Abstract
Rheumatoid arthritis (RA) is a significant global health issue. Recent research highlights the gut microbiota’s critical role in RA’s development, noting how dietary factors can alter these microbial communities. This has led to an increased focus on how the gut microbiota (GM) influences RA and the potential for dietary ingredients to offer anti-RA benefits by modifying GM. This review presents a concise examination of the GM associated with RA, identifying specific microbial taxa at various levels that are implicated in the disease. It delves into dietary components known for their anti-RA properties through GM modulation and their mechanisms. Findings from numerous studies, including both animal and human research, show significant differences in the GM composition between individuals with early and established RA. Certain microbes like Tenericutes, Synergistetes, and Proteobacteria have been linked to RA progression, whereas Bacteroidetes and some strains of Lactobacillus are shown to have protective effects against RA. Dietary elements such as fibers, polysaccharides, resistant starch, and peptides have been identified as influential in combating RA. These components work by altering the GM’s metabolites and impacting immune cells related to the GM. This review suggests the potential for developing functional foods aimed at treating RA by targeting GM.
1. Introduction
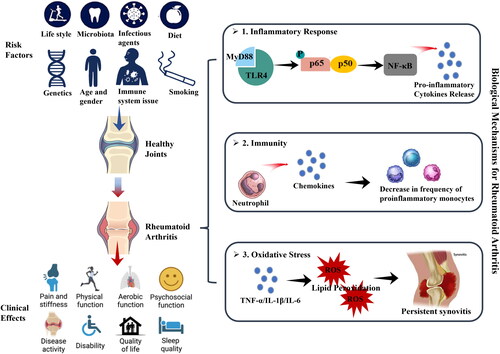
The World Health Organization (WHO) acknowledges rheumatoid arthritis (RA) as a significant contributor to the global disease burden, emphasizing its nature as a systemic autoimmune disease affecting millions (Wang et al. Citation2021). Characterized by inflammation of synovial joint tissue and extra-articular manifestations that lead to pain, joint damage, and disability, RA’s impact is profound, with an estimated 18 million sufferers worldwide as of 2019 and a documented global prevalence of 0.24% in the early twenty first century (Marita Cross et al. Citation2014; Vos et al. Citation2020; Reyes-Castillo et al. Citation2021; Wang et al. Citation2021; Zhang et al. Citation2023). The increased mortality risk among individuals with RA, being 1.5 times higher than the general population, has prompted a surge in research efforts aimed at enhancing RA management (Dadoun et al. Citation2013). The general approach to treating RA includes both nonpharmacological and pharmacological therapies (Radu and Bungau Citation2021). Nonpharmacological approaches aim to decrease anxiety and depression, reduce pain, and increase mobility. Polyunsaturated fatty acids (PUFAs) have gained attention due to their links to various brain disorders, including anxiety and depression (Liao et al. Citation2019). In RA patients, these conditions, along with pain, are associated with disease activity and poor functional status (Garip et al. Citation2015). According to existing medical evidence, PUFAs can be useful in controlling these symptoms, but additional studies are needed. Rest, physical exercise, and surgery can also be beneficial (Radu and Bungau Citation2021). Most studies evaluating the role of physical activity and psychological interventions for RA-related fatigue have demonstrated their effectiveness (Cramp Citation2019). Combined with rest, these interventions may relieve stress on inflamed tissues and slow disease progression (Hewlett et al. Citation2011). Joint surgery is reserved for severe stages of RA, providing pain relief and restoring joint function (Radu and Bungau Citation2021). However, nonpharmacological interventions have not fundamentally cured RA, and the inflammatory response often continues to intensify. Continuous improvements in procedures and drug design strategies have led to considerable progress in pharmacological approaches toward finding a cure for RA. Current treatment options, following the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) recommendations, manage RA from two perspectives: symptomatic treatment (NSAIDs and GCs) and disease-modifying management (DMARDs) (Smolen et al. Citation2020; Fraenkel et al. Citation2021). Nonsteroidal anti-inflammatory drugs (NSAIDs) are used in the acute phase to reduce pain by decreasing inflammation (Radu and Bungau Citation2021). Glucocorticoids (GCs) have greater potency and efficacy than NSAIDs due to their complex anti-inflammatory and immunosuppressive mechanisms, although NSAIDs have a slightly better safety profile (Radu and Bungau Citation2021). Disease-modifying antirheumatic drugs (DMARDs) are pharmacological agents used to promote remission by suppressing autoimmune activity and delaying or preventing joint degeneration. However, the risk of harm should be considered, as the inhibition of prostaglandins can lead to serious side effects, such as bleeding, gastrointestinal ulceration, renal failure, heart failure, and seizures (Crofford Citation2013). Long-term side effects of GCs include weight gain, water retention, muscle weakness, diabetes, and bone thinning (Radu and Bungau Citation2021). Recent trends in RA treatment research focus on targeted therapies, exploring the role of the gut as the largest immune organ, and the potential of gut microbiota modulation to improve RA management while mitigating the adverse effects of conventional treatments (Guo et al. Citation2018; Köhler et al. Citation2019; Radu and Bungau Citation2021). While the precise etiology of RA remains incompletely understood, there is consensus that its development involves a complex interplay between genetic and environmental factors () (Scherer, Häupl, and Burmester Citation2020). Among the various risk factors identified, intestinal dysbiosis—defined as alterations in the composition and/or function of the gut microbiota—has been extensively documented in the development process of RA (Scherer, Häupl, and Burmester Citation2020). It is commonly postulated that the continual exposure of mucosal sites in the oral cavity, lungs, and gastrointestinal tract to a high load of microbial antigens may contribute to the loss of immune tolerance and the onset of RA () (Holers et al. Citation2018). The plasticity of the microbiota presents an opportunity to modulate disease pathways independently or in conjunction with immune-modulating drugs (Horta-Baas, Sandoval-Cabrera, and Romero-Figueroa Citation2021). Recognizing the potential for therapeutic intervention, the microbiome emerges as an attractive target (Larsen Citation2017). It can be influenced through various means, including pharmaceuticals, dietary adjustments, probiotics, prebiotics, and fecal transplantation () (Larsen Citation2017).
Our review meticulously navigates through the wealth of research literature examining the link between the gut microbiota and RA, methodically shedding light on how dietary interventions might influence specific aspects of the gut microbiota to either prevent or mitigate the effects of RA. This comprehensive analysis heralds the potential of dietary components to act as functional foods, offering a promising avenue to enhance RA management through nutrition. To construct this narrative review, we engaged in a thorough examination of both original research and review articles. Our research endeavor spanned several esteemed databases, including PubMed, Elsevier, Springer, the American Chemical Society (ACS), the Royal Society of Chemistry (RSC), Wiley, Taylor & Francis, and Google Scholar. We employed a strategic search methodology, using keywords such as “rheumatoid arthritis” in conjunction with “gut microbiota” or “intestinal,” aiming to amass pertinent data that shed light on this intricate relationship. This diligent search process enabled us to aggregate and analyze critical findings that underscore the significant role of diet in influencing the gut microbiota, thereby impacting the development and progression of RA.
2. The influence of gut microbiota on the development of Rheumatoid arthritis
The gastrointestinal tract is essential to the immune system, providing a habitat for a broad and diverse array of microorganisms (Reyes-Castillo et al. Citation2021). Its main role is to act as a protective barrier, blocking pathogenic bacteria from reaching internal tissues (Ahluwalia, Magnusson, and Öhman Citation2017). Located within the submucosa, the gut-associated lymphoid tissue (GALT) is comprised of structured cell formations such as Peyer’s patches and lymph nodes, along with individual cells including intestinal intraepithelial lymphocytes (IELs), macrophages, dendritic cells (DCs), natural killer (NK) cells, polymorphonuclear leukocytes (PMN), and innate lymphoid cells (ILCs) (Geuking et al. Citation2014; Thaiss et al. Citation2016). The GALT plays a crucial role in regulating immune responses, and defending against intestinal pathogens while fostering tolerance toward harmless commensal bacteria, food substances, and self-proteins (Ahluwalia, Magnusson, and Öhman Citation2017). The gut microbiota, which includes trillions of bacteria, archaea, fungi, protozoa, and viruses, establishes a symbiotic relationship with the host (Geuking et al. Citation2014). The genetic material of this microbiome encodes more than eight million bacterial proteins, thereby extending our genome and supporting metabolic, neuroendocrine, and immunological functions (Li et al. Citation2014). Vital for the development of the immune system, the gut microbiota affects the GALT both directly, through pattern recognition receptors on immune cells and indirectly, via the secretion of metabolites that influence cell physiology within the intestines and beyond (Round and Mazmanian Citation2009; Arpaia et al. Citation2013). We summarize the role of gut microbiota in the overall progression of RA in .
2.1. Impact of gut microbiota on Rheumatoid arthritis development: influences on inflammatory immune mechanisms
RA progression is influenced by infections or bacterial imbalances at mucosal sites. It’s essential to highlight that anti-citrullinated peptide antibodies (ACPAs), produced through citrullination, serve as crucial biomarkers for predicting RA in some patients (Reyes-Castillo et al. Citation2021). ACPAs play a significant role in RA pathogenesis by forming immune complexes, initiating inflammatory responses in innate immune cells, and promoting osteoclastogenesis, which many RA medications target (Clavel et al. Citation2008; Harre et al. Citation2012). Citrullination is triggered by the overactivation of peptidyl arginine deiminases (PADs) due to cell damage, necrosis, or NETosis, a process intensified by certain factors, including specific mucosa-associated bacteria (Darrah and Andrade Citation2018). A previous study identified Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans in the oral cavity as promoters of citrullination, expressing enzymes similar to PADs and producing leucotoxin A (LtxA), a bacterial toxin that creates pores in the neutrophil membrane and induces a calcium-dependent overactivation of intracellular PADs (Darrah and Andrade Citation2018). Another significant discovery is the linkage of P. gingivalis to RA pathogenesis through its alteration of the gut microbiota and increase in intestinal permeability when given orally to arthritis-prone mice (Nakajima et al. Citation2015; Flak et al. Citation2019). The gut microbiota may induce changes in the host proteome’s self-proteins, potentially triggering the production of other autoantibody systems in RA (Juarez et al. Citation2016). For instance, anti-acetylated protein antibodies were found in approximately 35% of early RA patients, with a specificity ranging from 65%-85% (Juarez et al. Citation2016). Mouse studies have shown that the microbiome affects acetylation patterns, and specific dietary components can regulate lysine acetylation levels (Kim et al. Citation2010; Simon, Cheng, and Gordon Citation2012). Although the precise mechanisms leading to the production of anti-APAs in RA and their biological and clinical relevance remain to be fully understood, these findings highlight a significant link between diet, gut microbiota, and protein acetylation as potential targets for antibody responses in RA, emphasizing the interconnectedness between oral and gut microbiota and its impact on systemic immune responses.
The contribution of gut microbiota alterations to RA progression involves an increase in intestinal permeability. Specifically, C. aerofaciens, found in high numbers in RA patients, is associated with increased permeability in the epithelial cell line by reducing the expression of tight junction proteins such as zonula occludens-1 (ZO-1) and occludin, while simultaneously enhancing the expression of pro-inflammatory mediators like Interleukin-17 (IL-17), chemokine (C-X-C motif) ligand (CXCL), and Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (Chen et al. Citation2016). Additionally, individuals at risk of RA show a decrease in interferon γ (IFN-γ)-producing innate lymphoid cells-1 (ILC1) cells, whereas early RA patients have an increase in IL-17-producing ILC3 cells, indicating immunophenotypic changes at the intestinal level in the disease’s early stages. Intestinal bacteria promote inflammation and intestinal permeability by influencing the GALT, leading to the loss of gut barrier integrity and allowing bacterial antigens to enter the systemic circulation, potentially triggering inflammatory immune responses at sites outside the intestine (Fine et al. Citation2020). RA is known to be driven by inflammatory immune processes, with growing evidence highlighting the critical role of inflammation in its development and progression. In a particular study, germ-free Sakai Koyasu Granulocyte (SKG) mice inoculated with fecal samples from RA patients, enriched with P. copri, showed an increase in intestinal T helper 17 (Th17) cells and subsequently developed severe RA (Maeda et al. Citation2016). Notably, microbial peptides strongly bind to human leukocyte antigen “shared epitope” molecules, the most significant genetic risk factor for ACPA-positive RA (Reyes-Castillo et al. Citation2021), potentially further activating T and B cell responses in individuals with RA.
2.2. Epidemiological insights into the gut microbiota’s contribution to Rheumatoid arthritis development
At various stages of RA development, the immune system plays a significant role that cannot be underestimated. The RA etiopathogenic mechanisms are complex, involving interactions between the innate and acquired immune responses, including antigen-presenting cells (APCs), autoreactive T cell formation, and the production of autoantibodies directed against the self-cellular structures, such as rheumatoid factor (RF) and ACPAs (Weyand and Goronzy Citation2021). These antibodies are often present in the blood long before any signs of joint inflammation, suggesting that the initiation of autoimmunity may occur in locations other than the joints, such as the gastrointestinal tract (Malmström, Catrina, and Klareskog Citation2017).
From an epidemiological perspective, the hygiene hypothesis suggests an inverse relationship between the frequency of infections and the prevalence of autoimmune diseases at the population level (Strachan Citation1989; Ege Citation2017). Current epidemiological evidence strongly underscores the significant role of the gut microbiota in the development and regulation of the immune system (Bach Citation2018). Additionally, the rise in immune-mediated diseases in recent decades has been partly attributed to widespread microbial deprivation (Bach Citation2018). Evidence from the literature emphasizes the critical role of the gastrointestinal tract in maintaining immune homeostasis and preventing autoimmunity through the production of inducible regulatory T cells (iTregs) and IL17-producing T cells (Th17) (Jiao et al. Citation2020). This complex equilibrium is largely driven by the synergistic effects of multiple bacterial strains, as opposed to the impact of individual species (Picchianti-Diamanti et al. Citation2018).
2.3. The impact of gut microbiota on Rheumatoid arthritis development: insights from clinical studies
From a clinical viewpoint, metagenomic studies on the gut microbiota in RA patients have identified prevalent microbial changes, including reduced diversity, altered functional profiles, and specific bacterial proliferation (Reyes-Castillo et al. Citation2021). Nonetheless, pinpointing a definitive microbial profile associated with RA has proven difficult due to methodological differences and the limitations of sample sizes in current studies (Reyes-Castillo et al. Citation2021). Detailed in , this review highlights the connections between RA and microbiota alterations. Contrary to initial assumptions, RA patients often exhibit increased levels of probiotics, which are traditionally viewed as beneficial. This finding challenges earlier suggestions that probiotics could improve RA symptoms (Liu, Zeng, et al. Citation2016). Importantly, supplementation with L. casei and L. acidophilus in RA patients has been shown to decrease inflammatory markers and disease activity, whereas other species such as L. rhamnosus and L. reuteri do not have significant effects on patients with active RA (Hatakka et al. Citation2003; de los Angeles Pineda et al. Citation2011; Vaghef-Mehrabany et al. Citation2014; Zamani et al. Citation2016). This emphasizes the species-specific nature of probiotic impacts (Sánchez et al. Citation2017; Picchianti-Diamanti et al. Citation2018). Despite the heterogeneity of studies, the existing research consistently shows alterations in the gut microbiota of RA patients across various stages of the disease.
Table 1. The significant changes of the microbiota composition in early RA patients, RA patients, and RA mice models.
3. Overview of gut microbiota imbalance in Rheumatoid arthritis development
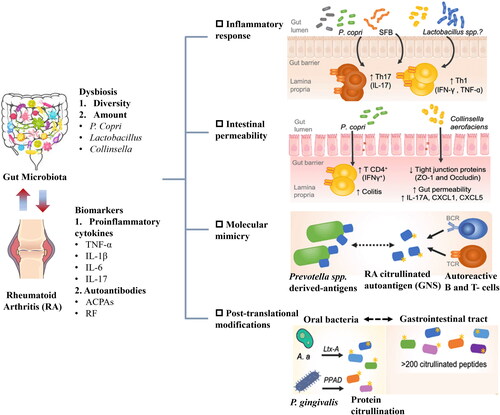
Dysbiosis, characterized by changes in the composition and function of the microbiota due to environmental and host-related factors, has been observed in both the oral cavity and intestines of individuals with RA (Zaiss et al. Citation2021). This supports the hypothesis that mucosal regions, which harbor specific microbial flora, are foundational to RA’s pathogenesis by potentially disrupting local and systemic immune responses (Holers et al. Citation2018; Zaiss et al. Citation2021). It’s critical to distinguish between microbiota alterations in the early stages of RA and those in established RA, as systemic inflammation and pharmacological treatments can affect microbial composition (Zaiss et al. Citation2021).
Oral and intestinal dysbiosis have been documented in patients with early and established RA. Notably, early RA patients without drug treatment show a significant increase in Lactobacillus compared to healthy individuals (Liu et al. Citation2013), a trend also seen in collagen-induced arthritis (CIA) mice (Li et al. Citation2022). Early RA patients also demonstrate a reduction in the Faecalibacterium genus and Parabacteroides distasonis species (Picchianti-Diamanti et al. Citation2018; Sun et al. Citation2023), which continues in established RA. The absence of these bacteria may lead to intestinal inflammation and compromised intestinal epithelial integrity (Lai et al. Citation2022). Interestingly, Bacteroides genus levels decrease in early RA but increase in established RA and CIA mice compared to healthy controls, possibly due to their role in promoting immunoglobulin M (IgM) to immunoglobulin A (IgA) class switching and increasing intestinal IgA (Yanagibashi et al. Citation2009; Ben-Amram et al. Citation2017; Nemoto et al. Citation2020; Zaiss et al. Citation2021). Some studies also show a reduction in Prevotellaceae in RA patients, though it is enriched in RA mice. Prevotellaceae’s link to RA pathogenesis is noted, with similar epitopes to human self-antigens found in RA patients (Pianta et al. Citation2017). The above content has been summarized in .
Figure 3. Dysbiosis in the gut microbiome is associated with the initiation and progression of rheumatoid arthritis (RA).
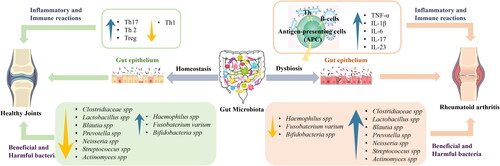
As shown in , the complexity of the gut microbiota suggests that RA susceptibility is not solely due to the abundance of a single species (Liu, Zeng, et al. Citation2016). Assessments typically measure species diversity using Chao1, Shannon, and Simpson indices in RA mouse gut microbiota research (Ma et al. Citation2023). Significant changes at the phylum level, particularly in Bacteroidetes, Firmicutes, and Proteobacteria, have been observed. Proteobacteria levels vary significantly across studies, suggesting its potential role as a marker for RA-associated intestinal disorders (Liu, Zeng, et al. Citation2016; Liu, Peng, et al. Citation2023; Ma et al. Citation2023). Firmicutes levels also vary, with some studies showing a decrease in CIA mice compared to healthy controls, and others an increase (Nemoto et al. Citation2020; Li et al. Citation2022; Ma et al. Citation2023). These differences may be due to the variability in beneficial versus non-beneficial bacteria within the Firmicutes phylum. Discrepancies at the order level, such as inconsistent trends within the same family or between the order and genus levels, highlight the diverse functionalities of bacterial families, even within the same order (Nemoto et al. Citation2020; Bai et al. Citation2021; Wu et al. Citation2022). In summary, modifications in the gut microbiota are evident in both early and established RA patients and CIA mice, with dysbiosis playing a crucial role at each stage of RA development.
4. Dietary influence on Rheumatoid arthritis: modulating gut microbiota for therapeutic effect
The intricate relationship between the intestinal microbiota and the management of RA has become a focal point of contemporary medical research. The recognition that various RA management strategies can induce changes in the gut microbiota composition underscores the potential of gut flora modulation as a novel approach to both prevent and treat RA. This insight has catalyzed a burgeoning interest in the scientific community toward leveraging dietary interventions as a means to address the underlying microbial imbalances associated with RA.
Recent years have witnessed a paradigm shift, with an increased emphasis on the development of food-based products specifically designed to target and ameliorate chronic diseases linked to gut dysbiosis, including RA. This approach is rooted in the understanding that the gut microbiome plays a crucial role in human health, affecting everything from immune system function to metabolic processes and even the body’s response to treatment. The premise is that by altering the gut microbiota through specific dietary interventions, it may be possible to mitigate the symptoms of RA, reduce disease activity, and potentially even alter the disease’s trajectory.
4.1. Modulating gut microbiota through dietary interventions: a novel approach to managing Rheumatoid arthritis
In this context, we have meticulously reviewed and summarized studies conducted over the last five years, aiming to shed light on how dietary modifications can positively influence RA outcomes by modulating the gut microbiota. The findings, detailed in and , cover a wide range of dietary components, including probiotics, prebiotics, specific dietary fibers, and whole-food plant-based diets. These dietary components have been shown to influence the composition and functionality of the gut microbiota, leading to an improved inflammatory profile, enhanced gut barrier function, and a reduction in the autoimmunity markers typically associated with RA. For example, research has demonstrated that the inclusion of certain probiotics in the diet can lead to an increase in beneficial gut bacteria, such as Lactobacillus and Bifidobacterium, which are known to produce anti-inflammatory compounds like short-chain fatty acids (SCFAs) (Li et al. Citation2022). These SCFAs can help modulate the immune system and reduce inflammation, offering relief to individuals with RA (Kim Citation2018). Similarly, diets rich in fibers and prebiotics can foster a gut environment that supports the growth of these and other beneficial microbes, further contributing to disease management. Moreover, specific dietary patterns, such as the Mediterranean diet, rich in fruits, vegetables, whole grains, and healthy fats, have been associated with a more favorable gut microbiota profile and lower levels of systemic inflammation in RA patients. These dietary strategies not only aim to modulate the gut microbiome but also offer broader health benefits, including improved cardiovascular health and reduced risk of other chronic conditions.
Figure 4. Nutrients and their food sources are involved in the development and progression of Rheumatoid arthritis (RA). TMAO: trimethylamine-N-oxide.
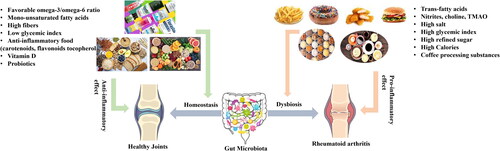
Table 2. The gut microbiota is modulated by dietary ingredients (references sourced from articles published after 2019).
The cumulative evidence from these studies underscores the promising role of dietary interventions in modulating the gut microbiota as a complementary approach to traditional RA treatments. It highlights the potential for diet to serve as a powerful tool in the holistic management of RA, paving the way for more personalized nutrition-based strategies in the future, as detailed in .
4.2. The meliorative effect of diet on Rheumatoid arthritis model by regulating intestinal microorganisms
In existing literature, the administration of the probiotics Faecalibacterium prausnitzii and Lactobacillus casei CCFM1074 has been found to effectively restore arthritis scores, joint tissue damage, and inflammatory responses in RA models (Fan et al. Citation2021; Moon et al. Citation2023). These positive outcomes can be attributed to the beneficial alterations in gut microbiota resulting from the consumption of these probiotics. While probiotic supplementation in RA patients has generally proven to be safe, further research is imperative to establish a robust rationale for determining the most suitable strains, considering the diverse clinical features of individual patients.
The intake of specific nutrients or foods, such as resistant starch, polysaccharides, and biopeptides, has demonstrated a notable impact on gut microbiota composition (Bai et al. Citation2021; Li et al. Citation2021; Zhang et al. Citation2022). The metabolites produced from gut microbiota play a pivotal role in alleviating RA symptoms. According to the literature, SCFAs, identified as crucial mediators in arthritis alleviation, are implicated in enhancing lipid metabolism, regulating energy consumption, preserving intestinal mucosal integrity, and controlling the immune system and inflammatory response in various animal models (Kim Citation2018). The significance of SCFAs in RA, associated with alterations in the intestinal flora, is underscored by studies conducted by Bai, Feng, and Zhang (Bai et al. Citation2021; Feng et al. Citation2022; Zhang et al. Citation2022). Their findings suggest that high-fiber diets, resistant starch, β-carotene, and biopeptides may alleviate inflammation in RA mice by modulating intestinal flora, subsequently influencing specific SCFAs production with immunomodulatory effects against RA. Specifically, a high-fiber diet (HFD) rich in resistant starch (RS) eliminated the increased abundance of Lactobacillus and Lachnoclostridium genera associated with RA (Bai et al. Citation2021). Concurrently, RS-HFD led to a predominance of Bacteroidetes and increased abundances of the Lachnospiraceae_NK4A136_group and Bacteroidales_S24-7_group genera in CIA mice (Bai et al. Citation2021). Additionally, β-carotene partially restored the decreased abundance of Muribaculaceae, Ruminococcaceae_UCG-014, and Lachnospiraceae_NK4A136_group induced by RA (Feng et al. Citation2022). Moreover, tuna elastin peptide-treated CIA mice showed reversed gut microbiota dysbiosis and an increased abundance of Lactobacillus and Clostridium (Zhang et al. Citation2022). While specific evidence regarding dietary nutritional supplementation with particular compounds is currently limited, dietary interventions as a complement to RA therapy hold promise not only in reestablishing dysbiosis associated with the disease but also in enhancing the metabolic profile in RA models.
Moreover, certain dietary plants and medicinal extracts from herbs emerge as functional foods or potential drugs for mitigating RA progression (Cong et al. Citation2023; Jianghui et al. Citation2023; Wang et al. Citation2023). The intervention with these ingredients consistently enhances the α-diversity of the gut microbial community and reverses changes in the overall structure and composition of the gut microbiota in RA mice (Cong et al. Citation2023; Jianghui et al. Citation2023; Wang et al. Citation2023). These bioactive ingredients not only significantly impact the aforementioned SCFAs but also are associated with inflammatory response pathways, such as the toll-like receptor 4/myeloid differentiation primary response 88/mitogen-activated protein kinase (TLR4/MyD88/MAPK) signaling pathways, by modulating intestinal flora. It is noteworthy that specific gut microbiota is linked to Th17 cells (Jianghui et al. Citation2023); for instance, Prevotella can increase the number of Th17 cells in the intestine, inducing macrophages and fibroblasts to produce substantial amounts of proinflammatory cytokines (Xu et al. Citation2020). Overall, modifying the gut microbiota of RA mice models through the use of probiotics, prebiotics, and dietary approaches appears to be a promising strategy for restoring gut dysbiosis, improving RA clinical parameters, and positively influencing metabolic profiles–one of the major comorbidities associated with RA.
5. Conclusion and perspective
In conclusion, the intricate relationship between gut microbiota and the development of RA has been extensively validated across molecular, clinical, and epidemiological fronts. The dynamic alterations observed in gut microbiota, spanning from phylum to species levels, correlate closely with the progression of RA. Notably, in larger statistical cohorts, gut microbiota emerges as a promising indicator for assessing RA severity. However, the subtle variations in gut microbiota changes among RA patients warrant further exploration.
Experimental models utilizing RA mice and rats have unveiled the potential of dietary interventions in mitigating RA symptoms, owing to their shared pathological and immunological characteristics with human RA. Specifically, interventions targeting gut microbiota modulation through probiotics, prebiotics, and bioactive compounds have shown promising results in altering RA progression, notably by regulating metabolites such as SCFAs and immune-related cell generation.
As per future direction, it is imperative to identify more beneficial gut microbiota strains with anti-RA properties and evaluate dietary components for their effects on gut microbiota. Moreover, deeper investigations into the interplay between genetics and the microbiome in RA development are crucial, alongside understanding the roles of other microbiota constituents in the etiology of RA. These endeavors will not only enhance our understanding of RA pathogenesis but also pave the way for more targeted and effective therapeutic interventions.
Authors’ contributions
Lingyue Shan: Writing-Original Draft, Drawing. Ramachandran Chelliah: Writing-Review & Editing. Deog Hwan Oh: Project administration and funding acquisition.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
All the datasets will be available to the reviewers and readers.
Additional information
Funding
References
- Ahluwalia, B., M. K. Magnusson, and L. Öhman. 2017. Mucosal immune system of the gastrointestinal tract: Maintaining balance between the good and the bad. Scandinavian Journal of Gastroenterology 52 (11):1185–93. doi:10.1080/00365521.2017.1349173.
- Arpaia, N., C. Campbell, X. Fan, S. Dikiy, J. van der Veeken, P. deRoos, H. Liu, J. R. Cross, K. Pfeffer, P. J. Coffer, et al. 2013. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504 (7480):451–5. doi:10.1038/nature12726.
- Bach, J. F. 2018. The hygiene hypothesis in autoimmunity: The role of pathogens and commensals. Nature Reviews. Immunology 18 (2):105–20. doi:10.1038/nri.2017.111.
- Bai, Y., Y. Li, T. Marion, Y. Tong, M. M. Zaiss, Z. Tang, Q. Zhang, Y. Liu, and Y. Luo. 2021. Resistant starch intake alleviates collagen-induced arthritis in mice by modulating gut microbiota and promoting concomitant propionate production. Journal of Autoimmunity 116:102564. doi:10.1016/j.jaut.2020.102564.
- Ben-Amram, H., T. Bashi, N. Werbner, H. Neuman, M. Fridkin, M. Blank, Y. Shoenfeld, and O. Koren. 2017. Tuftsin-phosphorylcholine maintains normal gut microbiota in collagen induced arthritic mice. Frontiers in Microbiology 8:1222. doi:10.3389/fmicb.2017.01222.
- Breban, M., J. Tap, A. Leboime, R. Said-Nahal, P. Langella, G. Chiocchia, J.-P. Furet, and H. Sokol. 2017. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Annals of the Rheumatic Diseases 76 (9):1614–22. doi:10.1136/annrheumdis-2016-211064.
- Chen, J., K. Wright, J. M. Davis, P. Jeraldo, E. V. Marietta, J. Murray, H. Nelson, E. L. Matteson, and V. Taneja. 2016. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Medicine 8 (1):43. doi:10.1186/s13073-016-0299-7.
- Clavel, C., L. Nogueira, L. Laurent, C. Iobagiu, C. Vincent, M. Sebbag, and G. Serre. 2008. Induction of macrophage secretion of tumor necrosis factor α through Fcγ receptor IIa engagement by rheumatoid arthritis–specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis and Rheumatism 58 (3):678–88. doi:10.1002/art.23284.
- Cong, S., L. Wang, Y. Meng, X. Cai, C. Zhang, Y. Gu, X. Ma, and L. Luo. 2023. Saussurea involucrata oral liquid regulates gut microbiota and serum metabolism during alleviation of collagen-induced arthritis in rats. Phytotherapy Research: PTR 37 (4):1242–59. doi:10.1002/ptr.7681.
- Cramp, F. 2019. The role of non-pharmacological interventions in the management of rheumatoid-arthritis-related fatigue. Rheumatology 58 (Supplement_5):v22–v28. doi:10.1093/rheumatology/kez310.
- Crofford, L. J. 2013. Use of NSAIDs in treating patients with arthritis. Arthritis Research & Therapy 15 (Suppl 3):S2. doi:10.1186/ar4174.
- Dadoun, S., N. Zeboulon-Ktorza, C. Combescure, M. Elhai, S. Rozenberg, L. Gossec, and B. J. J. B. S. Fautrel. 2013. Mortality in rheumatoid arthritis over the last fifty years: Systematic review and meta-analysis. Joint Bone Spine 80 (1):29–33. doi:10.1016/j.jbspin.2012.02.005.
- Darrah, E., and F. Andrade. 2018. Rheumatoid arthritis and citrullination. Current Opinion in Rheumatology 30 (1):72–8. doi:10.1097/BOR.0000000000000452.
- de los Angeles Pineda, M., S. F. Thompson, K. Summers, F. de Leon, J. Pope, and G. Reid. 2011. A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 17 (6):CR347–CR354. doi:10.12659/MSM.881808.
- Ege, M. J. 2017. The hygiene hypothesis in the age of the microbiome. Annals of the American Thoracic Society 14 (Supplement_5):S348–S353. doi:10.1513/AnnalsATS.201702-139AW.
- Fan, Z., R. P. Ross, C. Stanton, B. Hou, J. Zhao, H. Zhang, B. Yang, and W. Chen. 2021. Lactobacillus casei CCFM1074 alleviates collagen-induced arthritis in rats via balancing Treg/Th17 and modulating the metabolites and gut microbiota. Frontiers in Immunology 12:680073. doi:10.1002/eji.200939578.
- Feng, Y., Y. Yu, Z. Chen, L. Wang, J. Ma, X. Bai, Y. Sun, and D. Wang. 2022. Effects of β-carotin and green tea powder diets on alleviating the symptoms of gouty arthritis and improving gut microbiota in C57BL/6 Mice. Frontiers in Microbiology 13:837182. doi:10.3389/fmicb.2022.837182.
- Fine, R. L., S. Manfredo Vieira, M. S. Gilmore, and M. A. Kriegel. 2020. Mechanisms and consequences of gut commensal translocation in chronic diseases. Gut Microbes 11 (2):217–30. doi:10.1080/19490976.2019.1629236.
- Flak, M. B., R. A. Colas, E. Muñoz-Atienza, M. A. Curtis, J. Dalli, and C. Pitzalis. 2019. Inflammatory arthritis disrupts gut resolution mechanisms, promoting barrier breakdown by Porphyromonas gingivalis. JCI Insight 4 (13):125191. doi:10.1172/jci.insight.125191.
- Fraenkel, L., J. M. Bathon, B. R. England, E. W. St.Clair, T. Arayssi, K. Carandang, K. D. Deane, M. Genovese, K. K. Huston, G. Kerr, et al. 2021. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis & Rheumatology 73 (7):1108–23. doi:10.1002/art.41752.
- Garip, Y., E. Filiz, A. Kiliçarslan, and H. Bodur. 2015. Prevalence of neuropathic pain in rheumatic disorders: Association with disease activity, functional status and quality of life. Archives of Rheumatology 30 (3):231–7. doi:10.5606/ArchRheumatol.2015.5295.
- Geuking, M. B., Y. Köller, S. Rupp, and K. D. McCoy. 2014. The interplay between the gut microbiota and the immune system. Gut Microbes 5 (3):411–8. doi:10.4161/gmic.29330.
- Guo, Q., Y. Wang, D. Xu, J. Nossent, N. J. Pavlos, and J. Xu. 2018. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Research 6 (1):15. doi:10.1038/s41413-018-0016-9.
- Harre, U., D. Georgess, H. Bang, A. Bozec, R. Axmann, E. Ossipova, P.-J. Jakobsson, W. Baum, F. Nimmerjahn, E. Szarka, et al. 2012. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. The Journal of Clinical Investigation 122 (5):1791–802. doi:10.1172/JCI60975.
- Hatakka, K., J. Martio, M. Korpela, M. Herranen, T. Poussa, T. Laasanen, M. Saxelin, H. Vapaatalo, E. Moilanen, and R. Korpela. 2003. Effects of probiotic therapy on the activity and activation of mild rheumatoid arthritis–a pilot study. Scandinavian Journal of Rheumatology 32 (4):211–5. doi:10.1080/03009740310003695.
- Hewlett, S., T. Chalder, E. Choy, F. Cramp, B. Davis, E. Dures, C. Nicholls, and J. Kirwan. 2011. Fatigue in rheumatoid arthritis: Time for a conceptual model. Rheumatology 50 (6):1004–6. doi:10.1093/rheumatology/keq282.
- Holers, V. M., M. K. Demoruelle, K. A. Kuhn, J. H. Buckner, W. H. Robinson, Y. Okamoto, J. M. Norris, and K. D. Deane. 2018. Rheumatoid arthritis and the mucosal origins hypothesis: Protection turns to destruction. Nature Reviews Rheumatology 14 (9):542–57. doi:10.1038/s41584-018-0070-0.
- Horta-Baas, G., A. Sandoval-Cabrera, and M. d S. Romero-Figueroa. 2021. Modification of gut microbiota in inflammatory arthritis: Highlights and future challenges. Current Rheumatology Reports 23 (8):67. doi:10.1007/s11926-021-01031-9.
- Jiang, Z.-M., S.-L. Zeng, T.-Q. Huang, Y. Lin, F.-F. Wang, X.-J. Gao, J. Li, P. Li, and E.-H. Liu. 2023. Sinomenine ameliorates rheumatoid arthritis by modulating tryptophan metabolism and activating aryl hydrocarbon receptor via gut microbiota regulation. Science Bulletin 68 (14):1540–55. doi:10.1016/j.scib.2023.06.027.
- Jianghui, H., N. Jimin, J. Zheng, G. Yanlei, Y. Yong, Y. Cheng, S. Xiongjie, X. Hui, L. Yanju, and L. Hongtao. 2023. Tripterygium hypoglaucum extract ameliorates adjuvant-induced arthritis in mice through the gut microbiota. Chinese Journal of Natural Medicines 21 (10):730–44. doi:10.1016/S1875-5364(23)60466-2.
- Jiao, Y., L. Wu, N. D. Huntington, and X. Zhang. 2020. Crosstalk between gut microbiota and innate immunity and its implication in autoimmune diseases. Frontiers in Immunology 11:282. doi:10.3389/fimmu.2020.00282.
- Juarez, M., H. Bang, F. Hammar, U. Reimer, B. Dyke, I. Sahbudin, C. D. Buckley, B. Fisher, A. Filer, and K. Raza. 2016. Identification of novel antiacetylated vimentin antibodies in patients with early inflammatory arthritis. Annals of the Rheumatic Diseases 75 (6):1099–107. doi:10.1136/annrheumdis-2014-206785.
- Kim, C. H. 2018. Microbiota or short-chain fatty acids: Which regulates diabetes? Cellular & Molecular Immunology 15 (2):88–91. doi:10.1038/cmi.2017.57.
- Kim, G.-W., G. Gocevski, C.-J. Wu, and X.-J. Yang. 2010. Dietary, metabolic, and potentially environmental modulation of the lysine acetylation machinery. International Journal of Cell Biology 2010:632739–14. doi:10.1155/2010/632739.
- Kishikawa, T., Y. Maeda, T. Nii, D. Motooka, Y. Matsumoto, M. Matsushita, H. Matsuoka, M. Yoshimura, S. Kawada, S. Teshigawara, et al. 2020. Metagenome-wide association study of gut microbiome revealed novel aetiology of rheumatoid arthritis in the Japanese population. Annals of the Rheumatic Diseases 79 (1):103–11. doi:10.1136/annrheumdis-2019-215743.
- Köhler, B. M., J. Günther, D. Kaudewitz, and H.-M. J. J. o c m Lorenz. 2019. Current therapeutic options in the treatment of rheumatoid arthritis. Journal of Clinical Medicine 8 (7):938. doi:10.3390/jcm8070938.
- Lai, W., C. Wang, R. Lai, X. Peng, and J. Luo. 2022. Lycium barbarum polysaccharide modulates gut microbiota to alleviate rheumatoid arthritis in a rat model. NPJ Science of Food 6 (1):34. doi:10.1038/s41538-022-00149-z.
- Larsen, J. M. 2017. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 151 (4):363–74. doi:10.1111/imm.12760.
- Li, J., H. Jia, X. Cai, H. Zhong, Q. Feng, S. Sunagawa, M. Arumugam, J. R. Kultima, E. Prifti, T. Nielsen, MetaHIT Consortium., et al. 2014. An integrated catalog of reference genes in the human gut microbiome. Nature Biotechnology 32 (8):834–41. doi:10.1038/nbt.2942.
- Li, N., X. Li, R. Su, R. Wu, H.-Q. Niu, J. Luo, C. Gao, X. Li, and C. Wang. 2022. Low-dose interleukin-2 altered gut microbiota and ameliorated collagen-induced arthritis. Journal of Inflammation Research 15:1365–79. doi:10.2147/JIR.S344393.
- Li, Y., M. Dai, L. Wang, and G. Wang. 2021. Polysaccharides and glycosides from Aralia echinocaulis protect rats from arthritis by modulating the gut microbiota composition. Journal of Ethnopharmacology 269:113749. doi:10.1016/j.jep.2020.113749.
- Liao, Y., B. Xie, H. Zhang, Q. He, L. Guo, M. Subramanieapillai, B. Fan, C. Lu, and R. S. McIntyre. 2019. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Translational Psychiatry 9 (1):190. doi:10.1038/s41398-019-0515-5.
- Liu, J., F. Peng, H. Cheng, D. Zhang, Y. Zhang, L. Wang, F. Tang, J. Wang, Y. Wan, J. Wu, et al. 2023. Chronic cold environment regulates rheumatoid arthritis through modulation of gut microbiota-derived bile acids. The Science of the Total Environment 903:166837. doi:10.1016/j.scitotenv.2023.166837.
- Liu, M., S. Li, N. Cao, Q. Wang, Y. Liu, Q. Xu, L. Zhang, C. Sun, X. Xiao, and J. Yao. 2023. Intestinal flora, intestinal metabolism, and intestinal immunity changes in complete Freud’s adjuvant-rheumatoid arthritis C57BL/6 mice. International Immunopharmacology 125 (Pt A):111090. doi:10.1016/j.intimp.2023.111090.
- Liu, X., B. Zeng, J. Zhang, W. Li, F. Mou, H. Wang, Q. Zou, B. Zhong, L. Wu, H. Wei, et al. 2016. Role of the gut microbiome in modulating arthritis progression in mice. Scientific Reports 6 (1):30594. doi:10.1038/srep30594.
- Liu, X., J. Zhang, Q. Zou, B. Zhong, H. Wang, F. Mou, L. Wu, and Y. Fang. 2016. Lactobacillus salivarius isolated from patients with rheumatoid arthritis suppresses collagen-induced arthritis and increases Treg frequency in mice. Journal of Interferon Cytokine Research 36 (12):706–12. doi:10.1089/jir.2016.0057.
- Liu, X., Q. Zou, B. Zeng, Y. Fang, and H. Wei. 2013. Analysis of fecal Lactobacillus community structure in patients with early rheumatoid arthritis. Current Microbiology 67 (2):170–6. doi:10.1007/s00284-013-0338-1.
- Ma, Y., W. Li, S. Niu, X. Zhu, M. Chu, W. Wang, W. Sun, X. Wei, J. Zhang, and Z. Zhang. 2023. BzATP reverses ferroptosis-induced gut microbiota disorders in collagen-induced arthritis mice. International Immunopharmacology 124 (Pt A):110885. doi:10.1016/j.intimp.2023.110885.
- Maeda, Y., T. Kurakawa, E. Umemoto, D. Motooka, Y. Ito, K. Gotoh, K. Hirota, M. Matsushita, Y. Furuta, M. Narazaki, et al. 2016. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis & Rheumatology (Hoboken, N.J.) 68 (11):2646–61. doi:10.1002/art.39783.
- Malmström, V., A. I. Catrina, and L. Klareskog. 2017. The immunopathogenesis of seropositive rheumatoid arthritis: From triggering to targeting. Nature Reviews. Immunology 17 (1):60–75. doi:10.1038/nri.2016.124.
- Marita Cross, E. S., D. Hoy, L. Carmona, F. Wolfe, T. Vos, B. Williams, S. Gabriel, M. Lassere, N. Johns, R. Buchbinder, et al. 2014. The global burden of rheumatoid arthritis: Estimates from the global burden of disease 2010 study. Annals of the Rheumatic Diseases 73 (7):1316–22. doi:10.1136/annrheumdis-2013-204627.
- Moon, J., A. R. Lee, H. Kim, J. Jhun, S.-Y. Lee, J. W. Choi, Y. Jeong, M. S. Park, G. E. Ji, and M.-L. Cho. 2023. Faecalibacterium prausnitzii alleviates inflammatory arthritis and regulates IL-17 production, short chain fatty acids, and the intestinal microbial flora in experimental mouse model for rheumatoid arthritis. Arthritis ResearchTherapy 25 (1):130. doi:10.1186/s13075-023-03118-3.
- Nakajima, M., K. Arimatsu, T. Kato, Y. Matsuda, T. Minagawa, N. Takahashi, H. Ohno, and K. Yamazaki. 2015. Oral administration of P. gingivalis induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of enterobacteria to the liver. PLoS One 10 (7): e0134234. doi:10.1371/journal.pone.0134234.
- Nemoto, N., Y. Takeda, H. Nara, A. Araki, M. Y. Gazi, Y. Takakubo, Y. Naganuma, M. Takagi, and H. Asao. 2020. Analysis of intestinal immunity and flora in a collagen-induced mouse arthritis model: Differences during arthritis progression. International Immunology 32 (1):49–56. doi:10.1093/intimm/dxz058.
- Pianta, A., S. L. Arvikar, K. Strle, E. E. Drouin, Q. Wang, C. E. Costello, and A. C. Steere. 2017. Two rheumatoid arthritis–specific autoantigens correlate microbial immunity with autoimmune responses in joints. The Journal of Clinical Investigation 127 (8):2946–56. doi:10.1172/JCI93450.
- Picchianti-Diamanti, A., C. Panebianco, S. Salemi, M. L. Sorgi, R. Di Rosa, A. Tropea, M. Sgrulletti, G. Salerno, F. Terracciano, R. D’Amelio, et al. 2018. Analysis of gut microbiota in rheumatoid arthritis patients: Disease-related dysbiosis and modifications induced by etanercept. International Journal of Molecular Sciences 19 (10):2938. doi:10.3390/ijms19102938.
- Radu, A.-F., and S. G. Bungau. 2021. Management of rheumatoid arthritis: An overview. Cells 10 (11):2857. doi:10.3390/cells10112857.
- Reyes-Castillo, Z., E. Valdés-Miramontes, M. Llamas-Covarrubias, and J. F. J. C. Muñoz-Valle. 2021. Troublesome friends within us: The role of gut microbiota on rheumatoid arthritis etiopathogenesis and its clinical and therapeutic relevance. Clinical and Experimental Medicine 21 (1):1–13. doi:10.1007/s10238-020-00647-y.
- Round, J. L., and S. K. Mazmanian. 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nature Reviews. Immunology 9 (5):313–23. doi:10.1038/nri2515.
- Sánchez, B., S. Delgado, A. Blanco-Míguez, A. Lourenço, M. Gueimonde, and A. Margolles. 2017. Probiotics, gut microbiota, and their influence on host health and disease. Molecular Nutrition & Food Research 61 (1):1600240. doi:10.1002/mnfr.201600240.
- Scher, J. U., A. Sczesnak, R. S. Longman, N. Segata, C. Ubeda, C. Bielski, T. Rostron, V. Cerundolo, E. G. Pamer, S. B. Abramson, et al. 2013. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2:e01202. doi:10.7554/eLife.01202.
- Scherer, H. U., T. Häupl, and G. R. Burmester. 2020. The etiology of rheumatoid arthritis. Journal of Autoimmunity 110:102400. doi:10.1016/j.jaut.2019.102400.
- Simon, G. M., J. Cheng, and J. I. Gordon. 2012. Quantitative assessment of the impact of the gut microbiota on lysine ε-acetylation of host proteins using gnotobiotic mice. Proceedings of the National Academy of Sciences 109 (28):11133–8. doi:10.1073/pnas.1208669109.
- Smolen, J. S., R. B. M. Landewé, J. W. J. Bijlsma, G. R. Burmester, M. Dougados, A. Kerschbaumer, I. B. McInnes, A. Sepriano, R. F. van Vollenhoven, M. de Wit, et al. 2020. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Annals of the Rheumatic Diseases 79 (6):685–99. doi:10.1136/annrheumdis-2011-70-08.
- Strachan, D. P. 1989. Hay fever, hygiene, and household size. BMJ (Clinical Research ed.) 299 (6710):1259–60. doi:10.1136/bmj.299.6710.1259.
- Sun, H., Y. Guo, H. Wang, A. Yin, J. Hu, T. Yuan, S. Zhou, W. Xu, P. Wei, S. Yin, et al. 2023. Gut commensal Parabacteroides distasonis alleviates inflammatory arthritis. Gut 72 (9):1664–77. doi:10.1136/gutjnl-2022-327756.
- Thaiss, C. A., N. Zmora, M. Levy, and E. Elinav. 2016. The microbiome and innate immunity. Nature 535 (7610):65–74. doi:10.1038/nature18847.
- Vaghef-Mehrabany, E., B. Alipour, A. Homayouni-Rad, S.-K. Sharif, M. Asghari-Jafarabadi, and S. Zavvari. 2014. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition (Burbank, Los Angeles County, Calif.) 30 (4):430–5. doi:10.1016/j.nut.2013.09.007.
- Vos, T., S. S. Lim, C. Abbafati, K. M. Abbas, M. Abbasi, M. Abbasifard, M. Abbasi-Kangevari, H. Abbastabar, F. Abd-Allah, and A. Abdelalim, GBD 2019 Diseases and Injuries Collaborators. 2020. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. The Lancet (London, England) 396 (10258):1204–22. doi:10.1016/S0140-6736(20)30925-9.
- Wang, X., D. Liu, D. Li, J. Yan, J. Yang, X. Zhong, Q. Xu, Y. Xu, Y. Xia, Q. Wang, et al. 2023. Combined treatment with glucosamine and chondroitin sulfate improves rheumatoid arthritis in rats by regulating the gut microbiota. Nutrition & Metabolism 20 (1):1–12. doi:10.1186/s12986-023-00735-2.
- Wang, Y., S. Chen, K. Du, C. Liang, S. Wang, E. Owusu Boadi, J. Li, X. Pang, J. He, and Y.-X. Chang. 2021. Traditional herbal medicine: Therapeutic potential in rheumatoid arthritis. Journal of Ethnopharmacology 279:114368. doi:10.1016/j.jep.2021.114368.
- Weyand, C. M., and J. J. Goronzy. 2021. The Immunology of Rheumatoid Arthritis. Nature immunology 22 (1):10–8.
- Wu, R., Q. Shen, P. Li, and N. Shang. 2022. Sturgeon chondroitin sulfate restores the balance of gut microbiota in colorectal cancer bearing mice. International Journal of Molecular Sciences 23 (7):3723. doi:10.3390/ijms23073723.
- Xu, H., H. Zhao, D. Fan, M. Liu, J. Cao, Y. Xia, D. Ju, C. Xiao, and Q. Guan. 2020. Interactions between gut microbiota and immunomodulatory cells in rheumatoid arthritis. Mediators of Inflammation 2020:1430605–14. doi:10.1155/2020/1430605.
- Yanagibashi, T., A. Hosono, A. Oyama, M. Tsuda, S. Hachimura, Y. Takahashi, K. Itoh, K. Hirayama, K. Takahashi, and S. Kaminogawa. 2009. Bacteroides induce higher IgA production than Lactobacillus by increasing activation-induced cytidine deaminase expression in B cells in murine Peyer’s patches. Bioscience, Biotechnology, and Biochemistry 73 (2):372–7. doi:10.1271/bbb.80612.
- Zaiss, M. M., H.-J. Joyce Wu, D. Mauro, G. Schett, and F. Ciccia. 2021. The gut–joint axis in rheumatoid arthritis. Nature Reviews Rheumatology 17 (4):224–37. doi:10.1038/s41584-021-00585-3.
- Zamani, B., H. R. Golkar, S. Farshbaf, M. Emadi-Baygi, M. Tajabadi-Ebrahimi, P. Jafari, R. Akhavan, M. Taghizadeh, M. R. Memarzadeh, and Z. Asemi. 2016. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: A randomized, double-blind, placebo-controlled trial. International Journal of Rheumatic Diseases 19 (9):869–79. doi:10.1111/1756-185X.12888.
- Zhang, F., A. H. Jonsson, A. Nathan, N. Millard, M. Curtis, Q. Xiao, M. Gutierrez-Arcelus, W. Apruzzese, G. F. Watts, and D. J. N. Weisenfeld. 2023. Deconstruction of Rheumatoid Arthritis Synovium Defines Inflammatory Subtypes. Nature 623 (7987):616–24.
- Zhang, X., D. Zhang, H. Jia, Q. Feng, D. Wang, D. Liang, X. Wu, J. Li, L. Tang, Y. Li, et al. 2015. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nature Medicine 21 (8):895–905. doi:10.1038/nm.3914.
- Zhang, Z., H. Wan, J. Han, X. Sun, R. Yu, B. Liu, C. Lu, J. Zhou, and X. Su. 2022. Ameliorative effect of tuna elastin peptides on AIA mice by regulating the composition of intestinal microorganisms and SCFAs. Journal of Functional Foods 92:105076. doi:10.1016/j.jff.2022.105076.