 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The current consumption trends, combined with the expected demographic growth in the coming years, call for a protein transition, i.e., the partial substitution of animal protein-rich foods with foods rich in proteins produced in a more sustainable way. Here, we have discussed some of the most common and promising protein sources alternative to animal proteins, namely: legumes, insects, and microorganisms (including microalgae and fungi). The primary objective was to assess their nutritional quality through the collection of digestible indispensable amino acid score (DIAAS) values available in the scientific literature. Protein digestibility corrected amino acid score (PDCAAS) values have been used where DIAAS values were not available. The ecological impact of each protein source, its nutritional quality and the potential applications in traditional foods or novel food concepts like meat analogues are also discussed. The data collected show that DIAAS values for animal proteins are higher than all the other protein sources. Soybean proteins, mycoproteins and proteins of some insects present relatively high DIAAS (or PDCAAS) values and must be considered proteins of good quality. This review also highlights the lack of DIAAS values for many potentially promising protein sources and the variability induced by the way the proteins are processed.
Introduction
In 2019, the EAT-Lancet commission on healthy diets from sustainable food systems highlighted the need to move toward a substantial global-scale dietary shift/transformation from current dietary patterns to healthier diets produced by more sustainable food systems for the achievement of the UN Sustainable Development Goals (Willett et al. Citation2019). More specifically the EAT-Lancet commission “emphasizes a plant-forward diet where whole grains, fruits, vegetables, nuts and legumes comprise a greater proportion of foods consumed. Meat and dairy constitute important parts of the diet but in significantly smaller proportions than whole grains, fruits, vegetables, nuts and legumes”.
One of the most debated issues relates to meat production and consumption. Whereas the superior nutritional value of meat for humans is indisputable, a long and growing list of potential problems and threats associated with the current livestock meat production systems and meat consumption patterns has been compiled and highlighted over the recent years. Meat conventional production (animal farming) and consumption pattern have imposed and will expectedly raise prime public concerns about the global risks of environmental deterioration (Godfray et al. Citation2018; Poore and Nemecek Citation2018; Sanchez-Sabate, Badilla-Briones, and Sabaté Citation2019; Humpenöder et al. Citation2022). The negative environmental impact of meat production on natural ecosystems, such as large carbon footprint/greenhouse gas emissions (GHG) and their contribution to global warming and climatic changes, excessive water footprint, biodiversity loss, pollution, freshwater eutrophication, deforestation, soil erosion and land system change has been clearly highlighted (Machovina, Feeley, and Ripple Citation2015; Herrero et al. Citation2016; Springmann et al. Citation2016; Van Mierlo, Rohmer, and Gerdessen Citation2017; Poore and Nemecek Citation2018; González et al. Citation2020; Humpenöder et al. Citation2022; Parlasca and Qaim Citation2022; Zhang et al. Citation2022). Other major debates are going on around potential health hazards of meat consumption (Wolk Citation2017; Richi et al. Citation2015; Lescinsky et al. Citation2022), and ethical/social dimensions related to animal welfare and slaughter procedures (He et al. Citation2020; Nezlek and Forestell Citation2022). A large body of epidemiological evidence suggests a link between meat consumption quantity and the risk of a variety of chronic diseases and certain cancers (Johnson Citation2017; Richi et al. Citation2015; Lescinsky et al. Citation2022). Furthermore, zoonosis, exposure to foodborne pathogens and chemicals/antibiotics used in livestock farming are among the other putative health hazards (Godfray et al. Citation2018; Parlasca and Qaim Citation2022). However, meat production is not the sole responsible factor for the impact of livestock on the ecosystem. It has been reported that 83% of the total GHG emissions can be ascribed to production of meat, dairy and eggs (Sandström et al. Citation2018). A more recent study revealed that livestock is responsible for about one-third of the total anthropogenic methane (CH4) emissions to the Earth’s atmosphere which is mainly attributed to the enteric fermentation of ruminants (Zhang et al. Citation2022).
In recent years, in order to facilitate protein transition, there has been a growing interest in alternative proteins sources including plants, insects and microorganisms. To judge the adequacy of an alternative protein source, different factors must be considered such as the resource availability, cost-effectiveness/economic viability, consumer acceptability and the potential environmental impacts/sustainability. One of the most important points, however, is the ability of the source to provide an appropriate protein quantity and quality to meet human nutritional requirements in any group of population. Proteins play a crucial role in a wide range of vital processes/functions related to human health and well-being. This highlights a need for a standard assessment approach for determining the nutritional quality of alternative protein sources. Indeed, a set of inherent (source-related) and extrinsic (process-induced) factors potentially affect the nutritional quality of proteins.
The World Health Organization (WHO) dietary protein recommendation and the dietary reference intakes (DRIs) values are among the well-established and internationally accepted standards which can be considered as a nutritional benchmark while considering alternative protein sources (Institute of Medicine Citation2005; WHO (World Health Organization) Citation2015; Ahnen, Jonnalagadda, and Slavin Citation2019). The protein DRI to meet the “Dietary Allowance” for protein (Institute of Medicine Citation2005) could serve as a comparative basis to evaluate nutritional quality of alternative proteins. However, protein quality, i.e., the ability of the unit mass of a protein to fulfill nutritional needs, is equally important. There are several approaches for determining and expressing the nutritional quality of protein (Ahnen, Jonnalagadda, and Slavin Citation2019). Protein digestibility corrected amino acid score (PDCAAS) as one of the first protein quality ranking systems, is a well-established, simple, generally-accepted and widely-used criterion approved by FAO/WHO to evaluate the nutritional quality of proteins. Although PDCAAS has been recognized as a practical tool for determining the protein quality, it has been criticized due to its drawbacks/limitations (see more on this in the next section) (Rutherfurd et al. Citation2015). While the PDCAAS scoring pattern approach still continues to have its application in some cases, it has been widely replaced by a new criterion. The most recent officially recognized system for analyzing the nutritional quality of protein in food products is the digestible indispensable amino acid score (DIAAS), which has been proposed by the Food and Agriculture Organization (FAO) of the United Nations (FAO, 2011). The formulas to calculate PDCAAS and DIAAS are thoroughly described in the next section.
A comprehensive assessment of the nutritional quality of proteins in alternative sources is therefore needed but, to the best of our knowledge, a comparative review on the nutritional quality of proteins in alternative sources, especially the state of the art with regard to DIAAS scoring system is currently lacking. We believe that providing a thorough insight into the nutritional quality of alternative proteins may help to improve stakeholders’ decision and consumer awareness when making a decision toward reducing animal-derived protein in their dietary patterns. This may also help alternative protein designers/producers to apply protein modification/fortification strategies to improve the nutritional quality of their final products.
Protein quality: PDCAAS and DIAAS
Protein quality of foods is an important criterion to ensure adequate nutrition and health, given the essential role played by proteins and their amino acids as structural and functional components of living beings. Several methods have been and still are used to assess protein quality. An overview of those methods is beyond the scope of the current review and can be found in other publications for a detailed comparison on different systems of protein quality ranking (Ahnen, Jonnalagadda, and Slavin Citation2019). In 1991, The Joint FAO/WHO Expert Consultation on Protein Quality Evaluation recommended the use of PDCAAS to assess protein quality (FAO/WHO Expert Consultation Citation1991). The PDCAAS is calculated as follows: first, a score is calculated for each essential amino acid from Equationequation 1(1)
(1) :
(1)
(1)
The reference protein was based on the essential amino acid requirements of preschool-age children as published in 1985 by FAO/WHO/United Nations University (FAO/WHO/UNU Citation1985). The smallest score determines the PDCAAS and the corresponding amino acid is defined as first limiting amino acid. Limitations of the PDCAAS method are that it is based on the assumption that all amino acids have the same digestibility as the crude protein, i.e., differences in bioavailability of individual amino acids are not taken into account. Next, PDCAAS makes use of total intestinal tract digestibility, i.e., it is calculated based on the amount of amino acids present in feces, which includes the contribution of proteins from the microbes inhabiting the small and large intestine and does not account for the bacterial utilization of dietary proteins in the large intestine. Finally, PDCAAS values are truncated at 1, so proteins that provide more essential amino acids than required cannot be compared and ranked. To avoid these limitations, FAO now recommends the use of an alternative method, the DIAAS (FAO Citation2013). DIAAS values are calculated by first calculating a score for each essential amino acid as reported in equation 2.
(2)
(2)
The smallest score determines the DIAAS for the test protein and the corresponding amino acid is defined as first limiting amino acid. Compared to PDCAAS, DIAAS is therefore calculated by considering the digestibility of each individual essential amino acid determined at the level of the ileum (true ileum digestibility) and the value is not truncated. DIAAS can be calculated for three different age groups using the appropriate amino acid requirement pattern: 1) infants from birth to 6 months, 2) children from 6 months to 3 years, and 3) children older than 3 years, adolescents, and adults. FAO has also set two threshold values for DIAAS which identify three quality classes for labeling purposes: A protein can be claimed to be excellent if DIAAS >100%; of good quality if DIAAS is ≥75% and <100%; a protein cannot be used as a sole source in a diet and must be integrated by other protein sources (and no nutrition claim should be allowed) if the DIAAS is <75%. For the determination of the DIAAS value, digestibility must be therefore based on true ileal digestibility, preferably in humans, and if this is not possible, in growing pigs or, as least preferred option, in growing rats (FAO Citation2017).
Besides the obvious importance of DIAAS for assessing and ranking protein quality, DIAAS may be used to correct the required level of protein intake. The current recommended daily allowance (RDA) for protein used in most countries is given as 0.83 g protein/kg bodyweight for adults of all ages, except pregnant and lactating women (Joint WHO/FAO/UNU Citation2007). This applies if the proteins in the diet can provide the adequate amount of each essential amino acid as set by the reference protein, i.e., if the PDCAAS is =1 or the DIAAS is =100%. If this is not the case, the RDA for proteins must be higher to compensate for the incomplete use of its amino acids. For instance, if the DIAAS of a diet is 80%, the actual protein requirement would be 1.04 mg/kg BW per day i.e., (0.83 mg/kg BW per day/0.8) to account for the fact that only 80% of the protein can be utilized. It has been reported that, when the average daily protein intakes are corrected for DIAAS, the average daily protein intake of an adult fell below the required level for most countries (Moughan Citation2021). It has been also suggested that, when corrected by the DIAAS, the environmental impact of a protein source may be different from that calculated by considering the protein as fully utilizable by the human body (i.e., DIAAS ≥100%) (Moughan Citation2021).
DIAAS and PDCAAS values for animal and alternative source of proteins
In the next sections, animal proteins and some of the most widespread and promising alternative sources of proteins are discussed, with special emphasis on the quality of proteins as expressed by DIAAS values published in the scientific literature. Where DIAAS values for a specific source could not be found, in vitro DIAAS and PDCAAS values are reported. DIAAS values for animal protein are reported in . DIAAS values and PDCAAS for grain legumes are reported in and those for insects, microalgae, fungi, other microbial proteins and plant-based meat analogues are reported in . Specific values are discussed in each of the section. A selection of DIAAS, PDCAAS and digestibility values from have been reported in for comparative purposes.
Figure 1. Representatives DIAAS values (panel a), PDCAAS values (panel b) and digestibility values (panel c) for the protein sources discussed in the text. From left to right: meat, soy, pea, other legumes, insects, microalgae, fungi, plant-based meat analogues. All the data are taken from . DIAAS values reported for the age group older children and adults; PDCAAS values reported for children >3 years; digestibility values only include true or standardized ileal digestibility.
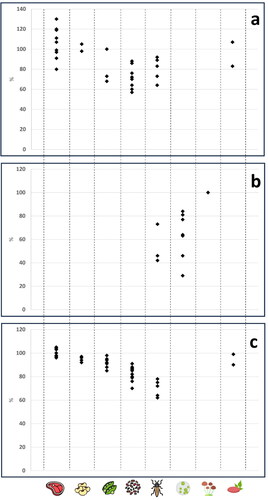
Table 1. Digestible indispensable amino acid score (DIAAS) values for animal proteins.
Table 2. Digestible indispensable amino acid score (DIAAS), protein digestibility corrected amino acid score (PDCAAS) for grain legumes.
Table 3. Digestible indispensable amino acid score (DIAAS), protein digestibility corrected amino acid score (PDCAAS) and in vitro DIAAS values for insect, microalgae, fungi and meat analogues.
Animal proteins
Animal proteins have long been considered paradigmatic in terms of protein quality given the high digestibility and the excellent amino acid profile. DIAAS values for meat, eggs, and dairy proteins almost always exceed 100% (). Whereas several DIAAS values are available for dairy proteins and meat proteins, relatively few data are available for egg proteins. No DIAAS value is currently available (at the best of the authors’ knowledge) on fish proteins but only values calculated from tabulated data (Shaheen et al. Citation2016) which show that fish protein may be of comparable quality as meat. Among meat proteins, differences among animal species as well as meat cuts must be acknowledged. A strong effect of domestic or industrial processing must be also mentioned. For instance, it is known that thermal treatment is essential for a proper utilization of egg proteins. True ileal digestibility of raw egg proteins, determined in human ileostomy patients, was only slightly >50% (Evenepoel et al. Citation1999) which no doubts have an impact on the DIAAS score of raw eggs. Cooking of meat may also have a large impact on the DIAAS score of meat proteins. The effect is mostly produced by loss of essential amino acids rather than by the decrease of protein digestibility (which is only limitedly affected by cooking) and can e.g., lower the DIAAS value of meat from 97 (raw bovine meat) to 80 (grilled meat) (Hodgkinson et al. Citation2018).
Grain legumes
Legumes are part of the family of Fabaceae or Leguminosae which is the third-largest family of flowering plants (Stagnari et al. Citation2017). The term legume is derived from the Latin legere which resembles the practice of gathering seeds by hand, while the term pulse is derived from the Latin puls or pultis, which means thick slurry (Kumar and Pandey Citation2020; Semba et al. Citation2021). Although frequently the terms legumes and pulses are used as synonyms, they have different meanings. Legumes represent the plant that includes leaves, stems, pods, or fruits (e.g., bean pods), while pulses, also called grain legumes, are the edible seeds or dry grains from the legume plant (e.g., beans inside the pod) (Harvard T.H. Chan School of Public Health Citation2023). Among the over 20000 species belonging to the Leguminosae family, just a limited number is cultivated for human consumption, including soybean (Glycine max), common bean (Phaseolus vulgaris), pea (Pisum sativum), faba bean (Vicia faba), lentil (Lens culinaris), chickpea (Cicer arietinum), cowpea (Vigna unguiculata), mung bean (Vigna radiata), and pigeon pea (Cajanus cajan) (Kumar and Pandey Citation2020; Vaz Patto et al. Citation2015).
Grain legumes have been cultivated worldwide and eaten as a protein source in the human diet in addition to proteins from animals. Soybean is the grain legume most widely cultivated, followed by peanut bean, pea, chickpea, cowpea, fava bean, lentil, pigeon pea, lupin, and Bambara bean (Semba et al. Citation2021). The cultivation of grain legumes is more sustainable than protein production from animals thanks to the lower water, energy, and fertilizer requirements and GHG production (Moughan Citation2021). The environmental advantage of legume cultivation is also related to its ability in fixing nitrogen, which, in turn, enhances soil quality by reducing the need for nitrogen fertilizers, improves crop production by reducing diseases thanks to intercropping or crop rotation, and is relatively inexpensive (Foyer et al. Citation2016). This, of course, does not apply for lands that cannot be tilled to grow legumes or marginal lands. For all these reasons and considering the global population increase, which will lead to a protein demand increase, a shift from livestock to legume production should be urgently considered (United Nations Citation2019). In addition, the evidence of health benefits associated with legume consumption rather than meat is getting stronger (Hidayat et al. Citation2022) giving another valid reason to increase the consumption of grain legumes in the diet as meat substitutes. The protein content of grain legumes (raw mature seeds, around 10% moisture content) ranges between 20 and 25% (with the exception of soybean that has 36.5% proteins). After boiling and draining, legumes have a protein content between 7 and 18% (USDA National Nutrient database, SR Legacy Foods accessed on January 15th 2024). This is quite comparable to most meat products where protein content also ranges between 17 and 20% for most beef-, pork- and chicken-based cuts (USDA National Nutrient database, SR Legacy Foods accessed on January 15th 2024).
DIAAS has been reported for a variety of grain legumes and often is available for the same grain legume differently processed or cooked. Nosworthy et al. (Citation2017c) reported DIAAS for 6 months-3 years aged children of 9 cooked Canadian beans, lentils, peas, and chickpeas ranging from 46% in split green peas to 73% in split yellow peas with methionine and cysteine as limiting amino acids for all pulses except chickpeas for which the limiting amino acid was tryptophan. For the same age group, Han et al. (Citation2020) reported DIAAS of cooked kidney beans, mung beans, adzuki beans, broad beans peas, and chickpeas ranging from 53% (broad beans) to 77% (kidney beans) with lysine as the limiting amino acid for all pulses except adzuki bean for which the limiting amino acid was leucine. DIAAS of the same pulses ranged from 60% (broad beans) to 88% (kidney beans), with lysine and leucine as limiting amino acids when calculated for the age group of infants >3 years and adults. Herreman et al. (Citation2020) listed DIAAS of fava bean (55%), lupin (68%), pea (70%), and soy (91%) analyzing intervention studies from the literature. Similar to Nosworthy et al. (Citation2017c) but differently from Han et al. (Citation2020), they determined cysteine and methionine as first limiting amino acids of grain legumes. shows that there is a lot of information related to DIAAS values of grain legumes, i.e., values reported for several legume species as well as for the same legume specie under different processing conditions. It can be noted that the grain legume with the highest DIAAS is soybean (>85%) and that most of the grain legumes have much lower DIAAS values compared to animal proteins (typically >100%). This stems from an incomplete amino acid profile and a lower digestibility compared to animal proteins (Fernandez et al. Citation2020). Particularly, the presence of a thick and resistant cell wall may limit protein digestibility when the legume tissue is consumed intact (Capuano et al. Citation2018). The presence of protease inhibitors, saponins, oxalate, phytic acid and tannins (historically referred to as anti-nutritional factors) further reduces the digestibility of grain legume proteins decreasing, consequently, their DIAAS value.
In some case, we noticed a large variety in the DIAAS values reported for the same grain legume (e.g., 46–82% for pea; 51–88% for kidney beans, etc.) and sometimes differences in the limiting amino acids. Such differences may arise from several factors such as the animal model used, the analytical method used for the quantification of individual amino acids, especially for the sulfur amino acids, growing conditions of the pulses and their variety (Nosworthy et al. Citation2017a, Citation2017b, Citation2017c, Citation2018). Clearly, the way proteins are produced and processed (e.g., whether consumed as isolated proteins or intact seed; level and type of cooking) further contributes to the variability in DIAAS values.
A strategy to compensate for the lower protein quality of pulses in terms of essential amino acids is their complementation, in the wider context of a diet, with other plant sources (e.g., cereals) which are rich in those amino acids that are limiting in grain legumes (Sá, Moreno, and Carciofi Citation2020). Other ways to improve DIAAS of pulse protein is to increase their digestibility, which is often <90% especially in whole beans and in some legumes like kidney bean, black bean, navy bean and adzuki bean. Digestibility can be increased by e.g., 1) reducing the content of the antinutritional factors through processing. Whereas heating is well known to drastically reduce the level of protease inhibitors, fermentation and germination are known to reduce the level of e.g., phytates through the action of microbial or endogenous phytates (Gilani, Cockell, and Sepehr Citation2005). Finally, removal of seed coat is a valuable strategy to reduce the level of e.g., phenolic compounds that are most concentrated there; 2) Reducing the level of encapsulation within intact cell walls, i.e., by processing them in flours, or by isolation of proteins (which is less environmental sustainable though) (Nosworthy, Tulbek, and House Citation2017d).
In addition to proteins, as reviewed by Ahnen, Jonnalagadda, and Slavin (Citation2019), Sá, Moreno, and Carciofi (Citation2020), and Vaz Patto et al. (Citation2015), grain legumes are also rich in other nutrients like dietary fiber, including resistant starch, non-starch polysaccharides and oligosaccharides but also vitamins and minerals as folate, potassium, magnesium, selenium, iron, and zinc; on the other hand, grain legumes are low in fat and phosphorus may be less bioavailable (mostly in the form of phytate) as well as iron and zinc since being partly chelated with phytates. These nutritional characteristics together with bioactive compounds like phenolic compounds and vitamins like tocopherols make grain legumes a protective food in helping prevent chronic diseases including heart disease, diabetes, and cancer (Ahnen, Jonnalagadda, and Slavin Citation2019). However, the amount of thermal- or oxygen-sensitive nutrients and bioactive compounds also depends on processing and storage conditions.
Insects
Entomophagy, consumption of edible insects is practiced by billions of humans around the world (Churchward-Venne et al. Citation2017; Moura et al. Citation2023). Environmental sustainability of insect protein production is partly reviewed by Akhtar and Isman (Citation2018). By compiling the results from several studies, they concluded that in comparison with conventional protein sources such as beef, the insect proteins (especially mealworms) could be potentially more environmentally sustainable in terms of GHG and NH3 production, land and water use (Akhtar and Isman Citation2018). More recently, Santo et al. (Citation2020) also concluded that compared to beef, pig and poultry meat, production of insect proteins results in a much smaller amount of CO2 eq., water use and land use per unit mass of proteins. Edible insects have experienced a remarkable surge of interest by food scientists interested in alternative sources of protein and food industry (Churchward-Venne et al. Citation2017). A large variety of insect species have been considered for potential use in food applications. As reported in Kurek et al. (Citation2022), among more than 2000 recorded edible insects, the most common species globally considered as alternative protein sources mainly belong to Coleoptera beetles, Lepidoptera caterpillars, Hemynoptera, wasps, bees, and ants. The insect species, rearing method, harvesting stage and processing techniques play significant roles in determining the ultimate nutritional quality as well as the potential health hazards of these source of proteins for humans (Akhtar and Isman Citation2018; Loveday Citation2019).
Most insect species are rich in nutrients and minerals (Akhtar and Isman Citation2018). Several studies have reviewed and highlighted the nutritional values of the insects commonly used in different parts of the world e.g., in African countries (Hlongwane, Slotow, and Munyai Citation2020), Australia (Xiaoming et al. Citation2010) and Europe (Kouřimská and Adámková Citation2016). Recently, Liceaga et al. (Citation2022) summarized the values of major nutritional components (i.e., % protein, fat and fiber) of major edible insect orders mainly consumed on a global scale (eight orders including Blattodea, Coleoptera, Diptera, Hemiptera, Hymenoptera, Lepidoptera, Odonata and Orthoptera). The highest average protein content on a dry weight basis (61.3%) was reported in Orthoptera (grasshoppers, crickets and locusts) followed by Odonata (55.2% in dragonflies and damselflies). The protein content for other orders varied between 35.1% to 49.5%. A large variation in the protein content of different species (from 20% to more than 70%) has been also reported elsewhere (Kurek et al. Citation2022). These values correspond to a protein content ranging from 10 to 25% of fresh weight and also dependent on the development stage of the insect (whether adults or pupae or larvae). The maximum fat content was reported in Coleoptera (33.4%) while Orthoptera and Odonata orders represented the lowest fat levels (13.4 and 19.8%, respectively). Edible insects are generally rich in unsaturated fatty acids and specific minerals e.g., Cu, Mg, Fe and Zn. Insect fatty acids are comparable in unsaturation levels to poultry and fish, but they contain higher levels of polyunsaturated fatty acids (PUFA) (Zielińska et al. Citation2015). The fiber content varied between 5.3% in Blattodea to 13.6% in Diptera (Liceaga et al. Citation2022) and is represented by the chitin of the insect exoskeleton.
A comprehensive collection of data on amino acid profile of selected insect species (provided by Hobbi et al. Citation2022) suggested that the amino acid profile and protein composition in many insects can potentially fulfill the criteria to adequately meet the metabolic requirements for humans and can be considered as high-quality dietary protein-rich source. Compared with the amino acid requirements of adults recommended by WHO, Akhtar and Isman (Citation2018) concluded that most of the edible insects contain satisfactory quantity of essential amino acids required for human nutrition. Next to this, insect proteins have suitable digestibility Liceaga et al. Citation2022 with values between 86% and 90% reported for insects in a rat model (Finke Citation2013).
DIAAS values for insect proteins are limited. Recently, Malla et al. (Citation2022) determined DIAAS values for five insect species (two mealworms, Alphitobius diaperinus or lesser mealworm and Tenebrio molitor or yellow mealworm; two crickets, Gryllodes sigillatus or banded cricket and Acheta domesticus or house cricket; and Hermetia illucens or black soldier fly) in ileal cannulated growing pigs. In this study, the commercially available insect products were added as powder to other feed ingredients of pig diet to maintain a crude protein concentration of 100 g/kg dry matter. They separately calculated and compared DIAAS of the insects for three categories including infants (birth to 6 months old), young children (6 months to 3 years old) and older children/adolescents/adults (>3 years old). The highest DIAAS for infants (57%) was reported for lesser mealworm, while the other examined species had DIAAS of 45–47%. For young children, the highest DIAAS values were found in crickets (78% and 76% for banded cricket and house cricket, respectively). For this age group, DIAAS in lesser mealworm, black soldier fly and yellow mealworm was reported at the levels of 71%, 57% and 54%, respectively. Banded cricket demonstrated the highest DIAAS for the third age group (92%) followed by DIAAS in house cricket (89%) and lesser mealworm (83%). The DIAAS in black soldier fly and yellow mealworm were 68% and 64%, respectively (Malla et al. Citation2022). The limiting amino acid(s) varies according to the age group considered. However, tryptophan and sulfur amino acids were determined as limiting in yellow and lesser mealworms and crickets, and lysine for black soldier fly. Notably, protein digestibility was rather low (standardized ileal digestibility of crude proteins ranging from 62% in black soldier fly to 78% in lesser mealworm). As DIAAS >75% in a given protein source is usually considered as “high-quality” or “good-quality” protein, Malla et al. (Citation2022) concluded that both crickets are potentially classified as good-quality protein for the ages older than 6 months and lesser mealworm can be qualified as a good-quality protein for older children/adolescents/adults (>3 years old) group.
In another recent study, in vitro and in vivo digestibility of black soldier fly (Hermetia illucens) larvae was investigated by Traksele et al. (Citation2021). In this study, the dried low-fat larvae of black soldier fly were added in the formulation of the test diet and the DIAAS was calculated using the reference pattern for young children (0.5 to 3 years old) in a rat model at the level of about 73% (Traksele et al. Citation2021). The observed difference between these results with those observed by Malla et al. (Citation2022) may be related to the different provision of raw material source, specific life stages, different processing method as well as the different model organisms considered for DIAAS calculation (rats and pigs) and the conditions under which insects were reared and produced. Whereas those described are the only studies reporting DIAAS values, few other studies reported PDCAAS values for other insect proteins. For instance, in a study by Oibiokpa et al. (Citation2018), the PDCAAS values for four insect species (Macrotermes nigeriensis or termite; Gryllus assimilis or cricket; Melanoplus foedus or grasshopper; and Cirina forda or moth caterpillar) were determined in rat model. In this study, the insect powders were added to other feed ingredients of young male weanling albino rat diet and the PDCAAS values were calculated for the age preschool children (age 2–5 years) age group. Each diet contained 100 g/kg of the test proteins. Cricket demonstrated the highest PDCAAS (73%, threonine as limiting amino acid) followed by PDCAAS in grasshopper (46%, isoleucine as limiting amino acid), moth (42%, sulfur amino acids as limiting amino acids), and termite (42%, sulfur amino acids as limiting amino acids) (Oibiokpa et al. Citation2018). All in all, the quality of insect proteins is highly variable and not all the insect species are source of good proteins but banded and house crickets have relatively high DIAAS values, 92% and 89% respectively. It is worth mentioning that DIAAS values have been collected for ingredients produced from whole insect and no report is available for isolated insect proteins. This may be relevant because chitin (the main component of insect exoskeleton) is reported to reduce digestibility of insect proteins in poultry feed (De Marco et al. Citation2015).
As an alternative protein source, insect flours, protein concentrates and isolates are becoming common functional ingredients to be used in food preparation (Gravel and Doyen Citation2020). Insect protein processing methods are almost similar to that of pulses. Gravel and Doyen (Citation2020) described and thoroughly reviewed a general 5-step procedure for production of insect flours, protein concentrates and isolates including pretreatment, defatting, solubilization and recovery of proteins, purification and drying. The major food applications of insect proteins involve incorporating these alternative proteins in food matrices for meat emulsion and analogues, snacks, pasta, bread and other bakery products (Gravel and Doyen Citation2020). Recently, attention was drawn to insect proteins in gluten-free food markets (da Rosa Machado and Thys Citation2019; Nissen et al. Citation2020). Gravel and Doyen (Citation2020) highlighted the main challenges in development of insect protein industry which need to be resolved. Some of those include dealing with consumer acceptance, finding optimal processing condition and provision of processing facilities on industrial scale, optimization of profitability and cost-effectiveness of rearing and processing procedures, focusing more detailed environmental impact analysis using standard methods such as Life Cycle Assessment (LCA). Above all, the major challenge of producing and using insects is to get authorization from each relevant national and international institution. For instance, the European Commission, under the regulation on novel food (European Parliament and Council of the European Union Citation2015), has, so far, only authorized the marketing of dried Tenebrio molitor larva, frozen, dried and powder form of Tenebrio molitor larva, frozen, dried and powder forms of Locusta migratoria, and frozen, dried and powder forms of Acheta domesticus as well as the frozen, paste, dried and powder forms of Alphitobius diaperionus. US, Canada, New Zealand and Australia do not consider edible insects a novel food and do not have restrictions for their consumption or market.
Microalgae
Generally, the term microalgae refer to a category of unicellular photosynthetic microorganisms (eukaryotic algae and prokaryotic cyanobacteria) widely recognized as phytoplankton. Due to their large biodiversity and thanks to technological advances, their taxonomic classification is under constant review and update (Lucakova, Branyikova, and Hayes Citation2022). Owing to the remarkable protein content (40–60% dry matter) as well as presence of broad-spectrum bioactive compounds, certain species of microalgae (especially blue-green and green algae) have recently received a lot of attention among food scientists especially as promising sources of (alternative) proteins (Chronakis and Madsen Citation2011). Furthermore, algae production offers several advantages in terms of economic and environmental sustainability (Bleakley and Hayes Citation2017). Natural potential for large and efficient biomass production, higher protein yield per unit area in comparison to terrestrial plants, much less water requirement in the cultivation/production process (vs. agriculture and in particular animal farming), no need for arable land and freshwater/potable water especially in marine strains, their potential for bioremediation of wastewater and ultimately the ability of these aquatic photosynthetic organisms to sequester CO2 and thus reducing the carbon footprint during their production process are considered as the major advantages of algae mass cultivation (Bleakley and Hayes Citation2017; Geada et al. Citation2021; Diaz et al. Citation2023). However, a study by Smetana et al. (Citation2017) revealed unexpected results when evaluating environmental impacts of autotrophic and heterotrophic cultivation of microalgae using the life cycle assessment methodology. They reported that the pilot industrial-scale cultivation of microalgae and production of microalgae protein powder had higher environmental impacts in comparison to production of several commonly used plant- and animal-based proteins. As an example, the global warming potential of Chlorella grown in a tubular reactor was estimated at 96.1 kg CO2 equivalents against 0.34–0.72 kg CO2 equivalents reported for soybean meal and 23.4 kg CO2 equivalents for egg proteins. The impact of Chlorella grown in open raceway pond is even higher, with a global warming potential estimated at 245.1 kg CO2 equivalents. The higher environmental impacts reported in this study are mainly related to the levels of heat and energy required in autotrophic microalgae production process and the glucose utilization (as a source of energy) in heterotrophic culture method (Smetana et al. Citation2017).
Microalgae consumption by humans is not new. Ancient evidence of microalgae utilization in local human communities (e.g., Nostoc species in China) has been documented in several parts of the world which in some cases dates back to centuries ago (Wang, Tibbetts, and McGinn Citation2021). Despite the extensive biodiversity of microalgae (about 200000 species) and the long history behind their consumption by humans, since the beginning of intensive cultivation of these microorganisms using modern technological approaches (the 1950s), only a limited number of species such as Arthrospira platensis (spirulina), Chlorella vulgaris (chlorella) and Aphanizomenon spp, have been introduced and widely adopted for human food purposes (Wang, Tibbetts, and McGinn Citation2021). At the moment, seven microalgae species are accepted as foods in the EU, namely: Aphanizomenon flos-aquae, Arthrospira platensis, Chlorella vulgaris, Chlorella luteoviridis and Chlorella pyrenoidosa, Odontella aurita, Tetraselmis chuii. The first five are listed in the EU novel foods catalogue as not novel food because consumed, albeit not extensively, in Europa before 1997. In US, several microalgal species are considered generally recognized as safe, including Arthrospira platensis, Chlamydomonas reinhardtii, Chlorella vulgaris, Auxenochlorella protothecoides, Euglena gracilis and Dunaniella bardawil. Microalgae have a rich profile of nutrients (i.e., proteins, lipids, carbohydrates, minerals and vitamins, PUFAs including omega-3 fatty acids such as eicosapentaenoic and docosahexaenoic acids) and bioactive molecules (e.g., antioxidants such as phenolic compounds, pigments such as carotenoids, phycobiliproteins, chlorophylls, phycocyanin, as well as inorganic elements such as iodine). A large variation in the nutritional profile of different microalgae species is related to genetics, culture medium (resource use), engineering aspects/considerations and environmental conditions during their cultivation (Conde et al. Citation2013; Amorim et al. Citation2021).
Recently, Li et al. (Citation2019) collected and presented the available data on the nutritional composition (i.e., protein, carbohydrate and lipid expressed in % of dry biomass) of ten microalgae species mainly consumed for food purposes (including A. maxima, C. vulgaris, Dunaliella sp., Haematococcus pluvialis, Nannochloropsis sp., Nitzschia sp., Phaeodactylum tricornutum, Scenedesmus obliquus and A. platensis). The highest average protein content (60–71%) was reported in A. maxima followed by A. platensis and C. vulgaris (46–63% and 51–58%, respectively). The protein content for other species varied between 17% to 57%. The maximum lipid content was reported in H. pluvialis (25%) while A. platensis represented the lowest lipid level (4–9%). The carbohydrate content varied between 7.8% in Nannochloropsis sp. to 37–40% in H. pluvialis (Li et al. Citation2019). However, it must be noted that the microalgal biomass contains up to 80% of moisture that needs to be removed for its utilization in foods and feeds. The available data on amino acid profiles of some species were comparatively analyzed and reviewed by Amorim et al. (Citation2021) to determine the quality of microalgae protein for human nutrition. They reported that, despite the presence of all essential amino acids in the selected algal species, some of them contain insufficient amounts of essential amino acids. For instance, Aphanizomenon sp. showed low contents of all essential amino acids when comparing to the reference protein recommended by FAO/WHO. In contrast, the other species mentioned there, C. vulgaris was slightly lacking adequate amounts of isoleucine (only about 5% deficiency in comparison to FAO/WHO reference protein). They also mentioned that the cultivation conditions play a significant role in shaping amino acid profile of microalgae. Optimization of the culture process is necessary for achieving the adequate amino acid profile in microalgae (Amorim et al. (Citation2021).
The data on DIAAS of microalgae are very limited and unavailable for most species. However, recently, a study by Li et al. (Citation2019) provided DIAAS for five microalgae species (including Chlorella sp., Nannochloropsis sp., P. tricornutum, S. obliquus and A. platensis) only for the reference population group of older children/adolescents/adults. Chlorella sp. presented the highest DIAAS (111%), while the other four species including Nannochloropsis sp. (23%), P. tricornutum (75%), S. obliquus (40%) and A. platensis (34%) had inferior protein quality (Li et al. Citation2019). However, it should be noted that these DIAAS values were not obtained in animal models but calculated from in vitro data and details of the calculation were not provided.
Whereas there is a lack of DIAAS for microalgae reported in the scientific literature, some PDCAAS values have been reported. Tessier et al. (Citation2021) reported a PDCAAS value for spirulina (Arthrospira sp.) of 84% with an excellent amino acids profile (chemical score of 0.98) and a digestibility comparable to other plant-based proteins (cecal digestibility of nitrogen= 90%). Wang et al. (Citation2020) reported PDCAAS for three green microalgal species including C. vulgaris, C. sorokiniana and Acutodesmus obliquus. In this study, male Sprague–Dawley rats were fed with trial diets containing 10% protein from one of the microalgal species. The microalgae ingredients were added to the basal diet in two forms including, undisrupted and mechanically cell (wall) ruptured (using high-pressure homogenization). The PDCAAS for undisrupted C. vulgaris, C. sorokiniana and A. obliquus were 63%, 64% and 29%, respectively. The authors reported a substantial improvement in amino acid bioavailability after the mechanical disruption of cell walls as the PDCAAS after this treatment were 77%, 81% and 46% for C. vulgaris, C. sorokiniana and A. obliquus, respectively (Wang et al. Citation2020). An increase in protein digestibility after disruption of the cell wall is also reported which may likely explain the increase in PDCAAS values.
All in all, we observed a large variety in protein quality across different species of microalgae, with Arthrospira platensis (spirulina) and Chlorella spp. as potentially good source of proteins. However, disruption of cell wall is one of the most essential steps for the full utilization of nutrients from microalgae including proteins (Amorim et al. Citation2021) and seems to be an essential step for the use of dry microalgal biomass. Employing efficient mechanical techniques for microalgae cell wall disruption, such as bead milling and homogenization, may qualitatively improve nutritional value of proteins (Li et al. Citation2019; Amorim et al. Citation2021). Another problem related to the use of microalgal biomass for food applications is that the biomass cannot be used as such but must be converted in a powder or the proteins been extracted (and dried) (Amorim et al. Citation2021). Proteins can potentially be extracted from the wet or dry algal biomass.
Proteins from fungi
This group of alternative proteins include protein mainly obtained from fermented biomass produced by common soil-dwelling filamentous eukaryotic nonpathogenic fungal microorganisms. The most well-known example of this group is Fusarium venenatum which is often referred to also as mycoproteins (Finnigan, Needham, and Abbott Citation2017). Commercial production of mycoprotein initiated in 1985 under QuornTM trade name (Wiebe Citation2004). For this reason, Fusarium venenatum is approved for food use in EU but it is listed in the EU catalogue of novel foods as not novel. It is also approved for use in several countries including US, Canada, UK and New Zealand. The industrial production of mycoprotein is carried out by the continuous flow culture of F. venenatum on a carbohydrate (e.g., glucose) substrate in air-lift sterile pressure cycle fermenters. At the end of the fermentation process, the obtained biomass should be thermally treated to reduce the RNA content to meet the food safety standards (Wiebe Citation2004). Detailed studies on environmental impacts of mycoprotein production are still scarce and its environmental impact still controversial. Some reviews, such as Finnigan, Needham, and Abbott (Citation2017) and Hashempour-Baltork et al. (Citation2020), concluded that mycoprotein could be potentially considered as an alternative protein source with low environmental impacts in terms of carbon footprint, water footprint and land use. However, most recent reviews criticized this view by employing more detailed LCA analysis as well as comparing mycoproteins to other novel protein sources. For instance, Smetana et al. (Citation2023) reported values for the global warming potential of mycoproteins in the range of 5.55-23.66 kg CO2 equivalents/kg proteins (i.e., comparable to egg proteins but substantially higher than soybean meal) and underlined that processing (e.g., drying) of the fungal biomass can double the environmental impact of mycoproteins. Another review by Mazac, Järviö, and Tuomisto (Citation2023) reported that mycoproteins ranked worse than fish and chicken meals in terms of global warming potential. Furthermore, LCA analysis of a number of meat alternatives using a “cradle-to-plate” methodology revealed high environmental impacts of mycoprotein-derived products, especially due to the high demand for energy used in cultivation process and medium (Smetana et al. Citation2015). Also, Souza Filho et al. (Citation2019) reviewed the environmental impacts of mycoprotein production and they noted that the results of LCA analyses showed that this alternative protein source causes environmental impacts similar to those reported for pork and chicken.
Mycoprotein has low fat, and is rich in proteins and dietary fiber, due to the presence of the fungal cell wall (Denny, Aisbitt, and Lunn Citation2008). Per 100 g of dry matter, about 45 g of proteins (corresponding to 11.5% on fresh weight), 13 g of fats and 25 g of fibers are reported as the main nutritional components of typical mycoproteins (Finnigan, Needham, and Abbott Citation2017). Also, a wide array of micronutrients e.g., vitamin B12, vitamin B9, Zn, Mg, Ca and P are present in mycoproteins (Derbyshire and Finnigan Citation2022). Mycoprotein lipids mainly include PUFAs, such as linoleic and linolenic acids (Hashempour-Baltork et al. Citation2020).
Only one study reported a PDCAAS of 100% (measured in ileostomy patients, true ileal digestibility estimated at 86%) which means that this alternative source can potentially be considered as a good quality (complete) protein (Edwards and Cummings Citation2010). No DIAAS values are so far reported for mycoproteins. However, recently a study by Ariëns et al. (Citation2021) provided in vitro digestible indispensable amino acid score (IVDIAAS) for mycoprotein. In this study, the authors used the harmonized INFOGEST static protocol to digest the proteins; then three methods were applied for the determination of the amino acid profile of the indigested fraction (that corresponding to the absorbable fraction in vivo) and hence the true ileal digestibility of the protein. In the first method, each individual amino acid (AA) in mg in the filtrate was subtracted by the same AA (mg) of the control ‘empty’ digest (containing and treated the same without the addition of a protein sample) and divided by that AA in the start protein sample. In the second method, the amount of an individual AA in the filtrate of a protein sample minus that AA of a control ‘empty’ digest was divided by the same AA in the digest (before filtration) minus the AA in digest control. In the third method, the filtrate was divided by the digest but without the subtraction of the control. The IVDIAAS values were computed for the age categories of young children (6–36 months old) and older children (3–10 years old), separately. For the age group 6–36 months old, the calculated IVDIAAS ranged from 33% to 42% For older children, the reported IVDIAAS ranged from 39% to 49% (Ariëns et al. Citation2021). These values were much lower than the PDCAAS value reported previously (Edwards and Cummings Citation2010) and let the authors question the reliability of the IVDIAAS produced in the ways described above (Ariëns et al. Citation2021).
Although F. venenatum represents the most well-known example of fungal proteins, other fungal species are emerging as potential source of alternative proteins including, among others, Yarrowia lipolytica, an oleaginous species of yeast, Saccharomyces ssp., or Paecilomyces variotii, the asexual state of Byssochlamys spectabilis, which is commercialized under the brand name PEKILO. Yarrowia lipolytica is authorized as novel food in EU only for use in food supplements. Paecilomyces variotii is considered novel food in EU but not authorized yet. Saccharomyces spp. is authorized for use in EU in food in the fresh and UV-irradiated form (this last as novel food). However, very little is known about the environmental impact as well as about the quality of the proteins of these fungal species. Among the very few studies about protein quality of yeasts, Pacheco, Caballero-Córdoba, and Sgarbieri (Citation1997) determined in vitro PDCAAS for yeast (Saccharomyces sp.) protein concentrates (including yeast cell biomass (YC), sodium perchlorate extracted and isoelectrically precipitated protein concentrate (P-PC) and sodium trimetaphosphate treated extract followed by isoelectrical precipitation (TMP-PC)) in an in vitro experimental design. They found fairly high PDCAAS values for the yeast as reported at the levels of 86%, 82% and 90% or YC, P-PC and TMP-PC, respectively. More recently IVDIAAS values ranging from 97% to 99% for older children and adults have been reported for Saccharomyces cerevisiae (Ariëns et al. Citation2021). In conclusion, whereas the quality of micoproteins from Fusarium venenatum are as good as some animal proteins (PDCAAS = 100%), there is still no DIAAS value available for Fusarium venenatum or any other fungal protein.
Other microbial proteins
If proteins from microscopic fungi or microalgae are well known to consumers, more innovative alternatives of animal proteins from microbes have been explored. Microbial protein produced for food or feed is commonly referred to as single cell protein (SCP), although microbes (and microalgae) do not always grow as isolated cells, but may also form colonies. This includes also autotrophic bacteria like microalgae and heterotrophic bacteria like fungi that have been described in previous chapters. Here we refer to autotrophic bacteria that are able to grow on a carbon source using gas as source of energy. The major applications so far involve knallgas bacteria, that use molecular hydrogen as energy source, and methanotrophic bacteria, that use methane (Nyyssölä et al. Citation2022). Whatever the source of energy used, proteins are produced in the form of a bacterial biomass, obtained by submersion or solid-state fermentation. This biomass typically contains a large amount of protein on a dry basis (30–80%). Proteins can be extracted or used in the form of a high protein flour for food applications. However, little is known about the techno-functional properties of these proteins. Very little is also known about the protein quality. One study has found that the essential amino acid score for H2-oxidizing autotrophic bacterial strains, Nocardioides nitrophenolicus KGS-27 and Rhodococcus opacus DSM 43205, is >1 for all the amino acid except tryptophan and that the amino acid content can vary depending on the cultivation conditions (Nyyssölä et al. Citation2021).
Another solution for producing proteins in a potentially more sustainable way by exploiting cellular agriculture consists in genetically engineering microbes. An example of this is the animal-free ice cream launched in 2019 in the US by the company Perfect Day, Inc. (www.perfectdayfoods.com). There are no studies at the moment that have investigated the quality of those proteins that are identical to animal proteins and that are produced from genetically modified microbes. Clearly, amino acid composition would be identical to the “natural”, animal counterpart and thus protein quality is expected to be high (i.e., high DIAAS). Protein digestibility would be mostly affected by the way protein-rich ingredients are isolated/purified and the industrial processes applied in food applications (e.g., extrusion, thermal treatments, etc.). An extra factor that can affect protein digestibility may be that the supramolecular organization of proteins produced from cellular agriculture may be different from that of the natural counterpart (e.g., casein micelles in milk compared to isolated casein molecules produced by the microbes).
Meat analogues
Meat analogues is a relatively new commercial category of food products where non-animal proteins are processed in a way to replicate the sensory properties, especially the texture, of real meat (Dekkers, Boom, and van der Goot Citation2018). Meat analogues must not be confused with cultured meat, which is based on tissue engineering principles using stem cells. At the moment, several alternative sources of proteins have been used to develop meat analogues including mycoproteins and other microbial proteins, insect proteins and algae proteins, often in combination with plant proteins. However, most of the commercially available meat analogues are based on legume proteins, primarily soybean and pea often in combination with wheat gluten. Proteins are usually extracted and isolated, and then processed to form the typical fibrous structure found in meat. Different techniques can be used to impart the typical fibrous structure to meat analogues, e.g., extrusion, shear cell technology, and 3D printing. By varying the processing conditions, for instance extrusion temperature (typically between 100 °C and 180 °C), or moisture content (typically between 30 and 70%), different textures can be achieved. Additives like colorants and thickeners can be incorporated to create the variety of meat analogues available in the market.
Despite their commercial success, little is known about how sustainable meat analogues are compared to meat and its derivatives. In a meta-analysis published in 2020 (Santo et al. Citation2020), the ecological impact of meat analogues was investigated based on various parameters. The result of the analysis shows that the ecological impact for plant-based meat analogues (PBMA) is significantly lower compared to meat, although it is higher than that of legumes since the production of meat analogues involves protein extraction and processing and addition of ingredients which production or purification has its own ecological impact.
Nutritional quality of PBMA generally reflects the nutritional composition of the ingredients used. Typically, they have a more unsaturated fatty acid profile compared to meat and contain some dietary fiber (either present in the protein ingredient or added as additive for structuring) (Bohrer Citation2019). Commercial PBMAs typically have lower content of iron, zinc and vitamin B12 compared to meat counterpart unless they are fortified but this occurs very seldom (Melville et al. Citation2023). Ongoing research is being conducted to evaluate the quality of proteins in PBMA. Protein quality of meat analogues would primarily depend on the amino acid composition of the plant proteins used. For instance, for the production of PBMA, the most commonly used plant-based ingredients are soy proteins isolates and pea protein isolates. Gluten is often used to improve the technological functionality of the product. Among these three protein sources, soybean has the best amino acid profile, whereas gluten proteins are of a much poorer quality. Recently, Cutroneo et al. (Citation2023) demonstrated that the sum of essential amino acids of some commercial plant-based burgers is able to satisfy the requirements for older children and adults (FAO Citation2013), even though they lack lysine compared to the amino acid scoring pattern set by FAO/WHO (for older children and adults). Secondly, protein quality in meat analogues would be affected by protein digestibility. Digestibility of proteins in meat analogues has been explored in vitro and in animal models and is generally reported as lower compared to the meat counterpart (Xie et al. Citation2022a; Xie et al. Citation2022b; Zhou et al. Citation2021; Chen, Capuano, and Stieger Citation2021). It must be mentioned however that extrusion conditions (temperature and time; moisture level; screw speed, etc.) may affect protein digestibility by producing different protein microstructures, which would reflect in different digestibility. Finally, the presence of other components in the ingredients of a meat analogue may also affect protein digestibility (e.g., antinutritional factors like polyphenols or soy saponins; dietary fiber) but this is little investigated and likely less important than protein microstructure and modifications. Clearly, extrusion conditions (and domestic preparation as well) may also result in degradation, racemization of essential amino acids and other oxidation damages (Duque-Estrada and Petersen Citation2023) which may lower protein quality. At the moment, the only DIAAS available is for PBMA (Fanelli et al. Citation2022). The authors have assessed the quality of proteins in two PBMA currently available on the market: the “Beyond Burger” (also sold in Europe) and the “Impossible Burger”, the first to be commercialized but currently available only in the United States. The authors used ileal cannulated gilts as animal model and the burgers were included in each diet as the sole source of crude protein and amino acids. The study shows that protein quality in PBMA varies significantly, with the Impossible Burger having a higher DIAAS (107%, close to that of meat) than the Beyond Burger (83%). One of the reasons for this difference is likely that “Beyond Burger” is produced with pea proteins compared to “Impossible Burger” which is based on soy proteins, with pea having a lower DIAAS compared to soy. Another reason may be the relatively lower digestibility of the Beyond Burger (standard ileal digestibility of crude protein = 90%) compared to the Impossible Burger (99%). This last study demonstrated that plant-based meat analogues might represent a good (or even excellent when soybean-based) source of proteins.
Conclusions
A rebalance of protein intake from animal-based proteins toward alternative and more sustainable sources seems to be needed to alleviate the environmental impact of the current food production system. This transition can be implemented by switching as much as possible to a plant-based diet, as well as to incorporate novel proteins sources in the diet. This report has discussed the protein quality of three possible alternative sources: grain legumes, insects and microorganisms.
In particular, this review shows that none of the examined alternative sources can produce DIAAS or PDCAAS values comparable to animal proteins, with the exception of soybean (DIAAS >90%), soybean-based meat analogues (DIAAS = 107%) and micoproteins from Fusarium venenatum (PDCAAS = 100%). However, some insect proteins can produce very high values, like house cricket (DIAAS = 89%) and banded cricket (DIAAS = 92%). Very little is currently known on microbial proteins, apart from algae proteins, the quality of which protein is unfortunately limited by the poor accessibility in intact algae cells.
From our analysis, it emerges that information on the quality of alternative proteins is largely available in terms of amino acid profile but there is a relative lack of DIAAS (but also of PDCAAS) values for most of the alternative protein sources reviewed here with the exception of grain legumes. Our review also underlines the importance of considering the final product rather than the protein ingredient in the assessment of the DIAAS (or any equivalent measure of protein quality). Insect protein may, for instance, be consumed as meal, formulated in bread or cookies, or extruded to produce the fibrous structure of meat analogues. Meat analogues can be prepared in a variety of ways at home, e.g., by grilling. Each industrial and domestic processing step will no doubt have an effect on protein digestibility and possibly on amino acid composition (degradation and racemization of essential amino acids). We therefore suggest that real food products are tested, with few key food categories that most contribute to the overall protein intake prioritized, and that the exact conditions for production of such foods are reported. In calling for more studies to broaden the list of DIAAS values and aware of the practical issues in producing them, due to the use of human or animal models for a large number of potential food products as discussed elsewhere, we stress the importance to develop more high-throughput, validated in vitro alternative methods, for the assessment of DIAAS values.
Finally, we highlight the application of further steps of purification or processing may often improve the protein quality of several alternative sources, for instance by removing antinutritional factors in grain legumes and possibly insects, or removing the barrier effect of cell walls in microalgae in the same time increasing the ecological fingerprint of the protein unit mass, an issue that has recently been indicated as “the sustainability paradox” (Duque-Estrada and Petersen Citation2023), i.e., “[the] fact that less processing most often results in lower protein nutritional quality compared to purified protein ingredients that are more processed”. However, it must be also stressed that whereas protein purification improves protein quality, it comes at the expenses of the loss of a variety of nutrients, e.g., dietary fiber, minerals and vitamins as well as bioactive compounds, e.g., phenolic compounds, which are richer in less refined ingredients, and contribute to human health. In the future, this balance between sustainability aspects and nutritional quality must be carefully considered in choosing appropriate alternative sources of proteins.
All in all, the information collected in this review in terms of DIAAS and PDCAAS values, environmental impact and nutritional quality (beside protein quality) may help stakeholders in the selection of alternative sources of proteins to be included in the future in the human diet as a (partial) replacement of animal proteins. Clearly, other factors must also be included, which could not be covered here, like the financial aspects as well as safety and toxicological issues such as, but not limited to, the potential allergenicity of the proteins.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahnen, R. T., S. S. Jonnalagadda, and J. L. Slavin. 2019. Role of plant protein in nutrition, wellness, and health. Nutrition Reviews 77 (11):735–47. doi: 10.1093/nutrit/nuz028.
- Akhtar, Y., and M. B. Isman. 2018. Insects as an alternative protein source. In Proteins in food processing, 263–88. Cambridge, UK: Woodhead Publishing.
- Amorim, M. L., J. Soares, J. S. D. R. Coimbra, M. D. O. Leite, L. F. T. Albino, and M. A. Martins. 2021. Microalgae proteins: Production, separation, isolation, quantification, and application in food and feed. Critical Reviews in Food Science and Nutrition 61 (12):1976–2002. doi: 10.1080/10408398.2020.1768046.
- Ariëns, R. M., S. Bastiaan-Net, D. B. Van de Berg-Somhorst, K. El Bachrioui, A. Boudewijn, R. T. van den Dool, G. A. De Jong, H. J. Wichers, and J. J. Mes. 2021. Comparing nutritional and digestibility aspects of sustainable proteins using the INFOGEST digestion protocol. Journal of Functional Foods 87:104748. doi: 10.1016/j.jff.2021.104748.
- Bailey, H. M., J. K. Mathai, E. P. Berg, and H. H. Stein. 2020. Most meat products have digestible indispensable amino acid scores that are greater than 100, but processing may increase or reduce protein quality. The British Journal of Nutrition 124 (1):14–22. doi: 10.1017/S0007114520000641.
- Bleakley, S., and M. Hayes. 2017. Algal proteins: Extraction, application, and challenges concerning production. Foods (Basel, Switzerland) 6 (5):33. doi: 10.3390/foods6050033.
- Bohrer, B. M. 2017. Nutrient density and nutritional value of meat products and non-meat foods high in protein. Trends in Food Science & Technology 65:103–12. doi: 10.1016/j.tifs.2017.04.016.
- Bohrer, B. M. 2019. An investigation of the formulation and nutritional composition of modern meat analogue products. Food Science and Human Wellness 8 (4):320–9. doi: 10.1016/j.fshw.2019.11.006.
- Burgos-Díaz, C., M. Opazo-Navarrete, T. Wandersleben, M. Soto-Añual, T. Barahona, and M. Bustamante. 2019. Chemical and nutritional evaluation of protein-rich ingredients obtained through a technological process from yellow lupin seeds (Lupinus luteus). Plant Foods for Human Nutrition 74 (4):508–17. doi: 10.1007/s11130-019-00768-0.
- Capuano, E., T. Oliviero, V. Fogliano, and N. Pellegrini. 2018. The role of food matrix and digestion on the calculation of the real energy content of food. Nutrition Reviews 76 (4):274–89. doi: 10.1093/nutrit/nux072.
- Chen, Y., E. Capuano, and M. Stieger. 2021. Chew on it: Influence of oral processing behaviour on in vitro protein digestion of chicken and soya-based vegetarian chicken. British Journal of Nutrition 126 (9):1408–19. doi: 10.1017/S0007114520005176.
- Chronakis, I. S., and M. Madsen. 2011. Algal proteins. In Handbook of food proteins, 353–94. Cambridge, UK: Woodhead Publishing.
- Churchward-Venne, T. A., P. J. Pinckaers, J. J. van Loon, and L. J. van Loon. 2017. Consideration of insects as a source of dietary protein for human consumption. Nutrition Reviews 75 (12):1035–45. doi: 10.1093/nutrit/nux057.
- Conde, E., E. M. Balboa, M. Parada, and E. Falqué. 2013. Algal proteins, peptides and amino acids. Functional ingredients from algae for foods and nutraceuticals, 135–80. Cambridge, UK: Woodhead Publishing.
- Cutroneo, S., B. Prandi, A. Faccini, N. Pellegrini, S. Sforza, and T. Tedeschi. 2023. Comparison of protein quality and digestibility between plant-based and meat-based burgers. Food Research International 172:113183. doi: 10.1016/j.foodres.2023.113183.
- da Rosa Machado, C., and R. C. S. Thys. 2019. Cricket powder (Gryllus assimilis) as a new alternative protein source for gluten-free breads. Innovative Food Science & Emerging Technologies 56:102180. doi: 10.1016/j.ifset.2019.102180.
- De Marco, M., S. Martínez, F. Hernandez, J. Madrid, F. Gai, L. Rotolo, M. Belforti, D. Bergero, H. Katz, S. Dabbou, et al. 2015. Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: Apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Animal Feed Science and Technology 209:211–8. doi: 10.1016/j.anifeedsci.2015.08.006.
- Dekkers, B. L., R. M. Boom, and A. J. van der Goot. 2018. Structuring processes for meat analogues. Trends in Food Science & Technology 81:25–36. doi: 10.1016/j.tifs.2018.08.011.
- Denny, A., B. Aisbitt, and J. Lunn. 2008. Mycoprotein and health. Nutrition Bulletin 33 (4):298–310. doi: 10.1111/j.1467-3010.2008.00730.x.
- Derbyshire, E. J., and T. J. Finnigan. 2022. Mycoprotein: A futuristic portrayal. In Future Foods. (pp. 287–303). Academic Press. doi: 10.1016/B978-0-323-91001-9.00037-2.
- Diaz, C. J., K. J. Douglas, K. Kang, A. L. Kolarik, R. Malinovski, Y. Torres-Tiji, J. V. Molino, A. Badary, and S. P. Mayfield. 2023. Developing algae as a sustainable food source. Frontiers in Nutrition 9:3147. doi: 10.3389/fnut.2022.1029841.
- Duque-Estrada, P., and I. L. Petersen. 2023. The sustainability paradox of processing plant proteins. Science of Food 7 (1):38.
- Edwards, D. G., and J. H. Cummings. 2010. The protein quality of mycoprotein. Proceedings of the Nutrition Society 69 (OCE4):E331. doi: 10.1017/S0029665110001400.
- European Parliament and Council of the European Union. 2015. Regulation (EU) 2015/2283 on novel foods. Official Journal of the European Union L327:1–22.
- Evenepoel, P., D. Claus, B. Geypens, M. Hiele, K. Geboes, P. Rutgeerts, and Ghoos, Y. 1999. Amount and fate of egg protein escaping assimilation in the small intestine of humans. American Journal of Physiology-Gastrointestinal and Liver Physiology 277 (5):G935–G943. doi: 10.1152/ajpgi.1999.277.5.G935.
- Fanelli, N. S., H. M. Bailey, T. W. Thompson, R. Delmore, M. N. Nair, and H. H. Stein. 2022. Digestible indispensable amino acid (DIAAS) is greater in animal-based burgers than in plant-based burgers if determined in pigs. European Journal of Nutrition 61 (1):461–75. doi: 10.1007/s00394-021-02658-1.
- FAO. 2013. Dietary protein quality evaluation in human nutrition: Report of an FAO expert consultation 2013. FAO Food and Nutrition Papers 92:1–66.
- FAO. 2017. Protein Quality Assessment in Follow-Up Formula for Young Children and Ready to Use Therapeutic Foods. Rome, Italy: Food and Agriculture Organization of the United Nations.
- FAO/WHO Expert Consultation. 1991. Protein quality evaluation report of the joint FAO/WHO expert consultation held in Bethesda, MD, USA, in 1989. Rome: FAO Food and Nutrition Paper 51.
- FAO/WHO/UNU. 1985. Energy and protein requirements. Report of a joint FAO/WHO/UNU expert consultation. Technical Report Series No. 724. Geneva: World Health Organization.
- Joint WHO/FAO/UNU Expert Consultation. 2007. Protein and amino acid requirements in human nutrition. World Health Organization Technical Report Series 935:1–265.
- Fernandez, M. A., R. F. Bertolo, A. M. Duncan, S. M. Phillips, R. Elango, D. W. Ma, S. Desroches, A. Grantham, and J. D. House. 2020. Translating “protein foods” from the new Canada’s Food Guide to consumers: Knowledge gaps and recommendations. Applied Physiology, Nutrition, and Metabolism 45 (12):1311–23. doi: 10.1139/apnm-2020-0192.
- Finke, M. D. 2013. Complete nutrient content of four species of feeder insects. Zoo Biology 32 (1):27–36. doi: 10.1002/zoo.21012.
- Finnigan, T., K. Mach, and A. Edlin. 2024. Mycoprotein: a healthy new protein with a low environmental impact. In Sustainable protein sources (pp. 539–566). Academic Press. doi: 10.1016/B978-0-323-91652-3.00011-3.
- Foyer, C. H., H.-M. Lam, H. T. Nguyen, K. H. M. Siddique, R. K. Varshney, T. D. Colmer, W. Cowling, H. Bramley, T. A. Mori, J. M. Hodgson, et al. 2016. Neglecting legumes has compromised human health and sustainable food production. Nature Plants 2 (8):16112. doi: 10.1038/nplants.2016.112.
- Frota, K. D. M. G., L. A. R. Lopes, I. C. V. Silva, and J. A. G. Arêas. 2017. Nutritional quality of the protein of Vigna unguiculata L. Walp and its protein isolate. Revista Ciência AGRONÔMICA 48 (5):792–8. doi: 10.5935/1806-6690.20170092.
- Geada, P., C. Moreira, M. Silva, R. Nunes, L. Madureira, C. M. Rocha, R. N. Pereira, A. A. Vicente, and J. A. Teixeira. 2021. Algal proteins: Production strategies and nutritional and functional properties. Bioresource Technology 332:125125. doi: 10.1016/j.biortech.2021.125125.
- Gilani, G. S., K. A. Cockell, and E. Sepehr. 2005. Effects of antinutritional factors on protein digestibility and amino acid availability in foods. Journal of AOAC International 88 (3):967–87. doi: 10.1093/jaoac/88.3.967.
- Godfray, H. C. J., P. Aveyard, T. Garnett, J. W. Hall, T. J. Key, J. Lorimer, R. T. Pierrehumbert, P. Scarborough, M. Springmann, and S. A. Jebb. 2018. Meat consumption, health, and the environment. Science 361 (6399):5324. doi: 10.1126/science.aam5324.
- González, N., M. Marquès, M. Nadal, and J. L. Domingo. 2020. Meat consumption: Which are the current global risks? A review of recent (2010–2020) evidences. Food Research International 137:109341. doi: 10.1016/j.foodres.2020.109341.
- Gravel, A., and A. Doyen. 2020. The use of edible insect proteins in food: Challenges and issues related to their functional properties. Innovative Food Science & Emerging Technologies 59:102272. doi: 10.1016/j.ifset.2019.102272.
- Guillin, F. M., C. Gaudichon, L. Guérin-Deremaux, C. Lefranc-Millot, G. Airinei, N. Khodorova, R. Benamouzig, P. H. Pomport, J. Martin, and J. Calvez. 2022. Real ileal amino acid digestibility of pea protein compared to casein in healthy humans: A randomized trial. The American Journal of Clinical Nutrition 115 (2):353–63. doi: 10.1093/ajcn/nqab354.
- Han, F., P. J. Moughan, J. Li, and S. Pang. 2020. Digestible indispensable amino acid (DIAAS) of six cooked Chinese pulses. Nutrients 12 (12):3831. doi: 10.3390/nu12123831.
- Harvard T.H. Chan School of Public Health. 2023. The nutrition source, legumes and pulses. Visited 14/06/2023. https://www.hsph.harvard.edu/nutritionsource/legumes-pulses/.
- Hashempour-Baltork, F., K. Khosravi-Darani, H. Hosseini, P. Farshi, and S. F. S. Reihani. 2020. Mycoproteins as safe meat substitutes. Journal of Cleaner Production 253:119958. doi: 10.1016/j.jclepro.2020.119958.
- He, J., N. M. Evans, H. Liu, and S. Shao. 2020. A review of research on plant-based meat alternatives: Driving forces, history, manufacturing, and consumer attitudes. Comprehensive Reviews in Food Science and Food Safety 19 (5):2639–56. doi: 10.1111/1541-4337.12610.
- Heo, J. M., E. Kiarie, R. K. Kahindi, P. Maiti, T. A. Woyengo, and C. M. Nyachoti. 2012. Standardized ileal amino acid digestibility in egg from hyperimmunized hens fed to weaned pigs. Journal of Animal Science 90 (Suppl_4):239–41. doi: 10.2527/jas.53983.
- Herreman, L., P. Nommensen, B. Pennings, and M. C. Laus. 2020. Comprehensive overview of the quality of plant-And animal-sourced proteins based on the digestible indispensable amino acid. Food Science & Nutrition 8 (10):5379–91. doi: 10.1002/fsn3.1809.
- Herrero, M., B. Henderson, P. Havlík, P. K. Thornton, R. T. Conant, P. Smith, S. Wirsenius, A. N. Hristov, P. Gerber, M. Gill, et al. 2016. Greenhouse gas mitigation potentials in the livestock sector. Nature Climate Change 6 (5):452–61. doi: 10.1038/nclimate2925.
- Hidayat, K., J. S. Chen, H. P. Wang, T. C. Wang, Y. J. Liu, X. Y. Zhang, C. P. Rao, J. W. Zhang, and L. Q. Qin. 2022. Is replacing red meat with other protein sources associated with lower risks of coronary heart disease and all-cause mortality? A meta-analysis of prospective studies. Nutrition Reviews 80 (9):1959–73. doi: 10.1093/nutrit/nuac017.
- Hlongwane, Z. T., R. Slotow, and T. C. Munyai. 2020. Nutritional composition of edible insects consumed in Africa: A systematic review. Nutrients 12 (9):2786. doi: 10.3390/nu12092786.
- Hobbi, P., A. E. D. A. Bekhit, F. Debaste, N. Lei, and A. Shavandi. 2022. Insect-Derived Protein as Food and Feed. In Alternative Proteins (pp. 85–132). Boca Raton, US: CRC Press.
- Hodgkinson, S. M., C. A. Montoya, P. T. Scholten, S. M. Rutherfurd, and P. J. Moughan. 2018. Cooking conditions affect the true ileal digestible amino acid content and digestible indispensable amino acid score (DIAAS) of bovine meat as determined in pigs. The Journal of Nutrition 148 (10):1564–9. doi: 10.1093/jn/nxy153.
- Humpenöder, F., B. L. Bodirsky, I. Weindl, H. Lotze-Campen, T. Linder, and A. Popp. 2022. Projected environmental benefits of replacing beef with microbial protein. Nature 605 (7908):90–6. doi: 10.1038/s41586-022-04629-w.
- Institute of Medicine. 2005. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington, DC: National Academies Press.
- Johnson, I. T. 2017. The cancer risk related to meat and meat products. British Medical Bulletin 121 (1):73–81. doi: 10.1093/bmb/ldw051.
- Kouřimská, L., and A. Adámková. 2016. Nutritional and sensory quality of edible insects. Nutrition and Food Science 4:22–6.
- Kumar, S., and G. Pandey. 2020. Biofortification of pulses and legumes to enhance nutrition. Heliyon 6 (3):e03682. doi: 10.1016/j.heliyon.2020.e03682.
- Kurek, M. A., A. Onopiuk, E. Pogorzelska-Nowicka, A. Szpicer, M. Zalewska, and A. Półtorak. 2022. Novel protein sources for applications in meat-alternative products—Insight and challenges. Foods (Basel, Switzerland) 11 (7):957. doi: 10.3390/foods11070957.
- Lescinsky, H., A. Afshin, C. Ashbaugh, C. Bisignano, M. Brauer, G. Ferrara, S. I. Hay, J. He, V. Iannucci, L. B. Marczak, et al. 2022. Health effects associated with consumption of unprocessed red meat: A Burden of Proof study. Nature Medicine 28 (10):2075–82. doi: 10.1038/s41591-022-01968-z.
- Li, Y., C. Lammi, G. Boschin, A. Arnoldi, and G. Aiello. 2019. Recent advances in microalgae peptides: Cardiovascular health benefits and analysis. Journal of Agricultural and Food Chemistry 67 (43):11825–38. doi: 10.1021/acs.jafc.9b03566.
- Liceaga, A. M., J. E. Aguilar-Toalá, B. Vallejo-Cordoba, A. F. González-Córdova, and A. Hernández-Mendoza. 2022. Insects as an alternative protein source. Annual Review of Food Science and Technology 13 (1):19–34. doi: 10.1146/annurev-food-052720-112443.
- Loveday, S. M. 2019. Food proteins: Technological, nutritional, and sustainability attributes of traditional and emerging proteins. Annual Review of Food Science and Technology 10 (1):311–39. doi: 10.1146/annurev-food-032818-121128.
- Lucakova, S., I. Branyikova, and M. Hayes. 2022. Microalgal proteins and bioactives for food, feed, and other applications. Applied Sciences 12 (9):4402. doi: 10.3390/app12094402.
- Machovina, B., K. J. Feeley, and W. J. Ripple. 2015. Biodiversity conservation: The key is reducing meat consumption. Science of the Total Environment 536:419–31. doi: 10.1016/j.scitotenv.2015.07.022.
- Malla, N., J. V. Nørgaard, H. N. Lærke, L. H. L. Heckmann, and N. Roos. 2022. Some insect species are good-quality protein sources for children and adults: Digestible indispensable amino acid (DIAAS) determined in growing pigs. The Journal of Nutrition 152 (4):1042–51. doi: 10.1093/jn/nxac019.
- Mathai, J. K., Y. Liu, and H. H. Stein. 2017. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). British Journal of Nutrition 117 (4):490–9. doi: 10.1017/S0007114517000125.
- Mathai, J. K. 2018. Digestible indispensable amino acid for food proteins. PhD dissertation. University of Illinois.
- Mazac, R., N. Järviö, and H. L. Tuomisto. 2023. Environmental and nutritional Life Cycle Assessment of novel foods in meals as transformative food for the future. Science of the Total Environment 876:162796. doi: 10.1016/j.scitotenv.2023.162796.
- Melville, H., M. Shahid, A. Gaines, B. L. McKenzie, R. Alessandrini, K. Trieu, J. H. Wu, E. Rosewarne, and D. H. Coyle. 2023. The nutritional profile of plant-based meat analogues available for sale in Australia. Nutrition & Dietetics 80 (2):211–22. doi: 10.1111/1747-0080.12793.
- Moughan, P. J. 2021. Population protein intakes and food sustainability indices: The metrics matter. Global Food Security 29:100548. doi: 10.1016/j.gfs.2021.100548.
- Moura, M. A. F. E., B. D. A. Martins, G. P. D. Oliveira, and J. A. Takahashi. 2023. Alternative protein sources of plant, algal, fungal and insect origins for dietary diversification in search of nutrition and health. Critical Reviews in Food Science and Nutrition 63 (31):10691–708. doi: 10.1080/10408398.2022.2085657.
- Mune, M. A. M., S. R. Minka, I. L. Mbome, and F. X. Etoa. 2011. Nutritional potential of Bambara bean protein concentrate. Pakistan Journal of Nutrition 10 (2): 112–19.
- Nezlek, J. B., and C. A. Forestell. 2022. Meat substitutes: Current status, potential benefits, and remaining challenges. Current Opinion in Food Science 47:100890. doi: 10.1016/j.cofs.2022.100890.
- Nissen, L., S. P. Samaei, E. Babini, and A. Gianotti. 2020. Gluten free sourdough bread enriched with cricket flour for protein fortification: Antioxidant improvement and volatilome characterization. Food Chemistry 333:127410. doi: 10.1016/j.foodchem.2020.127410.
- Nosworthy, M. G., A. J. Franczyk, G. Medina, J. Neufeld, P. Appah, A. Utioh, P. Frohlich, and J. D. House. 2017a. Effect of processing on the in vitro and in vivo protein quality of yellow and green split peas (Pisum sativum). Journal of Agricultural and Food Chemistry 65 (35):7790–6. doi: 10.1021/acs.jafc.7b03597.
- Nosworthy, M. G., A. Franczyk, A. Zimoch-Korzycka, P. Appah, A. Utioh, J. Neufeld, and J. D. House. 2017b. Impact of processing on the protein quality of pinto bean (Phaseolus vulgaris) and buckwheat (Fagopyrum esculentum Moench) flours and blends, as determined by in vitro and in vivo methodologies. Journal of Agricultural and Food Chemistry 65 (19):3919–25. doi: 10.1021/acs.jafc.7b00697.
- Nosworthy, M. G., G. Medina, A. J. Franczyk, J. Neufeld, P. Appah, A. Utioh, P. Frohlich, and J. D. House. 2018. Effect of processing on the in vitro and in vivo protein quality of beans (Phaseolus vulgaris and Vicia Faba). Nutrients 10 (6):671. doi: 10.3390/nu10060671.
- Nosworthy, M. G., J. Neufeld, P. Frohlich, G. Young, L. Malcolmson, and J. D. House. 2017c. Determination of the protein quality of cooked Canadian pulses. Food Science & Nutrition 5 (4):896–903. doi: 10.1002/fsn3.473.
- Nosworthy, M. G., M. C. Tulbek, and J. D. House. 2017d. Does the concentration, isolation, or deflavoring of pea, lentil, and faba bean protein alter protein quality? Cereal Foods World 62 (4):139–42. doi: 10.1094/CFW-62-4-0139.
- Nyyssölä, A., L. S. Ojala, M. Wuokko, G. Peddinti, A. Tamminen, I. Tsitko, E. Nordlund, and M. Lienemann. 2021. Production of endotoxin-free microbial biomass for food applications by gas fermentation of gram-positive H2-oxidizing bacteria. ACS Food Science & Technology 1 (3):470–9. doi: 10.1021/acsfoodscitech.0c00129.
- Nyyssölä, A., A. Suhonen, A. Ritala, and K. M. Oksman-Caldentey. 2022. The role of single cell protein in cellular agriculture. Current Opinion in Biotechnology 75:102686. doi: 10.1016/j.copbio.2022.102686.
- Oibiokpa, F. I., H. O. Akanya, A. A. Jigam, A. N. Saidu, and Egwim, E. C. 2018. Protein quality of four indigenous edible insect species in Nigeria. Food Science and Human Wellness 7 (2):175–83. doi: 10.1016/j.fshw.2018.05.003.
- Pacheco, M. T., G. M. Caballero-Córdoba, and V. C. Sgarbieri. 1997. Composition and nutritive value of yeast biomass and yeast protein concentrates. Journal of Nutritional Science and Vitaminology 43 (6):601–12. doi: 10.3177/jnsv.43.601.
- Parlasca, M. C., and M. Qaim. 2022. Meat consumption and sustainability. Annual Review of Resource Economics 14 (1):17–41. doi: 10.1146/annurev-resource-111820-032340.
- Poore, J., and T. Nemecek. 2018. Reducing food’s environmental impacts through producers and consumers. Science (New York, N.Y.) 360 (6392):987–92. doi: 10.1126/science.aaq0216.
- Richi, E. B., B. Baumer, B. Conrad, R. Darioli, A. Schmid, and U. Keller. 2015. Health risks associated with meat consumption: A review of epidemiological studies. International Journal for Vitamin and Nutrition Research 85 (1-2):70–8. doi: 10.1024/0300-9831/a000224.
- Rutherfurd, S. M., A. C. Fanning, B. J. Miller, and P. J. Moughan. 2015. Protein digestibility-corrected amino acid and digestible indispensable amino acid differentially describe protein quality in growing male rats. The Journal of Nutrition 145 (2):372–9. doi: 10.3945/jn.114.195438.
- Sá, A. G. A., Y. M. F. Moreno, and B. A. M. Carciofi. 2020. Plant proteins as high-quality nutritional source for human diet. Trends in Food Science & Technology 97:170–84. doi: 10.1016/j.tifs.2020.01.011.
- Sanchez-Sabate, R., Y. Badilla-Briones, and J. Sabaté. 2019. Understanding attitudes towards reducing meat consumption for environmental reasons. A qualitative synthesis review. Sustainability 11(22):6295. doi: 10.3390/su11226295.
- Sandström, V., H. Valin, T. Krisztin, P. Havlík, M. Herrero, and T. Kastner. 2018. The role of trade in the greenhouse gas footprints of EU diets. Global Food Security 19:48–55. doi: 10.1016/j.gfs.2018.08.007.
- Santo, R. E., B. F. Kim, S. E. Goldman, J. Dutkiewicz, E. M. B. Biehl, M. W. Bloem, R. A. Neff, and K. E. Nachman. 2020. Considering plant-based meat substitutes and cell-based meats: A public health and food systems perspective. Frontiers in Sustainable Food Systems 4:134. doi: 10.3389/fsufs.2020.00134.
- Semba, R. D., R. Ramsing, N. Rahman, K. Kraemer, and M. W. Bloem. 2021. Legumes as a sustainable source of protein in human diets. Global Food Security 28:100520. doi: 10.1016/j.gfs.2021.100520.
- Shaheen, N., S. Islam, S. Munmun, M. Mohiduzzaman, and T. Longvah. 2016. Amino acid profiles and digestible indispensable amino acid scores of proteins from the prioritized key foods in Bangladesh. Food Chemistry 213:83–9. doi: 10.1016/j.foodchem.2016.06.057.
- Smetana, S., A. Mathys, A. Knoch, and V. Heinz. 2015. Meat alternatives: Life cycle assessment of most known meat substitutes. The International Journal of Life Cycle Assessment 20 (9):1254–67. doi: 10.1007/s11367-015-0931-6.
- Smetana, S., D. Ristic, D. Pleissner, H. L. Tuomisto, O. Parniakov, and V. Heinz. 2023. Meat substitutes: Resource demands and environmental footprints. Resources, Conservation and Recycling 190:106831. doi: 10.1016/j.resconrec.2022.106831.
- Smetana, S., M. Sandmann, S. Rohn, D. Pleissner, and V. Heinz. 2017. Autotrophic and heterotrophic microalgae and cyanobacteria cultivation for food and feed: Life cycle assessment. Bioresource Technology 245 (Pt A):162–70. doi: 10.1016/j.biortech.2017.08.113.
- Souza Filho, P. F., D. Andersson, J. A. Ferreira, and M. J. Taherzadeh. 2019. Mycoprotein: Environmental impact and health aspects. World Journal of Microbiology & Biotechnology 35 (10):147. doi: 10.1007/s11274-019-2723-9.
- Springmann, M., H. C. J. Godfray, M. Rayner, and P. Scarborough. 2016. Analysis and valuation of the health and climate change cobenefits of dietary change. Proceedings of the National Academy of Sciences 113 (15):4146–51. doi: 10.1073/pnas.1523119113.
- Stagnari, F., A. Maggio, A. Galieni, and M. Pisante. 2017. Multiple benefits of legumes for agriculture sustainability: An overview. Chemical and Biological Technologies in Agriculture 4 (1):1–13. doi: 10.1186/s40538-016-0085-1.
- Tessier, R., J. Calvez, N. Khodorova, and C. Gaudichon. 2021. Protein and amino acid digestibility of 15N Spirulina in rats. European Journal of Nutrition 60 (4):2263–9. doi: 10.1007/s00394-020-02368-0.
- Traksele, L., V. Speiciene, R. Smicius, G. Alencikiene, A. Salaseviciene, G. Garmiene, V. Zigmantaite, R. Grigaleviciute, and A. Kucinskas. 2021. Investigation of in vitro and in vivo digestibility of black soldier fly (Hermetia illucens L.) larvae protein. Journal of Functional Foods 79:104402. doi: 10.1016/j.jff.2021.104402.
- United Nations. 2019. World population prospects 2019: Highlights. Department of Economic and Social Affairs. PopulationDivision. Retrieved at: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf
- USDA National Nutrients Database. 2024. https://fdc.nal.usda.gov/
- Use Therapeutic Foods: Report of the FAO Expert Working Group. 2017. Rome, Italy: Food and Agriculture Organization (FAO).
- van den Berg, L. A., J. J. Mes, M. Mensink, and A. J. Wanders. 2022. Protein quality of soy and the effect of processing: A quantitative review. Frontiers in Nutrition 9:2148. doi: 10.3389/fnut.2022.1004754.
- Van Mierlo, K., S. Rohmer, and J. C. Gerdessen. 2017. A model for composing meat replacers: Reducing the environmental impact of our food consumption pattern while retaining its nutritional value. Journal of Cleaner Production 165:930–50. doi: 10.1016/j.jclepro.2017.07.098.
- Vaz Patto, M. C., R. Amarowicz, A. N. A. Aryee, J. I. Boye, H.-J. Chung, M. A. Martín-Cabrejas, and C. Domoney. 2015. Achievements and challenges in improving the nutritional quality of food legumes. Critical Reviews in Plant Sciences 34 (1-3):105–43. doi: 10.1080/07352689.2014.897907.
- Wang, Y., S. M. Tibbetts, F. Berrue, P. J. McGinn, S. P. MacQuarrie, A. Puttaswamy, S. Patelakis, D. Schmidt, R. Melanson, and S. E. MacKenzie. 2020. A rat study to evaluate the protein quality of three green microalgal species and the impact of mechanical cell wall disruption. Foods (Basel, Switzerland) 9 (11):1531. doi: 10.3390/foods9111531.
- Wang, Y., S. M. Tibbetts, and P. J. McGinn. 2021. Microalgae as sources of high-quality protein for human food and protein supplements. Foods (Basel, Switzerland) 10 (12):3002. doi: 10.3390/foods10123002.
- WHO (World Health Organization). 2015. Protein and amino acid requirements in human nutrition.
- Wiebe, M. G. 2004. QuornTM Myco-protein-Overview of a successful fungal product. Mycologist 18 (1):17–20. doi: 10.1017/S0269915X04001089.
- Willett, W., J. Rockström, B. Loken, M. Springmann, T. Lang, S. Vermeulen, T. Garnett, D. Tilman, F. DeClerck, A.Wood, et al. 2019. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet (London, England) 393 (10170):447–92. doi: 10.1016/S0140-6736(18)31788-4.
- Wolk, A. 2017. Potential health hazards of eating red meat. Journal of Internal Medicine 281 (2):106–22. doi: 10.1111/joim.12543.
- Xiaoming, C., F. Ying, Z. Hong, and C. Zhiyong. 2010. Review of the nutritive value of edible insects. In Forest insects as food: Humans bite back, Proceedings of a workshop on Asia-Pacific resources and their potential for development , Chiang Mai, Thailand, 19–21 February, 2008, 85.
- Xie, Y., L. Cai, Z. Huang, K. Shan, X. Xu, G. Zhou, and C. Li. 2022b. Plant-based meat analogues weaken gastrointestinal digestive function and show less digestibility than real meat in mice. Journal of Agricultural and Food Chemistry 70 (39):12442–55. doi: 10.1021/acs.jafc.2c04246.
- Xie, Y., L. Cai, D. Zhao, H. Liu, X. Xu, G. Zhou, and C. Li. 2022a. Real meat and plant-based meat analogues have different in vitro protein digestibility properties. Food Chemistry 387:132917. doi: 10.1016/j.foodchem.2022.132917.
- Zhang, L., H. Tian, H. Shi, S. Pan, J. Chang, S. R. Dangal, X. Qin, S. Wang, F. N. Tubiello, J. G. Canadell, et al. 2022. A 130-year global inventory of methane emissions from livestock: Trends, patterns, and drivers. Global Change Biology 28 (17):5142–58. doi: 10.1111/gcb.16280.
- Zhou, H., Y. Hu, Y. Tan, Z. Zhang, and D. J. McClements. 2021. Digestibility and gastrointestinal fate of meat versus plant-based meat analogs: An in vitro comparison. Food Chemistry 364:130439. doi: 10.1016/j.foodchem.2021.130439.
- Zhou, Y., D. Wang, S. Zhou, H. Duan, J. Guo, and W. Yan. 2022. Nutritional composition, health benefits, and application value of edible insects: A review. Foods (Basel, Switzerland) 11 (24):3961. doi: 10.3390/foods11243961.
- Zielińska, E., B. Baraniak, M. Karaś, K. Rybczyńska, and A. Jakubczyk. 2015. Selected species of edible insects as a source of nutrient composition. Food Research International 77:460–6. doi: 10.1016/j.foodres.2015.09.008.
