Abstract
Carrots are main dietary sources of several potential anti-cancer compounds, including polyacetylenes, while β-carotene has shown no benefits in controlled cancer trials. Accordingly, associations between carrot intake and cancer incidence were quantified, where necessary using α-carotene as a non-causal biomarker of carrot consumption, by searching for studies published before June 2022 reporting risk estimates for relationships of cancer incidence with carrot intake or α-carotene intake or α-carotene plasma concentration, supplemented with hand searches of included studies and reviews. Meta-analyses comparing highest and lowest reported intakes in prospective studies using a random-effects model estimated summary relative risks (RRs) with 95% confidence intervals (CIs), separately for carrot intake or α-carotene plasma concentration, and the corresponding dose-responses. Of 198 observational studies, in 50 prospective studies with 52000 cases recording carrot intake, the cancer-risk was substantially reduced (RR 0.90, 95% CI 0.87–0.94, p ˂ 0·00004). In 30 prospective studies with 9331 cases reporting plasma α-carotene levels, summary RR was 0.80 (0.72–0.89, p ˂ 0·00006). For both exposure types, inter-study heterogeneity was moderate, interaction with cancer types insignificant, and the dose-response significant (p ˂ 0·01). In conclusion, carrot consumption is robustly associated with decreased cancer-risk; carrot consumption should be encouraged, and the causal mechanisms further investigated.
Introduction
Increased intake of fruit and vegetables is recognized to reduce the risk of cancer (WCRF/AICR Citation2018). However, for total fruit and vegetables this association is rather weak (Aune et al. Citation2017). A seminal study (Peto et al. Citation1981) concluded that dietary β-carotene either could materially reduce cancer rates (which should be tested in controlled trials) or was associated with ‘some truly protective factor’ in vegetables. Since then, randomized controlled trials of β-carotene, as well as other vegetable constituents that are not unique to carrots, such as polyphenols and fiber, have shown only limited benefits, if any (Yao et al. Citation2017; Moorthy et al. Citation2020; Zhang et al. Citation2023), while little research has focused on the other option (a cancer-preventive phytochemical strongly associated with dietary β-carotene). We therefore hypothesize that carrots are unique among most vegetables and fruits due to their content of one or more specific non-nutrient bioactive secondary metabolites, where carrot is the major dietary source. This applies to specific polyacetylenes and isocoumarins, each of which have been implicated as potential anti-cancer constituents based on in vitro studies or animal trials (Snene et al. Citation2017; Kobaek-Larsen et al. Citation2019; Alfurayhi, Huang, and Brandt Citation2023). Carrots provide approximately 85% (range 82–94%) of dietary α-carotene across different food cultures, providing even stronger correlations with carrot intake than β-carotene (O’Neill et al. Citation2001; Hendrickson et al. Citation2013; Lee et al. Citation2013). We are not aware of any controlled studies demonstrating sufficient differences in effect between α-carotene and β-carotene to justify considering α-carotene a likely candidate as cancer-preventive compound. However, its intake or plasma concentration are frequently reported, providing a surrogate measure/biomarker of carrot intake in papers where this is not reported directly, thus increasing the power of our analysis. Previous meta-analyses of carrot intake and cancer incidence (Xu et al. Citation2014; Fallahzadeh et al. Citation2015; Chen et al. Citation2018; Xu et al. Citation2019) each included only one cancer type (prostate, gastric, breast and lung cancer, respectively) and only retrospective studies. A previous meta-analysis of 55 studies on associations between dietary carotenoids and cancer (Musa-Veloso et al. Citation2009) concluded that the effects found in studies with retrospective designs were strongly affected by bias, in contrast to prospective studies, where this analysis did not observe significance. While several other meta-analyses have found significant associations of dietary alpha-carotene and reduced cancer incidence, their interpretations focused on carotenes as either having a potentially causal role (Musa-Veloso et al. Citation2009) or as markers of general vegetable intake (Aune et al. Citation2018), therefore not specifically addressing the role of carrots. The primary objective of our meta-analysis was to quantify the association between carrot intake and cancer incidence across all cancer types, focusing on high-quality (prospective) data. The secondary objective was to estimate dose dependency, to facilitate the development of quantitative recommendations, and also to allow comparison of separate datasets to assess the robustness of the outcomes. Specifically, the analysis aimed to ‘provide evidence for a dose–response relationship and for consistency of the… association across studies’, as required by the European Food Safety Authority (EFSA) (EFSA Panel on Dietetic Products Citation2021) as one (of several) prerequisites for a health claim for carrot consumption to reduce the risk of cancer.
Methods
Data sources, search strategy and study selection
PubMed, Cochrane Library, Web of Science, Scopus, EBSCO and JSTOR were searched from database inception to June 9, 2022, for published studies of any design, observational or intervention, which related human consumption of carrots (reported directly as carrot intake, or indirectly as intake or plasma concentration of α-carotene) with an incidence of any type of cancer, where risk estimates (e.g., odds ratios or equivalent data) were available. We hand-searched the reference lists of the identified articles and published reviews, and reported the results according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al. Citation2009). See the Supplementary Information for the search terms used and the PRISMA checklist (Table S19). We included studies of any geographical location or language. A study that was reported in Lithuanian (Zickute et al. Citation2005) was translated by a native speaker of this language. We considered each type of cancer a separate study if separate data were available, even when recorded from the same study population. When data on the same cases were reported in more than one article, we selected the newest publication with the largest number of cases. Each title and abstract were reviewed independently by two of three investigators (KB, CO and GO), and disagreements resolved jointly. URL for the published protocol: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=124009 (Ojobor et al. Citation2019)
Data extraction and analysis
The extracted data included: publication year; study design; country; numbers of cancer cases and controls; age range; sex; dietary assessment method; levels of exposure; exposure type; study duration or follow-up; risk estimates and confidence intervals; statistical method and adjustment variables. We contacted the corresponding authors for missing data or unpublished relevant details. Two authors (CO and GO) extracted data independently and discrepancies were resolved in consultation with a third author (KB). The extracted dataset is available in a repository (Ojobor et al. Citation2023).
Quality assessment of the included studies
The 80 studies with prospective design were assessed for study quality using the Newcastle-Ottawa scale (Wells et al. Citation2015). Two authors (CO and GO) scored the studies independently and discrepancies were resolved in consultation with a third author (KB).
Primary meta-analysis
The primary endpoint was the association between carrot intake and cancer incidence in humans. Where risk estimates were shown separately with different adjustments, we chose the value with the highest number of adjustment variables. We derived the summary RRs and 95% CIs for the highest vs. lowest levels of carrot intake using a random-effects model to account for anticipated heterogeneity among the studies (DerSimonian and Laird Citation1986). The natural logarithms of the RRs were weighted by the method of DerSimonian and Laird, and then pooled across studies (DerSimonian and Laird Citation1986). For studies reporting results only as more than one risk ratio (e.g., for raw and cooked carrots separately), we pooled them into a single risk ratio using a fixed-effects meta-analysis before subsequent pooling with other studies, to ensure that between-study heterogeneity was not underestimated (Vieira et al. Citation2016). Authors of studies reporting α-carotene intake from diet were asked for the underlying carrot intake data; these were provided for one study (Parent et al. Citation2018) only.
We assessed the heterogeneity among studies with the Q-test and I2 statistic (Higgins and Thompson Citation2002) and used subgroup analyses to explore the variation of the effects across specific variables: study design (prospective or retrospective), exposure type, gender, cancer types, geographical regions, exposure assessment method and adjustments for confounding factors. Due to consistently significant effects of study design (prospective v. retrospective) and exposure type (intake (of either carrots or α-carotene) v. plasma α-carotene concentration), the subsequent analyses were done separately for the subgroups defined by these factors, and analyses of data from retrospective studies were only shown in the Supplementary Information. However, the exposure types ‘carrot intake’ and ‘α-carotene intake’ were pooled, once the initial analysis confirmed our expectation that these could be treated as equivalent.
We did a ‘one-study removed’ sensitivity analysis to test for single study effects on the overall risk estimate for the prospective studies, separately for carrot/α-carotene intake and for plasma α-carotene exposure types. Potential publication bias was assessed using visual inspection of the funnel plots and tests for small-study effects (Egger et al. Citation1997). We used STATA, version 17 and R-Studio Statistical software for the statistical analyses, and significance was considered at p < 0.05 or I2 > 50%.
Dose-response analysis
For prospective studies that reported at least three categories of intake and the number of cases and person-years or non-cases per category, we estimated the dose-response relationships using the method described by Greenland (Greenland and Longnecker Citation1992; Orsini et al. Citation2012). We assigned the mean or median intake by category to the corresponding RRs, and assigned the midpoint of the upper and lower boundaries in each category as the average value of intakes for studies that only reported a range of intakes by category. When an intake range was open-ended, we assumed the width of the interval to be the same as in the preceding category. The potential nonlinear dose–response relationship between the intakes and cancer risks was examined by modeling exposure intakes using restricted cubic splines with three knots at the 25th, 50th, and 75th percentiles of the distribution (Harrell, Lee, and Pollock Citation1988). For studies that reported intake categories in servings or times or mg α-carotene, we converted the values into standard servings using 80 g carrot as a serving size (He, Nowson, and MacGregor Citation2006) and 5.5 mg α-carotene/100g carrot (Holden et al. Citation1999).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
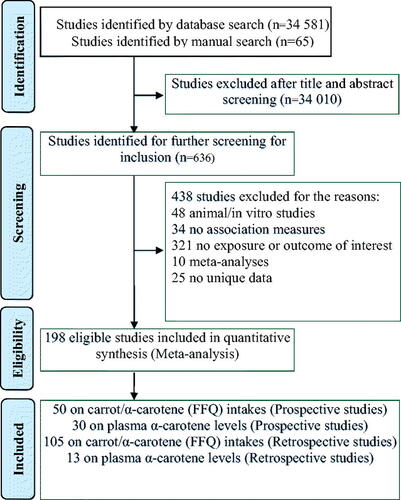
The 198 eligible studies () included 138917 cancer cases and 4707643 participants, with details shown in the Supplementary Information Tables S1–S6.
Table 1. Summary of the individual dietary intakes and cancer risk.
Study design and carrot exposure measurement method substantially affected the RR for the highest compared with the lowest intakes of carrots ().
Prospective studies reporting intake of carrots or α-carotene
The overall effect was moderate (RR = 0·90, 95% CI 0.87–94) but significant (t(49) = −4.53, p < 0·00004). It was very consistent (), with no significant interactions across the different subgroups (), including the exposure type, cancer type, geographical region (for gender and various confounding factors, see Supplementary Information Table S8). Due to the complete absence of interaction with exposure type (p = 0·99), we have shown only the combined dataset. Analyses of carrot intake and α-carotene intake separately are shown in Supplementary Information Figures S5 and S7. A sensitivity analysis (Supplementary Information Figure S2) showed that no individual study had a dominant effect on the outcome and also there was no effect (p = 0.25) of study quality (Supplementary Information Figure S12).
Figure 2. Analysis of highest compared to lowest carrot/α-carotene intake and cancer risk in 50 prospective studies. The squares represent the RR for each study and the horizontal lines are the 95% confidence interval around this estimate. The area of each square is proportional to its weighting in the meta-analysis. The diamond is the pooled estimate, with 95% CI. Circles indicate studies at risk of contribution to publication bias, see .
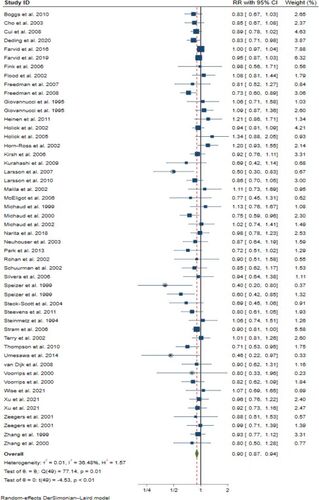
Table 2. Subgroup analyses of association between carrot/α-carotene intake and risk of cancers in 50 prospective studies.
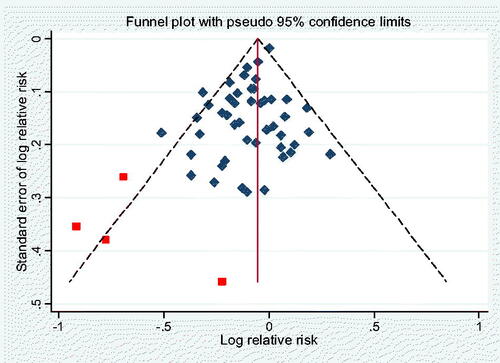
We found some evidence of publication bias (). Omitting 4 outlying studies reduced the heterogeneity (to I2 = 26%) and the overall association (to RR 0·92, 95% CI 0·88–0·95) (see Supplementary Information Figure S1), without substantially affecting its significance (p < 0·00002). A sensitivity analysis after removal of the 4 studies (see Supplementary Information Figure S3) confirmed that no individual study had a dominant effect on the outcome.
Prospective studies reporting plasma α-carotene
This dataset showed a stronger effect (RR = 0·80, 95% CIs 0·72–0·89, z= −4.03, p < 0·00006), and also here the subgroup analysis results () showed no significant interactions across the different subgroups, including the cancer types and geographical populations; see Supplementary Information Table S10 for subgroup results based on gender and various other factors. The sensitivity analysis (Supplementary Information Figure S4) also here showed no indication of single study dominance, and we found no significant evidence of publication bias as indicated by the symmetrical funnel plot () nor of study quality (p = 0.45) (Supplementary Information Figure S13).
Figure 4. Analysis of highest compared to lowest plasma α-carotene levels and cancer risk in 30 prospective studies. The squares represent the RR for each study and the horizontal lines are the 95% confidence interval around this estimate. The area of each square is proportional to its weighting in the meta-analysis. The diamond is the pooled estimate, with 95% CI.
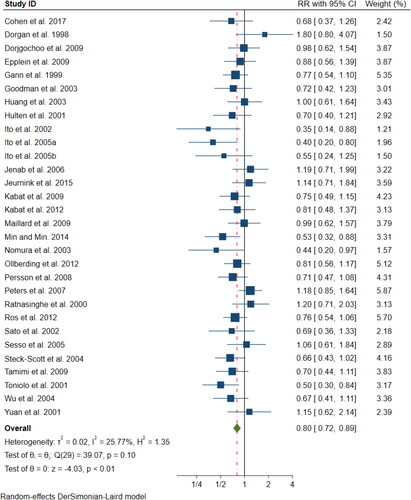
Figure 5. Egger’s funnel plot for publication bias in the meta-analysis of 30 prospective studies on plasma α-carotene levels (p = 0·114).
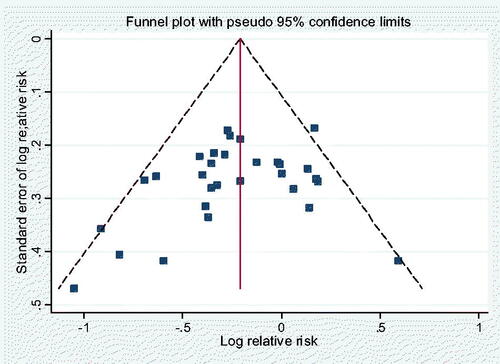
Table 3. Subgroup results for plasma α-carotene levels and cancer risks in 30 prospective studies.
Comparing the two independent prospective datasets measuring carrot exposure either as intake or by the biomarker plasma α-carotene, the RR estimates of 0·90 and 0·80 are significantly different (p = 0·04), so we did not combine them.
Dose-response analyses
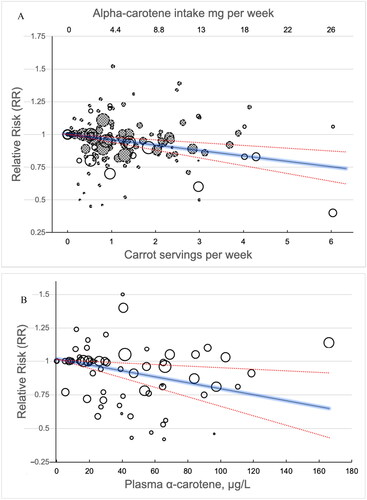
Studies on carrot/α-carotene intake showed a significant linear dose–response relationship (p < 0·0001) with 4 ± 2% lower risk for a carrot intake of one serving per week, and a 20 ± 10% lower cancer risk for 5 servings per week (400 g, 60 g per day) (). We also found a linear dose-response relationship between plasma α-carotene and cancer risk (p = 0·0058), with a 50 μg/L increase in plasma levels of α-carotene corresponding to a 9 ± 7% lower risk of cancer incidence in the overall population (). A spline model fitted to the data also provided a significant non-linear association between carrot intake and risk of cancer. However, due to extensive overlap of the confidence intervals for the predictions, we only report the results from the linear models here, being a simpler model and easier to interpret. Plots from the spline model are shown in the Supplementary Information, Figures S10 and S11.
Figure 6. A, B. Dose–response analyses with estimated RRs for cancer risks by doses of carrot (open circles)/α-carotene (hatched circles) intake in 33 studies (a), and plasma α-carotene in 17 studies (B). the solid line represents the estimated relative risks, and the dotted lines the 95% confidence intervals. The diameter of each data point is proportional to the weight of the corresponding data point in the analysis.
Discussion
Our analyses of prospective studies, where carrot consumption was assessed using Food Frequency Questionnaires (FFQs) and reported either as carrot intake or as α-carotene intake, showed highly significant identical RRs of 0·90, with 95% CIs of 0.84–0.97 and 0.86–0.96, respectively. The effect was highly consistent, without interaction with any of the tested subgroups, notably cancer type and geographical region. Testing a separate dataset where carrot consumption was assessed by the plasma α-carotene concentration, we found a similarly consistent and even stronger effect with RR = 0·80, 95% CIs 0·72–0·89. Both exposure types showed significant linear dose-response.
Assessment of quality and bias in the included studies
More than 90% of the prospective studies were rated ‘High’ (7 or more) on the Newcastle-Ottawa scale, whether carrot intake was assessed by FFQ or as plasma concentrations (Supplementary Information Figures S12 and S13) and these scores did not affect the RR. The high scores reflected quite extensive adjustments for potential confounders such as education, social group or other measures, that are known to be correlated with a generally healthy lifestyle. These adjustments reduce the risk that the observed correlation could be caused by confounding with another element of healthy lifestyle, although few of the studies adjusted for overall vegetable intake, which would have been the ultimate test in this regard. We noticed that several studies interpreted their outcomes as supporting a causal role of carotenes in cancer prevention; however, since this did not directly affect our analysis, we did not attempt to investigate this potential bias (Tatsioni, Bonitsis, and Ioannidis Citation2007) further.
Assessment of appropriateness of the meta-analysis methods
The meta-analysis comprised 50 prospective studies that had collected prospective diet information including carrot intake using FFQs. If all these studies had reported the cancer incidence data according to categories of carrot intake, either in their publications or when we contacted them about this, then the meta-analysis would have been simple and as accurate as possible with these data. However, for 35 of the 50 studies the results were only available as α-carotene intake. We are aware that our choice to use α-carotene intake data as an equivalent estimate of carrot intake was the ‘second best’ option; however, we find it unlikely that the approx. 15% of α-carotene intake from other foods than carrots would cause the ranking of carrot intake in that population to substantially deviate from the ranking of α-carotene intake.
However, based on this experience, we encourage all researchers who are in possession of such datasets (containing individual dietary items linked with subsequent health outcomes) to either make the full dataset available for research in an appropriately anonymized format, or to publish analyses of the associations of every individual food or drink for which data are available (whether the effects are significant or not). This will facilitate future more extensive and accurate hypothesis-directed meta-analyses, for carrots as well as many other foods with suspected positive or negative health impact, and make the research more cost-effective by reducing the need for collection of new data.
To the best of our knowledge, this is the first comprehensive meta-analysis to establish the association between carrot consumption and reduced cancer incidence across all cancer types and several measures of exposure. It updates and extends previous meta-analyses of individual cancer types, mostly based on retrospective studies. Our study substantially extends the review and meta-analysis on α-carotene and incidence of all cancers by Musa-Veloso et al. (Citation2009), which observed substantial evidence of bias in studies with a retrospective design, and recommended to focusing on more reliable data from prospective studies, despite not observing a significant effect of those in their dataset. Our results () confirmed the tendency for unrealistically low RRs in retrospective studies for all 3 exposure types, so we did not use these results further. While the RR values we observed from prospective studies were similar to those of Musa-Veloso et al. (Citation2009), our inclusion of additional studies provided sufficient statistical power to estimate significant consistent protective effects across the prospective studies, leading to a different conclusion.
While the protective efficacy of vegetable intake against cancer has long been recognized (Williams Citation1898), the operationalization of this insight regarding public health has been held back by difficulties in identifying protective mechanisms/constituents (WCRF/AICR Citation2018). Specifically, it is our impression that the demonstration of the absence of protective effects in randomized controlled trials of vitamins A, C and E and β-carotene (Bjelakovic, Nikolova, and Gluud Citation2013) hampered confidence in recommendations to increase consumption of vegetables in general and carrots in particular. Such apparent contradictions may be a contributory factor regarding how several public health initiatives in this area have only partially met their objectives (Wallace et al. Citation2020). Improved understanding of the roles and importance of relevant non-nutrient phytochemicals would allow more accurate estimates of benefits and more focused recommendations of individual vegetable types, which may then improve adherence and effectiveness of interventions (Wallace et al. Citation2020). While our analysis used α-carotene as a marker of carrot intake, the observed association could be caused by any carrot constituent that is not lost during cooking. Specifically, we have serious concerns about the applicability of the (mostly in vitro) evidence presented (Saini et al. Citation2020) as supporting causal roles of α-carotene and other carotenoids in cancer prevention. Regarding β-carotene, clinical trials showed harmful effects in doses exceeding nutritional (vitamin A) requirements (Zhang et al. Citation2023), and benefits were consistently absent in Mendelian Randomization studies of ovarian (Guo, Lu, and Jin Citation2020), colorectal (Tsilidis et al. Citation2021), digestive system (Zhang et al. Citation2022), endometrial (Wang, Glubb, and O’Mara Citation2023) and breast (Zhao et al. Citation2023) cancers; we are not aware of any reason to expect that α-carotene would have substantially different effects than β-carotene. Apart from α- and β-carotene, to our knowledge the only proposed anti-cancer phytochemicals of which carrots are major sources (more than 50% of dietary intake) are the polyacetylenes falcarinol, falcarindiol and falcarindiol-3-acetate (Christensen and Brandt Citation2006; Kobaek-Larsen et al. Citation2019; Alfurayhi, Huang, and Brandt Citation2023) and the isocoumarin 6-methoxymellein (Snene et al. Citation2017). Other apiaceous vegetables also contain polyacetylenes such as falcarinol and falcarindiol (Chen et al. Citation2015), but are consumed in much smaller amounts than carrots.
The almost identical results in the subgroups reporting carrot intake either directly or indirectly as α-carotene intake (calculated from FFQ results) support that these studies are equivalent and our pooling of the data justified. However, carrot intake and plasma α-carotene were associated with significantly different RRs of 0·90 (95%CIs 0·87–0·94) or 0·80 (0·72–0·89), respectively. While α-carotene intakes from FFQs are only moderately correlated with plasma concentrations (Al-Delaimy et al. Citation2005; Aune et al. Citation2012), our results confirm Aune et al.’s observation (2012) that the lower RR for the plasma data may be the more accurate value, indicating that the α-carotene concentration in plasma is a more precise marker of an individual’s long-term carrot intake than the direct, but imprecise, measurement using FFQs. In the present analysis, the plasma α-carotene values were only used as a ‘reality check’, to provide an independent dataset assessing the same variable (carrot intake). However, the consistent null findings from Mendelian Randomization studies are intriguing and warrant a future analysis where the Mendelian Randomization approach could be used ‘in reverse’, to refine the correlation with carrot intake by adjusting plasma concentrations in individuals for the effect of genetically determined differences in plasma carotene levels. If this enhances the association with cancer incidence, then it will be an independent indicator that the effect of carrot intake is not causally related to the presence of carotenes in the plasma.
The relatively uniform effect across different cancer types indicates a mechanism of action that is not confined to a particular organ, such as modulation of inflammation (Alfurayhi, Huang, and Brandt Citation2023). The inter-study heterogeneity in the meta-analysis was moderate in the data on carrot/α-carotene intakes () and low in the data on plasma α-carotene (), respectively. This indicates high stability in our results, that neither any individual studies nor the between-studies heterogeneity affected the meta-analysis results.
The robust linear relationships between carrot exposure and cancer risk reduction (, ) can be used directly for public health recommendations. When five servings per week reduced the risk of cancer by 20 ± 10%, then ‘a carrot a day’ really did keep the oncologist at some distance!
Table 4. Estimated RRs from linear dose-response relationships in prospective studies.
Conclusion
Carrot consumption was consistently negatively associated with cancer incidence across a wide range of geographical regions, exposure types and cancer types, which provided robust statistical power to quantify associations precisely. These findings provide enhanced support for safe and cost-effective public health recommendations and interventions to increase carrot intake, as part of the overall consumption of fruit and vegetables, in order to reduce the risk of cancer and other diet-related diseases. The outcomes justify additional research, such as randomized human clinical trials with carrots and/or their constituents (in diet-achievable doses), to more directly assess their potentials for primary or secondary prevention. The results also highlight the need to investigate the roles of a wider range of vegetable phytochemicals, specifically polyacetylenes and isocoumarins, in pre-clinical studies regarding cancer-related effects.
Authors’ contributions
All authors contributed to the study conception and design. Searches were done by Charles Ojobor, study selection and study quality scoring by Charles Ojobor, Gerard O’Brien and Kirsten Brandt. Data extraction was done by Charles Ojobor and Gerard O’Brien, and data analyses were performed by Charles Ojobor and Chibueze Ogbonnaya, with advice from Kirsten Brandt and Mario Siervo. The first draft of the manuscript was written by Charles Ojobor and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
| Abbreviations | ||
| CI | = | Confidence Interval |
| FFQ | = | Food Frequency Questionnaire |
| RR | = | Relative Risk |
Supplemental Material
Download MS Word (1.7 MB)Acknowledgements
We thank Ms Julija Simpson for translating a paper (Zickute et al. Citation2005) from Lithuanian to English.
Disclosure statement
The authors have no relevant financial or non-financial interests to disclose.
Data availability statement
The included studies are tabulated in the Supplementary Information (Tables S1–S6) as well as additional analyses, while the full datasets are deposited at Newcastle University’s data repository https://data.ncl.ac.uk/ with the DOI: 10.25405/data.ncl.21931533 (Ojobor et al. Citation2023).
Additional information
Funding
References
- Al-Delaimy, W. K., P. Ferrari, N. Slimani, V. Pala, I. Johansson, S. Nilsson, I. Mattisson, E. Wirfalt, R. Galasso, D. Palli, et al. 2005. Plasma carotenoids as biomarkers of intake of fruits and vegetables: Individual-level correlations in the european prospective investigation into cancer and nutrition (epic). European Journal of Clinical Nutrition 59 (12):1387–96. doi:10.1038/sj.ejcn.1602252.
- Alfurayhi, R., L. Huang, and K. Brandt. 2023. Pathways affected by falcarinol-type polyacetylenes and implications for their anti-inflammatory function and potential in cancer chemoprevention. Foods (Basel, Switzerland) 12 (6):1192. doi:10.3390/foods12061192.
- Aune, D., D. S. M. Chan, A. R. Vieira, D. A. Navarro Rosenblatt, R. Vieira, D. C. Greenwood, and T. Norat. 2012. Dietary compared with blood concentrations of carotenoids and breast cancer risk: A systematic review and meta-analysis of prospective studies. The American Journal of Clinical Nutrition 96 (2):356–73. doi:10.3945/ajcn.112.034165.
- Aune, D., E. Giovannucci, P. Boffetta, L. T. Fadnes, N. Keum, T. Norat, D. C. Greenwood, E. Riboli, L. J. Vatten, S. Tonstad, et al. 2017. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. International Journal of Epidemiology 46 (3):1029–56. doi:10.1093/ije/dyw319.
- Aune, D., N. Keum, E. Giovannucci, L. T. Fadnes, P. Boffetta, D. C. Greenwood, S. Tonstad, L. J. Vatten, E. Riboli, T. Norat, et al. 2018. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. The American Journal of Clinical Nutrition 108 (5):1069–91. doi:10.1093/ajcn/nqy097.
- Bjelakovic, G., D. Nikolova, and C. Gluud. 2013. Antioxidant supplements to prevent mortality. Jama 310 (11):1178–9. doi:10.1001/jama.2013.277028.
- Chen, H., F. Shao, F. Zhang, and Q. Miao. 2018. Association between dietary carrot intake and breast cancer a meta-analysis. Medicine 97 (37):e12164. doi:10.1097/MD.0000000000012164.
- Chen, Y., S. Peng, Q. Luo, J. Zhang, Q. Guo, Y. Zhang, and X. Chai. 2015. Chemical and pharmacological progress on polyacetylenes isolated from the family apiaceae. Chemistry & Biodiversity 12 (4):474–502. doi:10.1002/cbdv.201300396.
- Christensen, L. P., and K. Brandt. 2006. Bioactive polyacetylenes in food plants of the apiaceae family: Occurrence, bioactivity and analysis. Journal of Pharmaceutical and Biomedical Analysis 41 (3):683–93. doi:10.1016/j.jpba.2006.01.057.
- DerSimonian, R., and N. Laird. 1986. Meta-analysis in clinical trials. Controlled Clinical Trials 7 (3):177–88. doi:10.1016/0197-2456(86)90046-2.
- EFSA Panel on Dietetic Products. 2021. General scientific guidance for stakeholders on health claim applications (revision 1). EFSA Journal 19 (3):6553–397.
- Egger, M., G. D. Smith, M. Schneider, and C. Minder. 1997. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research ed.) 315 (7109):629–34. doi:10.1136/bmj.315.7109.629.
- Fallahzadeh, H., A. Jalali, M. Momayyezi, and S. Bazm. 2015. Effect of carrot intake in the prevention of gastric cancer: A meta-analysis. Journal of Gastric Cancer 15 (4):256–61. doi:10.5230/jgc.2015.15.4.256.
- Greenland, S., and M. P. Longnecker. 1992. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. American Journal of Epidemiology 135 (11):1301–9. doi:10.1093/oxfordjournals.aje.a116237.
- Guo, Y., Y. L. Lu, and H. C. Jin. 2020. Appraising the role of circulating concentrations of micro-nutrients in epithelial ovarian cancer risk: A Mendelian Randomization analysis. Scientific Reports 10 (1):7356. doi:10.1038/s41598-020-63909-5.
- Harrell, F. E., Jr., K. L. Lee, and B. G. Pollock. 1988. Regression models in clinical studies: Determining relationships between predictors and response. Journal of the National Cancer Institute 80 (15):1198–202. doi:10.1093/jnci/80.15.1198.
- He, F. J., C. A. Nowson, and G. A. MacGregor. 2006. Fruit and vegetable consumption and stroke: Meta-analysis of cohort studies. Lancet (London, England) 367 (9507):320–6. doi:10.1016/S0140-6736(06)68069-0.
- Hendrickson, S. J., W. C. Willett, B. A. Rosner, and A. H. Eliassen. 2013. Food predictors of plasma carotenoids. Nutrients 5 (10):4051–66. doi:10.3390/nu5104051.
- Higgins, J. P., and S. G. Thompson. 2002. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 21 (11):1539–58. doi:10.1002/sim.1186.
- Holden, J. M., A. L. Eldridge, G. R. Beecher, I. Marilyn Buzzard, S. Bhagwat, C. S. Davis, L. W. Douglass, S. Gebhardt, D. Haytowitz, S. Schakel, et al. 1999. Carotenoid content of U.S. Foods: An update of the database. Journal of Food Composition and Analysis 12 (3):169–96. doi:10.1006/jfca.1999.0827.
- Kobaek-Larsen, M., G. Baatrup, M. KhataeiNotabi, R. B. El-Houri, E. Pipó-Ollé, E. Christensen Arnspang, and L. P. Christensen. 2019. Dietary polyacetylenic oxylipins falcarinol and falcarindiol prevent inflammation and colorectal neoplastic transformation: A mechanistic and dose-response study in a rat model. Nutrients 11 (9):2223. doi:10.3390/nu11092223.
- Lee, H.-S., Y.-H. Cho, J. Park, H.-R. Shin, and M.-K. Sung. 2013. Dietary intake of phytonutrients in relation to fruit and vegetable consumption in Korea. Journal of the Academy of Nutrition and Dietetics 113 (9):1194–9. doi:10.1016/j.jand.2013.04.022.
- Moher, D., A. Liberati, J. Tetzlaff, D. Altman, and P. Grp. 2009. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine 6 (7):e1000097. doi:10.1371/journal.pmed.1000097.
- Moorthy, M., N. Chaiyakunapruk, S. A. Jacob, and U. D. Palanisamy. 2020. Prebiotic potential of polyphenols, its effect on gut microbiota and anthropometric/clinical markers: A systematic review of randomised controlled trials. Trends in Food Science & Technology 99:634–49. doi:10.1016/j.tifs.2020.03.036.
- Musa-Veloso, K., J. Card, A. Wong, and D. Cooper. 2009. Influence of observational study design on the interpretation of cancer risk reduction by carotenoids. Nutrition Reviews 67 (9):527–45. doi:10.1111/j.1753-4887.2009.00225.x.
- O’Neill, M. E., Y. Carroll, B. Corridan, B. Olmedilla, F. Granado, I. Blanco, H. Van den Berg, I. Hininger, A. M. Rousell, M. Chopra, et al. 2001. A European carotenoid database to assess carotenoid intakes and its use in a five-country comparative study. The British Journal of Nutrition 85 (4):499–507. doi:10.1079/bjn2000284.
- Ojobor, C., G. M. O’Brien, M. Siervo, C. Ogbonnaya, and K. Brandt. 2023. Data on carrot intake (measured directly or as intake or plasma/serum concentration of alpha-carotene) and cancer incidence from a systematic review and meta-analysis of observational studies. Newcastle University doi:10.25405/data.ncl.21931533.
- Ojobor, C., M. Siervo, G. O’Brien, and K. Brandt. 2019. A systematic review and meta-analysis of associations between carrot consumption and cancer risks. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=124009.
- Orsini, N., R. Li, A. Wolk, P. Khudyakov, and D. Spiegelman. 2012. Meta-analysis for linear and nonlinear dose-response relations: Examples, an evaluation of approximations, and software. American Journal of Epidemiology 175 (1):66–73. doi:10.1093/aje/kwr265.
- Parent, M. E., H. Richard, M. C. Rousseau, and K. Trudeau. 2018. Vitamin C intake and risk of prostate cancer: The Montreal Proteus study. Frontiers in Physiology 9:1218. doi:10.3389/fphys.2018.01218.
- Peto, R., R. Doll, J. D. Buckley, and M. B. Sporn. 1981. Can dietary beta-carotene materially reduce human cancer rates? Nature 290 (5803):201–8. doi:10.1038/290201a0.
- Saini, R. K., Y. S. Keum, M. Daglia, and K. R. R. Rengasamy. 2020. Dietary carotenoids in cancer chemoprevention and chemotherapy: A review of emerging evidence. Pharmacological Research 157:104830. doi:10.1016/j.phrs.2020.104830.
- Snene, A., C. Sirignano, D. Rigano, C. Formisano, R. El Mokni, G. Ercolano, H. Dhaouadi, A. Ianaro, S. Hammami, O. Taglialatela-Scafati, et al. 2017. Antiproliferative metabolites from the Northern African endemic plant Daucus virgatus (Apiaceae). Phytochemistry 143:194–8. doi:10.1016/j.phytochem.2017.08.010.
- Tatsioni, A., N. G. Bonitsis, and J. P. A. Ioannidis. 2007. Persistence of contradicted claims in the literature. Jama 298 (21):2517–26. doi:10.1001/jama.298.21.2517.
- Tsilidis, K. K., N. Papadimitriou, N. Dimou, D. Gill, S. J. Lewis, R. M. Martin, N. Murphy, G. Markozannes, V. Zuber, A. J. Cross, et al. 2021. Genetically predicted circulating concentrations of micronutrients and risk of colorectal cancer among individuals of European descent: A Mendelian Randomization study. The American Journal of Clinical Nutrition 113 (6):1490–502. doi:10.1093/ajcn/nqab003.
- Vieira, A. R., L. Abar, S. Vingeliene, D. S. M. Chan, D. Aune, D. Navarro-Rosenblatt, C. Stevens, D. Greenwood, and T. Norat. 2016. Fruits, vegetables and lung cancer risk: A systematic review and meta-analysis. Annals of Oncology: Official Journal of the European Society for Medical Oncology 27 (1):81–96. doi:10.1093/annonc/mdv381.
- Wallace, T. C., R. L. Bailey, J. B. Blumberg, B. Burton-Freeman, C.-Y O. Chen, K. M. Crowe-White, A. Drewnowski, S. Hooshmand, E. Johnson, R. Lewis, et al. 2020. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Critical Reviews in Food Science and Nutrition 60 (13):2174–211. doi:10.1080/10408398.2019.1632258.
- Wang, X. M., D. M. Glubb, and T. A. O’Mara. 2023. Dietary factors and endometrial cancer risk: A Mendelian Randomization study. Nutrients 15 (3):603. doi:10.3390/nu15030603.
- WCRF/AICR. 2018. Wholegrains, vegetables and fruit and the risk of cancer. Continuous update project expert report 2018. World Cancer Research Fund/American Institute for Cancer Research. Available at https://www.wcrf.org/wp-content/uploads/2020/12/Wholegrains-veg-and-fruit.pdf
- Wells, G. A., B. Shea, D. O’Connell, J. Peterson, V. Welch, M. Losos, et al. 2015. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Williams, W. R. 1898. Remarks on the mortality from cancer. The Lancet 152 (3912):481–2.
- Xu, H., H. Jiang, W. Yang, F. Song, S. Yan, C. Wang, W. Fu, H. Li, C. Lyu, Y. Gan, et al. 2019. Is carrot consumption associated with a decreased risk of lung cancer? A meta-analysis of observational studies. The British Journal of Nutrition 122 (5):488–98. doi:10.1017/S0007114519001107.
- Xu, X., Y. Cheng, S. Li, Y. Zhu, X. Xu, X. Zheng, Q. Mao, and L. Xie. 2014. Dietary carrot consumption and the risk of prostate cancer. European Journal of Nutrition 53 (8):1615–23. doi:10.1007/s00394-014-0667-2.
- Yao, Y., T. Suo, R. Andersson, Y. Cao, C. Wang, J. Lu, and E. Chui. 2017. Dietary fibre for the prevention of recurrent colorectal adenomas and carcinomas. The Cochrane Database of Systematic Reviews 1 (1):CD003430. doi:10.1002/14651858.CD003430.pub2.
- Zhang, X., H. Zhao, J. Man, X. Yin, T. Zhang, X. Yang, and M. Lu. 2022. Investigating causal associations of diet-derived circulating antioxidants with the risk of digestive system cancers: A Mendelian Randomization study. Nutrients 14 (15):3237. doi:10.3390/nu14153237.
- Zhang, Y., J. Yang, X. Na, and A. Zhao. 2023. Association between β-carotene supplementation and risk of cancer: A meta-analysis of randomized controlled trials. Nutrition Reviews 81 (9):1118–30. doi:10.1093/nutrit/nuac110.
- Zhao, H., S. N. Wu, H. L. Liu, Z. K. Luo, J. W. Sun, and X. L. Jin. 2023. Relationship between food-derived antioxidant vitamin intake and breast cancer risk: A Mendelian Randomized study. European Journal of Nutrition 62 (6):2365–73. doi:10.1007/s00394-023-03158-0.
- Zickute, J., L. Strumylaite, L. Dregval, J. Petrauskiene, J. Dudzevicius, and E. Stratilatovas. 2005. Daržovių bei vaisių vartojimas ir skrandžio vėžio rizika. [vegetables and fruits and risk of stomach cancer]. Medicina (Kaunas, Lithuania) 41 (9):733–40.
References to the studies included in the meta-analysis
- Boggs, D. A., J. R. Palmer, L. A. Wise, D. Spiegelman, M. J. Stampfer, L. L. Adams-Campbell, and L. Rosenberg. 2010. Fruit and vegetable intake in relation to risk of breast cancer in the Black Women’s Health Study. American Journal of Epidemiology 172 (11):1268–79. doi:10.1093/aje/kwq293.
- Cho, E., D. Spiegelman, D. J. Hunter, et al. 2003. Premenopausal intakes of vitamins A, C, and E, folate, and carotenoids, and risk of breast cancer. Cancer Epidemiology and Prevention Biomarkers 12 (8):713–20.
- Cohen, K., Y. Liu, J. Luo, C. M. Appleton, and G. A. Colditz. 2017. Plasma carotenoids and the risk of premalignant breast disease in women aged 50 and younger: A nested case–control study. Breast Cancer Research and Treatment 162 (3):571–80. doi:10.1007/s10549-017-4152-5.
- Cui, Y., J. M. Shikany, S. Liu, Y. Shagufta, and T. E. Rohan. 2008. Selected antioxidants and risk of hormone receptor-defined invasive breast cancers among postmenopausal women in the Women’s Health Initiative Observational Study. The American Journal of Clinical Nutrition 87 (4):1009–18. doi:10.1093/ajcn/87.4.1009.
- Deding, U., G. Baatrup, L. P. Christensen, and M. Kobaek-Larsen. 2020. Carrot intake and risk of colorectal cancer: A prospective cohort study of 57,053 Danes. Nutrients 12 (2):332. doi:10.3390/nu12020332.
- Dorgan, J. F., A. Sowell, C. A. Swanson, N. Potischman, R. Miller, N. Schussler, and H. E. Stephenson. 1998. Relationships of serum carotenoids, retinol, alpha-tocopherol, and selenium with breast cancer risk: Results from a prospective study in Columbia, Missouri (United States). Cancer Causes & Control: CCC 9 (1):89–97. doi:10.1023/a:1008857521992.
- Dorjgochoo, T., Y.-T. Gao, W.-H. Chow, X.-O. Shu, H. Li, G. Yang, Q. Cai, N. Rothman, H. Cai, A. A. Franke, et al. 2009. Plasma carotenoids, tocopherols, retinol and breast cancer risk: Results from the Shanghai Women Health Study (SWHS). Breast Cancer Research and Treatment 117 (2):381–9. doi:10.1007/s10549-008-0270-4.
- Epplein, M., Y. B. Shvetsov, L. R. Wilkens, A. A. Franke, R. V. Cooney, L. Le Marchand, B. E. Henderson, L. N. Kolonel, and M. T. Goodman. 2009. Plasma carotenoids, retinol, and tocopherols and postmenopausal breast cancer risk in the Multiethnic Cohort Study: A nested case-control study. Breast Cancer Research: BCR 11 (4):R49. doi:10.1186/bcr2338.
- Farvid, M. S., W. Y. Chen, K. B. Michels, E. Cho, W. C. Willett, and A. H. Eliassen. 2016. Fruit and vegetable consumption in adolescence and early adulthood and risk of breast cancer: Population based cohort study. BMJ (Clinical Research ed.) 353: I 2343. doi:10.1136/bmj.i2343.
- Farvid, M. S., W. Y. Chen, B. A. Rosner, R. M. Tamimi, W. C. Willett, and A. H. Eliassen. 2019. Fruit and vegetable consumption and breast cancer incidence: Repeated measures over 30 years of follow‐up. International Journal of Cancer 144 (7):1496–510. doi:10.1002/ijc.31653.
- Fink, B. N., M. M. Gaudet, J. A. Britton, P. E. Abrahamson, S. L. Teitelbaum, J. Jacobson, P. Bell, J. A. Thomas, G. C. Kabat, A. I. Neugut, et al. 2006. Fruits, vegetables, and micronutrient intake in relation to breast cancer survival. Breast Cancer Research and Treatment 98 (2):199–208. doi:10.1007/s10549-005-9150-3.
- Flood, A., E. M. Velie, N. Chaterjee, A. F. Subar, F. E. Thompson, J. V. Lacey, C. Schairer, R. Troisi, and A. Schatzkin. 2002. Fruit and vegetable intakes and the risk of colorectal cancer in the Breast Cancer Detection Demonstration Project follow-up cohort. The American Journal of Clinical Nutrition 75 (5):936–43. doi:10.1093/ajcn/75.5.936.
- Freedman, N. D., Y. Park, A. F. Subar, A. R. Hollenbeck, M. F. Leitzmann, A. Schatzkin, and C. C. Abnet. 2008. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. International Journal of Cancer 122 (10):2330–6. doi:10.1002/ijc.23319.
- Freedman, N. D., Y. Park, A. F. Subar, A. R. Hollenbeck, M. F. Leitzmann, A. Schatzkin, and C. C. Abnet. 2007. Fruit and vegetable intake and esophageal cancer in a large prospective cohort study. International Journal of Cancer 121 (12):2753–60. doi:10.1002/ijc.22993.
- Gann, P. H., J. Ma, E. L. Giovannucci, et al. 1999. Lower prostate cancer risk in men with elevated plasma lycopene levels: Results of a prospective analysis. Cancer Research 59 (6):1225–30.
- Giovannucci, E., A. Ascherio, E. B. Rimm, M. J. Stampfer, G. A. Colditz, and W. C. Willett. 1995. Intake of carotenoids and retino in relation to risk of prostate cancer. Journal of the National Cancer Institute 87 (23):1767–76. doi:10.1093/jnci/87.23.1767.
- Goodman, G. E., S. Schaffer, G. S. Omenn, C. Chen, and I. King. 2003. Association between lung and prostate cancer risk and serum micronutrients: Results and lessons learned from B-carotene and retinol efficacy trial. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 12 (6):518–26.
- Heinen, M. M., B. A. Verhage, R. A. Goldbohm, and P. A. van den Brandt. 2012. Intake of vegetables, fruits, carotenoids, vitamins C, E, and pancreatic cancer risk in The Netherlands Cohort Study. International Journal of Cancer 130 (1):147–58. doi:10.1002/ijc.25989.
- Holick, C. N., I. De Vivo, D. Feskanich, E. Giovannucci, M. Stampfer, and D. S. Michaud. 2005. Intake of fruits and vegetables, carotenoids, folate, and vitamins A, C, E and risk of bladder cancer among women (United States). Cancer Causes & Control: CCC 16 (10):1135–45. doi:10.1007/s10552-005-0337-z.
- Holick, C. N., D. S. Michaud, R. Stolzenberg-Solomon, S. T. Mayne, P. Pietinen, P. R. Taylor, J. Virtamo, and D. Albanes. 2002. Dietary carotenoids, serum beta-carotene, and retinol and risk of lung cancer in the alpha-tocopherol, beta-carotene cohort study. American Journal of Epidemiology 156 (6):536–47. doi:10.1093/aje/kwf072.
- Horn-Ross, P. L., K. J. Hoggatt, D. W. West, M. R. Krone, S. L. Stewart, H. Anton, C. L. Bernstei, D. Deapen, D. Peel, R. Pinder, et al. 2002. Recent diet and breast cancer risk: The California Teachers Study (USA). Cancer Causes & Control: CCC 13 (5):407–15. doi:10.1023/a:1015786030864.
- Huang, H. Y., A. J. Alberg, E. P. Norkus, S. C. Hoffman, G. W. Comstock, and K. J. Helzlsouer. 2003. Prospective study of antioxidant micronutrients in the blood and the risk of developing prostate cancer. American Journal of Epidemiology 157 (4):335–44. doi:10.1093/aje/kwf210.
- Hultén, K., A. L. Van Kappel, A. Winkvist, R. Kaaks, G. Hallmans, P. Lenner, and E. Riboli. 2001. Carotenoids, alpha-tocopherols, and retinol in plasma and breast cancer risk in northern Sweden. Cancer Causes & Control: CCC 12 (6):529–37. doi:10.1023/a:1011271222153.
- Ito, Y., M. Kurata, R. Hioki, K. Suzuki, J. Ochiai, and K. Aoki. 2005b. Cancer mortality and serum levels of carotenoids, retinol, and tocopherol: A population-based follow-up study of inhabitants of a rural area of Japan. Asian Pacific Journal of Cancer Prevention 6 (1):10–5.
- Ito, Y., K. Wakai, K. Suzuki, K. Ozasa, Y. Watanabe, N. Seki, M. Ando, Y. Nishino, T. Kondo, Y. Ohno, et al. 2005a. Lung cancer mortality and serum levels of carotenoids, retinol, tocopherols, and folic acid in men and women: A case-control study nested in the JACC Study. Journal of Epidemiology 15 Suppl 2 (Suppl II):S140–S149. doi:10.2188/jea.15.s140.
- Ito, Y., K. Wakai, K. Suzuki, A. Tamakoshi, N. Seki, M. Ando, Y. Nishino, T. Kondo, Y. Watanabe, K. Ozasa, Study., et al. 2003. Serum carotenoids and mortality from lung cancer: A case‐control study nested in the Japan Collaborative Cohort (JACC. Cancer Science 94 (1):57–63.) doi:10.1111/j.1349-7006.2003.tb01352.x.
- Jansen, R. J., D. P. Robinson, R. Z. Stolzenberg-Solomon, W. R. Bamlet, M. de Andrade, A. L. Oberg, K. G. Rabe, K. E. Anderson, J. E. Olson, R. Sinha, et al. 2013. Nutrients from fruit and vegetable consumption reduce the risk of pancreatic cancer. Journal of Gastrointestinal Cancer 44 (2):152–61. doi:10.1007/s12029-012-9441-y.
- Jenab, M., E. Riboli, P. Ferrari, M. Friesen, J. Sabate, T. Norat, N. Slimani, A. Tjønneland, A. Olsen, K. Overvad, et al. 2006. Plasma and dietary carotenoid, retinol and tocopherol levels and the risk of gastric adenocarcinomas in the European prospective investigation into cancer and nutrition. British Journal of Cancer 95 (3):406–15. doi:10.1038/sj.bjc.6603266.
- Jeurnink, S. M., M. M. Ros, M. Leenders, F. J. B. van Duijnhoven, P. D. Siersema, E. H. J. M. Jansen, C. H. van Gils, M. F. Bakker, K. Overvad, N. Roswall, et al. 2015. Plasma carotenoids, vitamin C, retinol and tocopherols levels and pancreatic cancer risk within the European Prospective Investigation into Cancer and Nutrition: A nested case–control study: Plasma micronutrients and pancreatic cancer risk. International Journal of Cancer 136 (6):E665–E676. doi:10.1002/ijc.29175.
- Kabat, G. C., M. Y. Kim, G. E. Sarto, J. M. Shikany, and T. E. Rohan. 2012. Repeated measurements of serum carotenoid, retinol and tocopherol levels in relation to colorectal cancer risk in the Women’s Health Initiative. European Journal of Clinical Nutrition 66 (5):549–54. doi:10.1038/ejcn.2011.207.
- Kabat, G. C., M. Kim, L. L. Adams-Campbell, B. J. Caan, R. T. Chlebowski, M. L. Neuhouser, J. M. Shikany, and T. E. Rohan, WHI Investigators. 2009. Longitudinal study of serum carotenoid, retinol, and tocopherol concentrations in relation to breast cancer risk among postmenopausal women. The American Journal of Clinical Nutrition 90 (1):162–9. doi:10.3945/ajcn.2009.27568.
- Kirsh, V. A., R. B. Hayes, S. T. Mayne, N. Chatterjee, A. F. Subar, L. B. Dixon, D. Albanes, G. L. Andriole, D. A. Urban, U. Peters, et al. 2006. Supplemental and dietary vitamin E, beta-carotene, and vitamin C intakes and prostate cancer risk. Journal of the National Cancer Institute 98 (4):245–54. doi:10.1093/jnci/djj050.
- Kurahashi, N., M. Inoue, M. Iwasaki, Y. Tanaka, M. Mizokami, and S. Tsugane, JPHC Study Group. 2009. Vegetable, fruit and antioxidant nutrient consumption and subsequent risk of hepatocellular carcinoma: A prospective cohort study in Japan. British Journal of Cancer 100 (1):181–4. doi:10.1038/sj.bjc.6604843.
- Larsson, S. C., L. Bergkvist, and A. Wolk. 2010. Dietary carotenoids and risk of hormone receptor-defined breast cancer in a prospective cohort of Swedish women. European Journal of Cancer (Oxford, England: 1990) 46 (6):1079–85. doi:10.1016/j.ejca.2010.01.004.
- Larsson, S. C., L. Bergkvist, I. Näslund, J. Rutegård, and A. Wolk. 2007. Vitamin A, retinol, and carotenoids and the risk of gastric cancer: A prospective cohort study. The American Journal of Clinical Nutrition 85 (2):497–503. doi:10.1093/ajcn/85.2.497.
- Maillard, V., K. Kuriki, B. Lefebvre, M.-C. Boutron-Ruault, G. M. Lenoir, V. Joulin, F. Clavel-Chapelon, and V. Chajès. 2010. Serum carotenoid, tocopherol and retinol concentrations and breast cancer risk in the E3N‐EPIC study. International Journal of Cancer 127 (5):1188–96. doi:10.1002/ijc.25138.
- Malila, N., J. Virtamo, M. Virtanen, P. Pietinen, D. Albanes, and L. Teppo. 2002. Dietary and serum alpha-tocopherol, beta-carotene and retinol, and risk for colorectal cancer in male smokers. European Journal of Clinical Nutrition 56 (7):615–21. doi:10.1038/sj.ejcn.1601366.
- McEligot, J. A., J. Largent, A. Ziogas, D. Peel, and H. Anton-Culver. 2006. Dietary fat, fiber, vegetable, and micronutrients are associated with overall survival in postmenopausal women diagnosed with breast cancer. Nutrition and Cancer 55 (2):132–40. doi:10.1207/s15327914nc5502_3.
- Michaud, D. S., P. Pietinen, P. R. Taylor, M. Virtanen, J. Virtamo, and D. Albanes. 2002. Intakes of fruits and vegetables, carotenoids and vitamins A, E, C in relation to the risk of bladder cancer in the ATBC cohort study. British Journal of Cancer 87 (9):960–5. doi:10.1038/sj.bjc.6600604.
- Michaud, D. S., D. Feskanich, E. B. Rimm, G. A. Colditz, F. E. Speizer, W. C. Willett, and E. Giovannucci. 2000. Intake of specific carotenoids and risk of lung cancer in 2 prospective US cohorts. The American Journal of Clinical Nutrition 72 (4):990–7. doi:10.1093/ajcn/72.4.990.
- Michaud, D. S., D. Spiegelman, S. K. Clinton, E. B. Rimm, W. C. Willett, and E. L. Giovannucci. 1999. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. Journal of the National Cancer Institute 91 (7):605–13. doi:10.1093/jnci/91.7.605.
- Min, K. B., and J. Y. Min. 2014. Serum carotenoid levels and risk of lung cancer death in US adults. Cancer Science 105 (6):736–43. doi:10.1111/cas.12405.
- Narita, S., E. Saito, N. Sawada, T. Shimazu, T. Yamaji, M. Iwasaki, J. Ishihara, R. Takachi, K. Shibuya, M. Inoue, et al. 2018. Dietary consumption of antioxidant vitamins and subsequent lung cancer risk: The Japan Public Health Center-based prospective study. International Journal of Cancer 142 (12):2441–60. doi:10.1002/ijc.31268.
- Neuhouser, M. L., R. E. Patterson, M. Thornquist, G. S. Omenn, I. B. King, and G. E. Goodman. 2003. Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the beta-carotene and retinol efficacy trial (CARET). Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 12 (4):350–8.
- Nomura, A. M., J. Lee, G. N. Stemmermann, and A. A. Franke. 2003. Serum vitamins and the subsequent risk of bladder cancer. The Journal of Urology 170 (4 Pt 1):1146–50. doi:10.1097/01.ju.0000086040.24795.ad.
- Ollberding, N. J., G. Maskarinec, S. M. Conroy, Y. Morimoto, A. A. Franke, R. V. Cooney, L. R. Wilkens, L. Le Marchand, M. T. Goodman, B. Y. Hernandez, et al. 2012. Prediagnostic circulating carotenoid levels and the risk of non-Hodgkin lymphoma: The multiethnic cohort. Blood 119 (24):5817–23. doi:10.1182/blood-2012-02-413609.
- Park, S. Y., N. J. Ollberding, C. G. Woolcott, L. R. Wilkens, B. E. Henderson, and L. N. Kolonel. 2013. Fruit and vegetable intakes are associated with lower risk of bladder cancer among women in the Multiethnic Cohort Study. The Journal of Nutrition 143 (8):1283–92. doi:10.3945/jn.113.174920.
- Persson, C., S. Sasazuki, M. Inoue, N. Kurahashi, M. Iwasaki, T. Miura, W. Ye, and S. Tsugane, JPHC Study Group. 2008. Plasma levels of carotenoids, retinol and tocopherol and the risk of gastric cancer in Japan: A nested case–control study. Carcinogenesis 29 (5):1042–8. doi:10.1093/carcin/bgn072.
- Peters, U., M. F. Leitzmann, N. Chatterjee, Y. Wang, D. Albanes, E. P. Gelmann, M. D. Friesen, E. Riboli, and R. B. Hayes. 2007. Serum lycopene, other carotenoids, and prostate cancer risk: A nested case-control study in the prostate, lung, colorectal and ovarian cancer screening trial. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 16 (5):962–8. doi:10.1158/1055-9965.EPI-06-0861.
- Ratnasinghe, D., M. R. Forman, J. A. Tangrea, Y. Qiao, S. X. Yao, E. W. Gunter, M. J. Barrett, C. A. Giffen, Y. Erozan, M. S. Tockman, et al. 2000. Serum carotenoids are associated with increased lung cancer risk among alcohol drinkers, but not among non-drinkers in a cohort of tin miners. Alcohol and Alcoholism (Oxford, Oxfordshire) 35 (4):355–60. doi:10.1093/alcalc/35.4.355.
- Rohan, T. E., M. Jain, G. R. Howe, and A. B. Miller. 2002. A cohort study of dietary carotenoids and lung cancer risk in women (Canada). Cancer Causes & Control: CCC 13 (3):231–7. doi:10.1023/a:1015048619413.
- Ros, M. M., H. B. Bueno-de-Mesquita, E. Kampman, K. K. H. Aben, F. L. Büchner, E. H. J. M. Jansen, C. H. van Gils, L. Egevad, K. Overvad, A. Tjønneland, et al. 2012. Plasma carotenoids and vitamin C concentrations and risk of urothelial cell carcinoma in the European Prospective Investigation into Cancer and Nutrition. The American Journal of Clinical Nutrition 96 (4):902–10. doi:10.3945/ajcn.111.032920.
- Sato, R., K. J. Helzlsouer, A. J. Alberg, S. C. Hoffman, E. P. Norkus, and G. W. Comstock. 2002. Prospective study of carotenoids, tocopherols, and retinoid concentrations and the risk of breast cancer. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 11 (5):451–7.
- Schuurman, A. G., R. A. Goldbohm, H. A. Brants, and P. A. van den Brandt. 2002. A prospective cohort study on intake of retinol, vitamins C and E, and carotenoids and prostate cancer risk (Netherlands). Cancer Causes & Control: CCC 13 (6):573–82. doi:10.1023/a:1016332208339.
- Sesso, H. D., J. E. Buring, S. M. Zhang, E. P. Norkus, and J. M. Gaziano. 2005. Dietary and plasma lycopene and the risk of breast cancer. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 14 (5):1074–81. doi:10.1158/1055-9965.EPI-04-0683.
- Silvera, S. A.., M. Jain, G. R. Howe, A. B. Miller, and T. E. Rohan. 2006. Carotenoid, vitamin A, vitamin C, and vitamin E intake and risk of ovarian cancer: A prospective cohort study. Cancer epidemiology, biomarkers & prevention 15 (2):395–397. doi:10.1158/1055-9965.EPI-05-0835.
- Speizer, F. E., G. A. Colditz, D. J. Hunter, B. Rosner, and C. Hennekens. 1999. Prospective study of smoking, antioxidant intake, and lung cancer in middle-aged women (USA). Cancer Causes & Control: CCC 10 (5):475–82. doi:10.1023/a:1008931526525.
- Steck-Scott, S., M. R. Forman, A. Sowell, C. B. Borkowf, P. S. Albert, M. Slattery, B. Brewer, B. Caan, E. Paskett, F. Iber, et al. 2004. Carotenoids, vitamin A and risk of adenomatous polyp recurrence in the polyp prevention trial. International Journal of Cancer 112 (2):295–305. doi:10.1002/ijc.20364.
- Steevens, J., L. J. Schouten, R. A. Goldbohm, and P. A. van den Brandt. 2011. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. International Journal of Cancer 129 (11):2681–93. doi:10.1002/ijc.25928.
- Steinmetz, K. A., L. H. Kushi, R. M. Bostick, A. R. Folsom, and J. D. Potter. 1994. Vegetables, fruit, and colon cancer in the Iowa Women’s Health Study: 4. Rehabilitation Oncology 12 (2):19. doi:10.1097/01893697-199412020-00021.
- Stram, D. O., J. H. Hankin, L. R. Wilkens, S. Park, B. E. Henderson, A. M. Y. Nomura, M. C. Pike, and L. N. Kolonel. 2006. Prostate cancer incidence and intake of fruits, vegetables and related micronutrients: The multiethnic cohort study*(United States). Cancer Causes & Control: CCC 17 (9):1193–207. doi:10.1007/s10552-006-0064-0.
- Tamimi, R. M., G. A. Colditz, and S. E. Hankinson. 2009. Circulating carotenoids, mammographic density, and subsequent risk of breast cancer. Cancer Research 69 (24):9323–9. doi:10.1158/0008-5472.CAN-09-1018.
- Terry, P., M. Jain, A. B. Miller, G. R. Howe, and T. E. Rohan. 2002. Dietary carotenoids and risk of breast cancer. The American Journal of Clinical Nutrition 76 (4):883–8. doi:10.1093/ajcn/76.4.883.
- Thompson, C. A., T. M. Habermann, A. H. Wang, R. A. Vierkant, A. R. Folsom, J. A. Ross, and J. R. Cerhan. 2010. Antioxidant intake from fruits, vegetables and other sources and risk of non‐Hodgkin’s lymphoma: The Iowa Women’s Health Study. International Journal of Cancer 126 (4):992–1003. doi:10.1002/ijc.24830.
- Toniolo, P., A. L. Van Kappel, A. Akhmedkhanov, P. Ferrari, I. Kato, R. E. Shore, and E. Riboli. 2001. Serum carotenoids and breast cancer. American Journal of Epidemiology 153 (12):1142–7. doi:10.1093/aje/153.12.1142.
- Umesawa, M., H. Iso, K. Mikami, T. Kubo, K. Suzuki, Y. Watanabe, M. Mori, T. Miki, and A. Tamakoshi, JACC Study Group. 2014. Relationship between vegetable and carotene intake and risk of prostate cancer: The JACC study. British Journal of Cancer 110 (3):792–6. doi:10.1038/bjc.2013.685.
- van Dijk, B. A. C., L. J. Schouten, E. Oosterwijk, C. A. Hulsbergen-van de Kaa, L. A. L. M. Kiemeney, R. A. Goldbohm, J. A. Schalken, and P. A. van den Brandt. 2008. Carotenoid and vitamin intake, von Hippel-Lindau gene mutations and sporadic renal cell carcinoma. Cancer Causes & Control: CCC 19 (2):125–34. doi:10.1007/s10552-007-9078-5.
- Voorrips, L. E., R. A. Goldbohm, H. A. Brants, et al. 2000. A prospective cohort study on antioxidant and folate intake and male lung cancer risk. Cancer Epidemiology and Prevention Biomarkers 9 (4):357–65.
- Voorrips, L. E., R. A. Goldbohm, D. T. Verhoeven, G. A. van Poppel, F. Sturmans, R. J. Hermus, and P. A. van den Brandt. 2000. Vegetable and fruit consumption and lung cancer risk in the Netherlands Cohort Study on diet and cancer. Cancer Causes & Control: CCC 11 (2):101–15. doi:10.1023/a:1008906706084.
- Wise, L. A., A. K. Wesselink, T. N. Bethea, T. M. Brasky, G. Wegienka, Q. Harmon, T. Block, and D. D. Baird. 2021. Intake of lycopene and other carotenoids and incidence of uterine leiomyomata: A prospective ultrasound study. Journal of the Academy of Nutrition and Dietetics 121 (1):92–104. doi:10.1016/j.jand.2020.08.013.
- Wu, K., J. W. Erdman, S. J. Schwartz, E. A. Platz, M. Leitzmann, S. K. Clinton, V. DeGroff, W. C. Willett, and E. Giovannucci. 2004. Plasma and dietary carotenoids, and the risk of prostate cancer: A nested case-control study. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 13 (2):260–9. doi:10.1158/1055-9965.epi-03-0012.
- Xu, X., Y. Zhu, S. Ye, S. Li, B. Xie, H. Meng, S. Wang, and D. Xia. 2021. Association of dietary carrot intake with bladder cancer risk in a prospective cohort of 99,650 individuals with 12.5 years of follow-up. Frontiers in Nutrition 8:669630. doi:10.3389/fnut.2021.669630.
- Yuan, J. M., R. K. Ross, X. D. Chu, Y. T. Gao, and M. C. Yu. 2001. Prediagnostic levels of serum beta-cryptoxanthin and retinol predict smoking-related lung cancer risk in Shanghai, China. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 10 (7):767–73.
- Zeegers, M. P. A., R. A. Goldbohm, and P. A. Van den Brandt. 2001. Are retinol, vitamin C, vitamin E, folate and carotenoids intakes associated with bladder cancer risk? Results from the Netherlands Cohort Study. British Journal of Cancer 85 (7):977–83. doi:10.1054/bjoc.2001.1968.
- Zeegers, M. P., R. A. Goldbohm, and P. A. Van den Brandt. 2001. Consumption of vegetables, fruits, and urothelial cancer incidence: A prospective study. Cancer Epidemiology and Prevention Biomarkers 10 (11):1121–8.
- Zhang, S. M., D. J. Hunter, B. A. Rosner, et al. 2000. Intakes of fruits, vegetables, and related nutrients and the risk of non-Hodgkin’s lymphoma among women. Cancer Epidemiology and Prevention Biomarkers 9 (5):477–85.
- Zhang, S., D. J. Hunter, M. R. Forman, B. A. Rosner, F. E. Speizer, G. A. Colditz, J. E. Manson, S. E. Hankinson, and W. C. Willett. 1999. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. Journal of the National Cancer Institute 91 (6):547–56. doi:10.1093/jnci/91.6.547.