams at every step, from production to utilization. For this scenario, there is an urgent requirement for suitable side streams management policies (Dahiya et al. Citation2018). Physical, chemical, and biological processes are often used in side stream treatment techniques, alone or in combination. Physical and chemical processes are often harsher and less ecological than biological processes like fermentation or enzymatic hydrolysis (Rai et al. Citation2011 Siddiqui et al., Citation2023). Conventional methods for valorizing discarded materials and seafood side streams depend on physical and chemical methods. There are always various restrictions to these processes, and these shortcomings have increased interest in greener alternatives, such as biorefineries (Venugopal and Sasidharan Citation2022).
The role of biorefineries in the valorization of seafood by-products
Biotechnology promises to produce goods with added value, ensure product quality, and develop new techniques for getting rid of side streams for recovering resources. Such methods have been made possible by new green technologies for industrial biomolecule recycling from side stream materials and seafood products (Venugopal Citation2021). Recovery using green process is possible; among the sources are microbial fermentation, enzymatic reactions, methanogenesis, photosynthesis and oil processing. (Venugopal and Sasidharan Citation2022). Biological procedures can be affordable, secure, and have little to no detrimental impact on the characteristics of isolated components. Consequently, these procedures offer a flexible and affordable method of concentrating side streams and effluent water into useful goods (Venugopal Citation2021). In particular, for the biorefinery, which seeks to increase the use of aquatic resources through the recovery of materials connected to seafood residues, resulting in production and addressing activities that enhance marine values (Veríssimo et al. Citation2021). By incorporating the biorefinery concept into the marine industry, it is possible to preserve natural resources and eliminate the production of seafood side streams while simultaneously generating employment and increasing profits. Side streams from fish processing may provide biorefineries with a viable renewable biomass source. Under the biorefineries method, value-added goods including industrial chemicals, biofuels, animal feed, organic fertilizers, nutrients, etc. are produced from fish side streams. Some key aspects of the process are low cost and ease of use, which are achieved by lowering labor costs, energy usage, and material costs while maintaining high production (Venugopal Citation2021). A complex matrix of macromolecules, natural polymers, and minerals makes up seafood side streams. As a result, the process of extracting by-products from these residues often involves several steps and separation processes. Depending on the traits and qualities provided by the new target components, as well as the required level of purity of the commercial goods, the extraction platform’s design will change (Veríssimo et al. Citation2021). Modern biorefineries are built exceptionally cost-effective and can handle untreated and dangerous source materials (such as industrial, residential, and agricultural side streams) while generating safe outputs (Venugopal Citation2021). A blue ocean economy is becoming a reality, thanks to significant advancements. Yet, we can only create a whole marine biorefinery when we combine each of these processes into a seamless and efficient production system (Veríssimo et al. Citation2021). The type and accessibility of raw materials will determine the amount of fuel that any biorefinery technology can generate. Consequently, if these technologies can be coupled under the integrated concept of side streams biorefining, mixed and diverse raw materials may be processed to generate many materials like power, food, heat, feed, and energy together with chemical products with added value (Nizami et al. Citation2017). By using biological treatment methods, side streams can be processed more effectively, and a variety of products, including biofuels (hydrogen, methane, and bioethanol), biomaterials, platform chemicals, biofertilizers, animal feed, biomass, and bioelectricity can be produced.
Microbial fermentation to recover valuable biomolecules
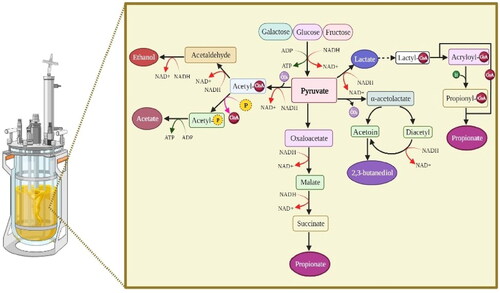
Due to its safety, energy production, lower environmental impact, and lower operating costs compared to chemical processes, fermentation has gained the interest of the food processing, pharmaceutical energy, and side streams treatment industries. It is described as the process of any natural substance reacting by means of microbial activity, which is facilitated by a range of enzymes (Chai et al. Citation2022; W. Sun, Shahrajabian, and Lin Citation2022). The use of fermentation can provide various advantages concerning hydrolysis by pH changes and has also been used for several years to produce valuable products from various side streams (Marti-Quijal et al. Citation2020; Venugopal and Sasidharan Citation2022). Fermentation is a biotechnological process that humans have been doing since ancient times. Today, many chemicals are manufactured using fermentation technology, which plays a significant role in industrial production. Living microorganisms (fungi, mycelium, bacteria, or microalgae) use raw resources in this procedure to produce high-quality goods (Venugopal Citation2021). Fermentation, in general, is an enzyme-driven process that breaks down complex organic compounds into simpler ones, like glucose, without oxygen. Less energy is produced during fermentation than during aerobic cellular respiration, which produces two ATP molecules. Depending on the organism, fermentation can produce a variety of other by-products. During fermentation, lactic acid, lactate, carbon dioxide (CO2), and water are produced by bacteria, fungi, protozoa, and animal cells. In yeast and even most plant cells, fermentation results in the production of ethyl alcohol, CO2, and water. Fermentation has also been utilized recently to produce valuable products from multiple side streams (Venugopal and Sasidharan Citation2022). The categorization of fermentation technologies is shown schematically in . The process of fermentation involves employing an endogenous electron acceptor, which is often an organic substance, to oxidize organic materials like carbohydrates in order to produce energy. The kinds might be conventional, biomass, or precise. Traditional fermentation has existed for thousands of years. Biomass fermentation has been employed in the food business since the 1980s to create cell mass that can be utilized as nutrients, biomaterials, enzymes, flavorings, fuel, and medicines. More recently, it has become a source of alternative proteins for the creation of cultured meat compositions (Venugopal Citation2021). Technology for microbial transformation offers an efficient method for isolating and using promising biotechnological substances (Dahiya et al. Citation2018; Venugopal Citation2021). Bacteria, fungi, and protozoa are examples of the microorganisms utilized for this function, which can be facultative, anaerobic, or aerobic. Anaerobic, batch, continuous, or fed-batch microbial fermentation processes are all possible in solid, submerged, or liquid media. By adding continuous or pulsed medium cultures until the desired volume is reached, fed-batch devices are frequently used to produce organic acids, ethanol, microbial biomass, enzymes, antibiotics, vitamins, and other compounds. Higher productivity, greater dissolved oxygen levels in the environment, better biodegradation rates, and shorter fermentation times are benefits of fed-batch processes over traditional non-continuous ones (Venugopal Citation2021). Compared to other biological platforms, anaerobic fermentation is attracting a lot of interest for the generation of biofuels and environmental chemicals from biogenic side streams, particularly food side streams (Dahiya et al. Citation2018). The fermentation of lactic acid bacteria (LAB) is a common technique for creating fermented fishery products (Venugopal Citation2021). Microbial fermentation using organisms generally recognized as safe has several commercial advantages. Now, this new initiative in the EU includes a minimum of 400 manufacturers (Nizami et al. Citation2017). Positive news is that sales of fortified/functional foods have increased dramatically globally in recent years, according to Euromonitor (Venugopal and Sasidharan Citation2022). By 2027, a large increase in the market for fish protein hydrolysis is anticipated. Fermentation has been used in recent years to transform various side streams into valuable products (Venugopal and Sasidharan Citation2022). Bioremediation of food side streams may supplement fossil-based remediation to some extent and address important drivers of the bioeconomy, resource security, ecosystem services, and climate by creating a wide variety of bio-based end products, including biofuels, product chemicals, and biological fertilizers. By biologically treating food side streams, we will soon achieve a sustainable path with minimal environmental effect (Dahiya et al. Citation2018).
Lactic acid fermentation to recover biomolecules
Researchers have looked at how LAB affects side streams stabilization and biomolecule recovery from side streams generated from animal and fish processing sectors (Coimbra Citation2016; Kumar et al. Citation2018; Marti-Quijal et al. Citation2020; Vázquez et al. Citation2019). Lactic acid fermentation is gaining popularity in the case of side streams since it is an ecologically acceptable process that produces a product that can be used straight as feed or as a neutralizer (Marti-Quijal et al. Citation2020). For example, extracting biomolecules from the processing of seafood side streams requires controlled fermentation. LAB fermentation has a greater impact than the acid approach. These microorganisms produce numerous compounds like diacetyl hydrogen peroxide, organic acids, and bacteriocins. They have a substantial effect on inhibiting spoilage microorganisms and improving food taste and texture. Commercial LAB strains are essential for the food and edible industries to benefit from fermentation (Özyurt et al. Citation2017). The kind of organism, the amount of inoculum, the pH at the beginning and during fermentation all have an impact on the formation of lactic acid. Lactic acid, which is created when sugar is broken down, causes a low pH, which reduces the development of bacteria and encourages the activity of acid proteases. These enzymes function best on the proteins found in seafood, many of which are chitin-, carotenoids-, and lipid-attached (Venugopal Citation2021). Fish and by-products from slaughterhouses have been successfully preserved using LAB fermentation (Marti-Quijal et al. Citation2020). The addition of an acceptable quantity of fermentable carbohydrates, decreasing the pH during fermentation and while storing the finished product, quick growth of the starting culture, and enough lactic acid generation are the three primary requirements for effective lactic acid fermentation. In 24 to 48 h, or even 3.9 in certain circumstances, a mixture of fish or animal by-products, carbohydrates, and a starting culture can ferment to produce a pH level ranging from 4.4 to 5.0. When the fermentation process of slaughter by-products becomes successful, the pH should be below 5.0 and remain there for less than one year. Fish fermentation, a common method for extending shelf life, creates the necessary bacterial metabolites. The generation of antioxidant chemicals, oil, and protein hydrolysates are all improved by fermentation in by-products (Marti-Quijal et al. Citation2020). LAB has long been used to create fermented fish products. Acidic proteases that work on proteins, lipids, and carotenoids that are bound to chitin are more active when the pH is low (Venugopal Citation2021). Fermentation can offer a number of benefits for hydrolysis by causing pH shifts. Among several of the principal advantages, fermentation boosts the efficacy of antioxidant peptides associated with glutathione to prevent oxidative stress damage. In addition, fermentation, which hydrolyzes and decomposes proteins to peptides and shorter amino acids, produces better digestible proteins when used as animal feed protein source. This makes it an excellent tool for enhancing fish nutritional content. In comparison to formic acid treatment, fermentation improves fish side streams oil quality (Marti-Quijal et al. Citation2020). Fermentation may thus result in the production of a variety of value added products, including enzymes, cell mass, flavors, and food additives. Fermentations brought on by LAB also result in a diversity of foods, which turn in edibles into edibles, preserve food, boost nutritional value, lower toxicity, and require less cooking time and energy (Venugopal Citation2021). This technique is advantageous, safe, and good for the environment. It offers the possibility of obtaining a wide range of compounds, in addition to not consuming much energy (Marti-Quijal et al. Citation2020; Venugopal Citation2021).
Various applications of biomolecules obtained from seafood by-products
Pigments, chitin, lipids, and proteins found in seafood offal can be recovered or used as inexpensive substrates to make a range of bioproducts (Veríssimo et al. Citation2021). Fish by-product fermentation results in high-quality proteins, oil, and antioxidants. The fermented product can be used as a nutrient for aquatic animals (Venugopal Citation2021). Also, fish side streams contain compounds rich in nitrogen, phosphorus, and calcium, which are suitable for preparing fertilizer. These fertilizers are currently available on the market and approved for organic farming (Marti-Quijal et al. Citation2020). With the help of a variety of microorganisms, it was possible to convert fish side streams into liquid fertilizer. This material demonstrated decay resistance for up to six months at room temperature (Venugopal Citation2021). Most often, a preliminary pretreatment is applied to these substrates in order to transform them into cultivable raw materials. The use of this pretreated solid product, which is rich in calcium, nitrogen, and phosphate, has helped tomato harvests, biopharming, and ice lettuce (Lactuca sativa L.) crops. On the other hand, culture experiments commonly use the nutrient-rich blue liquid supernatant as a starting medium to produce a variety of biomolecules. (Veríssimo et al. Citation2021). Rashid, Jung, and Kim (Citation2018) used Bacillus cereus to ferment shrimp shell powder to create sugars, antioxidants, and DNA-protective chemicals. Higher results were reported when comparing fed-batch fermentation to batch biological degradation (Rashid, Jung, and Kim Citation2018; Venugopal Citation2021). At STP conditions, 1 kg of food side streams can generate 385 g of propionic acid, 916 g of butyric acid, and 624.96 g of acetic acid (Dahiya et al. Citation2018; Nizami et al. Citation2017). In addition to biological molecules, energy can also be obtained from the by-products of marine products. Food side streams at the STP has a calorific value of 5350 KJ/kg, suggesting that it has the prospective to be used as a resource of bioenergy. One gram of glucose should completely oxidize to provide 16 KJ of energy. At a conversion efficiency of 50%, one kg of COD from food side streams produces 15.62 mol of methanol (350 L methanol), which results in 13,882 KJ of energy production and 3.85 kWh of power production (Dahiya et al. Citation2018). The growth of the bioeconomy and environmental sustainability can both be considerably aided by the usage of energy-intensive and economically viable goods made from food side streams. Also, combining several methods in order to maximize resource recovery with co-production lessens the detrimental effects of food side streams on the environment to a certain level (Dahiya et al. Citation2018). summarizes some other possible uses for fermentation technology.
Table 1. Fermentation technologies for potential application.
Application of fermentation technology for recovery of protein from seafood side streams
Among the six essential nutrients for life, protein is a crucial bioactive molecule (Mao et al. Citation2017). Living organisms require nutrients, proteins, and bioactive peptides to grow and develop (Ucak et al. Citation2021; Välimaa et al. Citation2019). To supply protein demand, sustainable approaches are needed. Promoting biodiversity in food production systems, creating substitute proteins through regenerative methods, and recovering wholesome and sustainable proteins from side streams are a few of these. Proteins may be found in food side streams, particularly seafood (Ucak et al. Citation2021). The basic protein content of fish side stream varies from 8 to 35%, based on the type of process and fish (Vicente et al. Citation2022). From seafood by-products, biologically active compounds may be isolated, including hydrolyzed proteins and bioactive peptides including components like, bone, internal organs, and collagen (Mao et al. Citation2017; Ucak et al. Citation2021).
Fermentation is an environmentally friendly, and economic method used in a wide range of applications throughout the past century. Fermentation produces a variety of valuable compounds, including proteins (Marti-Quijal et al. Citation2020). Proteins extracted from fermentation seafood side streams contain essential and non-essential amino acids (Mao et al. Citation2017; Ucak et al. Citation2021). They have comparable nutritional value to meat and egg proteins (Ucak et al. Citation2021). Seafood side streams contain various protein compounds that can be recovered by fermentation and used for numerous applications. Hydrolysis of muscle proteins can result in bioactive peptides with physiological and technical qualities beneficial in the food and pharmaceutical sector (Ucak et al. Citation2021). Gelatin has applications in protein-based composite biomaterials such as films, emulsions, fibers, foams, hydrogels, and furthermore. Due to their distinct structural and functional properties, fish collagen and gelatin are employed in the therapeutic, biomedical, cosmetic, and biotechnology sectors. They may be employed as food emulsifiers, foaming agents, stabilizers, nutritional microencapsulates, and to improve the sensory qualities of low-fat meals (Venugopal and Sasidharan Citation2022). In addition, These proteins regulate the metabolism of glucose, have favorable effects on lipid profiles, and control blood pressure, among other beneficial metabolic effects (Ucak et al. Citation2021). Instead of conventional fermentation, these advancements employ "biomass fermentation." Backbone fermentation is a brand-new alternative protein being researched and released on the market. Depending on the microorganism, fermentation can lead to the manufacture of hydrolytic enzymes (proteases, lipases and chitinases), as well as other enzymes. Microbial fermentation, which uses fish side streams as a carbon-nitrogen source, is an economic, secure, and environmentally friendly method of extracting a varied extent of useful materials from seafood side streams (Venugopal and Sasidharan Citation2022). Biomolecule recovery requires the employment of nonpathogenic bacterial enzymes (Rai et al. Citation2011). These microorganisms release hydrolytic enzymes, which break down specific source material constituents to create hydrolysates () (Gao et al. Citation2021). As opposed to proteases derived from other sources, microbial proteases generated by various microorganisms offer several benefits. They include inexpensive production costs, the potential for mass production, quicker enzyme isolation times, and the capacity of organisms to respond to genetic engineering that increases enzyme effectiveness. Bacillus spp., Bifidobacterium spp., and lactic acid bacteria are the most frequently used microbial proteases (Godinho et al. Citation2016). Proteases demineralize and deproteinize food side streams (Ucak et al. Citation2021). Microorganisms that occur naturally or added to hydrolyze proteins and sugars are required for fermentation. Two growth parameters that influence the production of hydrolyzed proteins are the inoculation circumstances and the peptide content of the medium. Extracellular microbial proteases hydrolyze a broad range of peptides differing in molecular weight and amino acid sequence. These bioactive peptides have fascinating features (Godinho et al. Citation2016). On the whole, peptides generated from seafood by-products often have biological qualities such as antimicrobial activity, antioxidant activity, antiproliferative impact, antihypertensive activity, anticoagulant action, calcium absorption, bone mineralization, and furthermore (Ucak et al. Citation2021). Recent studies by several authors have focused on the utilization of microbes to manufacture bioactive peptides through proteolysis (Godinho et al. Citation2016). Protein hydrolysates produced from fish flesh using A26 were investigated by Jemil et al. (Citation2014) for their functional, antibacterial, and antioxidant properties. It demonstrated the beneficial solubility, emulsification stability, and foaming capabilities of the hydrolyzed proteins generated by fermentation utilizing various fish meat. The proteolytic enzymes of Bacillus subtilis A26 produced peptides during the biorefinery of fish proteins that have antioxidant and antibacterial activities (Jemil et al. Citation2014). In one more study, the result of Bacillus subtilis fermentation by means of lactic acid bacteria isolated from freshwater fish head side streams to recover lipids and proteins simultaneously was reported by Ruthu et al. (Citation2014). The findings indicated that fermentation had no impact on fatty acid or lipid profiles. Fish head proteins that are antibacterial and antioxidant may be used as functional additives in food compositions. Hence, it could promote human health and/or lengthen the food’s shelf life. The fermented hydrolyzed protein has shown antibacterial properties against diverse bacterial species. Its usage may be seen in the pharmaceutical business, nutritional supplements, and food preservation (Ruthu et al. Citation2014). Hydrolysates made from soup fish skin fermentation with Aspergillus oryzae, according to Fang et al. (Citation2017), also exhibit strong antioxidant activity. It may be an inexpensive source of antioxidants with a large range of prospective applications in the food and medicinal sectors. These applications include those for food preservation and health promotion (Fang et al. Citation2017). Finally, fermentation bioprocesses contribute to clean production by minimizing environmental impacts while transforming fish side streams into valuable functional materials. shows the different applications of fermentation to enhance the valorizing of seafood by-products. So, it appears certain that fermentation is a method that suggests many options and can be employed to get various compounds of benefits (Marti-Quijal et al. Citation2020).
Table 2. Fermentation technologies for recovery of protein from seafood side streams.
Application of fermentation technology for recovery of lipids from seafood side streams
Fermentation could be considered as a suitable means in modifying and improving value of lipid waste. As a result of process of fermentation there is a hydrolyzed protein rich fraction as well as oil and chitin or collagen according to the type of waste. Other than protein from fish industry waste lipid base compounds include fish oil, poly unsaturated fatty acids (PUFA), phospholipids and cholesterol. The fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are well known source of nutritionally valuable component which are by-products/discards from fish processing. The PUFAs have got the advantage of reducing hypertension, risk of cardiovascular disease, inflammatory disease as well as autoimmune diseases. (Kim and Mendis Citation2006). Over the recent years, the economic worth of fish oil has seen a decline, primarily attributed to issues related to irregularities in demand and supply. Projections suggest that this trend is likely to persist in the foreseeable future (Turchini, Torstensen, and Ng Citation2009). There is a growing imperative to swiftly adopt and identify viable alternatives to fish oil to address the challenges posed by the escalating demand. Consequently, there is a heightened focus on exploring supplementary sources of fish oil. In fish industry lipids regained from fish industry side stream can be used to as an excellent replacement to fish oil as well as meet the demand for fish oil to a certain extent. Biological method like fermentation is gaining importance in this context. Fermentation is considered as microorganisms’ growth in food waste enable bioconversion which provide safe as well as effective release of components from food matrices (S. F. Bruno et al. Citation2019; Dessie et al. Citation2020; Shavandi et al. Citation2019). Lactic acid bacteria (LAB) dependent bioconversions have the advantage that it makes inedible fish edible, fewer toxicity, conserve and reduce cooking time and nutrition. LAB fermentation enables breakdown of sugars to lactic acid which cause pH to lower 3.5, as a result due to low pH. Lipases behave as triacylglycerol hydrolases which can also catalyze the production of ester complexes. Fish viscera are a good source of 19 to 21 lipids. Lipids up to 85% can be recovered by fermentation (Rai et al. Citation2010).
In fish industry waste, lipid content is reported to be in between 4 and 43.8%. These lipids are a considerable source of polyunsaturated fatty acids (PUFA) which are susceptible to oxidation. Fish visceral waste and peroxide value is seen to increase up to three to four days of fermentation during autolysis of fish visceral waste. Fish oil recovery conventional method involves physical treatments such as heating as well as separate oil by centrifugation. From fish visceral waste, solvent can be employed for removal of oil which is not a cost-effective approach. Reclamation of oil from fish visceral side stream can be studied using lactic acid fermentation (Rai et al. Citation2011). During fermentation the quality of oil recovered shows that lactic acid fermentation (LAF) is considered as a better methodology for regaining of oil with less effect on quality. The fatty acid composition of oil recovered through fermentation is indistinguishable from that obtained through solvent extraction. In terms of fatty acid composition, there is an even distribution of both saturated and unsaturated fatty acids (Rai et al. Citation2011). In case of fish processing side stream, oil recovered can be a competent alternative sources of fish oils which are rich in unsaturated fatty acids.
In comparison to meat, lipids extracted from the viscera and head of both freshwater and marine fishes exhibit higher levels (Rai et al. Citation2011; Swapna et al. Citation2010). A promising substitute for fish oil lies in the fatty acid composition derived from these fish-derived lipids. These lipids, along with carotenoids, offer diverse bio functionalities. Microorganisms play a crucial role in the fermentation process, including aerobic, anaerobic, or facultative bacteria, fungi, mycelium, or microalgae. These all enable production of hydrolytic enzymes which include lipase, chitinase and proteases. which depends on microorganisms involved in the replacement of alkali during conventional treatment for proteolysis which help in demineralization and deproteination of waste. Lactic acid bacteria help in bioconversion, helps in nutrition enhancement which makes inedible food edible, preserve, and decrease cooking time, less toxic. LAB fermentation helps breakdown of sugar to lactic acid which result in low PH as 3.5. The low PH helps in optimal activity of enzymes such as acid proteases which will release protein bound to carbohydrates, lipids, carotenoids, and minerals in the waste. The acid will react with the calcium carbonate which is the main mineral component which form calcium lactate which result in control growth of the contaminant microorganisms as well as the demineralization of cells. Microbial fermentation is favored for its simplicity, rapidity, easy handling, and controllability, with minimal organic solvent requirements. It offers safety, low energy consumption, and environmental friendliness. Chitin bio-extraction, a cleaner, green, economical process, benefits from fermentation, enhancing the quality of protein hydrolysates, chitin, and oil, while producing antioxidants (Marti-Quijal et al. Citation2020). Lactic acid fermentation (LAF) has been employed for chitin production since the 2000s, and fish viscera fermentation can recover lipids, constituting 19% to 21% of the content (Rai et al. Citation2010). depicts the major fermentation technologies adopted for the recovery of lipids from seafood side streams.
Table 3. Fermentation technologies for recovery of lipid from seafood side streams.
Extraction of bioactive peptides from seafood side streams using fermentation method
Fermentation is a very well accepted process to produce bioactive peptides (BPs) from organic substrates imparting chemical changes through action of enzymes produced by microorganism. Fermentation process on the basis of use of water can be divided in to two types such as solid-state fermentation (SSF) and submerged fermentation (SmF). In the SSF process, microorganisms are allowed to grow on organic materials/solid surface with no free water availability for the product formation. However, in SmF, microorganisms are cultured in a water rich nutrient medium where the substrate is consumed rapidly, and the BPs are synthesized and secreted to the culture medium. Depending upon the nature of the microorganism used, there are anaerobic, continuous, or fed batch fermentation processes (Mauerhofer et al. Citation2019). Fish processing industry waste is one of the important organic substrates of high protein content used for production BPs through all these fermentation processes (Abu Yazid et al. Citation2017; Chandrasekharan Citation2015; Mohapatra et al. Citation2017; Rashid, Jung, and Kim Citation2018). The complex protein molecules are broken down by the action of proteolytic enzymes produced by the microorganisms to short amino acid sequences of 2 to 20 residues. These are low molecular-weight compounds of less than 6,000 Da and often possess N-terminal and C-terminal amino acid residues (Saadi et al. Citation2015). These short chain amino acids, for their structural features exhibit functional characteristics as BPs (Chaudhary et al. Citation2021). The BPs are known for their antioxidative, antidiabetic, antimicrobial, antihypertensive, anticancer and immunomodulatory properties (Kang et al. Citation2019; Najafian and Babji Citation2012; Ngo et al. Citation2016; Nirmal et al. Citation2022; Phadke et al. Citation2021; Yaghoubzadeh et al. Citation2020) is represented in . Fermentation process employs different microorganism such as yeast, fungus and bacteria for the degradation of complex biomaterials. The lactic acid bacteria (LAB) are very commonly used for the fermentation of fish waste (Venugopal and Sasidharan Citation2022). The LAB, Gram positive bacilli are the eco-friendly microbes generally regarded as safe (GRAS) especially used for fermentation of fish processing waste.
Table 4. Fermentation process for recovery of bioactive peptides from seafood side streams.
Fermentation and antioxidant peptides
Lactobacillus, utilizing cell envelope proteinases (CEP), hydrolyzes proteins bound with various nutrients in seafood side streams, releasing peptides and free amino acids into the fermentation media (Savijoki, Ingmer, and Varmanen Citation2006). The resulting lactic acid production reduces media pH, controlling contaminant microorganism proliferation. In a study by Ruthu et al. (Citation2014), three LAB strains isolated from fish waste were evaluated for fermenting freshwater fish head waste. Conditions included 10% (w/w) glucose, 2% (w/w) NaCl, and 10% (v/w) LAB cultures at 37 °C. P. acidilactici NCIM5368 exhibited a significantly lower degree of hydrolysis compared to the other groups, all of which showed similar degrees of hydrolysis. The hydrolysates from all three groups demonstrated good antioxidant activity, confirmed through total antioxidant activity (TAO) and DPHH radical scavenging activity analysis. The LAB fermentation is also considered as the cost-effective method for fish waste valorization and production of BPs. A study conducted by Rajendran et al. (Citation2018) demonstrated that fermentation of fish waste taking mixture of two strains of LAB such as Lactobacillus plantarum CNCM MA 18/5 U (>1 3 1010 cfu/g), and Pediococcus acidilactici CNCM MA 18/5 M (>1 3 1010 cfu/g) in a media of deproteinized whey 5% (wt/wt) had a clear edge over enzymatic and intentional acidification for production of antioxidant peptides. However, the authors reported that the generation of antioxidant peptides from fish waste were because of inherent proteases in the tissues of the fish residue and was not of LAB origin. The fermentation of sea bass by-products (skin, head, tail, thorns, and backbone) with lactic acid bacteria (LAB) isolated from the three regions of the digestive system (stomach, small intestine, and colon) resulted in the production of DL-3-phenyl-lactic acid and benzoic acid (MartiQuijal, Remize, et al. Citation2020). They obtained the best antioxidant activity in the extracts of fermented fish by-products broth by bacteria isolated from the colon (6502 μM TE) and stomach (4797 μM TE). Fish sauce is a fermented fish product and very popular in Southeast Asian countries. The fish sauce by-product (FSB) is discarded after collection of high value fish sauce. A study conducted by Choksawangkarn et al. (Citation2018) found that FSB is a good source of protein and bioactive compounds especially antioxidant peptides. The crude protein content was 10% in FSB and quality of protein in fish sauce and FSB were the same. The FSB was rich in tyrosine confirmed through paper chromatographic studies. The anti-oxidation property in FSB could be due to the presence of tyrosine. Because the phenolic side chains found in the structure of tyrosine is a free radical scavenger and stops the continuous reaction of radicals (Lassoued et al. Citation2015). The DPPH radical scavenging assay also revealed the presence of low molecular weight antioxidant molecules in the FSB extract which were identified as PQLLLLLL and LLLLLLL peptide. These extracted molecules could be used as functional ingredients in other food/feed products. Japanese fish miso, made by fermenting fish paste with A. oryzae-inoculated koji, is a rich source of antioxidants. Giri, Nasu, and Ohshima (Citation2012) assessed the antioxidant activity of matured fish miso using a linoleic acid oxidation model, attributing radical-scavenging activity and linoleic acid oxidation inhibition to antioxidative peptides (<500 Da) formed during fermentation. The study concluded that various discarded fish types can be fermented with A. oryzae to create functional fish miso. Fang et al. (Citation2017) found that fermenting discarded turbot skin with Saccharomyces cerevisiae, A. oryzae, and Streptococcus thermophilus, with A. oryzae yielding the highest skin protein hydrolysis. A. oryzae’s potential for fermentation lies in its multiple protease-coding genes, enhancing protein component hydrolysis (Vishwanatha, Rao, and Singh Citation2009). The fermented hydrolysates exhibited DPPH inhibitory activity, with A. oryzae fermented hydrolysate displaying the highest antioxidant activity due to its unique amino acid profile. Lactic acid fermentation of shrimp waste using innovative substrates like whey and molasses yields proteins, lipids, and carotenoids in the fermentation liquor (Cabanillas-Bojórquez et al. Citation2021). The antioxidant activity of the liquor, attributed to carotenoids, measures 54.43 ± 4.73 µM Trolox equivalent/g via the ABTS method. Shrimp waste’s principal carotenoid, astaxanthin, demonstrates remarkable antioxidant potency, surpassing β-carotene by 10-fold and vitamin E by over 500 times (Afonso et al. Citation2016; Higuera-Ciapara, Felix-Valenzuela, and Goycoolea Citation2006). Shrimp fermentation waste shows promise for feed and pharmaceutical applications. Cabanillas-Bojórquez et al. (Citation2021) optimized supercritical CO2 conditions for astaxanthin extraction from lactic acid-fermented shrimp waste, extracting 0.6353 µg/g astaxanthin under 300 bar pressure, 60 °C temperature, and 6 mL/min flow rate. The extracted liquor exhibits antioxidant activity against various free radicals, suitable for food supplements. Shrimp by-products, rich in bioactive compounds, can be employed in food, feed, pharmaceutical, textile, and agricultural industries, challenging the perception of these by-products as "waste" (Seedevi et al. Citation2017). Bioprocessing shrimp by-products with proteolytic microorganisms is gaining traction, producing metabolites with high bioactivity potential (Mechri et al. Citation2019). Proper drying of fermented shrimp processing by-products preserves their functional properties, making them valuable in fee d applications (Ghorbel-Bellaaj et al. Citation2018).
Fermentation and antimicrobial peptides
Antimicrobial peptides (AMPs) are very diverse group of molecules. It is less than 10 KDa weight with average 33.19 amino acid residues (Baco et al. Citation2022; S. Wang et al. Citation2016). The AMPs are part of the innate immune defense system and protects the host from broad range of microorganisms, including bacteria, fungi, parasites and viruses (Das, Pradhan, and Pillai Citation2022; Mohammed, Said, and Dua Citation2017). AMPs exhibit amphipathic conformation as both hydrophobic and hydrophilic groups attached with it (L. T. Nguyen, Haney, and Vogel Citation2011). These peptides neutralize the pathogenic microbes by blocking membrane ion gradients, interacting with the cytoplasmic membrane, forming pores and even altering cell metabolism (Hoelscher et al. Citation2022; Q. Y. Zhang et al. Citation2021). The antimicrobial peptides are also known for their inhibition activity to Gram-position and Gram-negative bacteria (Huan et al. Citation2020).
The anti-microbial resistance of microorganisms is a serious concern due to rampant use of antibiotics in agriculture and animal husbandry sectors worldwide (Manyi-Loh, et al. Citation2018). There is an urgent need of antibiotic alternatives. Antimicrobial peptides are suitable alternatives to antibiotics as they are effective against multi drug resistance pathogens (Rima et al. Citation2021). Several antibacterial peptides have been derived and reported from sea foods and sea food side streams. Enzymatic hydrolysis is one of the commonly adopted processes to derive bioactive peptides from the sea food by-products. The common enzymes which are used for this purpose are pronase, bromelain, protease A and N, orientase, neutrase, protamex, validase, pancreatin, alcalase, trypsin, papain, pancreatin, and thermolysin and flavourzyme (Abuine, Rathnayake, and Byun Citation2019; Lorenzo et al. Citation2019). However, fermentation employing microbes is also suitable for the production of AMPs. Among microbes, Bacillus species and Aspergillus spp. are proficient in production of enzymes suitable for the fermentation of sea food side stream and production of AMPs. Ruthu et al. (Citation2014) fermented fish waste with three strains Bacillus (Pediococcus acidilactici NCIM5368, Enterococcus faecium NCIM5335 and Pediococcus acidilactici FD3) isolated from fish processing wastes the obtained hydrolysate exhibited good antibacterial activity and it was ascertained through checking against a number of Gram-positive and Gram-negative microbes. The fermented hydrolysate from all three groups also showed antifungal activity. The extract fermentation with E. faecium NCIM5335 was found to be efficient and effective compared to P. acidilactici FD3 and P. acidilactici NCIM5368 against Penicillium compared to A. oryzae and A. ochareus. This work is one of the few reports of LAB fermentation produced both antibacterial (bacteriocin) and antifungal peptides from fish waste.
Fish side streams, including skin, scale, and bone, are rich sources of valuable products such as collagen and peptone. Collagen extraction methods include using collagenolytic protease (CP) bacteria, as demonstrated by R. Ahmed et al. (Citation2018) with Bacillus cereus FORC005 and Bacillus cereus FRCY9-2, yielding higher collagen (188 g/kg waste) compared to bacteria with acid (177 g/kg) or acid extraction alone (134.5 g/kg). Enzymatic hydrolysis of collagen produces the antimicrobial peptide collagencin, which exhibits broad-spectrum antibacterial activity through a carpet mechanism. Collagen-derived peptides from fish, such as SIFIQRFTT, RKSGDPLGR, AKPDGAGSGPR, and GLPGLGPAGPK, demonstrate antibacterial properties, with GLPGLGPAGPK showing activity against both Gram-positive and Gram-negative bacteria (Ennaas et al. Citation2015). Peptone, another valuable product from fish waste, is widely used in microbiological media for microbial synthesis of antimicrobial peptides and metabolites. Fish-derived peptone, obtained through enzyme hydrolysis, is comparable to commercial peptone, and peptones from specific fish sources, like river pangasius catfish and magur catfish, may even be more effective for bacterial growth than commercial counterparts (Setijawati et al. Citation2020). Maky and Zendo hydrolyzed fish samples using pepsin enzyme, yielding a peptide effective against a wide spectrum of foodborne pathogens and spoilage bacteria.
Enzymatic hydrolysis and bioactive peptides
Solvent extraction, enzymatic hydrolysis, and microbial fermentation are key methods for extracting bioactive peptides (BPs) from marine proteins. Microbial fermentation yields silages and fish protein hydrolysates containing antioxidants and antimicrobial peptides. Lactic acid fermentation, while generating lipids, poses challenges in lipid removal from the hydrolysate, making it suitable for animal feed. In food and pharmaceutical industries, enzymatic hydrolysis is preferred for its absence of residual organic solvents or toxic chemicals (Ryan et al. Citation2011). Acid and alkali hydrolysis have drawbacks, as they destroy certain valuable amino acids, diminishing bioactivity potential. Acid treatment, for instance, completely destroys tryptophan and causes varying degrees of destruction to serine, threonine, asparagine, and glutamine (H. Gu et al. Citation2015). In contrast, enzymatic hydrolysis, conducted under controlled pH and temperature conditions, is well-suited for producing low molecular weight bioactive peptides with unique amino acid sequences. Crude hydrolysates are subsequently fractionated to separate individual peptides using different techniques, mainly reverse phase high performance liquid chromatography (RP-HPLC) or gel permeation chromatography (Ryan et al. Citation2011). The bioactive peptides generate through enzymatic hydrolysis show calcium binding, antidiabetic, antihypertensive and anticancer properties and that has been described in this section.
Calcium-binding peptide
Calcium (Ca) is a very important mineral for animal as well as human nutrition. The bioavailability of Ca can be enhanced when provided along with a protein phosphopeptide complex or by simple fortification in the staple food. Fish scales are good source of protein and have strong affinity to bind with Ca. In this regard, Y. Lu et al. (Citation2016) conducted experiment to find out Ca-binding peptide from tilapia scale protein hydrolysates on calcium absorption in Caco-2 cells. Tilapia scale protein hydrolysates were prepared by treating the scale powder with pepsin, trypsin and flavourzyme with suitable quantity and at proper pH and temperature. The isolation of Ca binding peptides was done with sephadex G-15, and the calcium uptake was estimated using Caco-2 cells in DMEM cell culture media. The Ca uptake increased by the calcium binding peptides than from CaCl2. The results suggest that peptide-calcium complex is a bioavailable form of Ca. The snapper fish scale protein hydrolysate-Ca complex also exhibited improved calcium cellular uptake and resisted the inhibitory effect of dietary inhibitors (Lin et al. Citation2020). The Ca binding peptides has been isolated from tilapia skin gelatin enzymatic hydrolysates. Bingtong, Yongliang, and Liping (Citation2020) purified two peptides such as Tyr-Gly-Thr-Gly-Leu (YGTGL, 509.25 Da) and Leu-Val-Phe-Leu (LVFL, 490.32 Da) with strong calcium-binding capacity from the different tilapia (Oreochromis niloticus) skin gelatin enzymatic hydrolysates.
Antidiabetic peptide
Numerous diabetes prevention strategies such as controlling carbohydrate rich food to insulin injection are followed by the patients. Bioactive food derived peptides has also been reported to maintain the glucose homeostasis due to their capacity to inhibit the digestive enzymes. Fish derived proteins and peptides are also very effective in increasing glucose uptake and glucose tolerance, reducing blood glucose concentrations, and up-regulating GLUT4 and PPAR-α (Zhou et al. Citation2021). Oral administration of 300 mg/day Atlantic salmon skin gelatin hydrolysate for five weeks in streptozotocin-induced diabetic rats inhibited dipeptidyl peptidase IV (DPP-IV) activity, increased plasma glucagon-like peptide-1 (GLP-1), insulin, and the insulin-to-glucagon ratio, suggesting its potential for regulating glucose metabolism (Hsieh et al. Citation2015). DPP-IV, a homodimeric serine peptidase, plays a crucial role in regulating glucose metabolism by interacting with peptides like GLP-1 and deactivating their insulinotropic activity (Liu, Cheng, and Wu Citation2019). So, inactivation of DPP-IV is the key target for the treatment of T2DM. As per a report, halibut and tilapia skin gelatin hydrolysates inhibited in vitro DPP-IV activity (38–51%) and increased the glucose tolerance in diabetic rats through the inhibition of plasma DPP-IV activity, increase of GLP-1 and insulin secretion when administered at a dose 750 mg/kg/day for 30 days (T. Y. Wang et al. Citation2015). Peptide fraction from Atlantic salmon (Salmo salar) frames improved the glucose tolerance and reduced the activation of TORC1/S6K1/IRS1 nutrient-sensing pathway in liver of male mice fed at a dose of 50% in the diet for 12 weeks (Chevrier et al. Citation2015). Decreased blood glucose level was found in mice with alloxan induced diabetes when provided with tilapia skin collagen peptides at dose of 0.85 and 1.7 g/kgbw for 25 days (R. Zhang et al. Citation2016). The peptides derived from Sardine pilchardus muscle proteins by hydrolyzing with a combination of three enzymes, such as subtilisin, trypsin and flavourzyme showed highest DPP-IV inhibitory activity with an IC50 value of 1.83 mg/ml (Rivero-Pino, Espejo-Carpio, and Guadix Citation2020). The discarded shrimp (Penaeus vannamei) head was hydrolyzed with five food grade proteases. The peptides generated were identified by LC MS/MS and four potential peptides YPGE, VPW, HPLY, YATP showed 40.90 ± 2.76 μM, 174.781 ± 5.08 μM, 461.89 ± 3.23 μM, 475.33 ±6.24 μM, IC50 value, respectively (Xiang Citation2021). The authors concluded that discarded P. vannamei head is a promising natural source of DPP-IV inhibitor and can improve glycemic control in Type 2 diabetes.
Antihypertensive peptides
According to WHO, approximately 1.28 billion adults aged 30–79 in low and middle-income countries have hypertension, necessitating various drug treatments. These include calcium channel blockers, renin-angiotensin system inhibitors, beta blockers, alpha blockers, diuretics, vasodilators, and ACE inhibitors, each with distinct mechanisms in controlling hypertension and potential side effects (Olowofela and Isah Citation2017). In hypertension prevention and treatment, natural products like fish-derived peptides have shown promise. These peptides, purified from seafood and side streams, are low in molecular weight (<1 kDa), with fewer than 20 amino acids, primarily consisting of arginine, valine, and leucine. Fish protein hydrolysates (FPH), prepared with food-grade enzymes, serve as good sources of ACE-I inhibitory peptides. ACE inhibitory peptides, extracted from various fish species and by-products, include those obtained from salmon pectoral fin through enzyme hydrolysis (Ahn et al. Citation2012). Purified ACE inhibitory peptides, such as Val-Trp-Asp-Pro-Pro-Lys-Phe-Asp (P1), Phe-Glu-Asp-Tyr-Val-Pro-Leu-Ser-Cys-Phe (P2), and Phe-Asn-Val-Pro-Leu-Tyr-Glu (P4), exhibit IC50 values against ACE activity (9.10 μM, 10.77 μM, and 7.72 μM, respectively). These peptides have been isolated from various salmon species (Y. Gu, Majumder, and Wu Citation2011; Neves et al. Citation2017; Ono et al. Citation2006). ACE-inhibitory peptides have been identified in marine species like krill (Park, Je, and Ahn Citation2016), pacific cod (Ngo et al. Citation2016), sardinelle (Jemil et al. Citation2017), and tuna (Martínez-Alvarez et al. Citation2016), as well as in freshwater fishes such as tilapia (Toopcham, Roytrakul, and Yongsawatdigul Citation2015) and grass carp. These peptides exhibit proven ACE-I inhibitory activity and antihypertensive effects, validated through in vitro and in vivo studies. Their potential as antihypertensive drugs should be highlighted.
Anti-cancer peptides
Cancer treatment involves various complex methods, such as chemotherapy, surgery, radiation, immunotherapy, gene therapy, and nanomedicine (Arruebo et al. Citation2011). Due to the side effects of chemotherapy and anticancer drugs, there’s a growing reliance on natural compounds with anticancer properties (S. Ahmed et al. Citation2021; Pangestuti and Kim Citation2017). Bioactive peptides from natural aquatic products can modulate molecular pathways related to DNA defense, cell-cycle control, and apoptosis initiation, making them potential candidates for cancer treatment (Kang et al. Citation2018). Protein hydrolysates from fish, amphibians, and turtles show potential in cancer treatment. Shark cartilage extract, particularly Neovastat (AE-941) from Squalus acanthias, demonstrates anti-tumor, anti-angiogenic, and anti-inflammatory properties. AE-941 disrupts the signaling pathway of vascular endothelial growth factor (VEGF) to its receptor (VEGFR), inducing apoptotic activities in endothelial cells (Gingras et al. Citation2003). Active oxygen species related oxidative stress can cause cancer in human being and antioxidants are potential candidates to control it (Umayaparvathi et al. Citation2014). The fish protein hydrolysate is a good source of antioxidant has been reported from different fish by-products (discussed earlier in this section). Halim et al. (Citation2018) fractionated eel protein hydrolysate (EPH) which exhibited antioxidant property and anticancer activity as well. The anticancer activity was determined by 3–4, 5-dimethylthiazol-2-yl-2, 5-diphenyltetrazolium bromide (MTT) assay using MCF-7 cell lines. The antioxidant and anticancer activities of bioactive peptide isolated from oyster (Saccostrea cucullata) protein hydrolysate were evaluated in vitro by Umayaparvathi et al. (Citation2014). The oyster peptide SCAP1 showed both antioxidant and anticancer activity against human colon carcinoma (HT-29) cell lines. Similarly, Yaghoubzadeh et al. (Citation2020) reported that antioxidant and anticancer properties of rainbow trout (Oncorhynchus mykiss) skin protein hydrolysate prepared using Alcalase (HA) and Flavourzyme (HF) enzymes. The antioxidant characteristics of hydrolysates ascertained through DPPH radical inhibitory power and ferric reducing assay. The anticancer properties of the hydrolysates were tested in MTT assay and using HCT-116 cell line. The isolated fractions on HCT-116 cancer cells showed cytotoxic properties and inhibit the growth of these cells in vitro.
Application potential of fermented seafood side streams in animal nutrition
Fish protein hydrolysates (FPH) are obtained by chemicals acid, enzymatic hydrolysis or by microbial fermentation of by-products. The amino acid composition of FPH is well-balanced, and highly digestible in animal feed (Ananey-Obiri, Matthews, and Tahergorabi Citation2019; Zheng et al. Citation2012). Seafood protein hydrolysates contain low molecular weight compounds and molecules having the ability to stimulate the production of insulin like growth factors that are favorable for growth, feed performance, immunity and survivability of the animals. The FPH industry is witnessing a growing demand from the feed manufacturers in order to reduce the production cost of feed. The use of FPH has potential to replace traditional protein sources such as FM and soybean meal from the diets of fish and animals (Siddik et al. Citation2021). The addition of FPH at appropriate concentration has been found to increase the growth rate of fish (Egerton et al. Citation2020), poultry bird, pig and sheep.
Application of FPH in aquafeed
The growing aquaculture practice is heavily dependent on continuous supply of good quality feed. Fishmeal (FM) is generally considered the benchmark ingredient in aquafeed formulations due to the balanced amino acid profile and other nutrients. However, due to over demand and escalating price the replacement of FM with alternative proteins is given a lot of emphasis. Fisheries and aquaculture industry generates 50% and 70% of the by-products typically consist of viscera, heads, skin, bones, and blood (Stevens et al. Citation2018). This inedible portion is increasingly being considered as a practical option after converting to silage, hydrolysate or even FM (low quality) to replace the use of high-quality FM (Egerton et al. Citation2020; Hua et al. Citation2019; Shao et al. Citation2020). Fermented fish waste has additional benefits because of the antioxidant and antimicrobial properties, making them suitable for feed applications (Ruthu et al. Citation2014).
Cancer treatment involves various complex methods, such as chemotherapy, surgery, radiation, immunotherapy, gene therapy, and nanomedicine (Arruebo et al. Citation2011). Due to the side effects of chemotherapy and anticancer drugs, there’s a growing reliance on natural compounds with anticancer properties (S. Ahmed et al. Citation2021; Pangestuti and Kim Citation2017). Bioactive peptides from natural aquatic products can modulate molecular pathways related to DNA defense, cell-cycle control, and apoptosis initiation, making them potential candidates for cancer treatment (Kang et al. Citation2018). Protein hydrolysates from fish, amphibians, and turtles show potential in cancer treatment. Shark cartilage extract, particularly Neovastat (AE-941) from S. acanthias, demonstrates anti-tumor, anti-angiogenic, and anti-inflammatory properties. AE-941 disrupts the signaling pathway of vascular endothelial growth factor (VEGF) to its receptor (VEGFR), inducing apoptotic activities in endothelial cells (Gingras et al. Citation2003). This indicates that the nutritional composition fish bio silage () was suitable for general metabolism, tissue structural integrity, nutritional and health status of fish. Bag et al. fed fermented fish offal to tilapia significantly increased growth, feed conversion ratio (FCR), protein efficiency ratio (PER), specific growth rate (SGR), hepatosomatic index (I) and gonadosomatic index (GSI) than the reference diet. In another study, fish silage fermented with lactobacillus species fed to tilapia fry as a substitute of FM for 84 days. The results showed up to 50% of FM replacement by fermented fish silage is possible without negative effects on growth and feed utilization and the feed cost reduced by 15.59% for the fish (Soltan et al. Citation2017).
Table 5. Chemical composition of FPH, FSWFS and FFWFS (%, as-fed basis).
FPH derived from enzymatic hydrolysis of fish waste has proven beneficial in farmed fish, positively impacting growth, feed intake, nutrient utilization, immune response, oxidative status, and disease resistance (Siddik et al. Citation2021). Its incorporation improves feed palatability and attraction in fish due to low molecular weight peptides (Tang et al. Citation2008). With a suitable nutrient profile and easy digestion, FPH is an attractive option for larval feed, especially when the digestive tract is not well developed at early developmental stages. FPH provides highly bioavailable nutrients, enhances digestive enzyme activity, protein metabolism, and intestinal health, thereby increasing larval survivability and development (Sheng et al. Citation2022). Reported benefits of FPH supplementation include enhanced larval survival and fry growth in various fish species, including Atlantic halibut, yellow croaker, sea bass, largemouth bass, Nile tilapia, and early post-larval Pacific white shrimp (Cai et al. Citation2015; Khieokhajonkhet and Surapon Citation2020; Kwasek et al. Citation2021; Niu et al. Citation2014; Tonheim et al. Citation2005). Fish silage and FPH are successful alternatives to FM in various species (Arruda, Borghesi, and Oetterer Citation2007; Elavarasan Citation2019). Moderate FPH inclusion (5–10% FM replacement) enhances growth performance (Egerton et al. Citation2020). Even at lower levels, FPH improves diet palatability and boosts fish growth (Wei et al. Citation2021; Xu et al. Citation2016). Salmon fed with 10% partly hydrolyzed fish protein and 80% plant protein exhibit comparable growth to a 35% FM diet (Egerton et al. Citation2020). Tiger puffer effectively utilizes FPH with plant ingredients, regulating 4E-PP1 expression to increase muscle protein synthesis (Wei et al. Citation2021). Dietary FPH benefits growth in black sea bream through TOR signaling pathway up-regulation (Irm et al. Citation2020). Stick water hydrolysate (SWH) enhances growth, immune function, and intestinal health in rice field eel when replacing 5–15% FM (Shi et al. Citation2019). However, FPH incorporation (>20%) decreases growth performance in various fish species (Xu et al. Citation2016). Nile tilapia and Japanese flounder showed reduced growth with over 10% APH and 16% FPH, respectively (Khieokhajonkhet and Surapon Citation2020). Turbot fed with 20% FPH also experienced significant growth reduction (Xu et al. Citation2016). The excess short-chain peptides and amino acids in hydrolyzed products may saturate intestinal peptide transport mechanisms, affecting growth in fish (Ospina-Salazar et al. Citation2016). Shrimp, however, exhibits better FPH utilization, as shown in a six-week trial with 20% tuna head hydrolysate improving growth and survival rates in Pacific white shrimp (H. T. M. Nguyen, Pérez-Gálvez, and Bergé Citation2012).
FPH supplementation in the diet of fish influences other biochemical responses such as hematological and immunological parameters. In red sea bream, fish fed diets containing roughly 4–5% of hydrolysates from whole Antarctic krill, white shrimp, or tilapia had considerably higher survival rates and total immunoglobulin levels than fish fed diets without these ingredients (Bui, et al. Citation2014). Additionally, they discovered that adding krill or tilapia hydrolysate to fish diets enhanced their resistance to Edwardsiella tarda. The availability of antimicrobial, anti-inflammatory, and antioxidant peptides in fish protein hydrolysates prepared with application of enzymes or through fermentation from side streams have been reported in several studies (Baco et al. Citation2022; Da Rocha et al. Citation2018). It is obvious that when these hydrolysates are provided as dietary components enhances health condition of fish. Additionally, Goosen, de Wet, and Görgens (Citation2014) observed that phagocytic activity of hemocytes increased by 18% in comparison to the control diet when low quantities of commercial fish protein hydrolysate (Actipal, 0.6%) were added to abalone diets. A study on red seabream, Pagrus major, found that replacing fishmeal (FM) with fish protein hydrolysate (FPH) increased hematocrit, hemoglobin, total protein, and cholesterol levels, while decreasing plasma glucose and triglyceride levels. This suggests that dietary FPH inclusion enhances protein absorption and overall fish health (Khosravi et al. Citation2015). Murrel (Channa striata) fingerlings fed with 10% FPH showed improved serum lysozyme and myeloperoxidase, along with up-regulation of IGF-I, enhancing growth performance and immune response (Siddaiah et al. Citation2022). Similarly, juvenile largemouth bass (Micropterus salmoides) fed with 30 g/kg dry matter of FPH replacing FM demonstrated no negative effects on growth and feed utilization, and FPH supplements improved intestinal immune mechanisms to address immunodeficiency caused by FM replacement (Fan et al. Citation2022).
Application of FPH in pig feed
FM and soybean meal (SBM) are the conventional protein ingredients in pig feeds owing to their balance of amino acids and other nutrients. These two ingredients are relatively expensive in comparison to other protein ingredients. The presence of antinutritional factors in SBM also affects health of weaning pig (Koepke et al. Citation2017). The replacement of FM with fish silage meal (FSM) has been successfully tried in pig diets. Thi Thuy, Lindberg, and Ogle (Citation2011) fed pigs with ensiled Tra catfish (Pangasius hypophthalmus) by-products (ECM) and found improved performance and meat quality of finishing pigs. However, feed intake was slightly affected due to ECM inclusion and the backfat thickness increased. The authors concluded that palatability difference due to the presence of FSM could be the reason for less feed intake with ECM and keeping the cost of production in view replacement of FM with FSM would still be profitable. Candido et al. reported that FSM, obtained from the mixture (1:1) of fish silage with corn, showed a quadratic effect on average daily gain, and the best result was obtained with the inclusion level of 25.83%. The results for feed conversion and economic viability indicate that up to 25% FSM, corresponding to 5.87% of fish silage based on dry matter, can be used in the pig growing and finishing phases. FPH is an emerging raw material in animal feed (Opheim et al. Citation2016). Weaning pigs’ fed with salmon protein hydrolysate (SPH) and FM resulted in 12–14% increased feed consumption than those containing soy protein concentrate. However, the higher feed intake did not change weight gain and feed utilization significantly among treatments. The higher feed intake due to addition of FPH could be due to the presence of flavor-like peptides in FPH which is often released in the hydrolysis process (B. J. Bruno, Miller, and Lim Citation2013). Similarly, Tucker et al. (Citation2011), observed that supplementation of up to 3% of salmon protein hydrolysate in diets does not affect the growth performance of weaning pigs. Furthermore, Opheim et al. reported that piglets fed with SPH in a weaning condition showed larger duodenal villi absorption area in comparison to the groups received plant and soy proteins. The authors also observed differences in intestinal microbiota community but no differences in growth performance between the experimental diets. The FPH hydrolysate is well accepted in pig and capable of replacement of SBM or FM but do not contribute to growth performance in general. N. Zhang et al. (Citation2022) found that addition of 5% FPH increased the average daily feed intake (ADFI), activities of total antioxidant capacity (T-AOC), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and immunoglobulin A (IgA). FPH in diet al.so resulted in high value of digestible energy and ileal digestible essential amino acids, improved nutrient digestibility, immunity, and intestinal health of piglets. But the piglets showed adverse nitrogen deposition could be the cause of decreased average daily gain (ADG) of piglets which was also obtained in the result. Contrastingly, when whole fish hydrolysate was fed replacing 75% and 100% FM significantly increased the ADG of piglets by 2.50% and 3.76% (Thuy, Joseph, and Ha Citation2016). This indicates FPH prepared from whole fish is superior to the FPH prepared from the side streams. The advantage of dietary fermented FM has also been noticed in weanling pigs. Addition of fermented FM to weanling pig diets increased average feed intake, final body weight, average daily gain, and gain: feed ratio improved blood hematocrit, monocyte, immunoglobulin G, and blood urea nitrogen levels. During the experimental period, diets with 0.2% and 0.5% fermented FM showed a reduction in Salmonella enterica and Escherichia coli populations which was also an indication of better gut health (H. J. Lee et al. Citation2017). Pigs, especially pregnant sows, are very sensitive to a lack of protein and good combinations of essential amino acids are very essential for their health. The fermented fish processing waste as a valuable nutrient source was tried in sows feeding by Nikulin, Nikulina, and Tsoy (Citation2021). They found that fermented fish paste based diet (fermentation process not mentioned) at a dose of 3 and 6.2% in feeding sows had a positive effect on their reproductive qualities, which allowed to increase the multiplicity of sows’ livestock by 3.8–9.7%, large-scale fertility by 1.7–5%, the average daily growth of piglets by 5.4–7.8%, and increase the safety of new born piglets by 2.2–4.4%. The results related to use of FPH, levels of incorporation of FPH and fermented fish waste in pig performance is inconsistent which requires more systematic studies and approaches.
Application of FPH in broiler/other bird feed
Fishmeal is an excellent source of nutrient in poultry feed and the inclusion is still recommended at 2– 10% in poultry diets (IFFO). The increasing cost of FM and presence of trimethylamine in fish meal which creates a residual fish smell and flavor in meat and eggs are the reasons of FM replacement from poultry diet (Leeson and Summers Citation2009). The use of fish silage as low-cost alternative to FM has also been studied in poultry diets (Johnson et al. Citation1985). However, replacement of soybean meal (SBM) with fermented fish waste and other plant ingredients are more recent trend. There is a huge reliance on soybean meal by poultry feed. The cost of soybean meal has increased manifold in recent years (The Economic Times). SBM, after fishmeal, is the most expensive ingredient in animal and aquafeed. The incorporation of fermented ingredients in broiler feed positively influences the gut health and production parameters (R. Zhang et al. Citation2016). Generally, the fermented feed contains high number of lactic acid bacteria (LAB), a low pH and a high concentration of organic acids (Canibe and Jensen Citation2012) which is beneficial in terms of protecting the feed from pathogen contamination, gut health, growth and development of birds (Missotten et al. Citation2013; Niba et al. Citation2009; Xie et al. Citation2016). In broiler chickens, replacing a part of SBM with fermented fish waste in the diet ensure improved growth performance and carcass characteristics (Shabani et al. Citation2016). Fish silage is a good source of highly available amino acids which helps for better growth and feed efficiency in young growing chickens (Panda et al. Citation2017). Enhanced growth performance, protein, and ether extract digestibility; increased activity of digestive enzymes and cecal beneficial bacteria populations; enhanced cecal short chain fatty acids (SCFA) contents (especially lactic acid and butyrate) and reduced excreta ammonia emission and cecal pathogenic bacteria populations in broiler chicks were reported by Shabani et al. (Citation2019) feeding fermented fish waste silage as a substitute of SBM. Similar observation was also reported by Shabani et al. (Citation2018) and Al-Marzooqi et al. (Citation2010), sardine fish silage replaced SBM with improved performance of broiler chickens and stated that sardine fish silage had a higher digestibility coefficient of amino acids relative to SBM. Similarly, various experiments have shown that fermented products could improve broiler performance (Ashayerizadeh et al. Citation2017; Chiang et al. Citation2009; Jazi et al. Citation2017; H. Sun et al. Citation2013). Lohmen brown chicken fed with 6% formic acid fermented fish silage adding into a commercial feed increased early egg lying, feed intake, body weight gain, egg production and average white eggs (Abelti Citation2018). Similarly, Opheim et al. (Citation2016) found that addition of SPH (salmon protein hydrolysate) 5 and 10% in the starter diet increased broiler chicken growth performance compared with either a plant protein-based diet or a FM diet. The inclusion of SPH in broiler chicken starter diet increased the villi height in the duodenum and ileum in comparison to plant protein diet. The early development of small intestine is an advantage for nutrient absorption thus growth performance at older ages of broiler birds (Wijtten et al. Citation2010).A study conducted by Biotai et al. found that broiler chicken fed with acid treated fish silage up to 10% did not show body weight gain. However, during starter phase, the birds exhibited improved feed conversion (FC) due to the incorporation of fish silage at both 5 and 10% however; no change in FCR was recorded in later growth stages. Ambarwati and Syah (Citation2022) evaluated the effect of dietary supplementation of flying fish silage on egg quality and duck performance. The results showed that 10% addition of flying fish silage to duck basal feed did not decrease egg quality and duck performance. The authors anticipated by increasing fish silage incorporation could increase egg quality and duck performance. The higher fish silage inclusion level was tried by Taufikrrahman et al. in duck ration and found that fermented fish waste can be used up to 20% without negative effects on interior quality of egg. A study was conducted by Tanuja et al. (Citation2017) by supplementing acid ensiled fish waste on production performance, egg quality and serum biochemistry in layer Japanese quail (Coturnix japonica). Acid ensiled silage was prepared from visceral waste (gut and gills) of freshwater fishes using 1.5% formic acid (v/w) and 1.5% hydrochloric acid (HCl). The results shows that acid ensiled fish waste could be used as a protein supplement in diet of layer Japanese quail birds up to a level of 12% without affecting their production performance and quality of eggs. Lipids that are recovered from the fish processing by products by lactic acid fermentation can be an alternative to vegetable oil and commercial fish oil and can be utilized as a feed component without compromising the growth performance and meat quality of broilers (Muhammed et al. Citation2015).
Ruminants
Fishmeal is not considered a major source of nutrient in the feed of ruminants. Inclusion level of FM in terrestrial livestock diets is usually remains below 5% on a dry matter basis (Cho and Kim Citation2011). However, the supplementation of FM has benefits in terms of improved milk yield and quality (Atwal and Erfle Citation1992; Koushki et al. Citation2019), fertility (Staples and Funge-Smith Citation2005), enhanced ruminal fermentation (Allison and Garnsworthy Citation2002), faster growth (Atti, Mahouachi, and Rouissi Citation2007), and meat enrichment (Mandell et al. Citation1997). The dietary protein in ruminants is degraded in to amino acids and ammonia and by ruminal microorganism then it is converted in to microbial protein (MCP) (Z. Lu et al. Citation2019). The higher MCP synthesis facilitates animal performance, reduces the protein waste and increases milk production. The protein which by passes the ruminal digestion or undegradable protein (UDP) reaches the intestine unmodified and digested by enzymatic hydrolysis. The UDP provides a direct source of amino acids for growth and metabolism of the ruminants. A lot of energy is saved in this process otherwise microbes need sufficient energy to synthesize MCP (Hackmann and Firkins Citation2015). Fish hydrolysate is a very good protein source but unlike FM it is easily degraded in the rumen as it is partially hydrolyzed and rich in peptides. The amino acids and peptides from the fish hydrolysate help the fibrolytic bacteria to grow and can better utilize fiber fractions in the diet. Ouellet et al. (Citation1997) compared the effect of supplementing grass silage with FM and FPH on growth, diet digestibility and rumen fermentation in beef cattle. The FM fed group showed 25% increased daily gains in comparison 11% with FPH. This suggests that the protein from the hydrolysate is wasted in the rumen due to high solubility. However, sheep fed with Lactobacillus plantarum fermented fish waste showed a net increase in weight in comparison to control diet (40% barley and 60% wheat bran) and enhancement of meat characteristics and carcass shape Rahmi et al. Citation2008). Dietary FM increased milk production with health fatty acids in cow was observed by Atwal and Erfle, (Citation1992). Cows fed with 1, 2, and 3% FM in their diet did not show more milk production, fat percentage and fat content of milk also were not affected by the diet composition. However, significant difference was observed than the control diet (Yazdani Citation2011). This is understood that when FM is utilized in cattle feed then fish silage or FPH hydrolysate could potentially be utilized for cattle performance.
Conclusion
The application of fermentation technology as a biorefining tool for recovering valuable nutrients from seafood side streams is a promising approach to address the issue of waste management and sustainable resource utilization in the seafood industry. This technology has been demonstrated to be effective in recovering high-value compounds such as proteins, lipids, and bioactive molecules from various seafood by-products, which can be further processed into various functional food and nutraceutical products. Fermentation technology offers several advantages over traditional chemical methods, including high selectivity, mild reaction conditions, and minimal environmental impact. Moreover, the use of microbial fermentation can provide a cost-effective and sustainable approach for recovering valuable compounds from seafood waste streams, as it can use low-cost substrates and operate at ambient temperatures and pressures. Several studies have demonstrated the potential of fermentation technology in extracting valuable compounds from different seafood by-products, such as fish skin, fish bones, and shrimp shells. For example, fish skin has been shown to be a rich source of collagen, which has been extracted through microbial fermentation and used as a functional food ingredient. Similarly, shrimp shells have been utilized as a source of chitin and chitosan, which have various applications in the food and pharmaceutical industries.
In addition to extracting valuable compounds, fermentation technology can also be used to produce value-added products from seafood by-products. For instance, several studies have reported the use of fish waste as a substrate for the production of biogas and biofuels through anaerobic digestion and microbial fermentation. This approach can provide a sustainable and cost-effective alternative to traditional fossil fuels, which are finite and contribute to environmental degradation. Moreover, the use of fermentation technology for recovering valuable compounds from seafood waste streams can contribute to the circular economy concept, which aims to minimize waste and maximize resource utilization. By extracting and utilizing high-value compounds from seafood by-products, fermentation technology can reduce the environmental impact of the seafood industry, while creating new economic opportunities and value chains. However, there are also some challenges associated with the application of fermentation technology in the seafood industry. One of the main challenges is the diversity of seafood waste streams, which vary in their composition and properties. Therefore, developing customized fermentation processes for each waste stream can be time-consuming and resource-intensive. Additionally, the scale-up of fermentation processes from laboratory to industrial scale can also be challenging, as it requires optimization of various parameters, such as substrate concentration, fermentation time, and reactor design.
Another challenge is the competition for substrates between different microbial species, which can lead to reduced yields and selectivity of the fermentation process. Therefore, identifying and optimizing the growth conditions for the desired microbial strains can be crucial for the success of the fermentation process. Despite these challenges, the potential benefits of fermentation technology as a biorefining tool for recovering valuable nutrients from seafood side streams outweigh the limitations. This technology has the potential to contribute to a more sustainable and circular seafood industry, while creating new economic opportunities and reducing the environmental impact of seafood waste disposal. In conclusion, the application of fermentation technology for recovering valuable compounds from seafood waste streams is a promising approach for addressing the challenges of waste management and sustainable resource utilization in the seafood industry. However, further research is needed to optimize the fermentation processes and scale up the technology for industrial applications. Moreover, promoting the utilization of fermented seafood by-products as value-added ingredients in various food and nutraceutical products can also enhance the economic viability of the technology. Overall, the application of fermentation technology as a biorefining tool for recovering valuable nutrients from seafood side streams can contribute to a more sustainable and circular seafood industry, while creating new economic opportunities and reducing the environmental impact of seafood waste disposal.
Author contributions
S.A.S.—Validation, Formal Analysis, Resources, Writing—Original Draft, Writing—Review and Editing, Visualization, Data Curation, Project administration, Methodology, Investigation, Supervision. D.L.—Writing—Original Draft. C.P.—Investigation, Writing—Original Draft. Z.F.—Writing—Original Draft. R.C-M.—Formal Analysis, Funding. A.S.—Conceptualization, Writing—Review and Editing, Supervision.
Acknowledgement
Financial support from Nobelium Joining Gdańsk Tech Research Community (contract number DEC 33/2022/IDUB/l.1; NOBELIUM nr 036236) is gratefully acknowledged. R. Castro-Muñoz also acknowledges the School of Engineering and Science and the FEMSA-Biotechnology Center at Tecnológico de Monterrey for their support through the Bioprocess (0020209I13) Focus Group.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abd Rashid, N. Y., M. A. Manan, K. F. Pa’ee, N. Saari, and F. W. Faizal Wong. 2022. Evaluation of antioxidant and antibacterial activities of fish protein hydrolysate produced from Malaysian fish sausage (Keropok Lekor) by-products by indigenous Lactobacillus casei fermentation. Journal of Cleaner Production 347:131303. doi: 10.1016/j.jclepro.2022.131303.
- Abelti, A. L. 2018. Evaluation of small barbus silage through inclusion into commercially formulated poultry feed. International Journal of Poultry and Fisheries Sciences 2 (1):1–7. doi: 10.15226/2578-1898/2/1/00105.
- Abuine, R., A. U. Rathnayake, and H. G. Byun. 2019. Biological activity of peptides purified from fish skin hydrolysates. Fisheries and Aquatic Sciences 22 (1):1–14. doi: 10.1186/s41240-019-0125-4.
- Abu Yazid, N., R. Barrena, D. Komilis, and A. Sánchez. 2017. Solid-state fermentation as a novel paradigm for organic waste valorisation: A review. Sustainability 9 (2):224. doi: 10.3390/su9020224.
- Afonso, C., N. M. Bandarra, L. Nunes, and C. Cardoso. 2016. Tocopherols in seafood and aquaculture products. Critical Reviews in Food Science and Nutrition 56 (1):128–40. doi: 10.1080/10408398.2012.694920.
- Ahmed, R., A. T. Getachew, Y. J. Cho, and B. S. Chun. 2018. Application of bacterial collagenolytic proteases for the extraction of type I collagen from the skin of bigeye tuna (Thunnus obesus). LWT 89:44–51. doi: 10.1016/j.lwt.2017.10.024.
- Ahmed, S., H. Mirzaei, M. Aschner, A. Khan, A. Al-Harrasi, and H. Khan. 2021. Marine peptides in breast cancer: Therapeutic and mechanistic understanding. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie 142:112038. doi: 10.1016/j.biopha.2021.112038.
- Ahn, C. B., Y. J. Jeon, Y. T. Kim, and J. Y. Je. 2012. Angiotensin I converting enzyme (ACE) inhibitory peptides from salmon byproduct protein hydrolysate by Alcalase hydrolysis. Process Biochemistry 47 (12):2240–5. doi: 10.1016/j.procbio.2012.08.019.
- Allegretti, C., E. Bellinetto, P. D’Arrigo, G. Griffini, S. Marzorati, L. A. M. Rossato, E. Ruffini, L. Schiavi, S. Serra, A. Strini, et al. 2022. Towards a complete exploitation of Brewers’ spent grain from a circular economy perspective. Fermentation 8 (4):151. doi: 10.3390/fermentation8040151.
- Allison, R. D., and P. C. Garnsworthy. 2002. Increasing the digestible undegraded protein intake of lactating dairy cows by feeding fishmeal or a rumen protected vegetable protein blend. Animal Feed Science and Technology 96 (1-2):69–81. doi: 10.1016/S0377-8401(01)00331-5.
- Al-Marzooqi, W., M. A. Al-Farsi, I. T. Kadim, O. Mahgoub, and J. S. Goddard. 2010. The effect of feeding different levels of sardine fish silage on broiler performance, meat quality and sensory characteristics under closed and open-sided housing systems. Asian-Australasian Journal of Animal Sciences 23 (12):1614–25. doi: 10.5713/ajas.2010.10119.
- Ambarwati, L., and S. P. Syah. 2022. Effect of dietary supplementation with flying fish (exocoetidae) silage on the egg quality and duck performance. IOP Conference Series: Earth and Environmental Science 1041 (1):012078. doi: 10.1088/1755-1315/1041/1/012078.
- Ananey-Obiri, D., L. G. Matthews, and R. Tahergorabi. 2019. Proteins from fish processing by-products. In C. M. Galanakis (Ed.) Proteins: Sustainable source, processing, and applications, 163–91. Academic Press. doi: 10.1016/B978-0-12-816695-6.00006-4.
- Arruda, L. F. D., R. Borghesi, A. Brum, M. R. D’Arce, and M. Oetterer. 2006. Nutritional aspects of Nile tilapia (Oreochromis niloticus) silage. Ciência e Tecnologia de Alimentos 26 (4):749–53. doi: 10.1590/S0101-20612006000400006.
- Arruda, L. F. D., R. Borghesi, and M. Oetterer. 2007. Use of fish waste as silage: A review. Brazilian Archives of Biology and Technology 50 (5):879–86. doi: 10.1590/S1516-89132007000500016.
- Arruebo, M., N. Vilaboa, B. Sáez-Gutierrez, J. Lambea, A. Tres, M. Valladares, and Á. González-Fernández. 2011. Assessment of the evolution of cancer treatment therapies. Cancers 3 (3):3279–330. doi: 10.3390/cancers3033279.
- Ashayerizadeh, A., B. Dastar, M. S. Shargh, A. S. Mahoonak, and S. Zerehdaran. 2017. Fermented rapeseed meal is effective in controlling Salmonella enterica serovar Typhimurium infection and improving growth performance in broiler chicks. Veterinary Microbiology 201:93–102. doi: 10.1016/j.vetmic.2017.01.007.
- Atti, N., M. Mahouachi, and H. Rouissi. 2007. Effects of fish meal in lamb diets on growth performance, carcass characteristics and subcutaneous fatty acid composition. Options Mediterraneenes, Series A 74:57–61.
- Atwal, A. S., and J. D. Erfle. 1992. Effects of feeding fish meal to cows on digestibility, milk production, and milk composition. Journal of Dairy Science 75 (2):502–507. doi: 10.3168/jds.S0022-0302(92)77787-X.
- Baco, N., S. N. H. Oslan, R. Shapawi, R. A. M. Mohhtar, W. N. M. Noordin, and N. Huda. 2022. Antibacterial activity of functional bioactive peptides derived from fish protein hydrolysate. IOP Conference Series: Earth and Environmental Science 967 (1):012019. doi: 10.1088/1755-1315/967/1/012019.
- Bingtong, L., Z. Yongliang, and S. Liping. 2020. Identification and characterization of the peptides with calcium‐binding capacity from tilapia (Oreochromis niloticus) skin gelatin enzymatic hydrolysates. Journal of Food Science 85 (1):114–22. doi: 10.1111/1750-3841.14975.
- Bruno, B. J., G. D. Miller, and C. S. Lim. 2013. Basics and recent advances in peptide and protein drug delivery. Therapeutic Delivery 4 (11):1443–67. doi: 10.4155/tde.13.104.
- Bruno, S. F., F. J. Ekorong, S. S. Karkal, M. S. Cathrine, and T. G. Kudre. 2019. Green and innovative techniques for recovery of valuable compounds from seafood by-products and discards: A review. Trends in Food Science & Technology 85:10–22. doi: 10.1016/j.tifs.2018.12.004.
- Bui, H. T. D., S. Khosravi, V. Fournier, M. Herault, and K. J. Lee. 2014. Growth performance, feed utilization, innate immunity, digestibility, and disease resistance of juvenile red seabream (Pagrus major) fed diets supplemented with protein hydrolysates. Aquaculture 418-419:11–6. doi: 10.1016/j.aquaculture.2013.09.046.
- Cabanillas-Bojórquez, L. A., E. P. Gutiérrez-Grijalva, R. I. Castillo-López, L. A. Contreras-Angulo, M. A. Angulo-Escalante, L. X. López-Martínez, E. Y. Rios-Iribe, and J. B. Heredia. 2021. Bioprocessing of shrimp waste using novel industrial by-products: effects on nutrients and lipophilic antioxidants. Fermentation 7 (4):312. doi: 10.3390/fermentation7040312.
- Cai, Z., W. Li, K. Mai, W. Xu, Y. Zhang, and Q. Ai. 2015. Effects of dietary size-fractionated fish hydrolysates on growth, activities of digestive enzymes and aminotransferases and expression of some protein metabolism related genes in large yellow croaker (Larimichthys crocea) larvae. Aquaculture 440:40–7. doi: 10.1016/j.aquaculture.2015.01.026.
- Canibe, N., and B. B. Jensen. 2012. Fermented liquid feed—Microbial and nutritional aspects and impact on enteric diseases in pigs. Animal Feed Science and Technology 173 (1-2):17–40. doi: 10.1016/j.anifeedsci.2011.12.021.
- Chai, W. Y., K. T. K. Teo, M. K. Tan, and H. J. Tham. 2022. Fermentation process control and optimization. Chemical Engineering & Technology 45 (10):1731–47. doi: 10.1002/ceat.202200029.
- Chandrasekharan, M. 2015. Biotechnology for utilization of marine byproducts. In Fish processing byproducts, ed. N. M. Sachindra and N. S. Mahendrakar, 43–62. Houston, TX: Studium Press.
- Chaudhary, A., S. Bhalla, S. Patiyal, G. P. Raghava, and G. Sahni. 2021. FermFooDb: A database of bioactive peptides derived from fermented foods. Heliyon 7 (4):e06668. doi: 10.1016/j.heliyon.2021.e06668.
- Chevrier, G., P. L. Mitchell, L. E. Rioux, F. Hasan, T. Jin, C. R. Roblet, A. Doyen, G. Pilon, P. St-Pierre, C. Lavigne, et al. 2015. Low-molecular-weight peptides from salmon protein prevent obesity-linked glucose intolerance, inflammation, and dyslipidemia in LDLR−/−/ApoB100/100 mice. The Journal of Nutrition 145 (7):1415–22. doi: 10.3945/jn.114.208215.
- Chiang, G., W. Q. Lu, X. S. Piao, J. K. Hu, L. M. Gong, and P. A. Thacker. 2009. Effects of feeding solid-state fermented rapeseed meal on performance, nutrient digestibility, intestinal ecology, and intestinal morphology of broiler chickens. Asian-Australasian Journal of Animal Sciences 23 (2):263–71. doi: 10.5713/ajas.2010.90145.
- Cho, J. H., and I. H. Kim. 2011. Fish meal–nutritive value. Journal of Animal Physiology and Animal Nutrition 95 (6):685–92. doi: 10.1111/j.1439-0396.2010.01109.x.
- Choksawangkarn, W., S. Phiphattananukoon, J. Jaresitthikunchai, and S. Roytrakul. 2018. Antioxidative peptides from fish sauce by-product: Isolation and characterization. Agriculture and Natural Resources 52 (5):460–6. doi: 10.1016/j.anres.2018.11.001.
- Coimbra, R. S. T. 2016. Marine by-products in Portugal: Sources, actual processing and alternative valorisation. Porto, Portugal: Universidade Do Algarve.
- Dahiya, S., A. N. Kumar, J. S. Sravan, S. Chatterjee, O. Sarkar, and S. V. Mohan. 2018. Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresource Technology 248 (Pt A):2–12. doi: 10.1016/j.biortech.2017.07.176.
- Da Rocha, M., A. Alemán, G. C. Baccan, M. E. López-Caballero, C. Gómez-Guillén, P. Montero, and C. Prentice. 2018. Anti-inflammatory, antioxidant, and antimicrobial effects of underutilized fish protein hydrolysate. Journal of Aquatic Food Product Technology 27 (5):592–608. doi: 10.1080/10498850.2018.1461160.
- Das, S., C. Pradhan, and D. Pillai. 2022. β-Defensin: An adroit saviour in teleosts. Fish & Shellfish Immunology 123:417–30. doi: 10.1016/j.fsi.2022.03.017.
- Dessie, W., X. Luo, M. Wang, L. Feng, Y. Liao, and Z. Wang. 2020. Current advances on waste biomass transformation into value-added products. Applied Microbiology and Biotechnology 104 (11):5647–63.
- Donzella, S., A. Fumagalli, S. Arioli, L. Pellegrino, P. D’Incecco, F. Molinari, G. Speranza, D. Ubiali, M. S. Robescu, and C. Compagno. 2022. Recycling food waste and saving water: optimization of the fermentation processes from cheese whey permeate to yeast oil. Fermentation 8 (7):341. doi: 10.3390/fermentation8070341.
- Dumay, J., C. Barthomeuf, and J. P. Berge. 2004. How enzymes may be helpful for upgrading fish by-products enhancement of fat extraction. Journal of Aquatic Food Product Technology 13 (2):69–84. doi: 10.1300/J030v13n02_07.
- Egerton, S., A. Wan, K. Murphy, F. Collins, G. Ahern, I. Sugrue, K. Busca, F. Egan, N. Muller, J. Whooley, et al. 2020. Replacing fishmeal with plant protein in Atlantic salmon (Salmo salar) diets by supplementation with fish protein hydrolysate. Scientific Reports 10 (1):4194. doi: 10.1038/s41598-020-60325-7.
- Elavarasan, K. 2019. Health benefits and potential applications of fish protein hydrolysate. ICAR-Central Institute of Fisheries Technology. India.
- Ennaas, N., R. Hammami, L. Beaulieu, and I. Fliss. 2015. Purification and characterization of four antibacterial peptides from protamex hydrolysate of Atlantic mackerel (Scomber scombrus) by-products. Biochemical and Biophysical Research Communications 462 (3):195–200. doi: 10.3390/md19020116.
- Esakkiraj, P., G. Austin Jeba Dhas, A. Palavesam, and G. Immanuel. 2010. Media preparation using tuna-processing wastes for improved lipase production by shrimp gut isolate Staphylococcus epidermidis CMST Pi 2. Applied Biochemistry and Biotechnology 160 (4):1254–65. doi: 10.1007/s12010-009-8632-x.
- Fan, Z., D. Wu, J. Li, Y. Zhang, Z. Cui, T. Li, X. Zheng, H. Liu, L. Wang, and H. Li. 2022. Assessment of fish protein hydrolysates in juvenile largemouth bass (Micropterus salmoides) diets: Effect on growth, intestinal antioxidant status, immunity, and microflora. Frontiers in Nutrition 9:816341. doi: 10.3389/fnut.2022.816341.
- Fang, B., J. Sun, P. Dong, C. Xue, and X. Mao. 2017. Conversion of turbot skin wastes into valuable functional substances with an eco-friendly fermentation technology. Journal of Cleaner Production 156:367–77. doi: 10.1016/j.jclepro.2017.04.055.
- Gao, R., Q. Yu, Y. Shen, Q. Chu, G. Chen, S. Fen, M. Yang, L. Yuan, D. J. McClements, and Q. Sun. 2021. Production, bioactive properties, and potential applications of fish protein hydrolysates: Developments and challenges. Trends in Food Science & Technology 110:687–99. doi: 10.1016/j.tifs.2021.02.031.
- Ghorbel-Bellaaj, O., H. Maalej, M. Nasri, and K. Jellouli. 2018. Fermented shrimp waste hydrolysates: Promising source of functional molecules with antioxidant properties. Journal of Culinary Science & Technology 16 (4):357–77. doi: 10.1080/15428052.2017.1394950.
- Gingras, D., D. Boivin, C. Deckers, S. Gendron, C. Barthomeuf, and R. Béliveau. 2003. Neovastat—A novel antiangiogenic drug for cancer therapy. Anti-Cancer Drugs 14 (2):91–6. doi: 10.1097/00001813-200302000-00001.
- Giri, A., M. Nasu, and T. Ohshima. 2012. Bioactive properties of Japanese fermented fish paste, fish miso, using koji inoculated with Aspergillus oryzae. International Journal of Nutrition and Food Sciences 1 (1):13–22. doi: 10.11648/j.ijnfs.20120102.12.
- Godinho, I., C. Pires, S. Pedro, B. Teixeira, R. Mendes, M. L. Nunes, and I. Batista. 2016. Antioxidant properties of fish protein hydrolysates prepared from cod protein hydrolysate by Bacillus sp. Applied Biochemistry and Biotechnology 178 (6):1095–112. doi: 10.1007/s12010-015-1931-5.
- González-Camejo, J., C. Andreola, V. Maceratesi, G. Toscano, A. L. Eusebi, and F. Fatone. 2023. Biorefineries to improve water and resource recovery in the seafood-processing industry. In A. Basile, A. Cassano, & C. Conidi (Eds.), Advanced Technologies in Wastewater Treatment, 127–54. Elsevier.
- Goosen, N. J., L. F. de Wet, and J. F. Görgens. 2014. The effects of protein hydrolysates on the immunity and growth of the abalone Haliotis midae. Aquaculture 428-429:243–8. doi: 10.1016/j.aquaculture.2014.03.018.
- Gottardi, D., M. Ciccone, L. Siroli, R. Lanciotti, and F. Patrignani. 2022. Use of Yarrowia lipolytica to obtain fish waste functional hydrolysates rich in flavoring compounds. Fermentation 8 (12):708. doi: 10.3390/fermentation8120708.
- Gu, H., J. Du, F. Carnevale Neto, P. A. Carroll, S. J. Turner, E. G. Chiorean, R. N. Eisenman, and D. Raftery. 2015. Metabolomics method to comprehensively analyze amino acids in different domains. The Analyst 140 (8):2726–34. doi: 10.1039/c4an02386b.
- Gu, Y., K. Majumder, and J. Wu. 2011. QSAR-aided in silico approach in evaluation of food proteins as precursors of ACE inhibitory peptides. Food Research International 44 (8):2465–74. doi: 10.1016/j.foodres.2011.01.051.
- Guo, N., J. Sun, Z. Zhang, and X. Mao. 2019. Recovery of chitin and protein from shrimp head waste by endogenous enzyme autolysis and fermentation. Journal of Ocean University of China 18 (3):719–26. doi: 10.1007/s11802-019-3867-9.
- Hackmann, T. J., and J. L. Firkins. 2015. Maximizing efficiency of rumen microbial protein production. Frontiers in Microbiology 6:465. doi: 10.3389/fmicb.2015.00465.
- Halim, N. R. A., A. Azlan, H. M. Yusof, and N. M. Sarbon. 2018. Antioxidant and anticancer activities of enzymatic eel (Monopterus sp) protein hydrolysate as influenced by different molecular weight. Biocatalysis and Agricultural Biotechnology 16:10–6. doi: 10.1016/j.bcab.2018.06.006.
- Harikrishna, N., S. Mahalakshmi, K. Kiran Kumar, and G. Reddy. 2017. Fish scales as potential substrate for production of alkaline protease and amino acid rich aqua hydrolyzate by Bacillus altitudinis GVC11. Indian Journal of Microbiology 57 (3):339–43. doi: 10.1007/s12088-017-0664-2.
- Higuera-Ciapara, I., L. Felix-Valenzuela, and F. M. Goycoolea. 2006. Astaxanthin: A review of its chemistry and applications. Critical Reviews in Food Science and Nutrition 46 (2):185–96. doi: 10.1080/10408690590957188.
- Hoelscher, M. P., J. Forner, S. Calderone, C. Krämer, Z. Taylor, F. V. Loiacono, S. Agrawal, D. Karcher, F. Moratti, X. Kroop, et al. 2022. Expression strategies for the efficient synthesis of antimicrobial peptides in plastids. Nature Communications 13 (1):5856. doi: 10.1038/s41467-022-33516-1.
- Hsieh, C. H., T. Y. Wang, C. C. Hung, M. C. Chen, and K. C. Hsu. 2015. Improvement of glycemic control in streptozotocin-induced diabetic rats by Atlantic salmon skin gelatin hydrolysate as the dipeptidyl-peptidase IV inhibitor. Food & Function 6 (6):1887–92. doi: 10.1039/C5FO00124B.
- Hu, J., J. Luo, Z. Zhu, B. Chen, X. Ye, P. Zhu, and B. Zhang. 2021. Multi-scale biosurfactant production by Bacillus subtilis using tuna fish waste as substrate. Catalysts 11 (4):456. doi: 10.3390/catal11040456.
- Hua, K., J. M. Cobcroft, A. Cole, K. Condon, D. R. Jerry, A. Mangott, C. Praege, M. J. Vucko, C. Zeng, K. Zenge, et al. 2019. The future of aquatic protein: Implications for protein sources in aquaculture diets. One Earth 1 (3):316–29. doi: 10.1016/j.oneear.2019.10.018.
- Huan, Y., Q. Kong, H. Mou, and H. Yi. 2020. Antimicrobial peptides: Classification, design, application, and research progress in multiple fields. Frontiers in Microbiology 11:582779. doi: 10.3389/fmicb.2020.582779.
- Irm, M., S. Taj, M. Jin, H. J. T. Andriamialinirina, X. Cheng, and Q. Zhou. 2020. Influence of dietary replacement of fish meal with fish soluble meal on growth and TOR signaling pathway in juvenile black sea bream (Acanthopagrus schlegelii). Fish & Shellfish Immunology 101:269–76. doi: 10.1016/j.fsi.2020.03.053.
- Jazi, V., F. Boldaji, B. Dastar, S. R. Hashemi, and A. Ashayerizadeh. 2017. Effects of fermented cottonseed meal on the growth performance, gastrointestinal microflora population and small intestinal morphology in broiler chickens. British Poultry Science 58 (4):402–8. doi: 10.1080/00071668.2017.1315051.
- Jemil, I., O. Abdelhedi, R. Nasri, L. Mora, M. Jridi, M. C. Aristoy, F. Toldrá, and M. Nasri. 2017. Novel bioactive peptides from enzymatic hydrolysate of Sardinelle (Sardinella aurita) muscle proteins hydrolysed by Bacillus subtilis A26 proteases. Food Research International 100 (Pt 1):121–33. doi: 10.1016/j.foodres.2017.06.018.
- Jemil, I., M. Jridi, R. Nasri, N. Ktari, R. B. S.-B. Salem, M. Mehiri, M. Hajji, and M. Nasri. 2014. Functional, antioxidant and antibacterial properties of protein hydrolysates prepared from fish meat fermented by Bacillus subtilis A26. Process Biochemistry 49 (6):963–72. doi: 10.1016/j.procbio.2014.03.004.
- Johnson, R. J., N. Brown, P. Eason, and J. Sumner. 1985. The nutritional quality of two types of fish silage for broiler chickens. Journal of the Science of Food and Agriculture 36 (11):1051–6. doi: 10.1002/jsfa.2740361105.
- Kang, H. K., M. C. Choi, C. H. Seo, and Y. Park. 2018. Therapeutic properties and biological benefits of marine-derived anticancer peptides. International Journal of Molecular Sciences 19 (3):919. doi: 10.3390/ijms19030919.
- Kang, H. K., H. H. Lee, C. H. Seo, and Y. Park. 2019. Antimicrobial and immunomodulatory properties and applications of marine-derived proteins and peptides. Marine Drugs 17 (6):350. doi: 10.3390/md17060350.
- Karimi, S., N. M. Soofiani, A. Mahboubi, J. A. Ferreira, T. Lundh, A. Kiessling, and M. J. Taherzadeh. 2021. Evaluation of nutritional composition of pure filamentous fungal biomass as a novel ingredient for fish feed. Fermentation 7 (3):152. doi: 10.3390/fermentation7030152.
- Khieokhajonkhet, A., and K. Surapon. 2020. Effects of fish protein hydrolysate on the growth performance, feed, and protein utilization of Nile tilapia (Oreochromis niloticus). International Journal of Agricultural Technology 16 (3):641–54.
- Khositanon, P., D. Inpratom, T. Somwang, P. Iawsipo, S. Roytrakul, and W. Choksawangkarn. 2018. Antibacterial and anticancer activities of protein hydrolysate from fish sauce byproduct. In 6th International Conference on Biochemistry and Molecular Biology, 20–2. https://www.scisoc.or.th/BMBThailand/images/BMB2018/S2-P-15.pdf
- Khosravi, S., S. Rahimnejad, M. Herault, V. Fournier, C. R. Lee, H. T. D. Bui, J. B. Jeong, and K. J. Lee. 2015. Effects of protein hydrolysates supplementation in low fish meal diets on growth performance, innate immunity, and disease resistance of red sea bream Pagrus major. Fish & Shellfish Immunology 45 (2):858–68. doi: 10.1016/j.fsi.2015.05.039.
- Kim, S. K., and E. Mendis. 2006. Bioactive compounds from marine processing by-products- A review. Food Research International. 39 (4):383–93. doi: 10.1016/j.foodres.2005.10.010.
- Koepke, J. R., R. S. Kaushik, W. R. Gibbons, M. Brown, and C. L. Levesque. 2017. Evaluation of a bioprocessed soybean meal on nursery pig performance and immune status. Journal of Animal Science 95 (11):5030–9. doi: 10.2527/jas2017.1679.
- Koushki, R., H. Mansoori Yarahmadi, M. Khaldari, J. Fakhraei, and K. Karkoodi. 2019. Milk yield and blood metabolite profile in late pregnancy in Lori ewes receiving diets containing undegradable protein sources. Iranian Journal of Applied Animal Science 9 (4):643–50.
- Kuley, E., G. Özyurt, I. Özogul, M. Boga, I. Akyol, J. M. Rocha, and F. Özogul. 2020. The role of selected lactic acid bacteria on organic acid accumulation during wet and spray-dried fish-based silages. Contributions to the winning combination of microbial food safety and environmental sustainability. Microorganisms 8 (2):172. doi: 10.3390/microorganisms8020172.
- Kumar, A., D. Kumar, N. George, P. Sharma, and N. Gupta. 2018. A process for complete biodegradation of shrimp waste by a novel marine isolate Paenibacillus sp. AD with simultaneous production of chitinase and chitin oligosaccharides. International Journal of Biological Macromolecules 109:263–72. doi: 10.1016/j.ijbiomac.2017.12.024.
- Kusumaningtyas, E., M. Nurilmala, and D. Sibarani. 2019. Antioxidant and antifungal activities of collagen hydrolysates from skin of milkfish (Chanos chanos) hydrolyzed using various bacillus proteases. IOP Conference Series: Earth and Environmental Science 278 (1):012040. doi: 10.1088/1755-1315/278/1/012040.
- Kwasek, K., C. Gonzalez, M. Wick, G. S. Molinari, and M. Wojno. 2021. Fish muscle hydrolysate obtained using largemouth bass Micropterus salmoides digestive enzymes improves largemouth bass performance in its larval stages. PLoS One 16 (12):e0261847. doi: 10.1371/journal.pone.0261847.
- Lassoued, I., L. Mora, R. Nasri, M. Aydi, F. Toldrá, M. C. Aristoy, A. Barkia, and M. Nasri. 2015. Characterization, antioxidative and ACE inhibitory properties of hydrolysates obtained from thornback ray (Raja clavata) muscle. Journal of Proteomics 128:458–68. doi: 10.1016/j.jprot.2015.05.007.
- Lee, D.-H., C. T. Doan, T. N. Tran, V. B. Nguyen, A. D. Nguyen, C.-L. Wang, and S.-L. Wang. 2021. Proteases production and chitin preparation from the liquid fermentation of chitinous fishery by-products by Paenibacillus elgii. Marine Drugs 19 (9):477. doi: 10.3390/md19090477.
- Lee, H. J., I. H. Choi, D. H. Kim, Y. H. Joo, and S. C. Kim. 2017. Influence of fermented fish meal supplementation on growth performance, blood metabolites, and fecal microflora of weaning pigs. Revista Brasileira de Zootecnia 46 (5):433–7. doi: 10.1590/s1806-92902017000500010.
- Leeson, S., and J. D. Summers. 2009. Commercial poultry nutrition. 3rd ed. Nottingham: Nottingham University Press.
- Leong, H. Y., C.-K. Chang, K. S. Khoo, K. W. Chew, S. R. Chia, J. W. Lim, J.-S. Chang, and P. L. Show. 2021. Waste biorefinery towards a sustainable circular bioeconomy: A solution to global issues. Biotechnology for Biofuels 14 (1):87. doi: 10.1186/s13068-021-01939-5.
- Lin, Y., X. Cai, X. Wu, S. Lin, and S. Wang. 2020. Fabrication of snapper fish scales protein hydrolysate-calcium complex and the promotion in calcium cellular uptake. Journal of Functional Foods 65:103717. doi: 10.1016/j.jff.2019.103717.
- Liu, R., J. Cheng, and H. Wu. 2019. Discovery of food-derived dipeptidyl peptidase IV inhibitory peptides: A review. International Journal of Molecular Sciences 20 (3):463. doi: 10.3390/ijms20030463.
- Lorenzo, J. M., A. Mousavi Khaneghah, M. Gavahian, K. Marszałek, I. Eş, P. E. Munekata, I. C. F. R. Ferreira, and F. J. Barba. 2019. Understanding the potential benefits of thyme and its derived products for food industry and consumer health: From extraction of value-added compounds to the evaluation of bioaccessibility, bioavailability, anti-inflammatory, and antimicrobial activities. Critical Reviews in Food Science and Nutrition 59 (18):2879–95. doi: 10.1080/10408398.2018.1477730.
- Lu, Y., R. Nie, F. Li, and Z. Liu. 2016. Effects of calcium-binding peptide from tilapia scale protein hydrolysates on calcium absorption in Caco-2 cells. Journal of Aquatic Food Product Technology 25 (8):1213–20. doi: 10.1080/10498850.2015.1051258.
- Lu, Z., Z. Xu, Z. Shen, Y. Tian, and H. Shen. 2019. Dietary energy level promotes rumen microbial protein synthesis by improving the energy productivity of the ruminal microbiome. Frontiers in Microbiology 10:847. doi: 10.3389/fmicb.2019.00847.
- Mandell, I. B., J. G. Buchanan-Smith, B. J. Holub, and C. P. Campbell. 1997. Effects of fish meal in beef cattle diets on growth performance, carcass characteristics, and fatty acid composition of longissimus muscle. Journal of Animal Science 75 (4):910–9. doi: 10.2527/1997.754910x.
- Manyi-Loh, C., S. Mamphweli, E. Meyer, and A. Okoh. 2018. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 23 (4):795. doi: 10.3390/molecules23040795.
- Mao, X., N. Guo, J. Sun, and C. Xue. 2017. Comprehensive utilization of shrimp waste based on biotechnological methods: A review. Journal of Cleaner Production 143:814–23. doi: 10.1016/j.jclepro.2016.12.042.
- Martínez-Alvarez, O., I. Batista, C. Ramos, and P. Montero. 2016. Enhancement of ACE and prolyl oligopeptidase inhibitory potency of protein hydrolysates from sardine and tuna by-products by simulated gastrointestinal digestion. Food & Function 7 (4):2066–73. doi: 10.1039/c5fo01603g.
- Marti-Quijal, F. J., F. Remize, G. Meca, E. Ferrer, M.-J. Ruiz, and F. J. Barba. 2020. Fermentation in fish and by-products processing: An overview of current research and future prospects. Current Opinion in Food Science 31:9–16. doi: 10.1016/j.cofs.2019.08.001.
- Martí-Quijal, F. J., A. Tornos, A. Príncep, C. Luz, G. Meca, P. Tedeschi, M. J. Ruiz, and F. J. Barba. 2020. Impact of fermentation on the recovery of antioxidant bioactive compounds from sea bass byproducts. Antioxidants 9 (3):239. doi: 10.3390/antiox9030239.
- Mauerhofer, L. M., P. Pappenreiter, C. Paulik, A. H. Seifert, S. Bernacchi, and S. K. M. R. Rittmann. 2019. Methods for quantification of growth and productivity in anaerobic microbiology and biotechnology. Folia Microbiologica 64 (3):321–60. doi: 10.1007/s12223-018-0658-4.
- Mechri, S., K. Bouacem, F. Jabeur, S. Mohamed, N. A. Addou, A. Dab, A. Bouraoui, A. Bouanane-Darenfed, S. Bejar, H. Hacène, et al. 2019. Purification and biochemical characterization of a novel thermostable and halotolerant subtilisin SAPN, a serine protease from Melghiribacillus thermohalophilus Nari2A T for chitin extraction from crab and shrimp shell by-products. Extremophiles: Life under Extreme Conditions 23 (5):529–47. doi: 10.1007/s00792-019-01105-8.
- Mechri, S., I. Sellem, K. Bouacem, F. Jabeur, H. Laribi-Habchi, L. Mellouli, H. Hacène, A. Bouanane-Darenfed, and B. Jaouadi. 2020. A biological clean processing approach for the valorization of speckled shrimp Metapenaeus monoceros by-product as a source of bioactive compounds. Environmental Science and Pollution Research International 27 (13):15842–55. doi: 10.1007/s11356-020-08076-w.
- Missotten, J. A., J. Michiels, N. Dierick, A. Ovyn, A. Akbarian, and S. De Smet. 2013. Effect of fermented moist feed on performance, gut bacteria, and gut histo-morphology in broilers. British Poultry Science 54 (5):627–34. doi: 10.1080/00071668.2013.811718.
- Mohammed, I., D. G. Said, and H. S. Dua. 2017. Human antimicrobial peptides in ocular surface defense. Progress in Retinal and Eye Research 61:1–22. doi: 10.1016/j.preteyeres.2017.03.004.
- Mohapatra, S., B. Sarkar, D. P. Samantaray, A. Daware, S. Maity, S. Pattnaik, and S. Bhattacharjee. 2017. Bioconversion of fish solid waste into PHB using Bacillus subtilis based submerged fermentation process. Environmental Technology 38 (24):3201–8. doi: 10.1080/09593330.2017.1291759.
- Muhammed, M. A., D. Domendra, S. P. Muthukumar, P. Z. Sakhare, and N. Bhaskar. 2015. Effects of fermentatively recovered fish waste lipids on the growth and composition of broiler meat. British Poultry Science 56 (1):79–87. doi: 10.1080/00071668.2014.980719.
- Najafian, L., and A. S. Babji. 2012. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 33 (1):178–85. doi: 10.1016/j.peptides.2011.11.013.
- Najafian, L., and A. S. Babji. 2019. Purification and identification of antioxidant peptides from fermented fish sauce (Budu). Journal of Aquatic Food Product Technology 28 (1):14–24. doi: 10.1080/10498850.2018.1559903.
- Neves, A. C., P. A. Harnedy, M. B. O’Keeffe, and R. J. FitzGerald. 2017. Bioactive peptides from Atlantic salmon (Salmo salar) with angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory, and antioxidant activities. Food Chemistry 218:396–405. doi: 10.1016/j.foodchem.2016.09.053.
- Ngo, D. H., T. S. Vo, B. Ryu, and S. K. Kim. 2016. Angiotensin-I-converting enzyme (ACE) inhibitory peptides from Pacific cod skin gelatin using ultrafiltration membranes. Process Biochemistry 51 (10):1622–8. doi: 10.1016/j.procbio.2016.07.006.
- Nguyen, H. T. M., R. Pérez-Gálvez, and J. P. Bergé. 2012. Effect of diets containing tuna head hydrolysates on the survival and growth of shrimp Penaeus vannamei. Aquaculture 324-325:127–34. doi: 10.1016/j.aquaculture.2011.11.014.
- Nguyen, L. T., E. F. Haney, and H. J. Vogel. 2011. The expanding scope of antimicrobial peptide structures and their modes of action. Trends in Biotechnology 29 (9):464–72. doi: 10.1016/j.tibtech.2011.05.001.
- Niba, A. T., J. D. Beal, A. C. Kudi, and P. H. Brooks. 2009. Potential of bacterial fermentation as a biosafe method of improving feeds for pigs and poultry. African Journal of Biotechnology 8 (9): 1758–1767.
- Nikulin, Y. P., O. A. Nikulina, and Z. V. Tsoy. 2021. The using of a paste of fermented fish feed in gestation sows feeding. IOP Conference Series: Earth and Environmental Science 677 (2):022030. doi: 10.1088/1755-1315/677/2/022030.
- Nirmal, N. P., C. Santivarangkna, S. Benjakul, and S. Maqsood. 2022. Fish protein hydrolysates as a health-promoting ingredient—Recent update. Nutrition Reviews 80 (5):1013–26. doi: 10.1093/nutrit/nuab065.
- Niu, J., Y. Q. Zhang, Y. J. Liu, L. X. Tian, H. Z. Lin, X. Chen, H. J. Yang, and G. Y. Liang. 2014. Effects of graded replacement of fish meal by fish protein hydrolysate on growth performance of early post-larval Pacific white shrimp (Litopenaeus vannamei, Boone). Journal of Applied Animal Research 42 (1):6–15. doi: 10.1080/09712119.2013.795897.
- Nizami, A. S., M. Rehan, M. Waqas, M. Naqvi, O. K. M. Ouda, K. Shahzad, R. Miandad, M. Z. Khan, M. Syamsiro, I. M. I. Ismail, et al. 2017. Waste biorefineries: Enabling circular economies in developing countries. Bioresource Technology 241:1101–17. doi: 10.1016/j.biortech.2017.05.097.
- Olowofela, A. O., and A. O. Isah. 2017. A profile of adverse effects of antihypertensive medicines in a tertiary care clinic in Nigeria. Annals of African Medicine 16 (3):114–9. doi: 10.4103/aam.aam_6_17.
- Ono, S., M. Hosokawa, K. Miyashita, and K. Takahashi. 2006. Inhibition properties of dipeptides from salmon muscle hydrolysate on angiotensin I-converting enzyme. International Journal of Food Science & Technology 41 (4):383–6. doi: 10.1111/j.1365-2621.2005.01080.x.
- Opheim, M., M. Lenz Strube, H. Sterten, M. Øverland, and N. P. Kjos. 2016. Atlantic salmon (Salmo salar) protein hydrolysate in diets for weaning piglets─ Effect on growth performance, intestinal morphometry, and microbiota composition. Archives of Animal Nutrition 70 (1):44–56. doi: 10.1080/1745039X.2015.1117694.
- Ospina-Salazar, G. H., M. G. Ríos-Durán, E. M. Toledo-Cuevas, and C. A. Martínez-Palacios. 2016. The effects of fish hydrolysate and soy protein isolate on the growth performance, body composition and digestibility of juvenile pike silverside, Chirostoma estor. Animal Feed Science and Technology 220:168–79. doi: 10.1016/j.anifeedsci.2016.08.011.
- Ouellet, D. R., J. R. Seoane, D. M. Veira, and J. G. Proulx. 1997. Effects of supplementation with fish meal or fish protein hydrolysate on growth, nutrient digestibility and rumen fermentation of growing cattle fed grass silage. Animal Feed Science and Technology 68 (3-4):307–26. doi: 10.1016/S0377-8401(97)00035-7.
- Ozyurt, C. E., E. K. Boga, A. S. Ozkutuk, Y. Ucar, M. Durmus, and G. Ozyurt. 2020. Bioconversion of discard fish (Equulites klunzingeri and Carassius gibelio) fermented with natural lactic acid bacteria; the chemical and microbiological quality of ensilage. Waste and Biomass Valorization 11 (4):1435–42. doi: 10.1007/s12649-018-0493-5.
- Özyurt, G., A. S. Özkütük, M. Boğa, M. Durmuş, and E. K. Boğa. 2017. Biotransformation of seafood processing wastes fermented with natural lactic acid bacteria. The Quality of Fermented Products and Their Use in Animal Feeding 555:543–55. doi: 10.4194/1303-2712-v17.
- Panda, S., L. K. Babu, A. K. Panda, S. Tanuja, A. Mohanty, K. K. Panigrahy, and P. Samal. 2017. Effect of dietary supplementation of fermented fish silage on serum biochemical parameters of broiler Japanese quails (Coturnix coturnix japonica). Veterinary World 10 (4):380–5. doi: 10.14202/vetworld.2017.380-385.
- Pangestuti, R., and S. K. Kim. 2017. Bioactive peptide of marine origin for the prevention and treatment of non-communicable diseases. Marine Drugs 15 (3):67. doi: 10.3390/md15030067.
- Park, S. Y., J. Y. Je, and C. B. Ahn. 2016. Protein hydrolysates and ultrafiltration fractions obtained from krill (Euphausia superba): Nutritional, functional, antioxidant, and ACE-inhibitory characterization. Journal of Aquatic Food Product Technology 25 (8):1266–77. doi: 10.1080/10498850.2015.1054539.
- Phadke, G. G., N. B. Rathod, F. Ozogul, K. Elavarasan, M. Karthikeyan, K. H. Shin, and S. K. Kim. 2021. Exploiting of secondary raw materials from fish processing industry as a source of bioactive peptide-rich protein hydrolysates. Marine Drugs 19 (9):480. doi: 10.3390/md19090480.
- Rahmi, M., M. Faid, M. ElYachioui, E. H. Berny, M. Fakir, and M. Ouhssine. 2008. Protein rich ingredients from fish waste for sheep feeding. African Journal of Microbiology Research 2 (4):73–7.
- Rai, A. K., R. Jini, H. C. Swapna, N. M. Sachindra, N. Bhaskar, and V. Baskaran. 2011. Application of native lactic acid bacteria (LAB) for fermentative recovery of lipids and proteins from fish processing wastes: Bioactivities of fermentation products. Journal of Aquatic Food Product Technology 20 (1):32–44. doi: 10.1080/10498850.2010.528174.
- Rai, A. K., H. C. Swapna, N. Bhaskar, P. M. Halami, and N. M. Sachindra. 2010. Effect of fermentation ensilaging on recovery of oils from freshwater fish viscera. Enzyme and Microbial Technology 46 (1):9–13. doi: 10.1016/j.enzmictec.2009.09.007.
- Rajendran, S. R. C. K., A. Mohan, Z. Khiari, C. C. Udenigwe, and B. Mason. 2018. Yield, physicochemical, and antioxidant properties of Atlantic salmon visceral hydrolysate: Comparison of lactic acid bacterial fermentation with Flavourzyme proteolysis and formic acid treatment. Journal of Food Processing and Preservation 42 (6):e13620. doi: 10.1111/jfpp.13620.
- Rashid, H. A., H. Y. Jung, and J. K. Kim. 2018. Enhanced reutilization value of shrimp-shell waste via fed-batch biodegradation with higher production of reducing sugar, antioxidant, and DNA protective compounds. Fish Aquatic Sci, 21–33, doi: 10.1186/s41240-018-0109-9.
- Rima, M., M. Rima, Z. Fajloun, J.-M. Sabatier, B. Bechinger, and T. Naas. 2021. Antimicrobial peptides: A potent alternative to antibiotics. Antibiotics 10 (9):1095. doi: 10.3390/antibiotics10091095.
- Rivero-Pino, F., F. J. Espejo-Carpio, and E. M. Guadix. 2020. Production and identification of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from discarded Sardine pilchardus protein. Food Chemistry 328:127096. doi: 10.1016/j.foodchem.2020.127096.
- Ruthu, P. S. Murthy, A. K. Rai, and N. Bhaskar, (2014). Fermentative recovery of lipids and proteins from freshwater fish head waste with reference to antimicrobial and antioxidant properties of protein hydrolysate. Journal of Food Science and Technology 51 (9): 1884–1892. doi: 10.1007/s13197-012-0730-z.
- Ryan, J. T., R. P. Ross, D. Bolton, G. F. Fitzgerald, and C. Stanton. 2011. Bioactive peptides from muscle sources: Meat and fish. Nutrients 3 (9):765–91. doi: 10.3390/nu3090765.
- Saadi, S., N. Saari, F. Anwar, A. A. Hamid, and H. M. Ghazali. 2015. Recent advances in food biopeptides: Production, biological functionalities, and therapeutic applications. Biotechnology Advances 33 (1):80–116. doi: 10.1016/j.biotechadv.2014.12.003.
- Sar, T., J. A. Ferreira, and M. J. Taherzadeh. 2021. Conversion of fish processing wastewater into fish feed ingredients through submerged cultivation of Aspergillus oryzae. Systems Microbiology and Biomanufacturing 1 (1):100–10. doi: 10.1007/s43393-020-00009-5.
- Savijoki, K., H. Ingmer, and P. Varmanen. 2006. Proteolytic systems of lactic acid bacteria. Applied Microbiology and Biotechnology 71 (4):394–406. doi: 10.1007/s00253-006-0427-1.
- Seedevi, P., M. Moovendhan, S. Vairamani, and A. Shanmugam. 2017. Evaluation of antioxidant activities and chemical analysis of sulfated chitosan from Sepia prashadi. International Journal of Biological Macromolecules 99:519–29. doi: 10.1016/j.ijbiomac.2017.03.012.
- Setiawan, A., W. Widyastuti, A. Irawan, O. S. Wijaya, A. Laila, W. A. Setiawan, N. L. G. R. Juliasih, K. Nonaka, M. Arai, and J. Hendri. 2021. Solid state fermentation of shrimp shell waste using Pseudonocardia carboxydivorans 18A13O1 to produce bioactive metabolites. Fermentation 7 (4):247. doi: 10.3390/fermentation7040247.
- Setijawati, D., A. A. Jaziri, H. S. Yufidasari, M. D. Pratomo, D. W. Wardani, D. Ersyah, and N. Huda. 2020. Characteristics and use of peptones from catfish (Clarias gariepinus) and pangas catfish (Pangasius pangasius) heads as bacterial growth media. Squalen Bulletin of Marine and Fisheries Postharvest and Biotechnology 15 (1):19–29. doi: 10.15578/squalen.v15i1.437.
- Shabani, A., F. Boldaji, B. Dastar, T. Ghorchi, and S. Zerehdaran. 2016. Effect of diets containing fermented fish waste on growth performance and carcass characteristics of broiler chickens. Animal Sciences Journal 29 (111):75–86.
- Shabani, A., F. Boldaji, B. Dastar, T. Ghoorchi, and S. Zerehdaran. 2018. Preparation of fish waste silage and its effect on the growth performance and meat quality of broiler chickens. Journal of the Science of Food and Agriculture 98 (11):4097–103. doi: 10.1002/jsfa.8926.
- Shabani, A., V. Jazi, A. Ashayerizadeh, and R. Barekatain. 2019. Inclusion of fish waste silage in broiler diets affects gut microflora, cecal short-chain fatty acids, digestive enzyme activity, nutrient digestibility, and excreta gas emission. Poultry Science 98 (10):4909–18. doi: 10.3382/ps/pez244.
- Shao, J., L. Wang, X. Shao, and M. Liu. 2020. Dietary different replacement levels of fishmeal by fish silage could influence growth of Litopenaeus vannamei by regulating mTOR at transcriptional level. Frontiers in Physiology 11:359. doi: 10.3389/fphys.2020.00359.
- Sharma, P., V. K. Gaur, S. H. Kim, and A. Pandey. 2020. Microbial strategies for bio-transforming food waste into resources. Bioresource Technology 299:122580.). doi: 10.1016/j.biortech.2019.122580.
- Shavandi, A., Y. Hou, A. Carne, M. Mc Connell, and A. E. Bekhit. 2019. Marine waste utilization as a source of functional and health compounds. Advances in Food and Nutrition Research 87:187–254.
- Sheng, Z., G. M. Turchini, J. Xu, Z. Fang, N. Chen, R. Xie, H. Zhang, and S. Li. 2022. Functional properties of protein hydrolysates on growth, digestive enzyme activities, protein metabolism, and intestinal health of larval largemouth bass (Micropterus salmoides). Frontiers in Immunology 13:913024. doi: 10.3389/fimmu.2022.913024.
- Shi, Y., L. Zhong, X. Ma, Y. Liu, T. Tang, and Y. Hu. 2019. Effect of replacing fishmeal with stickwater hydrolysate on the growth, serum biochemical indexes, immune indexes, intestinal histology and microbiota of rice field eel (Monopterus albus). Aquaculture Reports 15:100223. doi: 10.1016/j.aqrep.2019.100223.
- Siddaiah, G. M., R. Kumar, R. Kumari, D. K. Damle, K. D. Rasal, V. Manohar, J. K. Sundaray, and B. R. Pillai. 2022. Dietary supplementation of fish protein hydrolysate improves growth, feed efficiency and immune response in freshwater carnivore fish, Channa striata fingerlings. Aquaculture Research 53 (9):3401–15. doi: 10.1111/are.15848.
- Siddik, M. A., J. Howieson, R. Fotedar, and G. J. Partridge. 2021. Enzymatic fish protein hydrolysates in finfish aquaculture: A review. Reviews in Aquaculture 13 (1):406–30. doi: 10.1111/raq.12481.
- Siddiqui, S. A.,Z. Erol,F. Taşçı,H. Ahu Kahraman,V. Toppi,L. Musa,G. Di Giacinto,Nur. Alim Bahmid,M. Mehdizadeh, andR. Castro-Muñoz. 2023. An overview of fermentation in the food industry- looking back from a new perspective. Bioresources and Bioprocessing 10 (85):1–47. doi: 10.1186/s40643-023-00702-y.
- Singh, S., T. Negi, N. A. Sagar, Y. Kumar, A. Tarafdar, R. Sirohi, R. Sindhu, and A. Pandey. 2022. Sustainable processes for treatment and management of seafood solid waste. The Science of the Total Environment 817:152951. doi: 10.1016/j.scitotenv.2022.152951.
- Soltan, M. A., I. M. Fouad, A. M. El-Zyat, and M. Y. Abou. 2017. Possibility of using fermented fish silage as feed ingredient in the diets of Nile Tilapia, Oreochromis niloticus. Global Veterinaria 18:59–67. doi: 10.5829/idosi.gv.2017.59.67.
- Song, Z., H. Liu, L. Chen, L. Chen, C. Zhou, P. Hong, and C. Deng. 2021. Characterization and comparison of collagen extracted from the skin of the Nile tilapia by fermentation and chemical pretreatment. Food Chemistry 340:128139. doi: 10.1016/j.foodchem.2020.128139.
- Staples, D., and S. Funge-Smith. 2005. Prized commodity: Low value/trash fish from marine fisheries in the Asia-pacific region. Fish for the People 3 (2):2–15.
- Stevens, J. R., R. W. Newton, M. Tlusty, and D. C. Little. 2018. The rise of aquaculture by-products: Increasing food production, value, and sustainability through strategic utilisation. Marine Policy 90:115–24. doi: 10.1016/j.marpol.2017.12.027.
- Sun, H., J. W. Tang, X. H. Yao, Y. F. Wu, X. Wang, and J. Feng. 2013. Effects of dietary inclusion of fermented cottonseed meal on growth, cecal microbial population, small intestinal morphology, and digestive enzyme activity of broilers. Tropical Animal Health and Production 45 (4):987–93. doi: 10.1007/s11250-012-0322-y.
- Sun, W., M. H. Shahrajabian, and M. Lin. 2022. Research progress of fermented functional foods and protein factory-microbial fermentation technology. Fermentation 8 (12):688. doi: 10.3390/fermentation8120688.
- Suryawanshi, N., and J. S. Eswari. 2022. Chitin from seafood waste: Particle swarm optimization and neural network study for the improved chitinase production. Journal of Chemical Technology & Biotechnology 97 (2):509–19. doi: 10.1002/jctb.6656.
- Swapna, H. C., K. R. Amit, N. Bhaskar, and N. M. Sachindra. 2010. Lipid classes and fatty acid profile of selected Indian freshwater fishes. Journal of Food Science and Technology 47 (4):394–400. doi: 10.1007/s13197-010-0065-6.
- Tang, H. G., T. X. Wu, Z. Y. Zhao, and X. D. Pan. 2008. Effects of fish protein hydrolysate on growth performance and humoral immune response in large yellow croaker (Pseudosciaena crocea R.). Journal of Zhejiang University Science B 9 (9):684–90. doi: 10.1631/jzus.B0820088.
- Tanuja, S., A. Kumar, S. K. Nayak, S. K. Behera, and A. Sarkar. 2017. Effect of dietary supplementation of acid ensiled fish waste on production performance, egg quality and serum biochemistry in layer Japanese quail (Coturnix coturnix japonica). Indian Journal of Animal Research 52 (5):740–3. doi: 10.18805/ijar.B-3276.
- Thi Thuy, N., J. E. Lindberg, and B. Ogle. 2011. Effects of replacing fish meal with ensiled catfish (Pangasius hypophthalmus) by-products on the performance and carcass quality of finishing pigs. Journal of Animal and Feed Sciences 20 (1):47–59. doi: 10.22358/jafs/66157/2011.
- Thuy, N. T., M. Joseph, and N. C. Ha. 2016. Effects of replacing marine fishmeal with graded levels of Tra Catfish by-product protein hydrolysate on the performance and meat quality of pigs. South African Journal of Animal Science 46 (3):221–9. doi: 10.4314/sajas.v46i3.1.
- Tonheim, S. K., M. Espe, K. Hamre, and I. Rønnestad. 2005. Pre-hydrolysis improves utilisation of dietary protein in the larval teleost Atlantic halibut (Hippoglossus hippoglossus L.). Journal of Experimental Marine Biology and Ecology 321 (1):19–34. doi: 10.1016/j.jembe.2004.12.036.
- Toopcham, T., S. Roytrakul, and J. Yongsawatdigul. 2015. Characterization and identification of angiotensin I-converting enzyme (ACE) inhibitory peptides derived from tilapia using Virgibacillus halodenitrificans SK1-3-7 proteinases. Journal of Functional Foods 14:435–44. doi: 10.1016/j.jff.2015.01.050.
- Tucker, J. L., V. D. Naranjo, T. D. Bidner, and L. L. Southern. 2011. Effect of salmon protein hydrolysate and spray-dried plasma protein on growth performance of weanling pigs. Journal of Animal Science 89 (5):1466–73. doi: 10.2527/jas.2010-3412.
- Turchini, G. M., B. E. Torstensen, and W. K. Ng. 2009. Fish oil replacement in finfish nutrition. Reviews in Aquaculture 1 (1):10–57. doi: 10.1111/j.1753-5131.2008.01001.x.
- Ucak, I., M. Afreen, D. Montesano, C. Carrillo, I. Tomasevic, J. Simal-Gandara, and F. J. Barba. 2021. Functional and bioactive properties of peptides derived from marine side streams. Marine Drugs 19(2):71. doi: 10.3390/MD19020071.
- Umayaparvathi, S., S. Meenakshi, V. Vimalraj, M. Arumugam, G. Sivagami, and T. Balasubramanian. 2014. Antioxidant activity and anticancer effect of bioactive peptide from enzymatic hydrolysate of oyster (Saccostrea cucullata). Biomedicine & Preventive Nutrition 4 (3):343–53. doi: 10.1016/j.bionut.2014.04.006.
- Välimaa, A.-L., S. Mäkinen, P. Mattila, P. Marnila, A. Pihlanto, M. Mäki, and J. Hiidenhovi. 2019. Fish and fish side streams are valuable sources of high-value components. Food Quality and Safety 3 (4):209–26. doi: 10.1093/fqsafe/fyz024.
- Vázquez, J. A., A. Meduíña, A. I. Durán, M. Nogueira, A. Fernández-Compás, R. I. Pérez-Martín, and I. Rodríguez-Amado. 2019. Production of valuable compounds and bioactive metabolites from by-products of fish discards using chemical processing, enzymatic hydrolysis, and bacterial fermentation. Marine Drugs 17 (3):139. doi: 10.3390/md17030139.
- Venugopal, V. 2021. Valorization of seafood processing discards: Bioconversion and bio-refinery approaches. Frontiers in Sustainable Food Systems 5:611835. doi: 10.3389/fsufs.2021.611835.
- Venugopal, V., and A. Sasidharan. 2022. Functional proteins through green refining of seafood side streams. Frontiers in Nutrition 9:974447. doi: 10.3389/fnut.2022.974447.
- Veríssimo, N. V., C. U. Mussagy, A. A. Oshiro, C. M. N. Mendonça, V. de Carvalho Santos-Ebinuma, A. Pessoa, R. P. de Souza Oliveira, and J. F. B. Pereira. 2021. From green to blue economy: Marine biorefineries for a sustainable ocean-based economy. Green Chemistry 23 (23):9377–400. doi: 10.1039/D1GC03191K.
- Vicente, F. A., S. P. M. Ventura, H. Passos, A. C. R. V. Dias, M. A. Torres-Acosta, U. Novak, and B. Likozar. 2022. Crustacean waste biorefinery as a sustainable cost-effective business model. Chemical Engineering Journal 442:135937. doi: 10.1016/j.cej.2022.135937.
- Vidotti, R. M., E. M. M. Viegas, and D. J. Carneiro. 2003. Amino acid composition of processed fish silage using different raw materials. Animal Feed Science and Technology 105 (1-4):199–204. doi: 10.1016/S0377-8401(03)00056-7.
- Vishwanatha, K. S., A. A. Rao, and S. A. Singh. 2009. Characterisation of acid protease expressed from Aspergillus oryzae MTCC 5341. Food Chemistry 114 (2):402–7. doi: 10.1016/j.foodchem.2008.09.070.
- Wang, S., X. Zeng, Q. Yang, and S. Qiao. 2016. Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. International Journal of Molecular Sciences 17 (5):603. doi: 10.3390/ijms17050603.
- Wang, T. Y., C. H. Hsieh, C. C. Hung, C. L. Jao, M. C. Chen, and K. C. Hsu. 2015. Fish skin gelatin hydrolysates as dipeptidyl peptidase IV inhibitors and glucagon-like peptide-1 stimulators improve glycaemic control in diabetic rats: A comparison between warm-and cold-water fish. Journal of Functional Foods 19:330–40. doi: 10.1016/j.jff.2015.09.037.
- Wei, Y., J. Wang, X. Zhang, M. Duan, L. Jia, H. Xu, M. Liang, and J. Liu. 2021. Fish protein hydrolysate supplementation in plant protein based diets for tiger puffer (Takifugu rubripes) is an effective strategy of fish meal sparing. Aquaculture Reports 20:100720. doi: 10.1016/j.aqrep.2021.100720.
- Widyastuti, W., F. Setiawan, C. Al Afandy, A. Irawan, A. Laila, N. L. G. R. Juliasih, W. A. Setiawan, M. Arai, J. Hendri, and A. Setiawan. 2022. Antifungal agent chitooligosaccharides derived from solid-state fermentation of shrimp shell waste by Pseudonocardia antitumoralis 18D36-A1. Fermentation 8 (8):353. doi: 10.3390/fermentation8080353.
- Wijtten, P. J. A., E. Hangoor, J. K. W. M. Sparla, and M. W. A. Verstegen. 2010. Dietary amino acid levels and feed restriction affect small intestinal development, mortality, and weight gain of male broilers. Poultry Science 89 (7):1424–39. doi: 10.3382/ps.2009-00626.
- Xiang, X., M. Lang, Y. Li, X. Zhao, H. Sun, W. Jiang, L. Ni, and Y. Song. 2021. Purification, identification and molecular mechanism of dipeptidyl peptidase IV inhibitory peptides from discarded shrimp (Penaeus vannamei) head. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 1186:122990. doi: 10.1016/j.jchromb.2021.122990.
- Xie, P. J., L. X. Huang, C. H. Zhang, and Y. L. Zhang. 2016. Nutrient assessment of olive leaf residues processed by solid‐state fermentation as an innovative feedstuff additive. Journal of Applied Microbiology 121 (1):28–40. doi: 10.1111/jam.13131.
- Ximenes, J. C. M., D. C. Hissa, L. H. Ribeiro, M. V. P. Rocha, E. G. Oliveira, and V. M. M. Melo. 2019. Sustainable recovery of protein-rich liquor from shrimp farming waste by lactic acid fermentation for application in tilapia feed. Brazilian Journal of Microbiology 50 (1):195–203. doi: 10.1007/s42770-018-0024-3.
- Xu, H., Y. Mu, Y. Zhang, J. Li, M. Liang, K. Zheng, and Y. Wei. 2016. Graded levels of fish protein hydrolysate in high plant diets for turbot (Scophthalmus maximus): Effects on growth performance and lipid accumulation. Aquaculture 454:140–7. doi: 10.1016/j.aquaculture.2015.12.006.
- Yaghoubzadeh, Z., F. Peyravii Ghadikolaii, H. Kaboosi, R. Safari, and E. Fattahi. 2020. Antioxidant activity and anticancer effect of bioactive peptides from rainbow trout (Oncorhynchus mykiss) skin hydrolysate. International Journal of Peptide Research and Therapeutics 26 (1):625–32. doi: 10.1007/s10989-019-09869-5.
- Yazdani, A. R. 2011. Effect of feeding fish meal on milk production and its composition in dairy cows. Indian Journal of Animal Sciences 81 (11):1161–4.
- Zhang, N., X. Song, W. Dong, L. Liu, Z. Cui, and Y. Ma. 2022. Nutritional evaluation of fish protein hydrolysate and its application in piglet production. Journal of Animal Science 100 (3):skab369. doi: 10.1093/jas/skab369.
- Zhang, Q. Y., Z. B. Yan, Y. M. Meng, X. Y. Hong, G. Shao, J. J. Ma, X. R. Cheng, J. Liu, J. Kang, and C. Y. Fu. 2021. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Military Medical Research 8 (1):48. doi: 10.1186/s40779-021-00343-2.
- Zhang, R., J. Chen, X. Jiang, L. Yin, and X. Zhang. 2016. Antioxidant and hypoglycaemic effects of tilapia skin collagen peptide in mice. International Journal of Food Science & Technology 51 (10):2157–63. doi: 10.1111/ijfs.13193.
- Zheng, K., M. Liang, H. Yao, J. Wang, and Q. Chang. 2012. Effect of dietary fish protein hydrolysate on growth, feed utilization and IGF‐I levels of Japanese flounder (Paralichthys olivaceus). Aquaculture Nutrition 18 (3):297–303. doi: 10.1111/j.1365-2095.2011.00896.x.
- Zhou, X., L. Chai, Q. Wu, Y. Wang, S. Li, and J. Chen. 2021. Anti-diabetic properties of bioactive components from fish and milk. Journal of Functional Foods 85:104669. doi: 10.1016/j.jff.2021.104669.