Abstract
Salt (sodium chloride) plays a key role in maintaining the textural, microbiological, and sensorial aspects of the foods. However high dietary salt intake in the population has led to a series of health problems. Currently manufacturers are under pressure to reduce the sodium levels in foods without compromising the consumer experience. Because of the clean salty taste produced by sodium chloride, it has been challenging for the food industry to develop a suitable salt substitute. Studies have shown that different components within a food matrix can influence the perception of saltiness. This review aims to comprehend the potential synergistic effect of compounds such as minerals and amino acids on the perception of saltiness and covers the mechanism of perception where relevant to taste resulting from sodium ions and other metallic ions (such as K, Mg, Ca), as well as various amino acids and their derivatives. Finally, the review summarizes various salt reduction strategies explored by researchers, government organizations and food industry, including the potential use of plant-based extracts.
Introduction
According to the Health Survey for England (2017), about 26.2% of England’s population, aged 16 years or older, had hypertension in 2017 (Public Health England Citation2020a). Even though the treatment and diagnosis of high blood pressure has improved in some high-income countries, the overall global burden of hypertension is still high, especially in low- and middle-income countries (Zhou et al. Citation2021). In 1995, a report published by the Committee on Medical Aspects of Food and Nutrition Policy (COMA), highlighted the relationship between salt intake and increased blood pressure, and consequently the increased risk of cardiovascular disease (Hunty Citation1995). Rucker, Rudemiller, and Crowley (Citation2018) highlighted that sodium accumulates in the bloodstream altering the blood cells (T cells and monocytes), thereby elevating blood pressure. Hence, there is global guidance on limiting salt intake; WHO suggests consumption is restricted to less than 5 g of salt (or 2 g of sodium) per day for an average adult. However, on average, adults consume about twice the amount of recommended salt, i.e., 9–12 g salt/day (World Health Organisation Citation2020). In the UK, approximately 67% of adults in England, and 70% in Scotland, consume more than 5 g salt/day (Purdy Citation2019). Therefore, Public Health England re-set salt reduction targets in 2020 for different food product categories such as meat products (e.g., bacon; maximum 2.59 g salt/100g bacon), breads (maximum 1.01 g/100g) and standard potato crisps (maximum 1.38 g/100g) to help consumers monitor their sodium intake. While achieving these salt levels on the labels or products is voluntary, a monitoring report is expected to take place in 2024 and 2025 (Public Health England Citation2020b).
Archaeological evidence suggests that the first human harvesting of salt happened in around 6000 BC at Lake Yuncheng in China and the first usage of salt in food was to preserve fish which is evidenced to around 2000 BC (Kurlansky Citation2002). Up to now, numerous studies have confirmed the essential role of sodium (Na+) in maintaining various functions of the body such as acid-base balance, functioning of cells, transmission of nerve impulses and maintenance of plasma volume (World Health Organisation Citation2020). Besides the importance of Na+ in human health, nutrition, and physiology, it has been a key ingredient in food manufacturing. Firstly, salt has been used as a preservative to extend the shelf life of food products since early times. Second, the salty taste is desirable in many products and can enhance other flavors in the food, and finally salt aids in the processing and handling of some products (Hutton Citation2002). However, due to the increased health concerns associated with high Na+ intake as mentioned above, policy makers have set up recommendations and guidelines for the food industry to reduce the levels of salt in food products. However, the role of salt is extremely diverse, and it can perform various functions in different food matrices. Its functions are inter-linked, which can make it challenging to reduce the amount of sodium chloride (NaCl) in food products. Therefore, there remains the need for on-going research on alternative salt reduction strategies as no single solutions are currently able to sufficiently reduce NaCl in all food systems. Substitutes often bring sensory defects in the final product, such as noticeable loss of salty taste, and or increased bitter or metallic taste (Vidal et al. Citation2020). NaCl also plays a key role in food processing, and reduction of salt can affect the textural and microbiological aspects of the product as well as their taste and flavor, which is often unacceptable to consumers.
This review aims to comprehend the role of different components known to contribute or enhance salty taste, such as alternative minerals and amino acids, and discuss the mechanisms by which they influence the perception of saltiness. It also considers specific molecules as well as alternative ingredients (such as plant-based substitutes) that may be present in a complex matrix. Hence, the review includes discussion of alternatives that may directly influence receptors (especially sodium channels) or may operate via a cross-modal effect (such as umami-salt taste interaction or odor-induced salt perception). This will expand our knowledge on the synergistic effect of these components and may pave the way for finding new substitutes to replace NaCl for the food industry.
Mechanism for saltiness perception by minerals
Taste transduction of sodium ion
Saltiness is a distinctive sensory quality linked to Na+ containing compounds and this quality is unique to only sodium and lithium. Other cations like potassium and calcium also exhibit salty taste, but it is not the dominant taste quality for these cations (Man Citation2007; van der Klaauw and Smith Citation1995). Salty taste transduction is a complex mechanism and is still under investigation. However, the two most widely studied pathways known to be involved in the perception of salty taste are amiloride sensitive (particularly the epithelial sodium channel, ENaC) and amiloride insensitive pathways.
The ENaC channel (an amiloride sensitive pathway)
The ENaC channel is a voltage-gated sodium ion (Na+) channel. When sodium chloride (NaCl) enters the taste bud, the Na+ interact with these ENaC receptors. This leads to depolarization, an increase in membrane potential inside the receptor cells which causes calcium ions to enter the taste receptor cells through specific voltage ion channels sensitive to calcium. This regulates the release of neurotransmitters and stimulates the sensory neurons, so that the signal travels to the cranial nerves and finally reaches the thalamus region of the brain. The thalamus sends these signals to the gustatory cortex which allows us to differentiate between the tastes, including salty taste () (Mattes Citation1997; Behrens and Meyerhof Citation2015; McCaughey and Scott Citation1998; McCaughey Citation2019). Besides Na+, other monovalent cations like lithium (Li+) and cesium (Cs+), can permeate through the ENaC channels, however their permeability is less than Na+, and these monovalent cations are usually not seen safe for human consumption (Bigiani Citation2016).
Figure 1. Depiction of tongue and taste buds in rats: (1) taste receptor cells within the papillae on the tongue and cross section of three types of papillae- circumvallate, foliate, fungiform. (2) Cross section of a taste bud with its components. (3) Cross section of ENaC channel present on the taste bud. Rats have 3 ENaC subunits, while in humans there is an additional ENaC-δ subunit present (adapted from McCaughey Citation2019; McCaughey and Scott Citation1998).
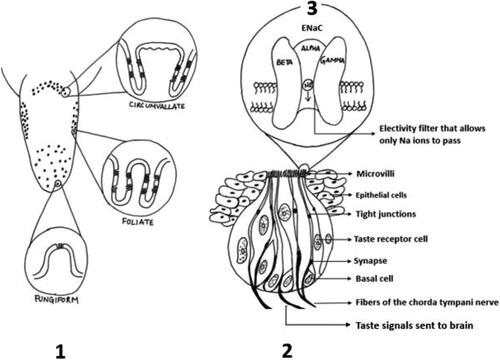
The ENaC is known to comprise of α-, β- and γ- and δ subunits. In humans it has been proposed that the ENaC-δ plays an important role in salty taste transduction (Coscoy et al. Citation1998). A recent review evaluating the role of ENaC in saltiness perception emphasized that the location of the subunits in the human taste system is also extremely important (Bigiani Citation2020). When the ENaC-δ is present at the apical membrane, then the transduction pathway is likely to be via the ENaC ion channels. Besides the location of the subunit, studies have reflected on the concentration of the subunits in the human taste system. Upon analysis it has been observed that among all the four subunits (α-, β-, γ- and δ-), the concentration of δ-ENaC in the taste system is extremely low (Stähler et al. Citation2008). There is still the uncertainty whether all four subunits, or a combination of the subunits, are synergistically playing a role in the Na+ transduction pathway.
However, the Na+ transduction can be hindered in the presence of a drug “amiloride,” which is a diuretic compound known to interfere with Na+ transduction, leading to lower perception of salty taste in mice and in humans. Schiffman, Lockhead, and Maes (Citation1983), examined the psychophysical and neurophysiological effects of amiloride on humans and observed significant reduction of salty taste in both studies. Interestingly the suppression of salty taste was only noticed on Na+ as well as lithium salts and not on potassium or calcium salts. With increasing interest in taste transduction and the role of amiloride, several studies confirmed the suppression of salty taste in the presence of amiloride. It has been observed that amiloride is attracted toward the Na+ channels due to several factors, such as the transmembrane voltage, extracellular pH, Na+ concentration and charge of the amiloride compound (only the cationic form of amiloride can bind to the ion channels). Therefore, it has been concluded that amiloride is capable of blocking the ion channels through attraction; thereby reducing the affinity of Na+ to the channels and eventually leading to a reduced saltiness perception (Smith and Ossebaard Citation1995; Avenet and Lindemann Citation1988; Kellenberger and Schild Citation2002; Chandrashekar et al. Citation2010; Garty and Palmer Citation1997).
Conversely, Halpern and Darlington (Citation1998) did not observe suppression of salty taste in the presence of amiloride at or below a certain concentration (i.e., 100 µM NaCl). This was further investigated by other researchers and led to an understanding of the role of different ENaC subunits. Thereby researchers proposed that it could be the ENaC-δ that affects the sensitivity of receptor to amiloride, however, to what extent ENaC-δ modulates the perception of saltiness is unclear (Ji et al. Citation2006; Stähler et al. Citation2008).
Amiloride insensitive pathway
Amiloride related research has provided evidence that receptors other than ENaC can be involved in salt taste transduction. In 2004, Lyall and coworkers reported the involvement of an “amiloride-insensitive” Na+ taste receptor. One predominant receptor cell involved is called the vanilloid receptor-1 (TRPV-1), which is an ion channel (further detailed in TRPV-1 section). There are other amiloride insensitive receptors involved in the taste perception, which is further discussed in the following sections. These amiloride-insensitive receptors are nonspecific to Na+ and have been shown to detect response from several mineral salts such as potassium, calcium, ammonium, and sodium salts (Lyall et al. Citation2004). However, more recent research has contradicted this and highlighted the importance of the anion. In mouse taste cells, Roebber, Roper, and Chaudhari (Citation2019) concluded that the amiloride-insensitive salt taste response can be generated by chloride and not sodium or any other cation.
Overall, to summarize the salty taste transduction mechanism, in humans, there is more than one mechanism for the perception of salty taste from NaCl, it can occur via the amiloride sensitive pathway, particularly the ENaC channels, or via other channels (like TRPV-1) which are not specific to Na+ and are insensitive to amiloride. Indeed, both above mechanisms could be activated during detection of NaCl. Moreover, the complexity of human salty taste system is not limited to the mechanism of NaCl perception. It is hypothesized that other factors such as protein derived compounds (amino acids, peptides) and minerals (other than Na+) might also contribute to salty taste perception, which will be discussed in the sections below.
Taste perception of minerals other than sodium
Mineral salts of copper, zinc, iron, calcium and magnesium are some of the most common mineral salts known to contribute to taste quality (Riera et al. Citation2007; Ahern et al. Citation2005; Lim and Lawless Citation2005). It is widely accepted that they are transduced by amiloride insensitive, or nonselective cation channels known as the “transient receptor potential” (TRP) family consisting of receptors including Transient receptor potential melastatin-5 (TRPM5), as well as the transient receptor potential vanilloid-1 (TRPV-1) discussed below. Initially, these receptors were only known to be activated by potentially harmful stimuli like capsaicin, noxious heat and protons (Ishida et al. Citation2002). However, later studies revealed minerals such as calcium, magnesium, potassium, and sodium (or their salts) could provoke additional activation of sweet, bitter and umami receptors, thereby contributing to the transduction of other taste qualities, though the mechanism for the activation of the specific receptor cells (i.e., TRPV-1 and TRPM5) might vary (Ahern et al. Citation2005; Lyall et al. Citation2004; Riera et al. Citation2009).
TRPV-1
The TRPV-1 () are abundantly located around the fungiform papillae () (Ishida et al. Citation2002). In addition to the evidence of anion response discussed above, TRPV-1 consists of two proton binding sites (E600 and E648), and an influx of divalent cations (e.g., Ca2+ and Mg2+) into the taste bud increases the extracellular membrane potential activating these two sites (Ahern et al. Citation2005). The interaction of the cations with E600 and E648 has been stated to be essential to fully activate the TRPV-1 receptor cells (), eventually stimulating the sensory neurons (Ahern et al. Citation2005). Once activated, the C terminal of the TRPV-1 interacts with the calcium sensor calmodium (CaM) which regulates the influx of the ions via the voltage gated Ca2+ channels and protects against the potential harmful effects of calcium overload (Aroke et al. Citation2020; Singh et al. Citation2018).
Figure 2. Transient receptor potential vanilloid-1 (TRPV1) [adapted from: Ahern et al. (Citation2005), and Aroke et al. (Citation2020)].
![Figure 2. Transient receptor potential vanilloid-1 (TRPV1) [adapted from: Ahern et al. (Citation2005), and Aroke et al. (Citation2020)].](/cms/asset/1125340d-079b-43c6-8ae9-3451940c6876/bfsn_a_2365962_f0002_b.jpg)
As mentioned in Amiloride Insensitive Pathway section, TRPV-1 is also known to be a major component of the amiloride insensitive pathway and can detect response from several mineral salts including Na+ salts, thereby contributing to the salt taste transduction (Smith et al. Citation2012; Lyall et al. Citation2004). This indicates that minerals other than Na+ can contribute to salty taste by the activation of the TRPV-1 receptors. Ahern et al. (Citation2005) conducted a study by injecting different minerals salts including magnesium chloride (MgCl2) and calcium chloride (CaCl2) on mice with and without TRPV-1 receptors, and patch clamp recordings were carried out to measure if current was generated upon the activation of the sensory neurons via TRPV-1 channels. Results demonstrated that even though all Na+, Ca2+ and Mg2+ cations could activate the TRPV-1 channels, the charge and the cationic density played essential roles in the activation of the TRPV-1 channels. Cations with a higher charge, like Ca2+ and Mg2+ (divalent cations) activated the channels more efficiently and quickly than monovalent cations such as Na+ (Ahern et al. Citation2005). Other divalent salts of zinc, copper and iron can also bind to the TRPV-1 activation sites, but their salty taste perception is less dominant compared to calcium and magnesium-based salts, perhaps because the former might not completely dissociate (Riera et al. Citation2007).
TRPM5
TRPM5 is another member of the transient receptor potential (TRP) family that functions as a nonspecific cation channel (Hofmann et al. Citation2003). When tastants containing monovalent cations like K+ and Na+ comes in contact with the taste buds, they can activate the TRPM5 receptor. There are three potential pathways; (i) as a response to increased calcium influx, (ii) responding in combination with G-protein receptors, or (iii) the coupling of TRPM5 with Inositol 1,4,5-trisphosphate receptor type III (IP3R3) and (or) indirectly by diacyl glycerol (DAG) (Pérez et al. Citation2002).
TRPM5 is extremely sensitive to calcium and even a minor change in the membrane potential or change in calcium influx can lead to the activation of this receptor (Hofmann et al. Citation2003; Prawitt et al. Citation2003). That means, even when ENaC is activated during the presence of Na+, there could be a possibility of activation of TRPM5 along with ENaC as TRPM5 is a sensitive nonspecific cation channel. Interestingly, a study by Nomura et al. (Citation2020) speculates that since both TRPM5 and ENaC share the similar calcium gated voltage channel facilitating the ATP neurotransmitter release, there could be a possibility of some sodium channels (ENaC) existing along with GPCRs or TRPs. Nonetheless, there is no evidence to verify this to date, and the current ENaC mechanism for salty taste perception (Na transduction) cannot be ignored, thus suggesting that it is independent of the activation of the TRPM5 (Zhang et al. Citation2003; Aroke et al. Citation2020). However, activation of TRPM5 has a direct association with the taste transduction of other taste qualities like sweet, bitter and umami (Talavera et al. Citation2008; Dutta Banik et al. Citation2018).
G Protein receptors (GPCR)
Some divalent cations like copper, magnesium, iron and zinc are detected by a combination of TRPM5 and the T1R3 G-protein receptor, and some salts at high concentrations have been shown to activate the T2R bitter receptors. The T1R3 is known to function as part of two heterodimers, T1R2-T1R3 for detecting sweet stimuli and T1R1-T1R3 for detecting umami stimuli. Interestingly, T1R3 is also known as the calcium tasting receptor, i.e., it is capable of detecting the taste of calcium based salts in the human taste system (Tordoff et al. Citation2012). The taste of calcium varies from salty to sour depending on the anion attached to it and leads to an undesirable chalky taste at high concentrations (Tordoff Citation1996). Besides activating TRPM5 and binding to T1R3, calcium also binds to the “calcium sensing receptor” (CaSr). Studies have shown that calcium can activate both T1R3 and CaSr receptors in the presence of magnesium (Tordoff et al. Citation2008). Additionally, CaSr is also activated by kokumi peptides (i.e., GSH, γ-Glu-Val-Gly and various γ-glutamyl peptides) upon which it imparts a distinct taste quality or sensation known as kokumi, i.e., the feeling of mouthfulness or thickness within flavor (Ohsu et al. Citation2010). These compounds do not substitute for salty taste, but the activation of CaSr by kokumi peptides in salt-reduced products might improve the overall perception/acceptance of the products.
Recently, a small-scale study by Wang et al. (Citation2019) analyzed the activation of another G-protein coupled receptor, “TAS2R7” in the presence of divalent mineral ions and found that calcium, magnesium, zinc, copper, manganese and aluminum all activated the TAS2R7. Since the T2R receptor family is responsible for bitter taste transduction, therefore, activation of TAS2R7 by the divalent ions leads to the perception of bitter taste (Chandrashekar et al. Citation2000). However, interaction of TAS2R7 with the CaSR or T1R3 receptors is unclear. Furthermore, in the presence of larger cationic molecules, such as salts of Fe3+ or ammonium chloride derivatives, the cations are not capable of binding to the receptor cells, and in these cases they are perceived by direct adsorption onto the apical membrane of the taste cells (Nakamura and Kurihara Citation1991). Subjects have reported quite a wide range of taste qualities resulting from magnesium and calcium salts, including bitter and salty with metallic and sour sensations, with bitterness being more dominant (Yang and Lawless Citation2005; Lim and Lawless Citation2005). Therefore, the involvement of the specific taste receptors like TRPM5 and T1R3, which are involved in the transduction of sweet, bitter and umami taste, may explain why the taste of some minerals can be confused between salty, bitter, sweet and umami, possibly depending on their concentration.
Taste perception of anions
Besides the activation of the taste receptor and the concentration of cation, studies show that anions also contribute to taste perception. In an early study, Murphy, Cardello, and Brand (Citation1981) concluded that salts of chloride, bromide and iodide exhibited unique taste properties, and noted that NaCl, NaBr, NaI did not all have the same taste. Their study also concludes that, even though it is not always a determining factor, the size and weight of the cation and anion can influence the perceived taste. Organic salts can lead to difference in perception; for example, organic salts of sodium and lithium exhibit slight saltiness but majorly sour taste (van der Klaauw and Smith Citation1995). Zinc also demonstrates both salty and bitter taste, and the resulting taste quality is affected by the anion attached to zinc. For example, “zinc iodide” exhibits sourness and astringency, whereas “zinc chloride” exhibits strong salty taste and further increasing the concentration increased the perception of the particular taste qualities (Keast Citation2003). Similarly, the chloride salts of calcium and magnesium also exhibit salty as well as bitter taste, depending on their concentration (van der Klaauw and Smith Citation1995; Lawless et al. Citation2003; Tordoff Citation1996). A recent study conducted both in human and mice identified a voltage dependent chloride specific channel which could be involved in salty taste perception, known as the transmembrane channel-like 4 (TMC4), which is amiloride insensitive (as discussed above). The function of the TMC4 channels showed similar properties in both mouse and humans (Kasahara et al. Citation2022) ().
Table 1. Key receptors activated by minerals and amino acid and the taste qualities elicited by them.
In summary, taste perception mechanisms for minerals other than Na+ are quite complex as there is not one single receptor involved. It is quite likely that we perceive the taste of minerals via different receptors, however, whether these receptors are activated all at the same time or within discrete combinations is not yet clear. The multiple receptors involved may partly explain why we may be unable to associate a unique taste quality to each mineral. Depending on the ion, minerals are either perceived as bitter, salty, sweet, umami or sour. However, bitter and salty are the most dominant taste qualities associated with mineral salts.
The role of free amino acids and peptides in taste transduction and interaction with salty taste
Taste perception of free amino acids and peptides
The contribution of amino acids to taste was first noticed by Ikeda in 1908 who termed another taste quality known as “umami” besides the four-basic tastes, i.e., sweet, salty, sour, and bitter. The major stimulus responsible for umami taste is the amino acid “glutamic acid or glutamate” (Ikeda Citation2002). Likewise, several studies have confirmed that most amino acids contribute to at least one of the five basic tastes. For example, L-lysine is perceived as salty-bitter-sweet, L-arginine is known to have a flat (i.e., virtually indistinguishable) bitter taste, whereas L-glycine, L-alanine, L-threonine are perceived as sweet (Schiffman, Sennewald, and Gagnon Citation1981; Delompré et al. Citation2019; Schmidt, Olsen, and Mouritsen Citation2020). Additionally, L-lysine, L-glutamic acid and L-aspartic acid were found to exhibit salty taste along with sweet/bitter/umami tastes in unstimulated saliva (Feron Citation2019). Kawai et al. (Citation2012) proposed that all amino acids exhibit at least one of the basic tastes, and there is a strong possibility that the taste of amino acids can be perceived as a combination of the five basic tastes. However, since the number of subjects (n = 8) in the above-mentioned study was substantially low, this theory could not be validated.
Many food products contain naturally occurring free amino acids, such as mushroom, vegetables, milk products, etc. (Sun et al. Citation2017; Górska-Warsewicz et al. Citation2018; Ito, Ueno, and Kikuzaki Citation2017). Usually, the savory taste of amino acids, resulting predominantly from L-glutamic acid and L-aspartic acid is categorized as “umami taste” and it has been observed, in certain foods, to increase the perception of saltiness (Kawai et al. Citation2012; Delompré et al. Citation2019), but can be argued as in certain foods the presence of L-glutamic acid doesn’t not lead to the perception of umami taste. Additionally, individual sensitivity can also lead to the association between umami and salty taste, (Hartley, Liem, and Keast Citation2019). Other amino acids might also play a role in mediating the salty taste (and/or other tastes) of foods, for example arginine and histidine have been used as flavor enhancers to enhance the salty taste of low Na+ meat products (da Silva et al. Citation2020). Processes such as fermentation, ripening and extraction, can break peptide bonds to release various free amino acids which subsequently can contribute to taste and lead to the improvement and enhancement of flavor of many food products; like sake, soy sauce, cheese, etc. (Ikeda Citation2002; Kirimura et al. Citation1969).
Besides the taste contribution of free amino acids, short peptides have also shown some contribution in taste perception, including salty taste enhancement. A recent study using both enzymatic hydrolysis and thermal reactions to prepare Maillard products from pea proteins, reported smaller peptides were quickly degraded into low molecular weight peptides (<1kDa), which were not salty on their own but played a role in salty and umami taste enhancement when added in a 0.5% NaCl solution. Furthermore, the study showed that only the Maillard reaction products [low molecular weight pea peptides (LPP) such as LPP-Glu] derived from hexoses (such as glucose) led to an increase in saltiness (by 8.03%), but saltiness enhancement was not observed with Maillard products derived from pentoses. This is related to the slower rate of Maillard reaction from hexoses compared to pentoses. Indeed the study suggests greater the degree of Maillard reaction the weaker the impact of the peptides or Maillard reaction products on saltiness or umami enhancement. (Yan et al. Citation2021). While this study mentions the low molecular weight compounds that could be generated it does not specify any further details of these products. Other researchers have also identified some specific umami, kokumi and salt enhancing peptides in soy sauce (Jünger et al. Citation2022), salt enhancing arginyl dipeptides in fermented fish sauce (Schindler et al. Citation2011) and some specific umami peptides with salt enhancing effects have also been identified in a variety of mushrooms (Wang et al. Citation2024). It is still unclear if the saltiness of peptides is perceived due to the activation of a specific receptor, or it is a result of activation of umami taste receptors leading to the perception of umami and subsequently enhancement of salty taste. As per a recent review by Le et al. (Citation2022), there is a strong possibility that peptides can activate one or more amino acid taste receptors in order to lead to saltiness perception.
Amino acid tase receptors
Studies confirm the most probable mechanism for the perception of L-glutamic acid and L-aspartic acid to be via the G protein coupled receptors, i.e., the T1R1 + T1R3 heterodimer and mGluR (Chaudhari, Landin, and Roper Citation2000). However, the mechanism for perception of other amino acids, such as lysine, is less clear. One possibility is that the receptors for the transduction of L-glutamic acid and L-aspartic acid amino acids, might also be responsible for the perception of lysine or any other amino acids (Wang et al. Citation2022). However, Smith (Citation2015) highlighted that the activation of T1R1 + T1R3 may not be necessary for the perception of all L-amino acids, thereby suggesting the possibility of the activation of other taste receptors. Stähler et al. (Citation2008) proposed other receptors to be involved in taste perception of amino acids. They reported that amino acids like L-lysine and L-arginine might lead to the activation of ENaC subunits, as they noticed an increased Na+ membrane current in oocytes for αβγ-and δβγ-ENaC units, which is usually observed in the presence of Na+. This might explain the salty taste associated with L-lysine, however further research is needed to verify this in association with the human taste system. It is interesting to note that there was no effect observed for L-glutamine upon interaction with the ENaC subunits in the above study, which further supports the most likely mechanism that glutamine perception is by the G-protein coupled receptors.
Furthermore, another G-protein coupled receptor in humans known as family C, subtype 6 A (hGPRC6A) has gained some attention in its ability to transduce the perception of amino acids, such as L-arginine and L-lysine. Genetically, the hGPRC6A has similarities with two other human taste receptors, i.e., 34% with CaSR [G Protein Receptors (GPCR) section] and 28% with T1R1 (Wellendorph et al. Citation2005). Recently, Jørgensen and Bräuner-Osborne (Citation2020) summarized the pharmacology and physiological function of hGPRC6A and Pallante et al. (Citation2021) highlights the role of hGPRC6A in the umami taste perception. However, there is still not enough understanding about the activation and mechanism of this specific receptor in the taste system, and if there is association with any other taste quality including salty taste.
G-protein receptors
As discussed earlier in G Protein Receptors (GPCR) section, the G-protein receptor heterodimer between T1R1 and T1R3 and is responsible for umami taste, comprising α, β- and γ-subunits which activates upon interaction with amino acids or nucleotide (Behrens and Meyerhof Citation2015). The activated α-subunit triggers signal transduction, elevating calcium influx and the opening of the “non-selective cation channel” TRPM5, which is crucial for umami taste perception (Zhang et al. Citation2003; Behrens and Meyerhof Citation2015; Li et al. Citation2002).
Furthermore, it is also proposed TIR3 may also form homodimers, binding to amino acids. (Nelson et al. Citation2001; Smith Citation2015; Zhao et al. Citation2003). A recent study by Banik et al. (Citation2020) identifies a “broadly responsive (BR)” type 3 cell with a transduction mechanism similar to T1R1/T1R3, influencing the perception of umami, bitter and sweet taste. However, it is yet fully understood if other L-amino acids can activate these cells and further if they are involved in the salty perception, or if the activation of these receptors leading umami is what leads to perception of enhanced salty taste.
Metabotropic glutamate receptors (mGluR)
Metabotropic glutamate receptors (mGluR1-8) are receptors known to be activated by L-glutamate and/or a mix of glutamate and inosine 5′-monophosphate (IMP) (Chaudhari, Landin, and Roper Citation2000; Pal Choudhuri, Delay, and Delay Citation2015). These receptors are known to be found within the circumvallate and foliate papillae of the posterior tongue. Activation of these receptor cells, similar to T1R1/T1R3, leads to the formation of IP3 which can boost calcium influx and thereby signaling the brain (Masu et al. Citation1991). Additionally, the signals can be further amplified in the presence of calcium homeostasis modulator 1(CALHM1), a voltage channel, helping facilitate the release of ATP in the taste buds (Lazutkaite et al. Citation2017; Taruno et al. Citation2013; Ma et al. Citation2018).
San Gabriel et al. (Citation2009) points out the possibility that mGluR may only be activated within specific concentration ranges of L-glutamate, i.e., when present in µmol/L. However, when the glutamate concentration is not enough to activate the mGluR, research suggests the synergetic involvement of both T1R1 + T1R3 and mGluR in the perception of amino acid (glutamate), thus confirming their significance in taste perception (Yasumatsu et al. Citation2015; Lazutkaite et al. Citation2017; Vandenbeuch and Kinnamon Citation2016). The current knowledge, only supports the activation of mGluR by glutamate, however, Pal Choudhuri, Delay, and Delay (Citation2015) puts forward a very fascinating possibility that the mGluR receptors (such as mGluR4, ) might form a complex with each other or with one of the T1Rs, which might act as a receptor for other L-amino acids, generating taste response. However, there is no such evidence to date.
In summary, amino acid derived umami perception is the result of the synergistic effect of T1R1 + T1R3 as well as mGluR taste receptor cells. Additionally, as mentioned previously, TRPM5 is sensitive to calcium, therefore, the release of calcium during the activation of T1R1, T1R3 and mGluR receptors, can also lead to the activation of TRPM5. The umami taste can arise from multiple sources including amino acids (i.e., L-glutamatic acid, L-aspartatic acid), peptides and nucleotides (i.e., IMP, GMP), and thus multiple binding sites are involved on these receptors. The current understanding suggests that umami taste derived from amino acids, which sometimes has been shown to lead to an increased perception of salty taste, is perceived either via the umami taste receptor pathway (mGluR) or the G-protein receptors (GPCRs) or can be a synergistic effect of the two (Wu et al. Citation2021). However, as previously stated, several studies have highlighted the possibility of amino acids other than glutamic acid and aspartic acid contributing to other basic tastes, suggesting the activation of other taste receptors which needs further investigation.
Current salt reduction strategies
Actions by government, restaurants and manufacturers
Consumer awareness is one of the key pillars in the WHO for action on salt reduction (WHO European Salt Action Network Citation2013). Besides setting up salt reduction targets, the governing bodies are making more efforts to educate consumers about the importance of reducing their consumption of salt. This is being carried out via campaigns in newspapers, television, radio, social media, press releases, etc. These campaigns include educating consumers about food labels, for example using color coded front of pack labeling on all products and dedicating national salt awareness days or weeks (WHO European Salt Action Network Citation2013). Annually, several countries around the world participate in “World Salt Awareness Week,” with a different theme each year, to educate consumers on the profound consequences of consuming too much salt (World Action on Salt Citation2021). Additionally, other non-governmental organizations, food manufacturers and retailers’ partner with the government to raise public awareness. However, education alone is not enough, caterers and food manufacturers have a key role in bringing awareness into action.
Consumers consider dining out as one of the challenges in limiting their salt intake, as such meals contribute to a significant amount of dietary Na+ intake. Except in Finland and the United States of America, implementing salt reduction guidelines is a voluntary action. The most common salt reduction strategies at restaurants include “menu labeling,” i.e., highlighting foods containing high Na+ (or salt). Additionally, some countries use symbols or have special campaigns highlighting foods with less salt, sugar and fat on their menus. Some countries with mandatory guidelines for salt reduction recommend reformulating products to ensure products are below maximum Na+ limits. Besides the guidelines, educating chefs on the effects of high salt in food and providing them with training on various low salt dishes are also carried out (Ding et al. Citation2020).
Furthermore, due to the increased consumer demand for clean label and minimally processed foods, food researchers and manufacturers have been exploring the use of non-thermal processing to extend the product shelf life by restricting the microbial growth, thereby, reducing the use of salt in the products. These technologies use very minimum heat, thus safeguarding the sensorial and nutritional properties while confirming the microbial safety of the food (Morris, Brody, and Wicker Citation2007). Non- thermal methods like “high pressure processing (HPP),” “ultrasound” and “pulse electric fields” have shown some positives results in extending the shelf life of food products, mainly meat products even in the presence of low salt (Parniakov et al. Citation2020; Inguglia et al. Citation2017). O’Flynn et al. (Citation2014) found that high pressure processed “low salt” sausages did not have a negative impact on the sensorial and textural properties of the sausages. Additionally, there was no negative impact on the pH and water holding capacity as well, which was not the case in “low salt” sausages without high pressure treatment. However, the application of these novel technologies is quite restricted to certain food products, potentially due to the high equipment cost and low production yield.
Available salt substitutes and their application
As noted earlier, salt has multiple properties in food products and there are different alternatives available that can partially impart some of these properties, however it remains extremely challenging to substitute NaCl with one specific ingredient. summarizes several combinations of salt substitutes which have been explored by the food industry so far. For example, the use of mineral salts (such as KCl, CaCl2 and MgCl2) which can activate the TRPV-1 receptor leading to the perception of salty taste or the use of amino acids (such as glutamic acid, arginine and histidine) which can activate the umami taste receptors like T1R1/T1R3 or mGluR leading to umami taste in sodium reduced products, subsequently leading to enhanced perception of saltiness. The interaction of different salt substitutes with taste receptors leading to enhanced salty taste perception is further discussed in more detail in Discussion section.
Table 2. Available salt substitutes and their applications.
Discussion
summarizes the key receptor cells which, when activated by minerals, amino acids other compounds, either directly contribute to salty taste or indirectly enhances the perception of saltiness in food products. Additionally, it is interesting to note that many of these receptors are not specific to single amino acids or minerals, they can be activated by wide range of stimuli. For example, TRPV-1 can be activated by both noxious heat stimuli contributing to bitter taste and by minerals like Na, K, Ca, contributing to salty taste. In the case of amino acids, one receptor is common for both sweet and umami taste, i.e., T1R3. Additionally, T1R3 (Tordoff et al. Citation2012; Tordoff Citation1996) and another G protein receptor, i.e., TAS2R7 (Wang et al. Citation2019) are also activated by minerals which have some salty taste like K, Ca, Mg and thus their activation can lead to enhanced salty taste perception. On the other hand, these minerals can also activate two or more of these taste receptors which might lead to the perception of one or more taste qualities. Thus, from our current knowledge we would expect these receptors, along with ENaC, could be activated when salt substitutes such as KCl, MgCl, CaCl2 are used in Na+ reduced products like cheese (Grummer et al. Citation2012; Horita et al. Citation2011) or meat products (Vidal et al. Citation2020).
There are some receptors which are not linked to salty taste, but their activation is necessary for the detection of other tastes involved with minerals, including umami, bitter and sweet taste, for example TRPM5. TRPM5 is identified as a key receptor in the transduction mechanism for both mineral salts as well as amino acids as its activation has been observed to be associated with the release of ATP, which is known to activate the sensory neurons, as discussed previously. There is a possibility of activation of the TRPM5 receptors along with the activation of the known mineral (TRPV-1) and amino acid (T1R1 + T1R3) receptors in cases where amino acids and mineral salts are present in the same food products. For instance, the study by da Silva et al. (Citation2020) used a combination of mineral salts (KCl) and amino acids (arginine and histidine) and Guo et al. (Citation2020) used a combination of NaCl and lysine. Therefore, the activation of both mineral and amino acid taste receptors, along with ENaC receptors are expected, in the presence of NaCl. However, it is still unclear if these receptors can be activated simultaneously, or if the saltiness is only perceived by the activation of one dominant receptor.
Furthermore, even though umami taste can be recognized as a different taste quality, it can play a key role in enhancing the saltiness perception of the food products. As we can see from , umami taste receptors including the heterodimer T1R1/T1R3, as well as TRPV-1 and CaSR, are activated by various stimuli, including mineral ions and umami tasting compounds like amino acids. Even though there are specific receptors for individual minerals (like sodium) and amino acids (like glutamate), there may be some overlap between the activation of some receptors (like CaSR) by calcium ions, as well as peptides eliciting salty/umami/kokumi taste (). Could this overlap be the reason why umami taste is associated with enhanced salty taste, or could it be that the umami taste and salty taste are cognitively associated to savory/salty taste? While this is still inconclusive, there are evidence () of the role of umami compounds in enhancing the perception of saltiness. Besides, it is also proposed that amino acids such as lysine can also work as a flavor enhancer to mask the bitter after-taste arising from potassium chloride. Studies highlighted in have explored the incorporation of umami taste compounds including amino acids (lysine, taurine, arginine, glutamic acid) and nucleotides (IMP and GMP) as flavor enhancers to mask the negative sensorial aspects arising by KCl (i.e., bitter, astringent and metallic taste) whilst maintaining the comparable saltiness of control samples (Campagnol et al. Citation2011; Rocha et al. Citation2020). Additionally, a very recent review highlights the promising aspect of using basic amino acids (like lysine, arginine, histidine) in low NaCl meat and fish products with sensory enhancement; indicating that amino acids can support salt reduction by being more than a taste enhancer (Zhang et al. Citation2022). However, these studies do not examine the effect of the salt substitutes at a receptor level, which is also highlighted by Zhang et al. (Citation2022); therefore, it is not clear if the perceived saltiness/other taste attribute is due to the addition of lysine, flavor enhancer or herbs and spices. Considering flavor enhancers in Na+ reduced products, a recent “salt-flip” theory was explored in food products available on the market, which involved switching to a Na+ salt with less Na+ than NaCl. In this case, MSG contains 12.2 g Na+/100g whereas NaCl contains 39.34 g Na+/100g, no undesirable flavors were observed and was accepted by the consumers (Halim et al. Citation2020). Since MSG is a Na+ salt of the glutamic acid, it will activate the umami taste receptors, i.e., T1R1 + T1R3, thereby leading to umami taste perception instead of salty taste (Halim et al. Citation2020). Although successful salt reduction using MSG was achieved with consumers’ acceptance; the acceptance was based on the “flavorful” and “savory” taste quality, rather than specifically on “salty”. The study did further confirm that MSG can be used as a flavor enhancer in salt reduced products. However, the consumers are hesitant in using MSG based products due to its negative reputation of causing harmful health effects. Thus, using MSG alone as a substitute to NaCl has not achieved success.
To cater for the aspect of consumer perception, an ingredient report by Mintel (Citation2017) highlights the presence of glutamic acid and its salts in various ingredients like yeast extract, soy extract, hydrolyzed vegetable protein and protein extracts, which can enhance saltiness perception. Consumers are more than likely to perceive these ingredients as more natural than MSG. Strong evidence has shown () the possibility of using ingredients like the yeast extracts and hydrolyzed vegetable protein for developing salt reduced products (). Mitchell, Brunton, and Wilkinson (Citation2011) used different forms of yeast extracts (autolyzed yeast, whole yeast cell, nucleotide yeast extract) and achieved 60% salt reduction with nucleotide yeast extracts, without having any undesirable impact on the sensorial properties of frozen ready meals. Protein extracts and hydrolates (in essence free amino acids or peptides) do not possess strong salty taste, but they can enhance the taste of NaCl and reduce the total amount of salt used. An interesting product in this category is a mycoprotein derivative, Mycoscent® (by-product from QuornTM), which is a natural flavor enhancer containing glutamic acid and nucleotides (Parniakov et al. Citation2020; Methven Citation2012). Industrial by-products from the wine industry have also been explored (Taladrid et al. Citation2020) as a potential option for salt reduction.
Studies have also explored the addition of herbs and spices as flavor enhancers in salt reduced products (). A review by Rhyu, Kim, and Lyall (Citation2021) suggests that the chemical components of herbs and spices activate the TRP channels such as TRPV-1 and TRPA-1 (Transient Receptor Potential ankyrin member 1), which might lead to an increase in the intracellular calcium concentration, thereby activating the TRPV-1 leading to stimulation of the sensory neurons (TRPV-1 section). As previously discussed, TRPV-1 is activated by several stimuli (like capsaicin and minerals), and is associated with salty, sweet, bitter and umami taste qualities. Therefore, a salt substitute mixture containing herbs and spices along with Na+ or other minerals, will activate TRPV-1 which might have an impact on the stimulation of ENaC. However, the relationship between the activation of TRPV-1 and TRPA-1 in the salt substitute mixture was not explained in the paper.
What remains unclear is where combination of salt substitutes or enhancers are present together in a food product, what receptors are being activated to determine the overall salty taste of the products? For example, to reduce NaCl by 50% in a low fat sausage, Dos Santos Alves et al. (Citation2017) successfully used a combination of KCl, amino acid (lysine), flavor enhancers (50% IMP + 50% GMP), liquid smoke, other spices and herbs (black pepper, coriander and garlic) to reduce 50% NaCl. The lysine was assumed to mask the bitterness of KCl. The current understanding of taste receptors indicates that all the ingredients would activate a combination of different receptors leading to salty and umami taste. For instance, herbs and spices are broadly known to activate TRPA-1 (), the umami taste of amino acids and some nucleotides are perceived by the activation of the heterodimer T1R1 + T1R3 and maybe the homodimer of T1R1 or T1R3 and hGPRC6A and it is known that NaCl can be perceived as slightly sweet in very low concentration (due to the activation of T1R2 + T1R3) and sour at very high concentration (McCaughey Citation2019). It is tempting to assume that other minerals salts and amino acids might behave the same way, but to different extents. Even through the understanding of the salty taste perception has evolved greatly in past decades, there are still a lot of uncertainties needing for further investigation. Therefore we can neither ignore the possibility that all these receptors can be activated at the same time, leading to overall salty taste, nor the possibility of dominating receptors for overall saltines perception Conversely, another interesting possibility which needs in depth exploration is at the neurological level than the receptor level, i.e., the involvement of a common calcium gated voltage channel involved in the release of neurotransmitter ATP in the perception of the different taste qualities via different receptor cells such GPCRs, TRPM5 and ENaC.
Conclusion
This paper summarizes the taste receptors involved in the perception of saltiness, highlighting the necessity to understand the activation of such receptors in developing optimum salt substitute strategies. Using herb and spice blends has been one of the most widely accepted strategies to reduce salt in various food products, but mainly used for flavor enhancement in low Na+ products. Other than imparting taste, salt plays many roles in forming the final product quality including textural and microbial stability. Therefore, depending on the food matrix, studies have explored using a combination of mineral salts with herb and spice blends to retain textural properties and avoid microbial deterioration.
However, with the increasing demand for using clean label and sustainably sourced ingredients, further studies are needed to gain a better understanding of the potential use of plant extracts or other industrial by-products. Additionally, since plants from the Salicornia species naturally grow around marshy land and have the potential to grow on infertile land not necessarily requiring fresh water, they may offer a solution to utilize land not used for other forms of agriculture. These underutilized plants may prove to be an asset to the food and beverage industry as sources of novel ingredients. Further, converting industrial by-products into food and flavorings ingredients is a smart technique to reduce food waste. Considering the composition of the plant extracts and by-products highlighted, we hypothesize that they will activate Na+, mineral and amino acid taste transduction pathways. So far there are only a limited number of studies exploring the use of plants and industrial by-products as salt replacers, and most research is limited to the investigation of sensorial attributes and elicitation of salty taste, rather than the fundament mechanisms for their taste perception. With the complexity involved in the perception of taste, it will be beneficial to have a greater understanding of the mechanisms responsible for saltiness perception of such ingredients to enable product optimization and improved consumer acceptance. Therefore, there is a need to understand the synergistic effect of the different components, particularly minerals and amino acids on the salty taste perception derived from natural salt-alternatives.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahern, G. P., I. M. Brooks, R. L. Miyares, and X. b Wang. 2005. Extracellular cations sensitize and gate capsaicin receptor TRPV1 modulating pain signaling. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 25 (21):5109–16. doi:10.1523/jneurosci.0237-05.2005.
- Ahmad, R., and J. E. Dalziel. 2020. G protein-coupled receptors in taste physiology and pharmacology. Frontiers in Pharmacology 11 (1771):587664. doi:10.3389/fphar.2020.587664.
- Aroke, E. N., K. L. Powell-Roach, R. B. Jaime-Lara, M. Tesfaye, A. Roy, P. Jackson, and P. V. Joseph. 2020. Taste the pain: The role of TRP channels in pain and taste perception. International Journal of Molecular Sciences 21 (16):5929. doi:10.3390/ijms21165929.
- Avenet, P., and B. Lindemann. 1988. Amiloride-blockable sodium currents in isolated taste receptor cells. The Journal of Membrane Biology 105 (3):245–55. doi:10.1007/BF01871001.
- Banik, D. D., E. D. Benfey, L. E. Martin, K. E. Kay, G. C. Loney, A. R. Nelson, Z. C. Ahart, B. T. Kemp, B. R. Kemp, A. M. Torregrossa, et al. 2020. A subset of broadly responsive Type III taste cells contribute to the detection of bitter, sweet and umami stimuli. PLoS Genetics 16 (8):e1008925. doi:10.1371/journal.pgen.1008925.
- Behrens, M., and W. Meyerhof. 2015. Taste receptors. In Flavour Development, Analysis and Perception in Food and Beverages, ed. J. K. Parker, J. S. Elmore, and L. Methven, 297–329. Woodhead Publishing.
- Bigiani, A. 2016. Electrophysiology of sodium receptors in taste cells. Journal of Biomedical Science and Engineering 09 (08):367–83. doi:10.4236/jbise.2016.98032.
- Bigiani, A. 2020. Does ENaC work as sodium taste receptor in humans? Nutrients 12 (4):1195. doi:10.3390/nu12041195.
- Campagnol, P. C. B., B. A. dos Santos, M. A. Morgano, N. N. Terra, and M. A. R. Pollonio. 2011. Application of lysine, taurine, disodium inosinate and disodium guanylate in fermented cooked sausages with 50% replacement of NaCl by KCl. Meat Science 87 (3):239–43. doi:10.1016/j.meatsci.2010.10.018.
- Chandrashekar, J., C. Kuhn, Y. Oka, D. A. Yarmolinsky, E. Hummler, N. J. P. Ryba, and C. S. Zuker. 2010. The cells and peripheral representation of sodium taste in mice. Nature 464 (7286):297–301. doi:10.1038/nature08783.
- Chandrashekar, J., K. L. Mueller, M. A. Hoon, E. Adler, L. Feng, W. Guo, C. S. Zuker, and N. J. Ryba. 2000. T2Rs function as bitter taste receptors. Cell 100 (6):703–11. doi:10.1016/s0092-8674(00)80706-0.
- Chaudhari, N., A. M. Landin, and S. D. Roper. 2000. A metabotropic glutamate receptor variant functions as a taste receptor. Nature Neuroscience 3 (2):113–9. doi:10.1038/72053.
- Coscoy, S., E. Lingueglia, M. Lazdunski, and P. Barbry. 1998. The Phe-Met-Arg-Phe-amide-activated sodium channel ss a tetramer. Journal of Biological Chemistry 273 (14):8317–22. doi:10.1074/jbc.273.14.8317.
- da Silva, S. L., J. M. Lorenzo, J. M. Machado, M. Manfio, A. J. Cichoski, L. L. M. Fries, M. A. Morgano, and P. C. B. Campagnol. 2020. Application of arginine and histidine to improve the technological and sensory properties of low-fat and low-sodium bologna-type sausages produced with high levels of KCl. Meat Science 159:107939. doi:10.1016/j.meatsci.2019.107939.
- Delompré, T., E. Guichard, L. Briand, and C. Salles. 2019. Taste perception of nutrients found in nutritional supplements: A review. Nutrients 11 (9):2050. doi:10.3390/nu11092050.
- Desmond, E. 2006. Reducing salt: A challenge for the meat industry. Meat Science 74 (1):188–96. doi:10.1016/j.meatsci.2006.04.014.
- Ding, J., Y. Sun, Y. Li, J. He, H. Sinclair, W. Du, H. Wang, and P. Zhang. 2020. Systematic review on international salt reduction policy in restaurants. International Journal of Environmental Research and Public Health 17 (24):9570. doi:10.3390/ijerph17249570.
- Dos Santos Alves, L. A. A., J. M. Lorenzo, C. A. Gonçalves, B. A. Dos Santos, R. T. Heck, A. J. Cichoski, and P. C. B. Campagnol. 2017. Impact of lysine and liquid smoke as flavor enhancers on the quality of low-fat Bologna-type sausages with 50% replacement of NaCl by KCl. Meat Science 123:50–6. doi:10.1016/j.meatsci.2016.09.001.
- Dutta Banik, D., L. E. Martin, M. Freichel, A.-M. Torregrossa, and K. F. Medler. 2018. TRPM4 and TRPM5 are both required for normal signaling in taste receptor cells. Proceedings of the National Academy of Sciences 115 (4):E772. doi:10.1073/pnas.1718802115.
- Feron, G. 2019. Unstimulated saliva: Background noise in taste molecules. Journal of Texture Studies 50 (1):6–18. doi:10.1111/jtxs.12369.
- García-Lomillo, J., M. a Luisa González-SanJosé, R. Del Pino-García, M. a Dolores Rivero-Pérez, and P. Muñiz-Rodríguez. 2017. Alternative natural seasoning to improve the microbial stability of low-salt beef patties. Food Chemistry 227:122–8. doi:10.1016/j.foodchem.2017.01.070.
- Garty, H., and L. G. Palmer. 1997. Epithelial sodium channels: Function, structure, and regulation. Physiological Reviews 77 (2):359–96. doi:10.1152/physrev.1997.77.2.359.
- Ghawi, S. K., I. Rowland, and L. Methven. 2014. Enhancing consumer liking of low salt tomato soup over repeated exposure by herb and spice seasonings. Appetite 81:20–9. doi:10.1016/j.appet.2014.05.029.
- Górska-Warsewicz, H., W. Laskowski, O. Kulykovets, A. Kudlińska-Chylak, M. Czeczotko, and K. Rejman. 2018. Food products as sources of protein and amino acids-the case of Poland. Nutrients 10 (12):1977. doi:10.3390/nu10121977.
- Grummer, J., M. Karalus, K. Zhang, Z. Vickers, and T. C. Schoenfuss. 2012. Manufacture of reduced-sodium Cheddar-style cheese with mineral salt replacers. Journal of Dairy Science 95 (6):2830–9. doi:10.3168/jds.2011-4851.
- Guo, X., S. Tao, J. Pan, X. Lin, C. Ji, H. Liang, X. Dong, and S. Li. 2020. Effects of L-Lysine on the physiochemical properties and sensory characteristics of salt-reduced reconstructed ham. Meat Science 166:108133. doi:10.1016/j.meatsci.2020.108133.
- Halim, J., A. Bouzari, D. Felder, and J.-X. Guinard. 2020. The Salt Flip: Sensory mitigation of salt (and sodium) reduction with monosodium glutamate (MSG) in “Better-for-You” foods. Journal of Food Science 85 (9):2902–14. doi:10.1111/1750-3841.15354.
- Halpern, B. P., and R. B. Darlington. 1998. Effects of amiloride on gustatory quality descriptions and temporal patterns produced by NaCl. Chemical Senses 23 (5):501–11. doi:10.1093/chemse/23.5.501.
- Hartley, I. E., D. G. Liem, and R. Keast. 2019. Umami as an ‘alimentary’ taste. A new perspective on taste classification. Nutrients 11 (1):182. doi:10.3390/nu11010182.
- Hofmann, T., V. Chubanov, T. Gudermann, and C. Montell. 2003. TRPM5 is a voltage-modulated and Ca2+-activated monovalent selective cation channel. Current Biology 13 (13):1153–8. doi:10.1016/S0960-9822(03)00431-7.
- Horita, C. N., M. A. Morgano, R. M. S. Celeghini, and M. A. R. Pollonio. 2011. Physico-chemical and sensory properties of reduced-fat mortadella prepared with blends of calcium, magnesium and potassium chloride as partial substitutes for sodium chloride. Meat Science 89 (4):426–33. doi:10.1016/j.meatsci.2011.05.010.
- Hunty, A. d l. 1995. The COMA report on nutritional aspects of cardiovascular disease: The scientific evidence. British Food Journal 97 (9):30–2. doi:10.1108/00070709510100145.
- Hutton, T. 2002. Sodium technological functions of salt in the manufacturing of food and drink products. British Food Journal 104 (2):126–52. doi:10.1108/00070700210423635.
- Ikeda, K. 2002. New seasonings. Chemical Senses 27 (9):847–9. doi:10.1093/chemse/27.9.847.
- Inguglia, E. S., Z. Zhang, B. K. Tiwari, J. P. Kerry, and C. M. Burgess. 2017. Salt reduction strategies in processed meat products – A review. Trends in Food Science & Technology 59:70–8. doi:10.1016/j.tifs.2016.10.016.
- Ishida, Y., S. Ugawa, T. Ueda, S. Murakami, and S. Shimada. 2002. Vanilloid receptor subtype-1 (VR1) is specifically localized to taste papillae. Brain Research. Molecular Brain Research 107 (1):17–22. doi:10.1016/S0169-328X(02)00441-2.
- Ito, H., H. Ueno, and H. Kikuzaki. 2017. Construction of a free-form amino acid database for vegetables and mushrooms. Integrative Food, Nutrition and Metabolism 4 (3):1–9. doi:10.15761/IFNM.1000186.
- Ji, H.-L., X.-F. Su, S. Kedar, J. Li, P. Barbry, P. R. Smith, S. Matalon, and D. J. Benos. 2006. δ-subunit confers novel biophysical features to αβγ-human epithelial sodium channel (ENaC) via a physical interaction. Journal of Biological Chemistry 281 (12):8233–41. doi:10.1074/jbc.M512293200.
- Jørgensen, C. V., and H. Bräuner-Osborne. 2020. Pharmacology and physiological function of the orphan GPRC6A receptor. Basic & Clinical Pharmacology & Toxicology 126 Suppl 6 (S6):77–87. doi:10.1111/bcpt.13397.
- Jünger, M., V. K. Mittermeier-Kleßinger, A. Farrenkopf, A. Dunkel, T. Stark, S. Fröhlich, V. Somoza, C. Dawid, and T. Hofmann. 2022. Sensoproteomic discovery of taste-modulating peptides and taste re-engineering of soy sauce. Journal of Agricultural and Food Chemistry 70 (21):6503–18. doi:10.1021/acs.jafc.2c01688.
- Kasahara, Y., M. Narukawa, A. Takeuchi, M. Tominaga, K. Abe, and T. Asakura. 2022. Molecular logic of salt taste reception in special reference to transmembrane channel-like 4 (TMC4). The Journal of Physiological Sciences: JPS 72 (1):31. doi:10.1186/s12576-022-00856-y.
- Kawai, M., Y. Sekine-Hayakawa, A. Okiyama, and Y. Ninomiya. 2012. Gustatory sensation of l- and d-amino acids in humans. Amino Acids 43 (6):2349–58. doi:10.1007/s00726-012-1315-x.
- Keast, R. S. J. 2003. The effect of zinc on human taste perception. Journal of Food Science 68 (5):1871–7. doi:10.1111/j.1365-2621.2003.tb12345.x.
- Kellenberger, S., and L. Schild. 2002. Epithelial sodium channel/degenerin family of ion channels: A variety of functions for a shared structure. Physiological Reviews 82 (3):735–67. doi:10.1152/physrev.00007.2002.
- Kirimura, J., A. Shimizu, A. Kimizuka, T. Ninomiya, and N. Katsuya. 1969. Contribution of peptides and amino acids to the taste of foods. Journal of Agricultural and Food Chemistry 17 (4):689–95. doi:10.1021/jf60164a031.
- Kurlansky, M. 2002. Salt: A world history." New York, NY: Walker and Co.
- Lawless, H. T., F. Rapacki, J. Horne, and A. Hayes. 2003. The taste of calcium and magnesium salts and anionic modifications. Food Quality and Preference 14 (4):319–25. doi:10.1016/S0950-3293(02)00128-3.
- Lazutkaite, G., A. Soldà, K. Lossow, W. Meyerhof, and N. Dale. 2017. Amino acid sensing in hypothalamic tanycytes via umami taste receptors. Molecular Metabolism 6 (11):1480–92. doi:10.1016/j.molmet.2017.08.015.
- Le, B., B. Yu, M. S. Amin, R. Liu, N. Zhang, O. P. Soladoye, R. E. Aluko, Y. Zhang, and Y. Fu. 2022. Salt taste receptors and associated salty/salt taste-enhancing peptides: A comprehensive review of structure and function. Trends in Food Science & Technology 129:657–66. doi:10.1016/j.tifs.2022.11.014.
- Lee, G. H. 2011. A salt substitute with low sodium content from plant aqueous extracts. Food Research International 44 (2):537–43. doi:10.1016/j.foodres.2010.11.018.
- Li, X., L. Staszewski, H. Xu, K. Durick, M. Zoller, and E. Adler. 2002. Human receptors for sweet and umami taste. Proceedings of the National Academy of Sciences 99 (7):4692–6. doi:10.1073/pnas.072090199.
- Lim, J., and H. T. Lawless. 2005. Qualitative differences of divalent salts: Multidimensional scaling and cluster analysis. Chemical Senses 30 (9):719–26. doi:10.1093/chemse/bji064.
- Lopes, C. d O., M. d F. P. Barcelos, N. A. A. Dias, J. d D. S. Carneiro, and W. C. de Abreu. 2014. Effect of the addition of spices on reducing the sodium content and increasing the antioxidant activity of margarine. LWT - Food Science and Technology 58 (1):63–70. doi:10.1016/j.lwt.2014.02.029.
- Lyall, V., G. L. Heck, A. K. Vinnikova, S. Ghosh, T. H. T. Phan, R. I. Alam, O. F. Russell, S. A. Malik, J. W. Bigbee, and J. A. DeSimone. 2004. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. The Journal of Physiology 558 (Pt 1):147–59. doi:10.1113/jphysiol.2004.065656.
- Ma, Z. M., A. Taruno, M. Ohmoto, M. Jyotaki, J. C. Lim, H. Miyazaki, N. Niisato, Y. Marunaka, R. J. Lee, H. Hoff, et al. 2018. CALHM3 is essential for rapid ion channel-mediated purinergic neurotransmission of GPCR-mediated tastes. Neuron 98 (3):547–61.e10. +. doi:10.1016/j.neuron.2018.03.043.
- Man, C. M. D. 2007. Technological functions of salt in food products. In Reducing salt in foods, ed. D. Kilcast and F. Angus157–73. Woodhead Publishing.
- Masu, M., Y. Tanabe, K. Tsuchida, R. Shigemoto, and S. Nakanishi. 1991. Sequence and expression of a metabotropic glutamate receptor. Nature 349 (6312):760–5. doi:10.1038/349760a0.
- Mattes, R. D. 1997. The taste for salt in humans. The American Journal of Clinical Nutrition 65 (2 Suppl):692S–7S. doi:10.1093/ajcn/65.2.692s.
- McCaughey, S. A. 2019. Dietary salt and flavour: Mechanisms of taste perception and physiological controls. In Reducing salt in foods. 2nd ed., ed. C. Beeren, K. Groves and P. M. Titoria, 45–70. Woodhead Publishing.
- McCaughey, S. A., and T. R. Scott. 1998. The taste of sodium. Neuroscience & Biobehavioral Reviews 22 (5):663–76. doi:10.1016/S0149-7634(97)00067-5.
- Methven, L. 2012. Natural food and beverage flavour enhancers. In Natural food additives, ingredients and flavourings, ed. D. Baines and R. Seal, 76–99. Cambridge: Woodhead Publ Ltd.
- Mintel. 2017. Ingredient insight: Salt and salt substitutes. Last Modified January 2017. Accessed April 18, 2021. https://www.mintel.com/products/reports/.
- Mitchell, M., N. P. Brunton, and M. G. Wilkinson. 2011. Current salt reduction strategies and their effect on sensory acceptability: A study with reduced salt ready-meals. European Food Research and Technology 232 (3):529–39. doi:10.1007/s00217-010-1420-6.
- Mitchell, M., N. P. Brutnon, R. J. Fitzgerald, and M. G. Wilkinson. 2013. The use of herbs, spices, and whey proteins as natural flavor enhancers and their effect on the sensory acceptability of reduced-salt chilled ready-meals. Journal of Culinary Science & Technology 11 (3):222–40. doi:10.1080/15428052.2013.769869.
- Morris, C., A. L. Brody, and L. Wicker. 2007. Non-thermal food processing/preservation technologies: A review with packaging implications. Packaging Technology and Science 20 (4):275–86. doi:10.1002/pts.789.
- Murphy, C., A. V. Cardello, and J. G. Brand. 1981. Tastes of 15 halide salts following water and NaCl- anion and cation effects. Physiology & Behavior 26 (6):1083–95. doi:10.1016/0031-9384(81)90213-4.
- Nakagawa, T., J. Kohori, S. Koike, Y. Katsuragi, and T. Shoji. 2014. Sodium aspartate as a specific enhancer of salty taste perception-sodium aspartate is a possible candidate to decrease excessive intake of dietary salt. Chemical Senses 39 (9):781–6. doi:10.1093/chemse/bju051.
- Nakamura, M., and K. Kurihara. 1991. Rat taste nerve responses to salts carrying cations of large molecular size; are the taste responses to the salts induced by cation transport across apical membranes of taste cells? Comparative Biochemistry and Physiology. A, Comparative Physiology 100 (3):661–5. doi:10.1016/0300-9629(91)90386-q.
- Nelson, G., J. Chandrashekar, M. A. Hoon, L. Feng, G. Zhao, N. J. P. Ryba, and C. S. Zuker. 2002. An amino-acid taste receptor. Nature 416 (6877):199–202. doi:10.1038/nature726.
- Nelson, G., M. A. Hoon, J. Chandrashekar, Y. Zhang, N. J. Ryba, and C. S. Zuker. 2001. Mammalian sweet taste receptors. Cell 106 (3):381–90. doi:10.1016/s0092-8674(01)00451-2.
- Nomura, K., M. Nakanishi, F. Ishidate, K. Iwata, and A. Taruno. 2020. All-electrical Ca2+ independent signal transduction mediates attractive sodium taste in taste buds. Neuron 106 (5):816–29.e6. doi:10.1016/j.neuron.2020.03.006.
- O’Flynn, C. C., M. C. Cruz-Romero, D. Troy, A. M. Mullen, and J. P. Kerry. 2014. The application of high-pressure treatment in the reduction of salt levels in reduced-phosphate breakfast sausages. Meat Science 96 (3):1266–74. doi:10.1016/j.meatsci.2013.11.010.
- Ohsu, T., Y. Amino, H. Nagasaki, T. Yamanaka, S. Takeshita, T. Hatanaka, Y. Maruyama, N. Miyamura, and Y. Eto. 2010. Involvement of the calcium-sensing receptor in human taste perception. The Journal of Biological Chemistry 285 (2):1016–22. doi:10.1074/jbc.M109.029165.
- Pal Choudhuri, S., R. J. Delay, and E. R. Delay. 2015. L-amino acids elicit diverse response patterns in taste sensory cells: A role for multiple receptors. PLoS One 10 (6):e0130088. doi:10.1371/journal.pone.0130088.
- Pallante, L., M. Malavolta, G. Grasso, A. Korfiati, S. Mavroudi, B. Mavkov, A. Kalogeras, C. Alexakos, V. Martos, D. Amoroso, et al. 2021. On the human taste perception: Molecular-level understanding empowered by computational methods. Trends in Food Science & Technology 116:445–59. doi:10.1016/j.tifs.2021.07.013.
- Parniakov, O., M. Mikhrovska, S. Toepfl, E. Rosello-Soto, C. A. Pinto, J. A. Saraiva, and F. J. Barba. 2020. Chapter 6 - Current and future strategies to reduce salt consumption. Agri-food industry strategies for healthy diets and sustainability: New challenges in nutrition and public health, ed. F. J. Barba, P. Putnik and D. B. Kovačević. Academic Press.
- Pérez, C. A., L. Huang, M. Rong, J. Ashot Kozak, A. K. Preuss, H. Zhang, M. Max, and R. F. Margolskee. 2002. A transient receptor potential channel expressed in taste receptor cells. Nature Neuroscience 5 (11):1169–76. doi:10.1038/nn952.
- Pires-Cabral, P., P. Pires-Cabral, and C. Quintas. 2021. Salicornia ramosissima as a salt substitute in the fermentation of white cabbage. Journal of Food Science and Technology 59 (2):597–605. doi:10.1007/s13197-021-05047-y.
- Prawitt, D., M. K. Monteilh-Zoller, L. Brixel, C. Spangenberg, B. Zabel, A. Fleig, and R. Penner. 2003. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proceedings of the National Academy of Sciences of the United States of America 100 (25):15166–71. doi:10.1073/pnas.2334624100.
- Public Health England. 2020a. Hypertension prevalence estimates in England, 2017. Accessed September 2021. https://www.gov.uk/government/publications/hypertension-prevalence-estimates-for-local-populations.
- Public Health England. 2020b. The salt reduction targets 2024. Accessed January 2022. https://www.gov.uk/government/publications/salt-reduction-targets-for-2024.
- Purdy, J. 2019. Dietary salt: Consumption, reduction strategies and consumer awareness. In Reducing salt in foods, ed. C.Beeren, K. Groves and P. M. Titori, 71–96. Woodhead Publishing.
- Rhyu, M.-R., Y. Kim, and V. Lyall. 2021. Interactions between chemesthesis and taste: Role of TRPA1 and TRPV1. International Journal of Molecular Sciences 22 (7):3360. doi:10.3390/ijms22073360.
- Riera, C. E., H. Vogel, S. A. Simon, S. Damak, and J. Le Coutre. 2009. Sensory attributes of complex tasting divalent salts are mediated by TRPM5 and TRPV1 channels. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 29 (8):2654–62. doi:10.1523/jneurosci.4694-08.2009.
- Riera, C. E., H. Vogel, S. A. Simon, and J. Le Coutre. 2007. Artificial sweeteners and salts producing a metallic taste sensation activate TRPV1 receptors. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 293 (2):R626–R634. doi:10.1152/ajpregu.00286.2007.
- Rocha, R. A. R., M. N. Ribeiro, G. A. Silva, L. C. R. Rocha, A. C. M. Pinheiro, C. A. Nunes, J. de, and D. S. Carneiro. 2020. Temporal profile of flavor enhancers MAG, MSG, GMP, and IMP, and their ability to enhance salty taste, in different reductions of sodium chloride. Journal of Food Science 85 (5):1565–75. doi:10.1111/1750-3841.15121.
- Roebber, J. K., S. D. Roper, and N. Chaudhari. 2019. The role of the anion in salt (NaCl) detection by mouse taste buds. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 39 (32):6224–32. doi:10.1523/JNEUROSCI.2367-18.2019.
- Rucker, A. J., N. P. Rudemiller, and S. D. Crowley. 2018. Salt, hypertension, and immunity. Annual Review of Physiology 80 (1):283–307. doi:10.1146/annurev-physiol-021317-121134.
- San Gabriel, A., T. Maekawa, H. Uneyama, and K. Torii. 2009. Metabotropic glutamate receptor type 1 in taste tissue. The American Journal of Clinical Nutrition 90 (3):743S–6S. doi:10.3945/ajcn.2009.27462I.
- Schiffman, S. S., E. Lockhead, and F. W. Maes. 1983. Amiloride reduces the taste intensity of Na + and Li + salts and sweeteners. Proceedings of the National Academy of Sciences 80 (19):6136–40. doi:10.1073/pnas.80.19.6136.
- Schiffman, S. S., K. Sennewald, and J. Gagnon. 1981. Comparison of taste qualities and thresholds of D- and L-amino acids. Physiology & Behavior 27 (1):51–9. doi:10.1016/0031-9384(81)90298-5.
- Schindler, A., A. Dunkel, F. Stähler, M. Backes, J. Ley, W. Meyerhof, and T. Hofmann. 2011. Discovery of salt taste enhancing arginyl dipeptides in protein digests and fermented fish sauces by means of a sensomics approach. Journal of Agricultural and Food Chemistry 59 (23):12578–88. doi:10.1021/jf2041593.
- Schmidt, C. V., K. Olsen, and O. G. Mouritsen. 2020. Umami synergy as the scientific principle behind taste-pairing champagne and oysters. Scientific Reports 10 (1):20077. doi:10.1038/s41598-020-77107-w.
- Singh, A. K., L. L. McGoldrick, E. C. Twomey, and A. I. Sobolevsky. 2018. Mechanism of calmodulin inactivation of the calcium-selective TRP channel TRPV6. Science Advances 4 (8):eaau6088. doi:10.1126/sciadv.aau6088.
- Smith, S. T., L. Metzger, and M. A. Drake. 2016. Evaluation of whey, milk, and delactosed permeates as salt substitutes. Journal of Dairy Science 99 (11):8687–98. doi:10.3168/jds.2016-10904.
- Smith, D. V., and C. A. Ossebaard. 1995. Amiloride suppression of the taste intensity of sodium chloride: Evidence from direct magnitude scaling. Physiology & Behavior 57 (4):773–7. doi:10.1016/0031-9384(94)00329-7.
- Smith, K. R., Y. Treesukosol, A. Brennan Paedae, R. J. Contreras, and A. C. Spector. 2012. Contribution of the TRPV1 channel to salt taste quality in mice as assessed by conditioned taste aversion generalization and chorda tympani nerve responses. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 303 (11):R1195–R1205. doi:10.1152/ajpregu.00154.2012.
- Smith, K. R. 2015. A psychophysical assessment of the role of the T1R proteins in the taste transduction of amino acids and maltodextrins, Ph.D., The Florida State University (10000570).
- Stähler, F., K. Riedel, S. Demgensky, K. Neumann, A. Dunkel, A. Täubert, B. Raab, M. Behrens, J.-D. Raguse, T. Hofmann, et al. 2008. A role of the epithelial sodium channel in human salt taste transduction? Chemosensory Perception 1 (1):78–90. doi:10.1007/s12078-008-9006-4.
- Sun, L., Q. Liu, C. Bao, and J. Fan. 2017. Comparison of free total amino acid compositions and their functional classifications in 13 wild edible mushrooms. Molecules (Basel, Switzerland) 22 (3):350. doi:10.3390/molecules22030350.
- Taladrid, D., L. Laguna, B. Bartolomé, and M. V. Moreno-Arribas. 2020. Plant-derived seasonings as sodium salt replacers in food. Trends in Food Science & Technology 99:194–202. doi:10.1016/j.tifs.2020.03.002.
- Talavera, K., K. Yasumatsu, R. Yoshida, R. F. Margolskee, T. Voets, Y. Ninomiya, and B. Nilius. 2008. "The taste transduction channel TRPM5 is a locus for bitter-sweet taste interactions. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 22 (5):1343–55. doi:10.1096/fj.07-9591com.
- Taruno, A., V. Vingtdeux, M. Ohmoto, Z. Ma, G. Dvoryanchikov, A. Li, L. Adrien, H. Zhao, S. Leung, M. Abernethy, et al. 2013. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 495 (7440):223–6. doi:10.1038/nature11906.
- Tordoff, M. G. 1996. Some basic psychophysics of calcium salt solutions. Chemical Senses 21 (4):417–24. doi:10.1093/chemse/21.4.417.
- Tordoff, M. G. L. K., Alarcón, S. Valmeki, and P. Jiang. 2012. T1R3: A human calcium taste receptor. Scientific Reports 2 (1):496. doi:10.1038/srep00496.
- Tordoff, M. G., H. Shao, L. K. Alarcón, R. F. Margolskee, B. Mosinger, A. A. Bachmanov, D. R. Reed, and S. McCaughey. 2008. Involvement of T1R3 in calcium-magnesium taste. Physiological Genomics 34 (3):338–48. doi:10.1152/physiolgenomics.90200.2008.
- van der Klaauw, N. J., and D. V. Smith. 1995. Taste quality profiles for 15 organic and inorganic salts. Physiology & Behavior 58 (2):295–306. doi:10.1016/0031-9384(95)00056-o.
- Vandenbeuch, A., and S. C. Kinnamon. 2016. Glutamate: Tastant and neuromodulator in taste buds. Advances in Nutrition (Bethesda, MD) 7 (4):823S–7S. doi:10.3945/an.115.011304.
- Vidal, V. A. S., J. B. Santana, C. S. Paglarini, M. A. A. P. da Silva, M. Q. Freitas, E. A. Esmerino, A. G. Cruz, and M. A. R. Pollonio. 2020. Adding lysine and yeast extract improves sensory properties of low sodium salted meat. Meat Science 159:107911. doi:10.1016/j.meatsci.2019.107911.
- Wang, Z., Y. Cheng, B. Muhoza, M. Sun, T. Feng, L. Yao, Q. Liu, and S. Song. 2024. Discovery of peptides with saltiness-enhancing effects in enzymatic hydrolyzed Agaricus bisporus protein and evaluation of their salt-reduction property. Food Research International (Ottawa, Ont.) 177:113917. doi:10.1016/j.foodres.2023.113917.
- Wang, C., Y. Lee, and S.-Y. Lee. 2014. Consumer acceptance of model soup system with varying levels of herbs and salt. Journal of Food Science 79 (10):S2098–S2106. doi:10.1111/1750-3841.12637.
- Wang, Q., X.-F. Liang, J. Gao, W. Cai, S. He, and W. Zhuang. 2022. Lysine regulates TOR and NPY through taste receptor T1R1 in Chinese perch (Siniperca chuatsi). Aquaculture 559:738445. doi:10.1016/j.aquaculture.2022.738445.
- Wang, Y., A. L. Zajac, W. Lei, C. M. Christensen, R. F. Margolskee, C. Bouysset, J. Golebiowski, H. Zhao, S. Fiorucci, and P. Jiang. 2019. Metal ions activate the human taste receptor TAS2R7. Chemical Senses 44 (5):339–47. doi:10.1093/chemse/bjz024.
- Wellendorph, P., K. B. Hansen, A. Balsgaard, J. R. Greenwood, J. Egebjerg, and H. Bräuner-Osborne. 2005. Deorphanization of GPRC6A: A promiscuous L-alpha-amino acid receptor with preference for basic amino acids. Molecular Pharmacology 67 (3):589–97. doi:10.1124/mol.104.007559.
- WHO European Salt Action Network. 2013. Mapping salt reduction initiatives in the WHO European Region.
- World Action on Salt. 2021. Salt awareness week. http://www.worldactiononsalt.com/awarenessweek/salt-awareness-week/.
- World Health Organisation. 2020. Salt reduction, fact sheets. Accessed November 3, 2020. https://www.who.int/news-room/fact-sheets/detail/salt-reduction.
- Wu, B., S. Eldeghaidy, C. Ayed, I. D. Fisk, L. Hewson, and Y. Liu. 2021. Mechanisms of umami taste perception: From molecular level to brain imaging. Critical Reviews in Food Science and Nutrition 62 (25):7015–24. doi:10.1080/10408398.2021.1909532.
- Yan, F., H. Cui, Q. Zhang, K. Hayat, J. Yu, S. Hussain, M. U. Tahir, X. Zhang, and C.-T. Ho. 2021. Small peptides hydrolyzed from pea protein and their maillard reaction products as taste modifiers: Saltiness, umami, and kokumi enhancement. Food and Bioprocess Technology 14 (6):1132–41. doi:10.1007/s11947-021-02630-1.
- Yang, H. H.-L., and H. T. Lawless. 2005. "Descriptive analysis of divalent salts. Journal of Sensory Studies 20 (2):97–113. doi:10.1111/j.1745-459X.2005.00005.x.
- Yasumatsu, K., T. Manabe, R. Yoshida, K. Iwatsuki, H. Uneyama, I. Takahashi, and Y. Ninomiya. 2015. Involvement of multiple taste receptors in umami taste: Analysis of gustatory nerve responses in metabotropic glutamate receptor 4 knockout mice. The Journal of Physiology 593 (4):1021–34. doi:10.1113/jphysiol.2014.284703.
- Zhang, Y., Q. Cheng, Y. Yao, X. Guo, R. Wang, and Z. Peng. 2014. A preliminary study: Saltiness and sodium content of aqueous extracts from plants and marine animal shells. European Food Research and Technology 238 (4):565–71. doi:10.1007/s00217-013-2136-1.
- Zhang, Y., X. Guo, Z. Peng, and M. A. Jamali. 2022. A review of recent progress in reducing NaCl content in meat and fish products using basic amino acids. Trends in Food Science & Technology 119:215–26. doi:10.1016/j.tifs.2021.12.009.
- Zhang, Y., M. A. Hoon, J. Chandrashekar, K. L. Mueller, B. Cook, D. Wu, C. S. Zuker, and N. J. P. Ryba. 2003. Coding of sweet, bitter, and umami tastes: Different receptor cells sharing similar signaling pathways. Cell 112 (3):293–301. doi:10.1016/S0092-8674(03)00071-0.
- Zhao, G. Q., Y. Zhang, M. A. Hoon, J. Chandrashekar, I. Erlenbach, N. J. P. Ryba, and C. S. Zuker. 2003. The receptors for mammalian sweet and umami taste. Cell 115 (3):255–66. doi:10.1016/s0092-8674(03)00844-4.
- Zhou, B., P. Perel, G. A. Mensah, and M. Ezzati. 2021. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nature Reviews. Cardiology 18 (11):785–802. doi:10.1038/s41569-021-00559-8.
