Abstract
The gastrointestinal immune system is crucial for overall health, safeguarding the human body against harmful substances and pathogens. One key player in this defense is dietary fiber pectin, which supports the gut’s immune barrier and fosters beneficial gut bacteria. Pectin’s composition, including degree of methylation (DM), RG-I, and neutral sugar content, influences its health benefits. This review assesses how pectin composition impacts the gastrointestinal immune barrier and what advantages specific chemistries of pectin has for metabolic, cardiovascular, and immune health. We delve into recent findings regarding pectin’s interactions with the immune system, including receptors like TLRs and galectin 3. Pectin is shown to fortify mucosal and epithelial layers, but the specific effects are structure dependent. Additionally, we explore potential strategies for enhancing the gut immune barrier function. Understanding how distinct pectin chemistries affect the gastrointestinal immune system is vital for developing preventive and therapeutic solutions for conditions related to microbiota imbalances and immune issues. Ultimately, this review offers insights into strategies to boost the gut immune barrier’s effectiveness, fostering better overall health by using specific pectins in the diet.
Introduction
Dietary fibers are plant-based carbohydrate structures that resist the digestive enzymes in the upper part of the human gastrointestinal tract and can reach the large intestine intact and serve as a fermentation substrate for the gut microbiota. These dietary fibers are considered to provide many health benefits (Anderson et al. Citation2009; Barber et al. Citation2020). It has been suggested that dietary fiber prevents or lowers the frequency of chronic diseases such as the risk for both type 1 and 2 diabetes and certain gastrointestinal diseases and they have been suggested to prevent or delay the development of heart failure and hypertension (Marques et al. Citation2017; Petruzziello et al. Citation2006). One such dietary fiber with health benefits is pectin. Pectin was traditionally seen as a gelling agent, thickening agent, and stabilizer in the food sector (Christensen Citation2020) and not so much as a health-promoting agent. In recent years it is more and more utilized as a fat replacer and health-promoting functional ingredient (Ciriminna et al. Citation2015; Min et al. Citation2010). Pectin has been shown to be instrumental in preventing gastrointestinal diseases (Fan et al. Citation2020; Jiang et al. Citation2016; Vogt et al. Citation2016), lower hyperglycemia (Hu et al. Citation2020; Liu et al. Citation2016), alleviate inflammatory processes (Hu et al. Citation2020; Vogt et al. Citation2016), and contribute to improved metabolic activities in cells such as by promoting mitochondrial health (Hu et al. Citation2020). Also, pectin is recognized for its beneficial effects on gut microbiota (Jiang et al. Citation2016). All these effects are highly dependent on the chemistry of the pectin applied. It has been shown that the diverse structural properties of pectin, such as degree of methylation (DM), RG-I, and neutral sugar composition, can contribute to their functional properties and final effect on the consumer’s health (Sousa et al. Citation2015). A major distinction in pectins is made in the DM but also other chemical characteristics as will be outlined in a dedicated section in this review have been shown to have a strong impact on its biological efficacy in promoting health. The current knowledge about this will be reviewed here.
Originally the health benefits were attributed to the possible prebiotic effects of pectin. Pectins have been shown to stimulate the growth of microbiota such as Ruminococcaceae, Succinivibrionaceae, Lachnospiraceae and Bacteroidaceae by supporting the adhesion of microbial cells to small intestinal cells (Zhao et al. Citation2018) and by stimulating production of fermentation products short-chain fatty acids (SCFAs) such as acetic and butyric acid (Bianchi et al. Citation2018; Slavin Citation2013). Enhanced consumption of citrus pectin with higher galacturonic acid (GalA) content (> 74%) was shown to alter the microbiota and its microbial metabolites (Li, Zhang, and Yang Citation2018). Especially the relative abundance of bacteroides, parabacteroides, olsenella, and bifidobacteria increased in the gut microbiota of mice on a high-fat diet, and also enhanced the production of SCFAs such as acetic acid and propionic acid (Li, Zhang, and Yang Citation2018). Pectin may also modulate microbiota composition by changing the ability of gut epithelial cells to allow adherence of gut bacteria. Early studies with crude pectin preparations have shown that some pectins may enhance the adhesion of Lactobacillus rhamnosus and Bifidobacterium bifidum to the gut epithelial cell line Caco-2 cells (Naqash et al. Citation2017; Parkar et al. Citation2010).
Not only pectin induced SCFA production but also pectin induced enhanced daily output of stool-neutral sterols and bile acids are considered to be health effects (Dongowski and Lorenz Citation2004; Reddy, Watanabe, and Sheinfil Citation1980). These effects are also pectin structure dependent as intestinal steroids in rats were higher with higher DM and higher molecular weights of pectin (Dongowski and Lorenz Citation2004). However, effects are not always dependent on the effects on gut microbiota. Also, direct effects on intestinal cells have been reported. Some pectins and in particular the lower DM pectins can stimulate the secretion of intestinal mucin by goblet cells and enhance the gut barrier function by forming a network of pectin with mucus which can exert a stronger protective effect toward toxins and pathogens (Hino et al. Citation2013; Liu et al. Citation2005). The gut immune barrier system plays a crucial role in preserving intestinal health and well-being (Bischoff Citation2011). During recent years more insight has become available on the interaction between pectin and different barrier layers within the gut immune barrier system. They do so by interacting with specific receptors on cells. It is now recognized that pectin interacts with different receptors such as pattern recognition receptors (PRR), galectin-3 (Gal-3) and pectin derived short chain fatty acids can bind to G protein-coupling receptors (GPCRs). In this review, we will discuss these interactions and how different types of pectins may interact with different layers of the gastrointestinal immune barrier. By reviewing the current knowledge on the impact of different pectin chemistries on the gastrointestinal immune barrier we hope to contribute to the development of customizing pectin formulations or developing products that can aid in preventing or delaying the onset of human disease.
Pectin and chemical variation in its composition
Pectin is a complex polysaccharide found in the primary cell walls of plants, where it provides structural support and plays a role in intercellular adhesion between plant cells (Caffall and Mohnen Citation2009). As pectin can enhance the viscosity of solutions, it is commonly used as a thickening agent (Chan et al. Citation2017; Ciriminna et al. Citation2015). Apple pomace and citrus peels are the main sources of commercially applied pectins. Other sources that have been considered include sugar beet, sunflower seed heads, and various fruits (May Citation1990). Pectin from sugar beet has several disadvantages as a commercial source due to its low gelling ability compared to pectin from apple and citrus sources. All these pectins may differ in their chemical structure and have different physicochemical effects on the product or on health benefits. Independent of the source, pectins can be found throughout the plant in different conformations and structures.
Structure of pectin
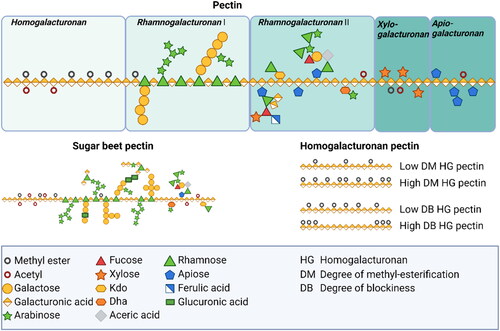
Pectin is composed of multiple domains, including homogalacturonan (HG), rhamnogalacturonan-I (RG-I), rhamnogalacturonan-II (RG-II), apiogalacturonan, and xylogalacturonan (). These domains can vary in their chemical structure and distribution within different plant organs and plant sources (Kontogiorgos Citation2020).
Figure 1. Schematic structure of pectin. Pectin molecules contain different regions such as homogalacturonan, rhamnogalacturonan I, rhamnogalacturonan II, xylogalacturonan, and apiogalacturonan. The primary structure that dominates the smooth pectin region is the linear, partially esterified HG backbone, accounting for approximately 60% of the total pectin. The hairy regions, on the other hand, consist primarily of highly branched RG I and RG II domains, with smaller contributions from xylogalacturonan and apiogalacturonan.
Galacturonans
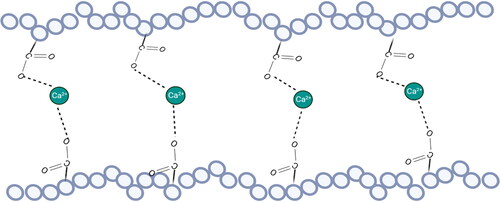
HG, as the smooth area of pectin, is a linear polymer of alpha-1,4-linked D-galacturonic acid residues with some of the acid groups being esterified with methyl groups (Mohnen Citation2008). It is thought to be a component of all primary cell walls and is involved in calcium-mediated gel formation, which contributes to maintenance of the integrity of the cell wall (). The GalA residues can be methyl-esterified at C-6. The de-esterification process is regulated by the action of pectin methyl esterase (PME), which removes methyl groups from the polymer (Mort, Qiu, and Maness Citation1993). This de-esterification process is complex but highly regulated. It results in the ability to stretch acidic residues that can associate with other HG chains through calcium cross-links (Wang, Wilson, and Cosgrove Citation2020). However, the functional properties of pectin are not only influenced by the degree of methyl esterification on the GalA backbone, but also by the distribution of non-esterified GalA residues which refers to the degree of blockiness (DB) or distribution of non-esterified GalA residues on the HG backbone (Willats, Knox, and Mikkelsen Citation2006). Also, in some plant sources such as sugar beet and spinach, the GalA residues in the HG backbone can be acetyl-esterified at O-2 and/or O-3 which has been shown to have a negative impact on the gelation property of pectin (Perrone et al. Citation2002; Ralet et al. Citation2003, Citation2005).
HG can also be substituted with other side chains such as β-d-apiofuranosyl residues and xylose to form apiogalacturonan and xylogalacturonan respectively, or even more complicated side chains termed A-F which contain thirteen different types of sugars attached to the GalA residues via twenty-one different glycosidic linkages which form RG-II (Kontogiorgos Citation2020; Mohnen Citation2008; Ndeh et al. Citation2017). RG-II is characterized by a short HG backbone with complex side chains. It is widely distributed in primary cell walls and is considered to be attached to HG (Vincken et al. Citation2003). RG-II is resistant to degradation by pectin-degrading enzymes, suggesting it has a distinct structural role in maintaining the integrity of the cell wall ().
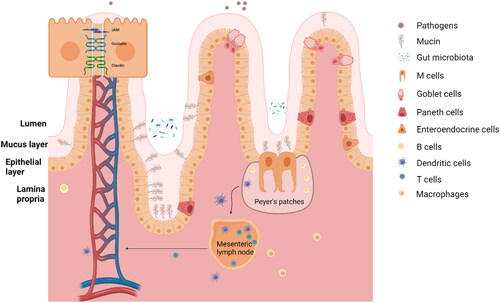
Figure 2. The gastrointestinal immune barrier. The gastrointestinal immune barrier is composed of three main layers: the mucus layer, the epithelial layer, and the lamina propria. These layers work together to protect the GI tract from harmful substances and to maintain immune homeostasis. (1) The mucus layer is the first line of defense in the GI immune barrier. It is composed of a gel-like matrix called mucus, which is produced by goblet cells. The mucus layer acts as a physical barrier, trapping pathogens, toxins, and other foreign particles. It also contains antimicrobial molecules that assist in neutralizing pathogens and prevent their entry into the underlying tissues. (2) The cellular layer is located beneath the mucus layer and consists of epithelial cells that form a tight barrier. These cells are connected by tight junctions, preventing the passage of harmful substances between them. The cellular layer also contains intraepithelial lymphocytes (IELs), specialized immune cells that monitor and protect the epithelium from invading pathogens. IELs can quickly respond to antigens and release immune mediators to eliminate threats. (3) The lamina propria is the third layer of the GI immune barrier, located beneath the cellular layer. It is a connective tissue layer containing a rich population of immune cells, such as lymphocytes, macrophages, and dendritic cells. These immune cells sample the luminal content, detect pathogens, and initiate immune responses when necessary. The lamina propria is also involved in immune tolerance, allowing beneficial nutrients and commensal bacteria to pass while preventing the invasion of harmful substances.
RG-I
The backbone may contain RG-I which is composed of alternating L-Rhamnose (Rha) and D-GalA residues. The backbone can be modified with various side chains, such as arabinan, galactan, and arabinogalactan, which contribute to the structural and functional diversity of pectin. These regions are known as the hairy area of the pectin (Kontogiorgos Citation2020). Most Rha residues were substituted at O-4 while less GalA residues are substituted at O-2 (Yapo Citation2011). The side chains various enormously based on the plant’s organ, type of plant, and development stages of the plant (Lerouge et al. Citation1993; Vincken et al. Citation2003). RG-I is highly variable in both its fine structure and distribution within the cell wall. It is composed of neutral oligosaccharides such as galactan and arabinan epitopes, but the relative proportions of these epitopes can vary within different regions of plant organs (Kontogiorgos Citation2020). RG-I is covalently attached to HG and possibly RG-II, but the precise nature of these connections is not well understood. RG-I has been implicated in a range of processes and health benefits ().
RG-II
RG-II is characterized by a highly complex and branched structure. Similar to RG-I, it consists of a backbone of alternating GalA and Rha residues. However, RG-II has additional structural features that distinguish it from RG-I, including unusual sugar residues, such as 2-keto-3-deoxy-d-lyxo-heptulosaric acid (KDO), apiose, and specific glycosidic linkages. RG-II can have various side chains attached to its backbone, including arabinan, arabinogalactan, and other sugar residues. These side chains can be further modified with ester and glycosidic linkages, forming intricate networks within the cell wall matrix. RG- II is known as a bioactive component as it attenuates proliferation of tumor cells (Ai et al. Citation2018; Park et al. Citation2013).
Apiogalacturonan and xylogalacturonan
Apiogalacturonan is primarily composed of linear chains of GalA residues and substituted with mono- or disaccharide apioduranosyl (Schols and Voragen Citation1996). Apiogalacturonan molecules can be linked together through HG regions or form cross-links with other pectic polysaccharides, such as RG-I and RG-II, to provide structural integrity to the cell wall. Xylogalacturonan is a complex polysaccharide that contains both GalA and xylose residues. It is a component of hemicellulose, which is another major constituent of the plant cell wall. Xylogalacturonan chains can be branched or linear, and their arrangement can vary between different plant species. Both apiogalacturonan and xylogalacturonan are involved in various biological processes, including cell growth, cell adhesion, and responses to environmental stresses (Popov and Ovodov Citation2013).
The connections and interactions within the pectic network are likely to be complex and multifaceted (Cardoso, Coimbra, and Lopes da Silva Citation2003). In addition to covalent attachment to other macromolecules in the cell wall, pectic polysaccharides have the potential to interact with ions and low-molecular-weight compounds. For example, acidic domains can bind cations such as calcium and aluminum, which can influence the conformational states and properties of the pectic network (Decreux and Messiaen Citation2005). Polyamines can also compete for calcium-binding sites on HG, which can affect the supramolecular assembly of pectins and the action of PMEs.
Molecular genetic approaches have been applied to provide insight into the structural chemistry of pectin. This includes the manipulation of pectin structure in planta, the isolation of mutants, and the elucidation of biosynthetic pathways. The identification and characterization of genes involved in pectin biosynthesis and modification has been instrumental for the development of e.g., specific monoclonal antibodies (Hu et al. Citation2017). However, the complexity and heterogeneity of pectin, as well as the indirect relationship between synthesis genes and carbohydrate structure, make the functional analysis of pectin a challenging task.
Gastrointestinal immune barrier system
Before reviewing current knowledge on how pectins impact the gastrointestinal immune barrier, it is needed to review the composition and organization of this crucial protective layer that protects the human body from undesired infectious agent present in the lumen of the intestine. The intestinal tract is one of the largest surfaces between the outside environment and inside of the body (Kagnoff Citation1993). A major function of the intestinal tract is to absorb nutrients and to transport them into the circulation. Another very important function is to serve as the gatekeeper for the human body. Many pathogens and toxins enter the human body via the gastrointestinal tract. The gastrointestinal immune barrier prevents these substances from entering the human system (Johansson, Larsson, et al. Citation2011; König et al. Citation2016). The gastrointestinal tract is the largest active immune organ of humans. It is 795.5 ± 129 cm in length and harbors the largest number of immune cells which is about 80% of all immune cells in the lamina propria (Hounnou et al. Citation2002; Mowat and Agace Citation2014). Combined with the lamina propria (LP), the gastrointestinal immune barrier is composed of an immune active part that serves to fight intruders such as pathogens, toxins and possible allergens and a barrier that is the true gatekeeper of the human body. From the lumen to the LP the gastrointestinal immune barrier is composed of three layers ().
The first layer is composed of a mucus layer, which plays a crucial role in the gastrointestinal immune barrier. This layer acts as a protective shield, trapping potential pathogens, toxins, and other harmful substances, preventing them from directly interacting with the epithelial cells (Allaire, Crowley, et al. Citation2018; Bourlioux et al. Citation2003). This layer is composed of a highly glycosylated polymeric protein called mucin, which forms a gel-like structure overlying the intestinal epithelium. The mucosal layer serves as a chemical barrier, limiting the contact between the microbiome and epithelial cells. The intestinal mucus layer serves as a barrier but is also a feeding layer for the gut microbiota (Paone and Cani Citation2020; Szentkuti et al. Citation1990). The mucus layer is different in composition in the small and large intestine (Ermund et al. Citation2013; Johansson, Ambort, et al. Citation2011). In the small intestine, the mucus layer is thinner (∼20 μm) and less dense compared to the large intestine (thickest in the colon, ∼830 μm) (Atuma et al. Citation2001). The tinner layer in the small intestine facilitates efficient absorption of nutrients and expedites the movement of digested food through the intestinal tract. The mucus layer in the large intestine is thicker and composed of a two-layered mucus system and is more densely packed. It contains a higher concentration of mucins, antimicrobial peptides, and other immune-related molecules. This thicker mucus layer provides enhanced protection and serves as a habitat for the gut microbiota, which plays a vital role in gut health and immune regulation (Johansson, Ambort, et al. Citation2011; Johansson, Larsson, et al. Citation2011).
The second layer of the barrier is the intestinal epithelial barrier, which is a crucial component of the gastrointestinal immune system that serves to protect the body from harmful substances and pathogens that enter the gut. Similar to the mucus layer, the composition and characteristics of the intestinal epithelial barrier also differ between the small and large intestine. The intestinal cellular layer is composed of a single epithelial layer and intraepithelial cells which includes M cells, goblet cells, Paneth cells, and enteroendocrine cells (Goto and Kiyono Citation2012). M cells, also known as microfold cells, are specialized epithelial cells found in the Peyer’s patches (PP) of the small intestine. They play an important role in the immune system by sampling antigens from the gut lumen and presenting them to immune cells in the underlying lymphoid tissue (Man, Prieto-Garcia, and Nicoletti Citation2004). Goblet cells, named for their distinctive shape, are responsible for the production and secretion of mucus which lubricates the gut and protects the mucosa from damage and infection (Allaire, Crowley, et al. Citation2018). Paneth cells, located at the base of the small intestine, produce a variety of antimicrobial peptides that defend against bacterial infections (Goto and Kiyono Citation2012). Paneth cells are uncommon in the colon, and the presence of intraepithelial lymphocytes is even scarcer compared to the small intestine. (Mowat and Agace Citation2014). Enteroendocrine cells are scattered throughout the gut and secrete hormones that regulate digestion and absorption. Columnar epithelial cells, which form the bulk of the intestinal epithelium, are involved in absorbing nutrients and electrolytes and in producing mucus. Epithelial cells form a continuous, polarized monolayer that functions as a physical barrier, facilitated by tight connections between each cell (Takiishi, Fenero, and Câmara Citation2017). These connections are formed by tight junction proteins, which seal the paracellular pathway and conduct gate and fence functions to restrict the passage of pathogenic molecules and bacteria.
The barrier function refers to the mucosal barrier, epithelial cell layer and the tight junctions between them. The permeability of the mucosal barrier is depending on the tight junction barrier function when the epithelial cell layer is intact. The intestinal epithelial cells serve as mediators between the mucosal immune system and luminal materials, which is pivotal for maintaining mucosal homeostasis and preventing diseases (Turner Citation2009). The intestinal epithelial cell layer not only provides a physical barrier but also serves as a biochemical barrier that maintains intestinal homeostasis between host tissue and commensal bacteria (Peterson and Artis Citation2014). Intestinal epithelial cells also maintain the pathways for the delivery of antigens to the antigen-presenting cells located in the lamina propia (Peterson and Artis Citation2014). Barrier dysfunction has been found in many diseases such as inflammatory bowel diseases, type 2 diabetes and type 1 diabetes, obesity, and celiac disease (Everard and Cani Citation2013; König et al. Citation2016; Schleimer and Berdnikovs Citation2017). Some studies showed that epithelial barrier disruption in IL10-/- mice increased the production of IFN-γ and TNF-γ, which may be considered a significant contributing factor in the development of colitis (Arrieta et al. Citation2009). Genetic factors could potentially play a role in the impairment of intestinal barrier function in families affected by inflammatory bowel disease as some studies showed that the presence of specific NOD2/CARD15 mutations genetically linked to barrier defects implies that immune activation could be responsible for early barrier disruptions observed in healthy relatives and in patients prior to disease reactivation (Buhner et al. Citation2006; Edelblum and Turner Citation2009). This might lead to barrier dysfunction and could potentially activate immunoregulatory responses.
The third defense barrier is the LP, which, together with the mesenteric lymph nodes (MLN) and PP are referred to as the gut-associated lymphoid tissue (GALT) which contains a large proportion of the gastrointestinal immune cells (Mörbe et al. Citation2021; Spahn and Kucharzik Citation2004). GALT contains both CD4+ and CD8+ T cells. Immune cells are another important component of the intestinal epithelial barrier, as they play a crucial role in the immune response and the tolerance of the host against external substances (Mörbe et al. Citation2021). These immune cells, including dendritic cells, T cells, B cells, and macrophages, work together with the epithelial cells and the mucosal layer to protect the body from potential threats (Forchielli and Walker Citation2005). The complex interplay between these different components of the gastrointestinal immune system is essential for maintaining the health and integrity of the intestinal barrier.
Gastrointestinal immune barrier and gut microbiota
The human gut is home to trillions of microorganisms, which together with the host form a superorganism in which energy and metabolites are exchanged and by which homeostasis is maintained in the immune system. The gut microbiota plays a crucial role in the development and function of the mucosal immune system, particularly during early life, and can influence the balance between pro-inflammatory and regulatory host responses (Lee and Mazmanian Citation2010). Alterations in the composition of the microbiota, known as dysbiosis, have been linked to the development of inflammatory disorders such as inflammatory bowel diseases (IBDs) (Nishida et al. Citation2017).
As outlined in the preceding section, the gut immune system is composed of both innate and adaptive immune mechanisms that cooperate to protect the host and maintain intestinal homeostasis (Faderl et al. Citation2015; Kayama and Takeda Citation2012). Epithelial cells, which form the first line of defense against the microbiota, express receptors for pathogen-associated molecular patterns (PAMPs) and produce antimicrobial products and chemokines in response to PAMPs signals (Kelly and Mulder Citation2012; R. Marques and Boneca Citation2011). The gut microbiota also stimulates the production of microbicidal peptides and secretory IgA by the host, which contain the microbiota within the intestinal lumen and neutralize PAMPs (Neu, Sharma, and Young Citation2010).
While some members of the microbiota have adopted peace-keeper activities to colonize the intestine, others are pro-inflammatory and can stimulate the production of effector immune responses by the host. One such group is segmented filamentous bacteria, which stimulate the postnatal maturation of immune responses in the mouse gut and are necessary for the control of colonization by the invasive pathogen Citrobacter rodentium (Cerf-Bensussan and Gaboriau-Routhiau Citation2010). The destruction of the microbiota following treatment with antibiotics can jeopardize innate immune responses in the gut and promote colonization by pathogens (Kim, Covington, and Pamer Citation2017; Thaiss et al. Citation2016).
Intestinal dysbiosis has been observed in patients with IBDs such as Crohn’s disease and ulcerative colitis (Maloy and Powrie Citation2011). Dysbiosis can arise from pro-inflammatory bacteria, such as enteroinvasive E. coli strains, or from reduced levels of resident Firmicutes spp. and Bacteroides spp. and an overgrowth of proteobacteria. However, it is difficult to assign a causative role for dysbiosis in IBD as host-predisposing factors and the reciprocal nature of the regulation of the immune system and microbial community structures must be considered (Kamada et al. Citation2013).
Therapeutic interventions targeting the microbiota, such as antibiotics, probiotics, and fecal microbiota transplantation, have shown promise in the treatment of IBD (Walker and Lawley Citation2013). Antibiotics can be used to eradicate the microbiota and reduce inflammation, but the long-term effects of antibiotic treatment on the microbiota and host immune system are unknown. Probiotics, which are live microorganisms that are similar to the beneficial microorganisms found in the human gut, have demonstrated some efficacy in the treatment of IBD but their mechanisms of action are not fully understood. Fecal microbiota transplantation, in which feces from a healthy donor are transplanted into the gut of a recipient, has shown promising results in the treatment of IBD and other diseases but there are concerns about the safety and long-term effects of the procedure (Rossen et al. Citation2015; Wang et al. Citation2019).
In conclusion, the gut immune barrier system plays a crucial role in the regulation of the gut microbiota and the maintenance of intestinal homeostasis (Goto and Kiyono Citation2012; Kamada et al. Citation2013; Okumura and Takeda Citation2018). Dysbiosis has been linked to the development of IBDs, and therapeutic interventions targeting the microbiota show promise in the treatment of these diseases. However, further research is needed to fully understand the complex interactions between the immune barrier system, microbiota, and host immunity in health and disease.
Possible mechanisms by which specific pectin types might support gastrointestinal health
As a dietary fiber, pectin has different functions in the digestion system. Due to its gelling ability, pectin can retain water and resist digestive enzymes in the small intestine, which could be a possible mechanism for how pectin slows down the absorption of glucose and lipid (Kontogiorgos Citation2020) and support metabolic health. By forming a coating on the mucosal surface, pectin effectively slows down the interaction between glucose and the mucosa, thereby delaying sugar absorption and improving glycemic control (Wikiera, Irla, and Mika Citation2014). This interaction with mucus is accomplished as follows. Pectin can bind with divalent cations such as Mg2+, Ca2+, Ba2+, or Zn2+. Pectin and divalent cations can form a strong inter-chain by cation bonding according to the so-called ‘shifted egg-box’ model (Braccini and Pérez Citation2001). This process is the basis of the gel formation (Christensen Citation2020; Thakur, Singh, and Handa Citation2009). These gels integrate into the mucus and lower glucose absorption. However, we have recently shown that this is not the only mechanism by which specific pectins can lower glucose absorption. Specific pectins such as DM19HB, DM18LB, DM84LB and DM88HB can also lower apical expression of glucose transporters and thereby lower glucose absorption (Tang et al. Citation2023).
While being present on mucus, pectin can also contribute to metabolic and immune health by serving as a substrate for gut microbiota. As mentioned above pectin cannot be digested in the small intestine but can be fermented by gut microbiota in the large intestine. Pectinolytic organisms in the colonic tract, such as the phyla Bacteroidetes and Firmicutes (Baylisss and Houston, Citation1984; Chung et al. Citation2016; Dongowski, Lorenz, and Anger Citation2000; Elshahed et al. Citation2021; Salyers et al. Citation1977) can ferment pectin into beneficial metabolites, which are SCFAs. SCFAs can be used by host cells to support metabolism and lower the Ph and by that inhibit adhesion and growth of pathogenic bacteria (Koh et al. Citation2016). It has been suggested that pectin supplementation influences the production and composition of SCFAs. The production of acetate and butyrate was increased after 6 h pectin exposure and was mediated by clostridium cluster XIV bacterial species (Bang et al. Citation2018).
Chemical structure
The chemical structure of pectin plays an important role in its fermentability and functionality. This includes characteristics such as RG-Ι content and DM (Mao et al. Citation2019; Tian et al. Citation2017; Y. Zhao et al. Citation2021). In a study involving 177 healthy individuals, it was found that carrot-derived RG-I (cRG-I) (1.5 g per day) exhibited significant immunomodulatory properties. It was shown that RG-I had the most potent effect on alleviating symptoms caused by rhinovirus 16 infection. Notably, RG-I facilitated a quicker interferon-induced response, stimulated the expression of EIF2AK2, a crucial antiviral gene, expedited viral clearance, and resulted in a reduction of both symptom severity (by 20%) and duration (by 25%) (Lutter et al. Citation2021). RG-I has also been studied for its gastroprotective activity. The administration of rhamnogalacturonan (RGal) was found to effectively inhibit the activity of MPO, which serves as a marker for neutrophil infiltration, as well as an agent that reduce TNF-α levels.(Maria-Ferreira et al. Citation2014) These findings indicate that RGal treatment reduces the migration of neutrophils to the ulcer site, thereby promoting an improvement in the gastric inflammatory process induced by acetic acid (Maria-Ferreira et al. Citation2014). Interestingly, citrus pectin with low DM fermented faster and more efficiently than high DM pectin in vitro and in vivo (Dongowski, Lorenz, and Proll Citation2002; Tian et al. Citation2017). However, M Gulfi et al. suggested that higher DM apple pectin fermented more thoroughly with slightly higher production of SCFAs than lower DM apple pectin (Gulfi, Arrigoni, and Amadò Citation2006). Thus, the fermentability of pectin could also be influenced by the source of pectin. Also, for soy and sugar beet pectin differences in production of SCFAs were observed (Tian et al. Citation2016). Acetate, lactate, formate, butyrate, and propionate are the main SCFA products and intermediates (Macfarlane and Macfarlane Citation2003). All these SCFAs are also recognized for their immunomodulatory potential. They are considered intermediaries that facilitate communication between the intestinal microbiome and the immune system.
Receptors involved in pectin signaling
Pectins or their fermentation products might bind and signal via different types of receptors and by that partially or completely exert their health benefits (). The fermentation products of pectin such as SCFAs have been shown to exhibit multiple mechanisms of action. SCFAs can e.g., inhibit histone deacetylase (HDAC), serve as an energy source and bind to GPCRs (Kootte et al. Citation2012; Lattimer and Haub Citation2010; Soliman Citation2019; Tolhurst et al. Citation2012). HDAC inhibition by pectin-derived SCFAs has been suggested as a potential therapeutic strategy in a variety of diseases (Blanco-Pérez et al. Citation2021). Also binding of pectin-derived SFCA to GPCRs has been suggested to be involved in many health benefits (Williams, Scott, and Wood Citation2019). via GPCRs SCFAs can activate signaling pathways that are involved in the resolution of inflammation in the gut (Lattimer and Haub Citation2010; Tolhurst et al. Citation2012). SCFAs can initiate the signal transmission by binding to Free fatty acids receptors (FFARs) such as FFAR2, FFAR3, hydroxycarboxylic acid receptor 2 (HCA2), Olfr-87(mice), and OR51E2 (humans) (Bolognini et al. Citation2016; Ohira, Tsutsui, and Fujioka Citation2017; Ratajczak et al. Citation2019). These receptors are not only distributed on the digestive tract epithelium but also on immune cells and smooth muscle cells of blood vessels (Ratajczak et al. Citation2019). The activity of the immune and inflammatory response is influenced by signaling through FFARs and HDAC activity (Kim and Betz Citation2018). SCFAs are believed to be responsible for maintaining the regulatory T cell (Treg) pools and its activity, which exert their effect by inhibiting HDAC () (Priyadarshini et al. Citation2021; Kim and Betz Citation2018).
Figure 4. Pectin and its currently known receptors in the gastrointestinal tract. Pectins and their fermentation products have various health benefits and can interact with different types of receptors. Short-chain fatty acids (SCFAs) derived from pectin fermentation can inhibit histone deacetylase (HDAC) and bind to G protein-coupled receptors (GPCRs), potentially providing therapeutic benefits for disease prevention and promoting the resolution of inflammation in the gut. SCFAs can activate signaling pathways through receptors such as FFAR2, FFAR3, HCA2, olfr-87 (in mice), and OR51E2 (in humans). Pectin can also interact with galectin-3 (gal-3) receptors, which play a role in various biological processes and have implications in preventing cancer progression and metastasis. Specific pectin chemistries can interact with pattern recognition receptors (PRRs) like TLR2 and TLR4, influencing immune responses. Pectins can also impact cholesterol metabolism and bile acid excretion. The effects of pectins on receptors and immune responses depend on their structure and composition. Pectin oligosaccharides can either enhance or downregulate inflammatory activities and immune activation, while low-DM citrus pectins can inhibit proinflammatory pathways while leaving tolerogenic pathways unaffected.
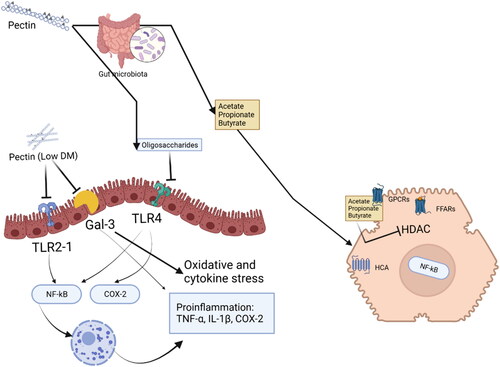
Pectin might also directly interact with receptors involved in metabolism and/or immunity. One of the best-recognized receptors for pectin is Gal-3. Gal-3 has a significant impact on a wide range of biological processes and appears to be implicated in various physiological and pathophysiological conditions (Dumic, Dabelic, and Flögel Citation2006). These conditions include development of organs, immune responses, as well as neoplastic transformation and metastasis (Dumic, Dabelic, and Flögel Citation2006). The initial discovery of Gal-3-pectin interaction by Gunning, A.P. et al. demonstrated the recognition of galactan components in pectin by the recombinant form of Gal-3 (Gunning, Bongaerts, and Morris Citation2009). This finding suggests potential implications in reduction of pectin on progression and metastasis formation of cancer (Gunning, Bongaerts, and Morris Citation2009). A few studies demonstrate that the supplementation of 1% modified citrus pectin (commercial pectin) resulted in a reduction of Gal-3 signaling which was accompanied by decreased renal fibrosis, attenuated macrophage infiltration, lower expression of pro-inflammatory cytokines, and reduced apoptosis (Kolatsi-Joannou et al. Citation2011). Studies in vivo demonstrate pro-inflammatory effects of Gal-3 which can be attenuated by specific pectins such as low-DM pectins (Hsu et al. Citation2000) Also, lower DM pectins such as lemon pectin DM5 can reduce oxidative and cytokine stress in insulin-producing beta-cells through a Gal-3 dependent mechanism (Hu et al. Citation2020). Potato and citrus pectins showed anticancer activity by specific binding to the human Gal-3 and impacting cell-cycle genes lowering metastasis of cancer cells (Gunning, Bongaerts, and Morris Citation2009).
Pectins also interact directly with immune receptors and by that participate in regulating immune responses. Our previous study shows that specific pectin chemistries allow interactions with PRR such as TLR (Beukema et al. Citation2021). Stimulation with lemon pectins induces activation of TLR2, TLR4 and NF-κB in a DM and DB dependent fashion (Beukema et al. Citation2021; Vogt et al. Citation2016). Pectin was also found to improve the 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoA) and hepatic activity of cholesterol 7α-hydroxylase activity by impacting fecal bile acid excretion (Garcia-Diez et al. Citation1996).
The immunological targets of pectin might be via several receptors. Current insight is that ability to bind to these receptors or a combination thereof is highly dependent on the structure of the applied pectin. Pectin oligosaccharides have been shown to bind to TLR4, which activates nuclear factor-kappa B (NF-κB) and COX-2 signaling pathways. They are associated with inflammatory activities and enhanced immune activation (Ishisono, Yabe, and Kitaguchi Citation2017). However, pectin oligosaccharides can also downregulate the expression of NF-κB and COX-2 during active inflammation leading to antioxidant and anti-inflammatory effects and supporting recovery from injury or immune attack. The binding between TLR4 and pectin oligosaccharides affects the activation of the TLR4/NF-κB/COX-2 signaling cascade (Chanput et al. Citation2010; Li et al. Citation2014; Ishisono, Yabe, and Kitaguchi Citation2017; Jia et al. Citation2018). Other pectins however are predominantly regulatory and prevent too strong immune responses. Such a characteristic is attributed to lower DM citrus pectins with a DM from 7–25 that inhibit the proinflammatory TLR2-1 pathway while leaving the tolerogenic TLR2-TLR6 pathway unaffected. They interact with TLR2 via electrostatic forces (Sahasrabudhe et al. Citation2018). This anti-inflammatory effect is TLR2-1 dependent and unrelated to SCFAs produced by microbiota, suggesting that low-DM pectins may reduce inflammation by directly interacting with TLR2-TLR1 receptors (Beukema et al. Citation2021; Sahasrabudhe et al. Citation2018).
Pectin’s health effects in different organs
Several studies have been shown that pectin is beneficial for health, such as for not only gastrointestinal health but during recent years there are more reports demonstrating also beneficial effects on cardiovascular health, digestive health, stabilizing blood sugar levels and for weight management (Blanco-Pérez et al. Citation2021; Brouns et al. Citation2011; Brown et al. Citation1999; Larsen et al. Citation2019; Liu et al. Citation2016; Wu et al. Citation2019). During the past years it has become recognized that also on an organ level specific chemistries of pectins are responsible for these health benefits. These chemistries include variations in DM, HG and RG-I content. More researchers are now focusing on the health-related effects of pectin’s structures. The subsequent subsections will delve into the effects that are dependent on this structural aspect and discuss impact on an organ level.
Gastrointestinal health
Gastrointestinal health was shown to improve by consumption of marsh cinquefoil Comarum palustre L. pectins (57% GalA residues) at doses of 50 and 100 mg/kg. This pectin attenuated tissue myeloperoxidase production and stimulated mucin secretion. By this, it alleviated experimental colitis in mice (Popov et al. Citation2006). Also, some studies show that specific pectins plays a vital role in maintaining the integrity and the functionality of the mucus layer. Especially, low DM citrus pectin stimulates the production of mucin 2 (Hino et al. Citation2013; Liu et al. Citation2005; Xie et al. Citation2020). Another study indicates that feeding pigs with 5% apple pectin leads to increased gene expression of mucin 2 and TFF3 in both the ileum and cecum. Additionally, this effect might associate with interactions between specific bacterial genera, particularly those involved in butyric acid production, and the modulation of mucosal immunity (Wu, Zheng, et al. Citation2020). RG-I enhanced the promotion of mucosal enterocytes and mucus-secreting goblet cells, alongside the preservation of collagen balances and augmentation of cell proliferation in Caco-2 cells (Maria-Ferreira et al. Citation2018).
Pectin offers additional health benefits beyond its protective effects on the mucus layer. It helps maintain the permeability of the epithelial cell layer by upregulating tight junctions and preserving their integrity (Beukema, Faas, and De Vos Citation2020). From our own previous study, we showed that lemon pectin DM19 and DM43 with high DB rescue A23187-induced intestinal epithelial barrier disruption (Tang et al. Citation2023). Mice with DSS-induced colitis, which received pretreatment of L102 pectin (DM 38.09 ± 0.78) at doses of 100 mg/kg and 200 mg/kg, exhibited increased expression of ZO-1 (Fan et al. Citation2020). Interestingly, one study found that in the cecum, low DM pectin increased the expression of claudin-1 and ZO-2 at both the protein and mRNA levels (Wu et al. Citation2019). However, in the colon, low DM pectin did not have the same effects on claudin-1 or occludin (Wu et al. Citation2019). Some studies showed that 5% (wt/wt) low DM pectin supplementation in female NOD/LtJ mice enhanced the barrier integrity and the length of the villus in non-obese diabetic mice (Wu et al. Citation2019). Apple-derived pectin (unknown composition) supplementation (5% wt/wt) for six weeks in rats improved the expression of intestinal alkaline phosphatase and the function of the gut epithelial barrier, specifically in the maintenance of tight junctions. This might contribute to the prevention of intestinal inflammatory disorders (Jiang et al. Citation2016). Compared to artichoke pectins, citrus pectin treatment in mice increased the expression of MUC-3, occludin and ZO-1, and decreased the expression of intercellular adhesion molecules 1 and inducible nitric oxide synthase, in colitis mice induced by dextran sulfate sodium (DSS), to maintain the barrier function (Sabater et al. Citation2019).
Pectin also exhibits benefits on immune cells in the LP. Some studies show that pectic polysaccharide fragments from Biophytum petersianum have immunomodulating activity on PP and macrophages by increasing IL-6 production, which also stimulates the growth of bone marrow cells (Grønhaug et al. Citation2011). It has been reported that an intact lemon pectin backbone induces the activation of TLR2, TLR4 and NF-κB in a DM-dependent way, and also shows epithelial barrier protective effects (Vogt et al. Citation2016). Emerging new insight indicate that RG-I in pectin exhibit significant activities that are associated with better human health (Wu et al. Citation2019; Cui et al. Citation2020; Ho et al. Citation2016; Dong et al. Citation2010). RG-I from Nerium indicum flowers contain high levels of arabinose and galactose and effectively stimulate macrophages to produce nitric oxide (NO), thereby facilitating immune system activation (Dong et al. Citation2010). High molecular RG-I containing polymers from elderflowers have high complement fixing activity and macrophage stimulating activities (Ho et al. Citation2016).
Also, clear chemistry dependent effects have been reported for effects of pectins on gut microbiota. A study found that exposing adult human microbiota to cRG-I resulted in increased levels of acetate, propionate, and butyrate (Abbeele et al. Citation2021). Certain bacteria, such as Bacteroides dorei and Prevotella species, played a key role in breaking down cRG-I, while Bifidobacterium longum fermented only specific components of cRG-I (Abbeele et al. Citation2021). The authors found that carrot RG-I supplementation can reduce interpersonal compositional differences in the gut microbiota by stimulating specific bacteria such as Bacteroides dorei/vulgatus and Bifidobacterium longum (Abbeele et al. Citation2023). Besides the carrot-derived RG-I, pepper-derived RG-I has been found to exhibit immunomodulatory effects on phagocytosis and to also have a modulatory effect on the microbiota, resulting in increased production of SCFAs (McKay et al. Citation2021). Pectin can be fermented by enzymes derived from various bacterial genera such as from Enterococcus, Bifidobacteria, Lactobacilli (Blanco-Pérez et al. Citation2021; Chung et al. Citation2016; Gómez et al. Citation2014). It has been reported that pectins selectively modulate the gut’s microbial communities, which is related to the presence of amide groups, the composition of neutral sugars, and the distribution of HG and rhamnogalacturonan fractions, but the DM plays a crucial role as the primary regulator of microbiota composition (Larsen et al. Citation2019). The effectiveness of low-DM pectin L13 (resource unknown) enhanced microbiota diversity and promoted the abundance of Clostridiaceae and Lachnospiraceae at the family level, and Bacteroides and Lachnospira at the genus level and supported gastrointestinal health (Huang et al. Citation2022).
Cardiovascular health
For cardiovascular health it has been shown that especially administration of high DM citrus pectin (DM 70) reduced low-density lipoprotein 6–7% (Brouns et al. Citation2011). A meta-analysis of a trial with 277 individuals that received 34 days of treatment of pectin demonstrated that consuming pectin-rich diets significantly decreased both total and low-density lipoprotein cholesterol levels (Brown et al. Citation1999).
Metabolism health
Also, metabolism health was shown to be improved by pectin consumption. Consumption of citrus pectin treatment (from 500 mg to 2000 mg/kg) with nonreported structure improved the glucose tolerance and reduced insulin resistance in diabetic rats (Liu et al. Citation2016). It was postulated that this might occur by the impact of pectin on PI3K/Akt signaling (Liu et al. Citation2016). Ren et al. corroborated this finding in a study with ginseng acidic polysaccharide which contains HG and RG-I-rich pectin and arabinogalactan enriched pectin which improved lipid metabolism in type 2 diabetic rats by regulating the gut microbiota composition by increasing SCFA production and enhancing the secretion of peptide tyrosine and glucagon-like peptide-1(Ren et al. Citation2023).
Immune health
Many have shown improved immune health after the consumption of pectin. In a study by Nascimento, G. E et al. it was shown that administration of 300 µg/mL DM36 citrus pectin can attenuate the production of the pro-inflammatory cytokines TNF-α and IL-1β induced by lipopolysaccharide (LPS) while promoting the production of the regulatory cytokine IL-10. It was shown that this effect was dependent on the degree of methyl esterification, molecular weight and presence of side chain (Nascimento et al. Citation2017). Sergey V.P et al. showed that lower DM citrus pectin can decrease the adhesion of peritoneal granulocytes and their ROS production and was found to improve the survival rate of mice subjected to a lethal dose of LPS (Popov et al. Citation2013). Lower DM 7 citrus pectin was shown to be more efficient in blocking TLR2-1 in both immune cells and in vivo in an ileitis mice model. It therewith alleviated intestinal inflammation (Sahasrabudhe et al. Citation2018). A study in non-obese type 1 diabetic mice showed that 5% low DM pectin (nonreported structure) supplementation suppress the activation of NOD like receptor protein 3 (NLRP3) inflammasome and increase the gene expression of the immune modulatory cytokine TGF-β1 (Wu et al. Citation2019). Gal-3 is pivotal in various cellular events and a key contributor to cancer cell apoptosis (Girotti et al. 2020). Pectin’s anticancer properties depend on its binding with Gal-3. Arabinoxylan/hemicellulose in pectin, containing the RG-I domain, serves as a natural ligand inhibiting the biological activity of Gal-3 (Prado et al. 2019).
General discussion and further perspectives
Strengthening the gut immune barrier has become a hot topic for not only gut health but also for immune health. This review discusses the current knowledge about the gut immune barrier, dietary fibers pectin and the interaction between them. It has become widely accepted that not just intake of more pectin as dietary fiber supports health but that specific chemistries must be consumed. The structure determines its function. The bioactivities of pectin are often associated with various side chains, such as by galactan and arabinogalactan, as well as by backbones like rhamnogalacturonan and HG, along with the effects of substituted groups, and their synergistic interactions. Different structural characteristics of pectins such as molecular weight, RG-I, DM and DB on the HG backbone, can contribute to the benefits to immune health and gut immune barrier function (Baggio et al. Citation2017.; Fan et al. Citation2020; Gunning, Bongaerts, and Morris Citation2009; Hu et al. Citation2020; Nascimento et al. Citation2013; Vogt et al. Citation2016; Wu et al. Citation2021). In our research we found that low DM or/and high DB lemon pectins have TLR2-1 dependent anti-inflammatory effects, and also had a protective effect on the gut barrier function by increasing the integrity of the gut barrier (Beukema et al. Citation2021; Vogt et al. Citation2016). The extent to which the protective effects of pectin on immune health or gut epithelial barrier, are applicable to other sources of pectin has not been extensively investigated but we found that the effects are rather dependent on DM and/or DB and to a lesser extend to the source when comparing orange and lemon pectins with similar chemical structures (Beukema et al. Citation2021).
Pectin, functioning as a soluble fiber, plays a crucial role in the fermentation process that occurs in the cecum and large intestine. This fermentation process results in the production of fermentation products that can stimulate the production of a thick, protective mucus layer, enhance bacterial adhesion, and promote the integrity of the gut epithelial barrier. As a result, pectin helps to strengthen the function of the intestinal barrier.
Pectin also has been shown to beneficially impact microbiota composition and to support production of beneficial fermentation products such as SCFAs. Dysbiosis of microbiota is known to be involved in disease severity in both diabetes and IBD (Metwaly, Reitmeier, and Haller Citation2022). Specific pectins, especially the higher DM that will be fermented have been shown to act as mediators in increasing the abundance of beneficial microbiota. However, impact of RG decoration and of e.g., molecular weight has not been studied in much detail and might be even more instrumental in supporting microbiota function.
The knowledge reviewed here demonstrates that specific pectin chemistries might have specific health benefits. It has been shown that with tailoring pectins with specific chemistries gastrointestinal, cardiovascular, metabolic and immune health might be stimulated and diseases might be prevented (Ho et al. Citation2016; Popov et al. Citation2013; Soliman Citation2019; Vogt et al. Citation2016). Although not studied yet, pectins might also be beneficial for brain or mental health as they support mediators such as SCFA but also indoles (Beukema et al. Citation2022) that are known to support brain health (Pappolla et al. Citation2021; Rajamma, Krishnaswami, and Kandasamy Citation2022). This is all dependent on specific chemistries pectins. Unfortunately, not all studies have measured the composition of the applied pectins. This is necessary to allow side-by-side comparison of data sets and to understand which chemistries need to be used in specific target groups. Current knowledge indicates that administration of pectins and dietary fibers in general is not a one-size fits all principle but that we need to use specific chemistries for specific target groups. This review hopefully contributes to expediting the process for tailoring specific pectin chemistries for supporting and preventing specific health issues.
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
References
- Abbeele, P. V. D., S. Deyaert, R. Albers, A. Baudot, and A. Mercenier. 2023. Carrot RG-I reduces interindividual differences between 24 adults through consistent effects on gut microbiota composition and function ex vivo. Nutrients 15 (9):2090. doi:10.3390/nu15092090.
- Abbeele, P. V. D., C. Duysburgh, I. Cleenwerck, R. Albers, M. Marzorati, and A. Mercenier. 2021. Consistent prebiotic effects of carrot Rg-I on the gut microbiota of four human adult donors in the shime® model despite baseline individual variability. Microorganisms 9 (10):2142. doi:10.3390/microorganisms9102142.
- Ai, L., Y.-C. Chung, S.-Y. Lin, K.-C. Lee, P. F.-H. Lai, Y. Xia, G. Wang, and S. W. Cui. 2018. Active pectin fragments of high in vitro antiproliferation activities toward human colon adenocarcinoma cells: Rhamnogalacturonan II. Food Hydrocolloids. 83(October): 239–45. doi:10.1016/j.foodhyd.2018.05.017.
- Allaire, J. M., S. M. Crowley, H. T. Law, S. Y. Chang, H. J. Ko, and B. A. Vallance. 2018. The intestinal epithelium: Central coordinator of mucosal immunity. Trends in Immunology 39 (9):677–96. doi:10.1016/j.it.2018.04.002.
- Allaire, J. M., V. Morampudi, S. M. Crowley, M. Stahl, H. B. Yu, K. Bhullar, L. A. Knodler, B. Bressler, K. Jacobson, and B. A. Vallance. 2018. Frontline defenders: Goblet cell mediators dictate host-microbe interactions in the intestinal tract during health and disease. American Journal of Physiology. Gastrointestinal and Liver Physiology 314 (3):G360–G377. doi:10.1152/ajpgi.00181.2017.
- Anderson, J. W., P. Baird, R. H. Davis, S. Ferreri, M. Knudtson, A. Koraym, V. Waters, and C. L. Williams. 2009. Health benefits of dietary fiber. Nutrition Reviews 67 (4):188–205. doi:10.1111/j.1753-4887.2009.00189.x.
- Arrieta, M. C., K. Madsen, J. Doyle, and J. Meddings. 2009. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut 58 (1):41–8. doi:10.1136/gut.2008.150888.
- Atuma, C., V. Strugala, A. Allen, and L. Holm. 2001. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. American Journal of Physiology - Gastrointestinal and Liver Physiology 280 (5):43–5.
- Baggio, C. H., F. Lopes, A. Nascimento, T. Cipriani, D. McKay, and W. MacNaughton. 2017. Modulation of intestinal epithelial barrier function by rhamnogalacturonan. The FASEB Journal 31 (S1):995–7. doi:10.1096/fasebj.31.1_supplement.995.7.
- Bang, S. J., G. Kim, M. Y. Lim, E. J. Song, D. H. Jung, J. S. Kum, Y. D. Nam, C. S. Park, and D. H. Seo. 2018. The influence of in vitro pectin fermentation on the human fecal microbiome. AMB Express 8 (1):98. doi:10.1186/s13568-018-0629-9.
- Barber, T. M., S. Kabisch, A. F. H. Pfeiffer, and M. O. Weickert. 2020. The health benefits of dietary fibre. Nutrients 2020, Vol. 12, Page 320912 (10):3209. doi:10.3390/nu12103209.
- Bayliss, C. E., and A. P. Houston. 1984. Characterization of plant polysaccharide- and mucin-fermenting anaerobic bacteria from human feces. Applied and Environmental Microbiology 48 (3):626–32. doi:10.1128/aem.48.3.626-632.1984.
- Beukema, M., M. M. Faas, and P. De Vos. 2020. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Experimental & Molecular Medicine 52 (9):1364–76. doi:10.1038/s12276-020-0449-2.
- Beukema, M., É. Jermendi, M. A. van den Berg, M. M. Faas, H. A. Schols, and P. de Vos. 2021. The impact of the level and distribution of methyl-esters of pectins on TLR2-1 dependent anti-inflammatory responses. Carbohydrate Polymers 251(January): 117093. doi:10.1016/j.carbpol.2020.117093.
- Beukema, M., É. Jermendi, M. M. P. Oerlemans, M. J. Logtenberg, R. Akkerman, R. An, M. A. van den Berg, E. G. Zoetendal, T. Koster, C. Kong, et al. 2022. The level and distribution of methyl-esters influence the impact of pectin on intestinal T cells, microbiota, and Ahr activation. Carbohydrate Polymers 286:119280. (June): doi:10.1016/j.carbpol.2022.119280.
- Bianchi, F., N. Larsen, T. D. M. Tieghi, M. A. T. Adorno, W. Kot, S. M. I. Saad, L. Jespersen, and K. Sivieri. 2018. Modulation of gut microbiota from obese individuals by in vitro fermentation of citrus pectin in combination with bifidobacterium longum BB-46. Applied Microbiology and Biotechnology 102 (20):8827–40. doi:10.1007/s00253-018-9234-8.
- Bischoff, S. C. 2011. Gut health: A new objective in medicine? BMC Medicine 9 (1):24. doi:10.1186/1741-7015-9-24.
- Blanco-Pérez, F., H. Steigerwald, S. Schülke, S. Vieths, M. Toda, and S. Scheurer. 2021. The dietary fiber pectin: Health benefits and potential for the treatment of allergies by modulation of gut microbiota. Current Allergy and Asthma Reports 21 (10):43. doi:10.1007/s11882-021-01020-z.
- Bolognini, D., A. B. Tobin, G. Milligan, and C. E. Moss. 2016. The pharmacology and function of receptors for short-chain fatty acids. Molecular Pharmacology 89 (3):388–98. doi:10.1124/mol.115.102301.
- Bourlioux, P., B. Koletzko, F. o Guarner, and V. Braesco. 2003. The intestine and its microflora are partners for the protection of the host: Report on the danone symposium ‘the intelligent intestine. The American Journal of Clinical Nutrition 78 (4):675–83. doi:10.1093/ajcn/78.4.675.
- Braccini, I., and S. Pérez. 2001. Molecular basis of Ca2+-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules 2 (4):1089–96. doi:10.1021/bm010008g.
- Brouns, F., E. Theuwissen, A. Adam, M. Bell, A. Berger, and R. P. Mensink. 2011. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. European Journal of Clinical Nutrition 2012 66:566 (5):591–9. doi:10.1038/ejcn.2011.208.
- Brown, L., B. Rosner, W. W. Willett, and F. M. Sacks. 1999. Cholesterol-lowering effects of dietary fiber: A meta-analysis. The American Journal of Clinical Nutrition 69 (1):30–42. doi:10.1093/ajcn/69.1.30.
- Buhner, S., C. Buning, J. Genschel, K. Kling, D. Herrmann, A. Dignass, I. Kuechler, S. Krueger, H. H.-J. Schmidt, and H. Lochs. 2006. Genetic basis for increased intestinal permeability in families with Crohn’s disease: Role of CARD15 3020insC mutation? Gut 55 (3):342–7. doi:10.1136/gut.2005.065557.
- Caffall, K. H., and D. Mohnen. 2009. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydrate Research 344 (14):1879–900. doi:10.1016/j.carres.2009.05.021.
- Cardoso, S. M., M. A. Coimbra, and J. A. Lopes da Silva. 2003. Temperature dependence of the formation and melting of pectin–Ca2+ networks: A rheological study. Food Hydrocolloids. 17 (6):801–7. doi:10.1016/S0268-005X(03)00101-2.
- Cerf-Bensussan, N., and V. Gaboriau-Routhiau. 2010. The immune system and the gut microbiota: Friends or foes? Nature Reviews. Immunology 10 (10):735–44. doi:10.1038/nri2850.
- Chan, S. Y., W. S. Choo, D. J. Young, and X. J. Loh. 2017. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydrate Polymers 161:118–39. doi:10.1016/j.carbpol.2016.12.033.
- Chanput, W., J. Mes, R. A. M. Vreeburg, H. F. J. Savelkoul, and H. J. Wichers. 2010. Transcription profiles of LPS-stimulated THP-1 monocytes and macrophages: A tool to study inflammation modulating effects of food-derived compounds. Food & Function 1 (3):254–61. 2010. doi:10.1039/c0fo00113a.
- Christensen, S. H. 2020. Pectins. Food Hydrocolloids. 205–30.
- Chung, W. S., Faith, A. W. Walker, P. Louis, J. Parkhill, J. Vermeiren, D. Bosscher, S. H. Duncan, and H. J. Flint. 2016. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biology 14 (1):3. doi:10.1186/s12915-015-0224-3.
- Ciriminna, R., N. Chavarría-Hernández, A. I. R. Hernández, and M. Pagliaro. 2015. Pectin: A new perspective from the biorefinery standpoint. Biofuels, Bioproducts and Biorefining 9 (4):368–77. doi:10.1002/bbb.1551.
- Cui, Y. S., Y. X. Li, S. L. Jiang, A. N. Song, Z. Fu, C. X. Dong, Z. Yao, and W. Qiao. 2020. Isolation, purification, and structural characterization of polysaccharides from atractylodis macrocephalae rhizoma and their immunostimulatory activity in RAW264.7 cells. International Journal of Biological Macromolecules 163(November): 270–8. doi:10.1016/j.ijbiomac.2020.06.269.
- Decreux, A., and J. Messiaen. 2005. Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant and Cell Physiology 46 (2):268–78. doi:10.1093/pcp/pci026.
- Dongowski, G., and A. Lorenz. 2004. Intestinal steroids in rats are influenced by the structural parameters of pectin. The Journal of Nutritional Biochemistry 15 (4):196–205. doi:10.1016/S0955-2863(03)00080-9.
- Dongowski, G., A. Lorenz, and H. Anger. 2000. Degradation of pectins with different degrees of esterification by bacteroides thetaiotaomicron isolated from human gut flora. Applied and Environmental Microbiology 66 (4):1321–7. doi:10.1128/AEM.66.4.1321-1327.2000.
- Dongowski, G., A. Lorenz, and J. Proll. 2002. The degree of methylation influences the degradation of pectin in the intestinal tract of rats and in vitro. The Journal of Nutrition 132 (7):1935–44. doi:10.1093/jn/132.7.1935.
- Dong, Q., X. Liu, J. Yao, X. T. Dong, C. Ma, Y. X. Xu, J. N. Fang, and K. Ding. 2010. Structural characterization of a pectic polysaccharide from nerium indicum flowers. Phytochemistry 71 (11-12):1430–7. doi:10.1016/j.phytochem.2010.05.019.
- do Prado, S. B. R., G. R. Santos, P. A. Mourão, and J. P. Fabi. Chelate-soluble pectin fraction from papaya pulp interacts with galectin-3 and inhibits colon cancer cell proliferation. International Journal of Biological Macromolecules 126:170–8. doi:10.1016/j.ijbiomac.2018.12.191.
- Dumic, J., S. Dabelic, and M. Flögel. 2006. Galectin-3: An open-ended story. Biochimica Et Biophysica Acta 1760 (4):616–35. doi:10.1016/j.bbagen.2005.12.020.
- Edelblum, K. L., and J. R. Turner. 2009. The tight junction in inflammatory disease: Communication breakdown. Current Opinion in Pharmacology 9 (6):715–20. doi:10.1016/j.coph.2009.06.022.
- Elshahed, M. S., A. Miron, A. C. Aprotosoaie, and M. A. Farag. 2021. Pectin in diet: Interactions with the human microbiome, role in gut homeostasis, and nutrient-drug interactions. Carbohydrate Polymers 255 (March):117388. doi:10.1016/j.carbpol.2020.117388.
- Ermund, A., A. Schütte, M. E. V. Johansson, J. K. Gustafsson, and G. C. Hansson. 2013. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the peyer’s patches. American Journal of Physiology - Gastrointestinal and Liver Physiology 305 (5):341–7.
- Everard, A., and P. D. Cani. 2013. Diabetes, obesity and gut microbiota. Best Practice & Research. Clinical Gastroenterology 27 (1):73–83. doi:10.1016/j.bpg.2013.03.007.
- Faderl, M., M. Noti, N. Corazza, and C. Mueller. 2015. Keeping bugs in check: The mucus layer as a critical component in maintaining intestinal homeostasis. IUBMB Life 67 (4):275–85. doi:10.1002/iub.1374.
- Fan, L. L., S. Zuo, H. Z. Tan, J. L. Hu, J. B. Cheng, Q. Y. Wu, and S. P. Nie. 2020. Preventive effects of pectin with various degrees of esterification on ulcerative colitis in mice. Food & Function 11 (4):2886–97. doi:10.1039/c9fo03068a.
- Forchielli, M. L., and W. A. Walker. 2005. The role of gut-associated lymphoid tissues and mucosal defence. The British Journal of Nutrition 93 Suppl 1 (S1):S41–S48. doi:10.1079/bjn20041356.
- Garcia-Diez, F., V. Garcia-Mediavilla, J. E. Bayon, and J. Gonzalez-Gallego. 1996. Pectin feeding influences fecal bile acid excretion, hepatic bile acid and cholesterol synthesis and serum cholesterol in rats. The Journal of Nutrition 126 (7):1766–71.
- Girotti, M. R., M. Salatino, T. Dalotto-Moreno, and G. A. Rabinovich. 2019. Sweetening the hallmarks of cancer: Galectins as multifunctional mediators of tumor progression. Journal of Experimental Medicine 217 (2):e20182041. doi:10.1084/jem.20182041.
- Gómez, B., B. Gullón, C. Remoroza, H. A. Schols, J. C. Parajó, and J. L. Alonso. 2014. Purification, characterization, and prebiotic properties of pectic oligosaccharides from orange peel wastes. Journal of Agricultural and Food Chemistry 62 (40):9769–82. doi:10.1021/jf503475b.
- Goto, Y., and H. Kiyono. 2012. Epithelial barrier: An interface for the cross-communication between gut flora and immune system. Immunological Reviews 245 (1):147–63. doi:10.1111/j.1600-065X.2011.01078.x.
- Grønhaug, T. E., H. Kiyohara, A. Sveaass, D. Diallo, H. Yamada, and B. S. Paulsen. 2011. Beta-d-(1 → 4)-galactan-containing side chains in RG-I regions of pectic polysaccharides from Biophytum Petersianum Klotzsch. contribute to expression of immunomodulating activity against intestinal Peyer’s patch cells and macrophages. Phytochemistry 72 (17):2139–47. doi:10.1016/j.phytochem.2011.08.011.
- Gulfi, M., E. Arrigoni, and R. Amadò. 2006. The chemical characteristics of apple pectin influence its fermentability in vitro. LWT - Food Science and Technology 39 (9):1001–4. doi:10.1016/j.lwt.2006.02.013.
- Gunning, A. P., R. J. M. Bongaerts, and V. J. Morris. 2009. Recognition of galactan components of pectin by galectin-3. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 23 (2):415–24. doi:10.1096/fj.08-106617.
- Hino, S., K. Sonoyama, H. Bito, H. Kawagishi, S. Aoe, and T. Morita. 2013. Low-methoxyl pectin stimulates small intestinal mucin secretion irrespective of goblet cell proliferation and is characterized by jejunum Muc2 upregulation in rats. The Journal of Nutrition 143 (1):34–40. doi:10.3945/jn.112.167064.
- Ho, G. T. T., Y. F. Zou, H. Wangensteen, and H. Barsett. 2016. RG-I regions from elderflower pectins substituted on GalA are strong immunomodulators. International Journal of Biological Macromolecules 92(November): 731–8. doi:10.1016/j.ijbiomac.2016.07.090.
- Hounnou, G., C. Destrieux, J. Desmé, P. Bertrand, and S. Velut. 2002. Anatomical study of the length of the human intestine. Surgical and Radiologic Anatomy: SRA 24 (5):290–4. doi:10.1007/s00276-002-0057-y.
- Hsu, D. K., R. Y. Yang, Z. Pan, L. Yu, D. R. Salomon, W. P. Fung-Leung, and F. T. Liu. 2000. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. The American Journal of Pathology 156 (3):1073–83. doi:10.1016/S0002-9440(10)64975-9.
- Huang, W. Q., Q. Y. Fang, L. L. Fan, T. Hong, H. Z. Tan, and S. P. Nie. 2022. Pectin with Various degrees of esterification differentially alters gut microbiota and metabolome of healthy adults. eFood 3 (1-2):e5. doi:10.1002/efd2.5.
- Hu, R., Y. Xu, C. J. Yu, K. He, Q. Tang, C. L. Jia, G. He, X. Y. Wang, Y. Z. Kong, and G. K. Zhou. 2017. Transcriptome analysis of genes involved in secondary cell wall biosynthesis in developing internodes of miscanthus lutarioriparius. Scientific Reports 7 (1):9034. doi:10.1038/s41598-017-08690-8.
- Hu, S. X., R. Kuwabara, M. Beukema, M. Ferrari, B. J. De Haan, M. T. Walvoort, P. De Vos, and A. M. Smink. 2020. Low methyl-esterified pectin protects pancreatic β-cells against diabetes-induced oxidative and inflammatory stress via galectin-3. Carbohydrate Polymers 249(December): 116863. doi:10.1016/j.carbpol.2020.116863.
- Ishisono, K., T. Yabe, and K. Kitaguchi. 2017. Citrus pectin attenuates endotoxin shock via suppression of toll-like receptor signaling in Peyer’s patch myeloid cells. The Journal of Nutritional Biochemistry 50(December): 38–45. doi:10.1016/j.jnutbio.2017.07.016.
- Jiang, T. T., X. J. Gao, C. Wu, F. Tian, Q. C. Lei, J. C. Bi, B. X. Xie, H. Y. Wang, S. Chen, and X. Y. Wang. 2016. Apple-derived pectin modulates gut microbiota, improves gut barrier function, and attenuates metabolic endotoxemia in rats with diet-induced obesity. Nutrients 8 (3):126. doi:10.3390/nu8030126.
- Jia, Z. Q., H. D. Tan, W. Chen, Q. S. Liu, G. J. Yang, and K. K. Li. 2018. Pectin oligosaccharides ameliorate colon cancer by regulating oxidative stress- and inflammation-activated signaling pathways 9: 1504.
- Johansson, M. E. V., D. Ambort, T. Pelaseyed, A. Schütte, J. K. Gustafsson, A. Ermund, D. B. Subramani, J. M. Holmén-Larsson, K. A. Thomsson, J. H. Bergström, et al. 2011. Composition and functional role of the mucus layers in the intestine. Cellular and Molecular Life Sciences: CMLS 68 (22):3635–41. doi:10.1007/s00018-011-0822-3.
- Johansson, M. E., J. M. H. Larsson, and G. C. Hansson. 2011. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host–microbial interactions. Proceedings of the National Academy of Sciences of the United States of America 108 Suppl 1 (Suppl 1):4659–65. doi:10.1073/pnas.1006451107.
- Kagnoff, M. F. 1993. Immunology of the intestinal tract. Gastroenterology 105 (5):1275–80. doi:10.1016/0016-5085(93)90128-y.
- Kamada, N., S. U. Seo, G. Y. Chen, and G. Núñez. 2013. Role of the gut microbiota in immunity and inflammatory disease. Nature Reviews. Immunology 13 (5):321–35. doi:10.1038/nri3430.
- Kayama, H., and K. Takeda. 2012. Regulation of intestinal homeostasis by innate and adaptive immunity. International Immunology 24 (11):673–80. doi:10.1093/intimm/dxs094.
- Kelly, D., and I. E. Mulder. 2012. Microbiome and immunological interactions. Nutrition Reviews 70 Suppl 1 (suppl_1):S18–S30. doi:10.1111/j.1753-4887.2012.00498.x.
- Kim, C. H., and J. Betz. 2018. Immune regulation by microbiome metabolites. Immunology 154 (2):220–9. doi:10.1111/imm.12930.
- Kim, S., A. Covington, and E. G. Pamer. 2017. The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunological Reviews 279 (1):90–105. doi:10.1111/imr.12563.
- Koh, A., F. De Vadder, P. Kovatcheva-Datchary, and F. Bäckhed. 2016. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 165 (6):1332–45. doi:10.1016/j.cell.2016.05.041.
- Kolatsi-Joannou, M., K. L. Price, P. J. Winyard, and D. A. Long. 2011. Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS One 6 (4):e18683. doi:10.1371/journal.pone.0018683.
- König, J., J. Wells, P. D. Cani, C. L. García-Ródenas, T. MacDonald, A. Mercenier, J. Whyte, F. Troost, and R. J. Brummer. 2016. Human intestinal barrier function in health and disease. Clinical and Translational Gastroenterology 7 (10):e196. doi:10.1038/ctg.2016.54.
- Kontogiorgos, V. 2020. Pectin: Technological and Physiological Properties. Pectin: Technological and Physiological Properties, October, 1–207.
- Kootte, R. S., A. Vrieze, F. Holleman, G. M. Dallinga-Thie, E. G. Zoetendal, W. M. de Vos, A. K. Groen, J. B. Hoekstra, E. S. Stroes, and M. Nieuwdorp. 2012. The therapeutic potential of manipulating gut microbiota in obesity and type 2 diabetes mellitus. Diabetes, Obesity & Metabolism 14 (2):112–20. doi:10.1111/j.1463-1326.2011.01483.x.
- Larsen, N., C. B. De Souza, L. Krych, T. B. Cahú, M. Wiese, W. Kot, K. M. Hansen, A. Blennow, K. Venema, and L. Jespersen. 2019. Potential of pectins to beneficially modulate the gut microbiota depends on their structural properties. Frontiers in Microbiology 10(FEB): 223. doi:10.3389/fmicb.2019.00223.
- Lattimer, J. M., and M. D. Haub. 2010. Effects of dietary fiber and its components on metabolic health. Nutrients 2 (12):1266–89. doi:10.3390/nu2121266.
- Lee, Y. K., and S. K. Mazmanian. 2010. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science (New York, N.Y.) 330 (6012):1768–73. doi:10.1126/science.1195568.
- Lerouge, P., M. A. O’Neill, A. G. Darvill, and P. Albersheim. 1993. Structural characterization of endo-glycanase-generated oligoglycosyl side chains of rhamnogalacturonan I. Carbohydrate Research 243 (2):359–71. doi:10.1016/0008-6215(93)87039-u.
- Liu, L. S., M. L. Fishman, K. B. Hicks, and M. Kende. 2005. Interaction of various pectin formulations with porcine colonic tissues. Biomaterials 26 (29):5907–16. doi:10.1016/j.biomaterials.2005.03.005.
- Liu, Y. L., M. Dong, Z. Y. Yang, and S. Y. Pan. 2016. Anti-diabetic effect of citrus pectin in diabetic rats and potential mechanism via PI3K/Akt signaling pathway. International Journal of Biological Macromolecules 89(August): 484–8. doi:10.1016/j.ijbiomac.2016.05.015.
- Li, W. F., K. Zhang, and H. Y. Yang. 2018. Pectin alleviates high fat (lard) diet-induced nonalcoholic fatty liver disease in mice: Possible role of short-chain fatty acids and gut microbiota regulated by pectin. Journal of Agricultural and Food Chemistry 66 (30):8015–25. doi:10.1021/acs.jafc.8b02979.
- Li, X., J. Jiang, S. Shi, S. W. A. Bligh, Y. Li, Y. Jiang, D. Huang, Y. Ke, and S. Wang. 2014. A RG-II type polysaccharide purified from aconitum coreanum alleviates lipopolysaccharide-induced inflammation by inhibiting the NF-KB signal pathway. PLoS One 9 (6):e99697. doi:10.1371/journal.pone.0099697.
- Lutter, R., A. Teitsma-Jansen, E. Floris, S. Lone-Latif, A. Ravi, Y. S. Sabogal Pineros, T. Dekker, B. Smids, R. Khurshid, M. Aparicio-Vergara, et al. 2021. The dietary intake of carrot‐derived rhamnogalacturonan‐I accelerates and augments the innate immune and anti‐viral interferon response to rhinovirus infection and reduces duration and severity of symptoms in humans in a randomized trial. Nutrients 13 (12):4395. doi:10.3390/nu13124395.
- Macfarlane, S., and G. T. Macfarlane. 2003. Regulation of short-chain fatty acid production. The Proceedings of the Nutrition Society 62 (1):67–72. doi:10.1079/PNS2002207.
- Maloy, K. J., and F. Powrie. 2011. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474 (7351):298–306. doi:10.1038/nature10208.
- Man, A. L., M. E. Prieto-Garcia, and C. Nicoletti. 2004. Improving M cell mediated transport across mucosal barriers: Do certain bacteria hold the keys? Immunology 113 (1):15–22. doi:10.1111/j.1365-2567.2004.01964.x.
- Mao, G. Z., S. Li, C. Orfila, X. M. Shen, S. Y. Zhou, R. J. Linhardt, X. Q. Ye, and S. G. Chen. 2019. Depolymerized RG-I-enriched pectin from citrus segment membranes modulates gut microbiota, increases SCFA production, and promotes the growth of Bifidobacterium Spp., Lactobacillus Spp. and Faecalibaculum Spp. Food & Function 10 (12):7828–43. doi:10.1039/c9fo01534e.
- Maria-Ferreira, D., A. M. Nascimento, T. R. Cipriani, A. P. Santana-Filho, P. D. S. Watanabe, D. D. M. G. S. Ana, F. B. Luciano, K. C. P. Bocate, R. M. Van, d Wijngaard, et al. 2018. Rhamnogalacturonan, a chemically-defined polysaccharide, improves intestinal barrier function in DSS-induced colitis in mice and human caco-2 cells. Scientific Reports 8 (1):12261. doi:10.1038/s41598-018-30526-2.
- Maria-Ferreira, D., L. M. da Silva, D. A. G. B. Mendes, D. d A. Cabrini, A. M. Nascimento, M. Iacomini, T. R. Cipriani, A. R. S. Santos, M. F. d P. Werner, and C. H. Baggio. 2014. Rhamnogalacturonan from Acmella oleracea (L.) R.K. Jansen: Gastroprotective and ulcer healing properties in rats. PLoS One 9 (1):e84762. doi:10.1371/journal.pone.0084762.
- Marques, F. Z., E. Nelson, P.-Y. Chu, D. Horlock, A. Fiedler, M. Ziemann, J. K. Tan, S. Kuruppu, N. W. Rajapakse, A. El-Osta, et al. 2017. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 135 (10):964–77. doi:10.1161/CIRCULATIONAHA.116.024545.
- Marques, R., and I. G. Boneca. 2011. Expression and functional importance of innate immune receptors by intestinal epithelial cells. Cellular and Molecular Life Sciences: CMLS 68 (22):3661–73. doi:10.1007/s00018-011-0829-9.
- May, C. D. 1990. Industrial pectins: Sources, production and applications. Carbohydrate Polymers 12 (1):79–99. doi:10.1016/0144-8617(90)90105-2.
- McKay, S., P. Oranje, J. Helin, J. H. Koek, E. Kreijveld, P. van den Abbeele, U. Pohl, G. Bothe, M. Tzoumaki, M. Aparicio-Vergara, et al. 2021. Development of an affordable, sustainable and efficacious plant-based immunomodulatory food ingredient based on Bell pepper or carrot RG-I pectic polysaccharides. Nutrients 13 (3):963. doi:10.3390/nu13030963.
- Metwaly, A., S. Reitmeier, and D. Haller. 2022. Microbiome risk profiles as biomarkers for inflammatory and metabolic disorders. Nature Reviews. Gastroenterology & Hepatology 19 (6):383–97. doi:10.1038/s41575-022-00581-2.
- Min, B., I. Y. Bae, H. G. Lee, S. H. Yoo, and S. Lee. 2010. Utilization of pectin-enriched materials from apple pomace as a fat replacer in a model food system. Bioresource Technology 101 (14):5414–8. doi:10.1016/j.biortech.2010.02.022.
- Mohnen, D. 2008. Pectin structure and biosynthesis. Current Opinion in Plant Biology 11 (3):266–77. doi:10.1016/j.pbi.2008.03.006.
- Mörbe, U. M., P. B. Jørgensen, T. M. Fenton, N. V. Burg, L. B. Riis, J. Spencer, and W. W. Agace. 2021. Human gut-associated lymphoid tissues (GALT); Diversity, structure, and function. Mucosal Immunology 14 (4):793–802. doi:10.1038/s41385-021-00389-4.
- Mort, A. J., F. Qiu, and N. O. Maness. 1993. Determination of the pattern of methyl esterification in pectin. Distribution of contiguous nonesterified residues. Carbohydrate Research 247 (C):21–35. doi:10.1016/0008-6215(93)84238-2.
- Mowat, A. M., and W. W. Agace. 2014. Regional specialization within the intestinal immune system. Nature Reviews. Immunology 14 (10):667–85. doi:10.1038/nri3738.
- Naqash, F., F. A. Masoodi, S. A. Rather, S. M. Wani, and A. Gani. 2017. Emerging concepts in the nutraceutical and functional properties of pectin—A review. Carbohydrate Polymers 168:227–39. doi:10.1016/j.carbpol.2017.03.058.
- Nascimento, A. M., L. M. de Souza, C. H. Baggio, M. F. d P. Werner, D. Maria-Ferreira, L. M. da Silva, G. L. Sassaki, P. A. J. Gorin, M. Iacomini, and T. R. Cipriani. 2013. Gastroprotective effect and structure of a rhamnogalacturonan from Acmella oleracea. Phytochemistry 85 (January):137–42. doi:10.1016/j.phytochem.2012.08.024.
- do Nascimento, G. E., S. M. B. Winnischofer, M. I. Ramirez, M. Iacomini, and L. M. C. Cordeiro. 2017. The influence of sweet pepper pectin structural characteristics on cytokine secretion by THP-1 macrophages. Food Research International (Ottawa, Ont.) 102(December): 588–94. doi:10.1016/j.foodres.2017.09.037.
- Ndeh, D., A. Rogowski, A. Cartmell, A. S. Luis, A. Baslé, J. Gray, I. Venditto, J. Briggs, X. Zhang, A. Labourel, et al. 2017. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature 544 (7648):65–70. doi:10.1038/nature21725.
- Neu, J., R. Sharma, and C. Young. 2010. Molecular modulation of intestinal epithelial barrier: Contribution of microbiota. Journal of Biomedicine & Biotechnology 2010:305879. 2010. doi:10.1155/2010/305879.
- Nishida, A., R. Inoue, O. Inatomi, S. Bamba, Y. Naito, and A. Andoh. 2017. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clinical Journal of Gastroenterology 2017 11:1, 11 (1):1–10. doi:10.1007/s12328-017-0813-5.
- Ohira, H., W. Tsutsui, and Y. Fujioka. 2017. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? Journal of Atherosclerosis and Thrombosis 24 (7):660–72. doi:10.5551/jat.RV17006.
- Okumura, R., and K. Takeda. 2018. Maintenance of intestinal homeostasis by mucosal barriers. Inflammation and Regeneration 38 (1):5. doi:10.1186/s41232-018-0063-z.
- Paone, P., and P. D. Cani. 2020. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 69 (12):2232–43. doi:10.1136/gutjnl-2020-322260.
- Pappolla, M. A., G. Perry, X. Fang, M. Zagorski, K. Sambamurti, and B. Poeggeler. 2021. Indoles as essential mediators in the gut-brain axis. Their role in Alzheimer’s disease. Neurobiology of Disease 156(August): 105403. doi:10.1016/j.nbd.2021.105403.
- Parkar, S. G., E. L. Redgate, R. Wibisono, X. X. Luo, E. T. Koh, and R. Schröder. 2010. Gut health benefits of kiwifruit pectins: Comparison with commercial functional polysaccharides. Journal of Functional Foods 2 (3):210–8. doi:10.1016/j.jff.2010.04.009.
- Park, S. N., K. T. Noh, Y.-I. Jeong, I. D. Jung, H. K. Kang, G. S. Cha, S. J. Lee, J. K. Seo, D. H. Kang, T.-H. Hwang, et al. 2013. Rhamnogalacturonan II is a toll-like receptor 4 agonist that inhibits tumor growth by activating dendritic cell-mediated CD8+ T cells. Experimental & Molecular Medicine 2013 45:2 45 (2):e8–e8–e8. doi:10.1038/emm.2013.14.
- Perrone, P., C. M. Hewage, A. R. Thomson, K. Bailey, I. H. Sadler, and S. C. Fry. 2002. Patterns of methyl and O-acetyl esterification in Spinach pectins: New complexity. Phytochemistry 60 (1):67–77. doi:10.1016/s0031-9422(02)00039-0.
- Peterson, L. W., and D. Artis. 2014. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nature Reviews. Immunology 14 (3):141–53. doi:10.1038/nri3608.
- Petruzziello, L., F. Iacopini, M. Bulajic, S. Shah, and G. Costamagna. 2006. Review article: Uncomplicated diverticular disease of the colon. Alimentary Pharmacology & Therapeutics 23 (10):1379–91. doi:10.1111/j.1365-2036.2006.02896.x.
- Popov, S. V., P. A. Markov, G. Y. Popova, I. R. Nikitina, L. Efimova, and Y. S. Ovodov. 2013. Anti-inflammatory activity of low and high methoxylated citrus pectins. Biomedicine & Preventive Nutrition 3 (1):59–63. doi:10.1016/j.bionut.2012.10.008.
- Popov, S. V., R. G. Ovodova, P. A. Markov, I. R. Nikitina, and Y. S. Ovodov. 2006. Protective effect of comaruman, a pectin of cinquefoil Comarum palustre L., on acetic acid-induced colitis in mice. Digestive Diseases and Sciences 51 (9):1532–7. doi:10.1007/s10620-005-9034-8.
- Popov, S. V., and Y. S. Ovodov. 2013. Polypotency of the immunomodulatory effect of pectins. Biochemistry. Biokhimiia 78 (7):823–35. doi:10.1134/S0006297913070134.
- Priyadarshini, M., K. Lednovich, K. Xu, S. Gough, B. Wicksteed, and B. T. Layden. 2021. FFAR from the gut microbiome crowd: SCFA receptors in T1D pathology. Metabolites 11 (5):302. doi:10.3390/metabo11050302.
- Rajamma, S. S., V. Krishnaswami, and R. Kandasamy. 2022. Functional role of prebiotic supplement in brain signalling. Microbiome-Gut-Brain Axis: Implications on Health 215–36. Singapore: Springer. doi:10.1007/978-981-16-1626-6_9.
- Ralet, M. C., J. C. Cabrera, E. Bonnin, B. Quéméner, P. Hellìn, and J. F. Thibault. 2005. Mapping sugar beet pectin acetylation pattern. Phytochemistry 66 (15):1832–43. doi:10.1016/j.phytochem.2005.06.003.
- Ralet, M. C., M. J. Crépeau, H. C. Buchholt, and J. F. Thibault. 2003. Polyelectrolyte behaviour and calcium binding properties of sugar beet pectins differing in their degrees of methylation and acetylation. Biochemical Engineering Journal 16 (2):191–201. doi:10.1016/S1369-703X(03)00037-8.
- Ratajczak, W., A. Rył, A. Mizerski, K. Walczakiewicz, O. Sipak, and M. Laszczyńska. 2019. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochimica Polonica 66 (1):1–12. doi:10.18388/abp.2018_2648.
- Reddy, B. S., K. Watanabe, and A. Sheinfil. 1980. Effect of dietary wheat bran, alfalfa, pectin and carrageenan on plasma cholesterol and fecal bile acid and neutral sterol excretion in rats. The Journal of Nutrition 110 (6):1247–54. doi:10.1093/jn/110.6.1247.
- Ren, T., F. R. Liu, D. X. Wang, B. Li, P. Jiang, J. M. Li, H. Li, C. B. Chen, W. Wu, and L. L. Jiao. 2023. Rhamnogalacturonan-I enriched pectin from steamed ginseng ameliorates lipid metabolism in type 2 diabetic rats via gut microbiota and AMPK pathway. Journal of Ethnopharmacology 301(January): 115862. doi:10.1016/j.jep.2022.115862.
- Rossen, N. G., J. K. MacDonald, E. M. de Vries, G. R. D’Haens, W. M. de Vos, E. G. Zoetendal, and C. Y. Ponsioen. 2015. Fecal microbiota transplantation as novel therapy in gastroenterology: A systematic review. World Journal of Gastroenterology 21 (17):5359–71. doi:10.3748/wjg.v21.i17.5359.
- Sabater, C., J. A. Molina-Tijeras, T. Vezza, N. Corzo, A. Montilla, and P. Utrilla. 2019. Intestinal anti-inflammatory effects of artichoke pectin and modified pectin fractions in the dextran sulfate sodium model of mice colitis. Artificial neural network modelling of inflammatory markers. Food & Function 10 (12):7793–805. doi:10.1039/c9fo02221j.
- Sahasrabudhe, N. M., M. Beukema, L. Tian, B. Troost, J. Scholte, E. Bruininx, G. Bruggeman, M. van den Berg, A. Scheurink, H. A. Schols, et al. 2018. Dietary fiber pectin directly blocks toll-like receptor 2-1 and prevents doxorubicin-induced ileitis. Frontiers in Immunology 9 (MAR):383. doi:10.3389/fimmu.2018.00383.
- Salyers, A. A., S. E. West, J. R. Vercellotti, and T. D. Wilkins. 1977. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Applied and Environmental Microbiology 34 (5):529–33. doi:10.1128/aem.34.5.529-533.1977.
- Schleimer, R. P., and S. Berdnikovs. 2017. Etiology of epithelial barrier dysfunction in patients with type 2 inflammatory diseases. The Journal of Allergy and Clinical Immunology 139 (6):1752–61. doi:10.1016/j.jaci.2017.04.010.
- Schols, H. A., and A. G. Voragen. 1996. Complex pectins: Structure elucidation using enzymes. Progress in Biotechnology 14 (C):3–19.
- Slavin, J. 2013. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 5 (4):1417–35. doi:10.3390/nu5041417.
- Soliman, G. A. 2019. Dietary fiber, atherosclerosis, and cardiovascular disease. Nutrients 11 (5):1155. doi:10.3390/nu11051155.
- Sousa, A. G., H. L. Nielsen, I. Armagan, J. Larsen, and S. O. Sørensen. 2015. The impact of rhamnogalacturonan-I side chain monosaccharides on the rheological properties of citrus pectin. Food Hydrocolloids. 47 (May):130–9. doi:10.1016/j.foodhyd.2015.01.013.
- Spahn, T. W., and T. Kucharzik. 2004. Modulating the intestinal immune system: The role of lymphotoxin and GALT organs. Gut 53 (3):456–65. doi:10.1136/gut.2003.023671.
- Szentkuti, L., H. Riedesel, M. L. Enss, K. Gaertner, and W. Von Engelhardt. 1990. Pre-epithelial mucus layer in the colon of conventional and germ-free rats. The Histochemical Journal 22 (9):491–7. doi:10.1007/BF01007234.
- Tang, X., M. Beukema, M. Ferrari, M. T. C. Walvoort, B. J. De Haan, and P. De Vos. 2023. Efficacy of pectins with different degree of methyl-esterification and of blockiness on preventing gut epithelial cell barrier disruption and impact on sodium-glucose co-transporter expression under low and high glucose conditions. Food & Function 14 (13):6226–35. doi:10.1039/d3fo01436c.
- Takiishi, T., C. I. M. Fenero, and N. O. S. Câmara. 2017. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 5 (4):e1373208. doi:10.1080/21688370.2017.1373208.
- Thaiss, C. A., N. Zmora, M. Levy, and E. Elinav. 2016. The microbiome and innate immunity. Nature 535 (7610):65–74. doi:10.1038/nature18847.
- Thakur, B. R., R. K. Singh, and A. K. Handa. 2009. Chemistry and uses of pectin—A review. Critical Reviews in Food Science and Nutrition 37 (1):47–73. doi:10.1080/10408399709527767.
- Tian, L. M., G. Bruggeman, M. Van den Berg, K. Borewicz, A. J. W. Scheurink, E. Bruininx, P. De Vos, H. Smidt, H. A. Schols, and H. Gruppen. 2017. Effects of pectin on fermentation characteristics, carbohydrate utilization, and microbial community composition in the gastrointestinal tract of weaning pigs. Molecular Nutrition & Food Research 61 (1):1600186. doi:10.1002/mnfr.201600186.
- Tian, L. M., J. Scholte, K. Borewicz, B. Van den Bogert, H. Smidt, A. J. Scheurink, H. Gruppen, and H. A. Schols. 2016. Effects of pectin supplementation on the fermentation patterns of different structural carbohydrates in rats. Molecular Nutrition & Food Research 60 (10):2256–66. doi:10.1002/mnfr.201600149.
- Tolhurst, G., H. Heffron, Y. S. Lam, H. E. Parker, A. M. Habib, E. Diakogiannaki, J. Cameron, J. Grosse, F. Reimann, and F. M. Gribble. 2012. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61 (2):364–71. doi:10.2337/db11-1019.
- Turner, J. R. 2009. Intestinal mucosal barrier function in health and disease. Nature Reviews. Immunology 9 (11):799–809. doi:10.1038/nri2653.
- Vincken, J. P., H. A. Schols, R. J. Oomen, M. C. McCann, P. Ulvskov, A. G. J. Voragen, and R. G. F. Visser. 2003. If homogalacturonan were a side chain of rhamnogalacturonan I. Implications for cell wall architecture. Plant Physiology 132 (4):1781–9. doi:10.1104/pp.103.022350.
- Visser, J., J. Rozing, A. Sapone, K. Lammers, and A. Fasano. 2009. Tight junctions, intestinal permeability, and autoimmunity. Annals of the New York Academy of Sciences 1165 (1):195–205. doi:10.1111/j.1749-6632.2009.04037.x.
- Vogt, L. M., N. M. Sahasrabudhe, U. Ramasamy, D. Meyer, G. Pullens, M. M. Faas, K. Venema, H. A. Schols, and P. De Vos. 2016. The impact of lemon pectin characteristics on TLR activation and T84 intestinal epithelial cell barrier function. Journal of Functional Foods 22(April): 398–407. doi:10.1016/j.jff.2016.02.002.
- Walker, A. W., and T. D. Lawley. 2013. Therapeutic modulation of intestinal dysbiosis. Pharmacological Research 69 (1):75–86. doi:10.1016/j.phrs.2012.09.008.
- Wang, J. W., C. H. Kuo, F. C. Kuo, Y. K. Wang, W. H. Hsu, F. J. Yu, H. M. Hu, P. I. Hsu, J. Y. Wang, and D. C. Wu. 2019. Fecal microbiota transplantation: Review and update. Journal of the Formosan Medical Association = Taiwan Yi Zhi 118 Suppl 1(March): S23–S31. doi:10.1016/j.jfma.2018.08.011.
- Wang, X., L. Wilson, and D. J. Cosgrove. 2020. Pectin methylesterase selectively softens the onion epidermal wall yet reduces acid-induced creep. Journal of Experimental Botany 71 (9):2629–40. doi:10.1093/jxb/eraa059.
- Wikiera, A., M. Irla, and M. Mika. 2014. Health-promoting properties of pectin. Postepy Higieny i Medycyny Doswiadczalnej (Online) 68 (January):590–6. doi:10.5604/17322693.1102342.
- Willats, W. G., J. P. Knox, and J. D. Mikkelsen. 2006. Pectin: New insights into an old polymer are starting to gel. Trends in Food Science & Technology 17 (3):97–104. doi:10.1016/j.tifs.2005.10.008.
- Williams, L. M., H. A. Scott, and L. G. Wood. 2019. Soluble fibre as a treatment for inflammation in asthma. Journal of Nutrition & Intermediary Metabolism 18(December): 100108. doi:10.1016/j.jnim.2019.100108.
- Wu, C., L.-L. Pan, Y. Luo, W. Niu, X. Fang, W. Liang, J. Li, H. Li, X. Pan, G. Yang, et al. 2019. Low methoxyl pectin protects against autoimmune diabetes and associated caecal dysfunction. Molecular Nutrition & Food Research 63 (21):e1900307. doi:10.1002/mnfr.201900307.
- Wu, D. M., X. Q. Ye, R. J. Linhardt, X. W. Liu, K. Zhu, C. X. Yu, T. Ding, D. H. Liu, Q. J. He, and S. G. Chen. 2021. Dietary pectic substances enhance gut health by its polycomponent: A review. Comprehensive Reviews in Food Science and Food Safety 20 (2):2015–39. doi:10.1111/1541-4337.12723.
- Wu, D. M., J. Q. Zheng, G. Z. Mao, W. W. Hu, X. Q. Ye, R. J. Linhardt, and S. G. Chen. 2020. Rethinking the impact of RG-I mainly from fruits and vegetables on dietary health. Critical Reviews in Food Science and Nutrition 60 (17):2938–60. doi:10.1080/10408398.2019.1672037.
- Wu, W. D., L. Zhang, B. Xia, S. L. Tang, J. J. Xie, and H. F. Zhang. 2020. Modulation of pectin on mucosal innate immune function in pigs mediated by gut microbiota. Microorganisms 8 (4):535. doi:10.3390/microorganisms8040535.
- Xie, J. L., R. X. Yu, J. M. Qi, G. W. Zhang, X. C. Peng, and J. M. Luo. 2020. Pectin and inulin stimulated the mucus formation at a similar level: An omics-based comparative analysis. Journal of Food Science 85 (6):1939–47. doi:10.1111/1750-3841.15163.
- Yapo, B. M. 2011. Rhamnogalacturonan-I: A structurally puzzling and functionally versatile polysaccharide from plant cell walls and mucilages. Polymer Reviews 51 (4):391–413. doi:10.1080/15583724.2011.615962.
- Zhao, L., F. Zhang, X. Ding, G. Wu, Y. Y. Lam, X. Wang, H. Fu, X. Xue, C. Lu, J. Ma, et al. 2018. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science (New York, N.Y.) 359 (6380):1151–6. doi:10.1126/science.aao5774.
- Zhao, Y. Y., J. F. Bi, J. Y. Yi, X. Y. Wu, Y. C. Ma, and R. P. Li. 2021. Pectin and homogalacturonan with small molecular mass modulate microbial community and generate high SCFAs via in vitro gut fermentation. Carbohydrate Polymers 269(October): 118326. doi:10.1016/j.carbpol.2021.118326.