Abstract
Ulva, a genus of green macroalgae commonly known as sea lettuce, has long been recognized for its nutritional benefits for food and feed. As the demand for sustainable food and feed sources continues to grow, so does the interest in alternative, plant-based protein sources. With its abundance along coastal waters and high protein content, Ulva spp. have emerged as promising candidates. While the use of Ulva in food and feed has its challenges, the utilization of Ulva in other industries, including in biomaterials, biostimulants, and biorefineries, has been growing. This review aims to provide a comprehensive overview of the current status, challenges and opportunities associated with using Ulva in food, feed, and beyond. Drawing on the expertise of leading researchers and industry professionals, it explores the latest knowledge on Ulva’s nutritional value, processing methods, and potential benefits for human nutrition, aquaculture feeds, terrestrial feeds, biomaterials, biostimulants and biorefineries. In addition, it examines the economic feasibility of incorporating Ulva into aquafeed. Through its comprehensive and insightful analysis, including a critical review of the challenges and future research needs, this review will be a valuable resource for anyone interested in sustainable aquaculture and Ulva’s role in food, feed, biomaterials, biostimulants and beyond.
1. Introduction
The world population is expected to reach 9.8 billion by 2050 (Population Reference Bureau, Citation2023). Accordingly, the agricultural food gap will increase due to climate change-induced constraints on natural resources, i.e., freshwater and farmland. Consequently, ensuring food security has become a global imperative. The oceans will play an increasingly important role in feeding the growing population with increasing demand for food and natural resources. Nevertheless, wild stocks cannot meet the increasing demand for fish or other biomass sources, including macroalgae. Therefore, seaweed cultivation may be essential for contributing to food security by provisioning food or feed ingredients (Araújo et al. Citation2022; Forster and Radulovich Citation2015; Radulovich et al. Citation2015).
Marine macroalgae, commonly known as seaweed, are considered the “promising plant of the millennium” (Dhargalkar and Neelam Citation2005) because of several advantages over land plants, such as no need for arable land, freshwater, fertilizer or pesticides to grow them, and the biomass can be utilized as food, feed, materials, gelling substances, and biofuels (e.g., Chapman and Chapman Citation1980). Furthermore, macroalgae grow more rapidly and occupy space more efficiently than terrestrial plants (Creed et al. Citation2019). In optimal conditions, macroalgae can produce higher dry biomass per unit area per year than fast-growing terrestrial crops such as sugar cane (Gao et al. Citation1994). Furthermore, macroalgae cultivation may help reduce greenhouse gas emissions in the food system by replacing food, feed, and materials with higher carbon footprints (Troell et al. Citation2022).
Seaweed production and processing can support the blue circular economy by contributing to the key drivers of the circular bio-based economy in the EU (Lange et al. Citation2021), namely bio-based products for health and new functionalities, primary production, land-use change, sustainable agriculture, biorefineries, and biomass supply for new biorefinery technologies. Further, many recent publications have promoted seaweed cultivation to meet many of the United Nations’ global sustainable development goals (UNSDGs), including reducing hunger, improving good health and well-being, providing affordable and clean energy, and mitigating climate change (Duarte, Bruhn, and Krause-Jensen 2022). Despite these potential contributions to the circular economy, seaweed production in Europe is lagging (Araújo et al. Citation2021), and many risks and benefits must be assessed before upscaling macroalgal productions into sustainable seaweed aquaculture. These include (i) food safety considerations in integrated multi-trophic aquaculture (IMTA)/waste streams, (ii) genetic interactions of wild crops with cultivated crops, (iii) impacts of seaweed aquaculture on the surrounding ecosystems, (iv) diseases and epiphytes, (v) area utilization from a marine spatial planning perspective, (vi) threats associated with climate change, (vii) using a precautionary approach during carbon accounting and blue carbon financing, (viii) technological advancement for upscaling, and (ix) overcoming legal and economic constraints (Bermejo et al. Citation2022; Chopin, Citation2021; Cottier-Cook et al. Citation2016; Hasselström et al. Citation2022; Loureiro, Gachon, and Rebours Citation2015; Rosa et al. Citation2020, Citation2019; Stévant, Rebours, and Chapman Citation2017; Troell et al. Citation2022). Although algal cultivation technology has improved in the last decade, there is still a need to optimize production for energy efficiency, product quality, consumer safety, and biomass utilization (Stévant, Rebours, and Chapman 2017). Green algae in the genera Ulva, due to the characteristics described below, show high potential for becoming ideal model organisms for innovative mariculture.
In the last 30 years, Ulva spp. have been extensively analyzed for their value as food, feed, food ingredients (e.g., protein, carbohydrates, pigments, antioxidants), chemical constituents and medicinal properties, and the number of scientific publications involving Ulva has increased from 2130 in 2000 to 6724 in 2023 (Google Scholar). Major advancements have been made in cultivation methods, molecular identification techniques, and in the fields of aqufeed, terrestrial feed, biostimulants, biomaterials, and biorefinery strategies. From a food and feed perspective, green algae in the genus Ulva contain suitable levels of proteins, vitamins, trace minerals, and dietary fibers (Toth et al. Citation2020; Trigo et al. Citation2021; Stedt, Trigo, et al. Citation2022; Stedt, Toth, et al. Citation2022; Steinhagen, Larsson, et al. Citation2022; Steinhagen, Enge, et al. Citation2021; Steinhagen, Enge, et al. Citation2022; Taboada, Millán, and MÃguez Citation2009) for human and animal consumption. The growing world population, environmental awareness and associated increased trends in vegetarianism and veganism, increasing demand for organic products, and global resource shortages are increasing the demand for sustainable marine crops and alternative proteins (Ismail et al. Citation2020; Faber et al. Citation2021; Yong et al. Citation2022; Duarte, Bruhn, and Krause-Jensen 2022). Furthermore, increasing environmental degradation and climate change awareness has actively encouraged health-promoting programs to link human diet and health with environmental sustainability (Patrick and Kingsley Citation2017). Considering that unhealthy diets primarily cause non-communicable diseases (NCDs), which are a leading cause of death (Lauber et al. Citation2020), health-promoting foods and lifestyles have attracted the world’s attention. Indeed, the increase in the consumption of plant (and algae)-derived foods is recommended, as they are usually healthier and more sustainable (Willett et al. Citation2019) protein sources. Nevertheless, the much-cited EAT-Lancet Commission work discussing the need for identifying alternative sustainable food sources in the Anthropocene pays little attention to algae, although aquatic habitats (accounting for 70% of the Earth) will be critical in identifying novel sustainable and health-promoting foods such as seaweed. With an amino acid composition comparable to soy or egg protein, and including all essential amino acids (except tryptophan), selected strains of Ulva bearing high protein contents can partially substitute less sustainable protein sources (Dominguez and Loret Citation2019), and high contents of essential dietary fiber and other bioactive substances render it a beneficial food item providing health and functional advantages (Rajapakse and Kim Citation2011; Holdt and Kraan Citation2011; Lopes et al. Citation2019; Moreira et al. Citation2022; Qi et al. Citation2005). In particular, phytochemicals (e.g., carotenoids, phenolics) have health-benefiting characteristics and can be used in the cosmeceutical and pharmaceutical sectors and as functional foods (Abd El-Baky, El-Baz, and El-Baroty Citation2009; Khairy and El-Sheikh Citation2015; Lanfer-Marquez, Barros, and Sinnecker Citation2005; Mapelli-Brahm et al. Citation2020; Meléndez-Martínez et al. Citation2021; Steinhagen, Enge, et al. Citation2021; Steinhagen, Larsson, et al. Citation2022; Steinhagen, Enge, et al. Citation2022), increasing the economic value of Ulva biomass.
While Ulva spp., and seaweed in general, remains a niche product in Europe, the European market may play an important role in the future of seaweed production and consumption. Currently, the main importing countries of seaweed in Europe are the UK, France and Germany according to the Center for the Promotion of Imports from developing countries (CBI Ministry of Foreign Affairs). Therefore, the aim of this review is to improve awareness of the seaweed Ulva in food, feed, and beyond and to provide a critical review of the current status, challenges and opportunities of incorporating this genus into the mainstream so that it may become tomorrow’s “wheat of the sea.” To this end, Ulva spp. can play a pivotal role in the sustainable and health-promoting food era, and its consumption and applications are expected to increase in the future. The sustainable exploitation of Ulva as food and feed can contribute to the demand for renewable and novel nutritious food sources, especially with vegetarian and vegan protein, emphasized by the UNSDGs (United Nations, Citation2015). Because of its valuable constituent composition, Ulva can be biorefined to obtain and valorize products, including food ingredients, materials, chemicals, and fuels, consistent with the new circular economy paradigm. Of course, consumption of seaweeds in general and Ulva in particular also raises safety concerns as it may entail microbiological or chemical risks.
This review explores contemporary Ulva research through a comprehensive analysis of their distinct chemical composition, production, uses as food, feed, and biomaterials, and other important aspects, such as nutritional value, food safety, and emerging research needs. This review is targeted toward not only scientists and industry professionals working with seaweed-based foods, feeds and biomaterials, but also food scientists, nutritionists, feed manufacturers, dietitians, cooks, and pharmacists less familiar with Ulva as an ingredient or additive. We provide a critical review of the current status, challenges and future needs that are necessary to bring Ulva and Ulva-based products closer to the forefront of food science and nutrition research.
2. Ulva – tomorrow’s “wheat of the sea”
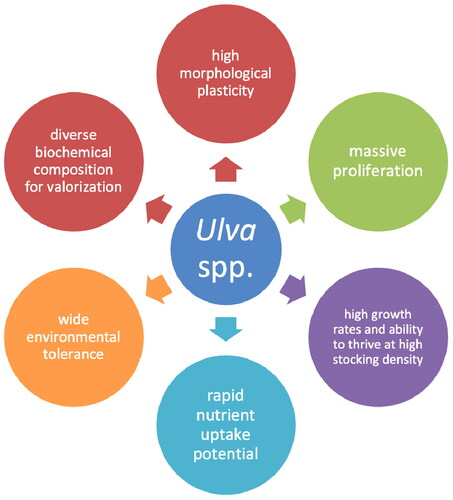
Marine aquaculture is the fastest-growing component of food production (>7%/year) (Lomartire and Gonçalves Citation2022; Duarte, Bruhn, and Krause-Jensen 2022; Moreira et al. Citation2022). Green seaweeds account for <0.1% of the total seaweed production (Bolton et al. Citation2016). Nevertheless, the ubiquitously distributed genus Ulva (Ulvales, Chlorophyta), widely known as sea lettuce, has received increasing attention. The distinct characteristics of the representative species of this ecologically and economically important genus are summarized in , which indicates the immense potential of Ulva species in playing a central role in the rapidly emerging European seaweed aquaculture industry as they can be cultivated in both on- and off-shore conditions (e.g., Bolton et al. Citation2009; Mata et al. Citation2016; Steinhagen, Enge, et al. Citation2021). The most notable characteristics include world-wide coastal distribution, fast growth rates, relatively simple life cycle, ease of culture, historical use in food and feed, documented bioactivity and efficiency as a biological filter (). Furthermore, it is the only species of macroalgae with a sequenced genome, which facilitates genetic transformation and presents the genus as an ideal model organism (Wichard et al. Citation2015; de Clerck et al. Citation2018; Blomme et al. Citation2023; Wichard Citation2023).
Figure 1. Exceptional characteristics of the genus Ulva, demonstrating the reasons for its increased attention in diverse industries. High morphological plasticity: (Blomster, Maggs, and Stanhope (Citation1999); Hayden et al. (Citation2003); Wichard et al. Citation2015; Steinhagen, Weinberger, and Karez (Citation2019); Steinhagen et al. (Citation2023); massive proliferation: (Charlier et al. (Citation2006); Charlier, Morand, and Finkl (Citation2008); Smetacek and Zingone (Citation2013); Gao et al. (Citation2010); Steinhagen Weinberger, and Karez (Citation2019)); high growth rates and ability to thrive at high stocking density: Mata, Schuenhoff, and Santos (Citation2010); Lawton et al. (Citation2013); Al-Hafedh, Alam, and Buschmann (Citation2014); Sebök, Herppich, and Hanelt (Citation2019); Stedt, Toth, et al. (Citation2022); rapid nutrient uptake potential: Gao et al. (Citation2013); Shahar et al. (Citation2020); rStedt et al. (Citation2022); wide environmental tolerance: Toth et al. (Citation2020); Kirst (Citation1990); Ghaderiardakani, Coates, and Wichard (Citation2017); Bao et al. (Citation2022); Thompson & Coates (Citation2017); Ghaderiardakani et al. (Citation2022); Steinhagen, Larsson, et al. (Citation2022); Simon, McHale, and Sulpice (Citation2022); Steinhagen, Enge et al. (Citation2021); Kraft, Kraft, and Waller (Citation2010).
2.1. Ulva taxonomy and identification
For most of taxonomic history, Ulva spp. have been discriminated using detailed morphological (blade shape or structure), anatomical (cell shape and size) and cellular (chloroplast position and appearance and the number of pyrenoids per cell) descriptions (Koeman and van den Hoek Citation1981). However, most species exhibit simple morphologies that are challenging to identify (Steinhagen et al. Citation2023; Hofmann et al. Citation2010; Kraft, Kraft, and Waller Citation2010), particularly due to phenotypic plasticity influenced by environmental parameters (Wolf et al. Citation2012) and the microbiome (Wichard et al. Citation2015). To complicate matters, names initially used to describe European species are now used worldwide for species with different biogeographies, giving the impression that many Ulva species are cosmopolitan (Hughey et al. Citation2019 for the example of U. lactuca (Linnaeus, 1753)). In short, according to the ‘International Code of Nomenclature for Algae, Fungi, and Plants’ (Turland et al. Citation2018), the former Ulva fasciata is now correctly known as Ulva lactuca, and the former Ulva lactuca is now correctly known as Ulva fenestrata (Hughey et al. Citation2019).
The correct identification of both parental wild stocks and cultivated Ulva spp. biomass is necessary, as the traits vary between the species (Fort et al. Citation2019; Olsson, Toth, et al. Citation2020; Olsson, Toth, et al. Citation2020; Cardoso et al. Citation2023), and is particularly important due to their prevalent application in commercial projects and industrial product labeling. In order to identify species and strains of particular commercial value, for example using species selection criteria, DNA barcoding, i.e., the amplification and sequencing of specific loci in the genome, must be used. With respect to EU legislation, the need for DNA barcoding to confirm the identity of commercially produced Ulva material is relevant, because only two species, Ulva lactuca, and the outdated genus “Enteromorpha” (Aonori), are is listed as acceptable non-novel food in the Novel Food catalogue of the Regulation (EU) 2017/2470 (Lähteenmäki-Uutela et al. Citation2021; Bolton, Citation2020; Barbier et al. Citation2019). Thus, several other Ulva species named differently may have been long used as foods and could also qualify for such status (Roleda et al. Citation2021; Barbier et al. Citation2019). Furthermore, several Ulva species are nuisance species in some coastal areas and negatively affect valuable coastal ecosystem functions when introduced to non-native areas (Charlier et al. Citation2006; Charlier, Morand, and Finkl Citation2008; Smetacek and Zingone Citation2013; Steinhagen, Weinberger, and Karez Citation2019; Fort, Mannion, et al. 2020). Therefore, correct species identification is critical to preventing invasive species propagation and introduction through aquaculture initiatives, and an integrative systematics approach is required to accurately identify Ulva spp., considering morphological characteristics, DNA sequencing of different markers, and species biogeography. However, due to a lack of algal barcode sequences from various geographical locations on public repositories (Bartolo et al. Citation2020) as well as sequence misidentifications in herbaria and online databases (Fort, McHale, et al. Citation2022), approximately 24–32% of foliose Ulva spp. in genetic databases are misidentified (Fort, McHale, et al. Citation2022). Thus, the taxonomy of Ulva, including identifying taxonomically valid names, species numbers and their circumscription, must be clarified using molecular methods with globally distributed specimens. Significant research has recently been conducted in Europe using foliose species, and currently both nomenclatural and taxonomic revisions of Ulva spp. are ongoing (Fort, McHale, et al. Citation2021; Fort et al. Citation2022; Hughey et al. Citation2019, Citation2020, Citation2021; Tran et al. Citation2022). Nevertheless, the names of Ulva species presented in this review should be taken with caution unless the authors have provided evidence of molecular identification confirmed by the type specimen. Possible alternatives to molecular approaches and their potential benefits and drawbacks, are comprehensively discussed by Tran et al. (Citation2022). Future investigations would ideally facilitate molecular species identification without requiring sequencing, as proposed by Fort et al. (Citation2021), who employed a restriction digest of the ITS1 PCR product to discriminate between the main foliose Ulva species.
2.2. Ulva production
The FAO database reports data on the production of green seaweed, such as sea lettuce, since 1979. Various denominations can be found, such as bright green nori (Enteromorpha clathrata), green laver (Monostroma nitidum; Monostroma is a green macroalgal genus similar in form to Ulva, but not closely related), lacy sea lettuce (Ulva pertusa), and sea lettuces nei (Ulva spp.). The FAO database records aquaculture of sea lettuce for Ulva spp. in South Africa (3715 t in 2020), Monostroma nitidum in South Korea (8286 t in 2020), and Ulva prolifera (as Enteromorpha prolifera) in China (200 t in 2020). The annual production rates of these taxa are shown in . The Republic of Korea’s 12,965 tonnes of green seaweed cultivation in 2019, including M. nitidum, Capsosiphon fulvescens, and Codium fragile, accounted for 78% of the global production. A recent report assessed that green macroalgae cultivation has recently decreased compared to the peak level of production, which occurred in the 1990s and early 2000s, depending on species (Cai Citation2021). This decrease can be seen in , most notably for M. nitidum in South Korea and U. prolifera in China. The 16,696 tonnes of global green macroalgae production recorded in 2019 (approximately 0.05% of global macroalgae production) was less than half of the peak level in 1992 (38,556 tonnes), as opposed to the rapid growth in the production of brown macroalgae (3-fold) and red macroalgae (15-fold) between 1992 and 2019 (Cai Citation2021; FAO Citation2021). The reasoning behind this is unclear; however, recent statistics show that growth rates of total seaweed production in the leading Asian countries have slowed since 2015, potentially due to climate change, arrival at maximum carrying capacity, changes in marine spatial planning, and the aging seaweed farming workforce (Rieve, Citation2023). The 2,155 tonnes of Ulva produced globally in 2019 was also less than its peak production between 1950 and 2019, with 14,074 tonnes in 2008. The decline primarily reflects the decrease in Ulva prolifera (as Enteromorpha prolifera) production in China from 12,540 tonnes in 2008 to almost zero in 2019, whereas the global Ulva production in 2019 was 2,155 tonnes exclusively from South Africa () (Cai, Citation2021; FAO, Citation2021). Nevertheless, the production numbers reported from South Africa may be overestimated, considering that only 2000 tons/year have been reported by Rothman et al. (Citation2020). Because the Ulva is used as feed on farms rather than sold, production numbers are only estimates. Portugal has reported Ulvophyceae (the class of green algae that includes the genera Ulva and Monostroma) production in Europe since 2014; in 2019, this production reached 35 t (wet weight, FAO estimates). This IMTA-produced Portuguese alga has been identified as Ulva lacinulata (formerly U. rigida), a non-sporulating species that grows well in floating culture and contributes to green tide formation in some regions. Surprisingly, the FAO database does not list any Ulva production from Israel, despite its largest company (Seakura, Israel) producing 50 tons FW/year. Within Europe, a recent assessment of the status of algal production in Europe reported 15 enterprises that cultivate Ulva spp. (Vazquez Calderon and Sanchez Lopez Citation2022). Nevertheless, not all European data are reported in the FAO database, which only includes data from Portugal and Spain. One of the leading European producers produced 150 tons of fresh weight in 2022, which is still only a fraction of the production rate (2000 tons/year) reported from abalone farms in South Africa (Bolton et al. Citation2016; Rothman et al. Citation2020). Considering that other countries may not be disclosing their production rates, the data currently available should be interpreted with caution and regarded as an underestimation.
Figure 2. Global production of green seaweeds reported in the FAO database since the 1970s reported by country; grey, Ulva spp. in South Africa; green, Ulva spp. in Vietnam; blue, M. nitidum in South Korea; orange, U. prolifera in China; not visible due to insignificant amounts: Ulva spp. produced in Portugal and Spain.
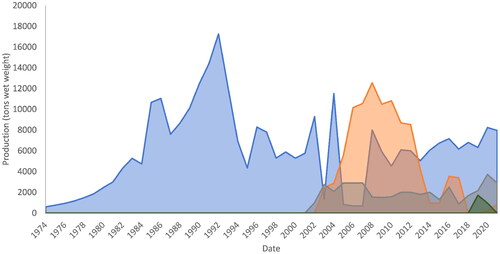
Table 1. Average and maximum global production of green seaweeds by country, 1950–2019 (Cai Citation2021; FAO Citation2022).
2.3. Nutritional content
The keywords “nutrient composition,” “Ulva,” “fatty acids,” “amino acids,” and “minerals” were searched through Scopus and Google Scholar to conduct a review on the nutritional content of Ulva spp. Special care was taken to include the species diversity of Ulva in all available data. The concentration ranges of proteins, carbohydrates, ash, lipids, amino acids, fatty acids, and minerals are summarized in and and Supplementary Tables 1 and 2. In some cases, concentration ranges are provided, as well as seasonal variations when available.
Table 2. Nutritional composition of different species of Ulva.
Table 3. Mineral composition of different species of Ulva.
The biochemical profiles of different Ulva species are characterized by 13–50% ash, 5–27% protein, 0.5–4% lipids, and 53–78% total carbohydrates, of which 37–61% are fiber (data referring to dry weight (DW), ). The protein content in sea lettuce is considered high among seaweeds; for instance, values >20% DW have been reported in Ulva reticulata and U. lactuca (Ortiz et al. Citation2006; Ratana-Arporn & Chirapart Citation2006) and can reach content >30%, more than double the natural content, when produced in nutrient-enriched environments (Viera et al. Citation2011). The levels found in high-protein terrestrial plants, such as soybeans, reach up to 40% DW (“USDA FoodData Central”). To measure the seaweed protein content, the nitrogen factor of 4.76 was used, which provides a lower nitrogen-to-protein ratio value (Angell et al. Citation2016). A conversion factor of 5 has been proposed to measure the nitrogen-to-protein ratio of seaweeds accurately.
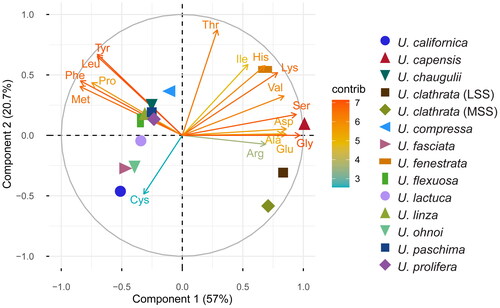
The amino acid profiles of Ulva species are characterized by the predominance of aspartic acid, glutamic acid, and alanine (Supplementary Table 3) (Ferreira et al. Citation2021; Maehre et al. Citation2014; Peña-Rodríguez et al. Citation2011; Shuuluka, Bolton, and Anderson Citation2013). The values presented are standardized to % of protein where possible, but, strong variability between studies and differences in the units reported (% of protein vs. dry weight) make comparisons difficult, suggesting the need for a more systematic, standardized analysis (see future research directions). A principal component analysis (PCA) of the amino acid content of various Ulva spp. from various geographic regions and cultivation conditions showed that the first two components accounted for 78% of the variability. The first principal component was primarily influenced by glycine, serine, leucine, and tyrosine, while the second component was notably affected by cysteine. Cysteine was detected in only some species, resulting in significant separations between them in the PCA. Notably, U. capensis exhibited an exceptionally high concentration of glycine, U. fenestrata showed a high concentration of histidine, and U. clathrata displayed elevated levels of both arginine and glycine (). The analysis also revealed co-occurrence patterns: glycine/serine (positive), leucine/tyrosine (positive), and cysteine/glycine (negative).
Figure 3. A principal component analysis of the amino acid profiles (% of protein) of various Ulva spp. from different geographic regions and cultivation conditions. Data are taken from Supplementary Table 3 and analyzed using JMP® pro v. 17 (SAS Institute Inc., Cary, NC, USA). Prior to analysis, data underwent a log + 0.1 transformation. The “contrib scale” indicates the contributions (in percentage) of the variables to the principal axes. LSS: large scale system; MSS: medium scale system (Peña-Rodríguez et al. Citation2011). Arg: arginine; ala: alanine; asp: aspartic acid; cys: cysteine; gly: glycine; glu: glutamic acid; his: histidine; ile: isoleucine; leu: leucine; lys: lysine; met: methionine; phe: phenylalanine; pro: proline; thr: threonine; tyr: tyrosine; ser: serine; val: valine.
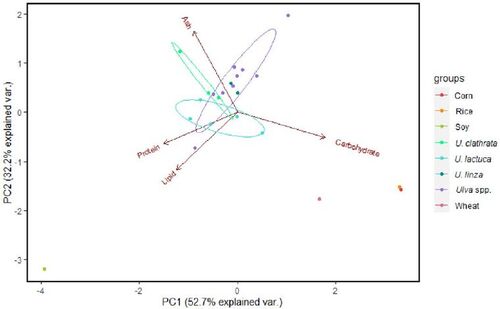
In order to compare the nutritional quality of Ulva spp. to terrestrial plants, we conducted a PCA of the nutritional content of Ulva spp. presented in and the nutritional content of soy, corn, rice and wheat from the USDA FoodData Central website. The results show that the Ulva spp. clustered more closely together than to the terrestrial plants, but all species clustered most closely to wheat, compared to corn, rice or soy (). The protein and lipid content separated Ulva spp. most strongly from soy. The implications of these results are discussed in the section on future research directions below.
Figure 4. Principle component analysis (Martin and Maes Citation1979; Becker and Venkataraman Citation1984; Venables Citation1997) of the nutritional content (ash, protein, lipids, carbohydrates) of different Ulva spp. and soy, corn, rice and wheat. Data for Ulva were taken from as percentages. When single values were missing, mean values were used. Data for soy, corn, rice and wheat were taken from the United States department of agriculture food data Central website (https://fdc.nal.usda.gov/index.html.) as percentages. The analysis was performed using the environment of R (version 4.02.), RStudio (version 2022.02.3). Data were not transformed prior to analysis. Percentages were transformed by arcsine-square-root transformation to correct for deficiencies of the proportions in normal distribution.
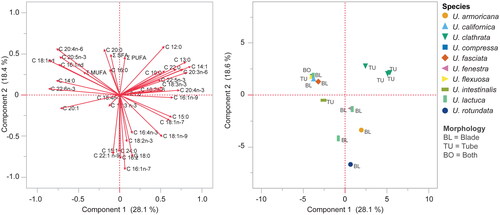
The lipid content of Ulva is relatively low (0.5–4%). The predominant fatty acid is the saturated fatty acid (SFA) palmitic acid (16:0), making up an average of 27.8% in the species presented in this review. Ulva also contains monounsaturated and polyunsaturated (PUFA) fatty acids, particularly the essential linoleic acid (18:2 n-6) and linolenic acid (18:3 n-3) (Supplementary Table 2; Cardoso et al. Citation2017; Maehre et al. Citation2014; Neto et al. Citation2018; Lopes et al. Citation2019). The SFA/USFA ratio of all species presented is below 1 (0.15–0.90), with the exception of cultivated U. clathrata from a large-scale cultivated system, which had a ratio of 2.00. The MUFA/PUFA ratios ranged from 0.3 to 2.02 and the omega 6/omega 3 ratios ranged from 0.2 to 2.7. Wild collected U. fenestrata, U. lactuca and U. rotundata had the highest omega-3 fatty acid content and U. fenestrata and U. rotundata had the highest UFSA/FSFA ratios. A PCA of the fatty acid data, which grouped species by morphology (tubular, foliose, or both), indicated that many blade-forming species clustered together (). On average, these foliose species (blades) exhibited higher total PUFA and SFA levels than the tubular species. Ulva clathrata was distinctive due to its particularly low MUFA content. Ulva lactuca demonstrated high variability, likely stemming from misidentification. Given that U. lactuca is not found in Europe, the data presented by van Ginneken et al. (Citation2011) probably pertain to U. fenestrata, which, as indicated by other studies (Colombo et al. Citation2006; Steinhagen, Kramár, and Toth Citation2022), has notably high PUFA levels compared to other Ulva species.
Figure 5. Principal component analysis (PCA) of the fatty acid profiles (left panel) of various Ulva spp. grouped by morphology (tubular or foliose/blade, right panel). Two species known to exhibit both morphologies were labeled as “both.” data were sourced from Supplementary Table 2 and analyzed using JMP® pro v. 17 (SAS Institute Inc., Cary, NC, USA). Prior to analysis, data underwent a log + 0.1 transformation. Trace values (less than 0.1%) were substituted with 0.001%.
The carbohydrate fraction characteristically contains sulfated polysaccharides, mainly ulvan, representing approximately 18–29% of the DW (Robic et al. Citation2009). Regarding micronutrients, Ulva species contain considerable amounts of potassium, magnesium, calcium, iron, and provitamin A (; Paiva et al. Citation2016). Ulva is also a rich source of bioactive compounds (Dominguez and Loret Citation2019). The large variations reported for different Ulva species and strains ( and and Supplementary Tables 1 and 2), even in samples with slight genetic variations (Fort et al. Citation2019; Ismail and Mohamed Citation2017; Roleda et al. Citation2021) are often due to different geographical locations (Tabarsa et al. Citation2012; Lee, Chang, and Lee Citation2014; Yaich et al. Citation2011; Mamede et al. Citation2021) characterized by different environmental conditions (temperature, irradiance, season, pH, salinity pCO2; Olsson, Toth, et al. Citation2020; Olsson, Toth, et al. Citation2020; Toth et al. Citation2020; Fort et al. Citation2019; Jansen et al. Citation2022; Lawton et al. Citation2021; Queirós et al. Citation2021; Steinhagen, Enge, et al. Citation2022). In addition, differences in the analytical methods contribute to variability, and the need for standardized methods is discussed below. While these variations can be used to produce more homogenous or optimized biomass (e.g., higher protein) in land-based cultivation systems with a controlled nitrogen source, nutrient flux, aeration regime, and other variables (Ben-Ari et al. Citation2014; Diamahesa et al. Citation2017; Shahar et al. Citation2020; Zertuche-González et al. Citation2021), further advancements in strain selection and a better understanding of the genes that control different phenotypes are needed in order to advance and optimize the aquaculture of Ulva spp. (Simon, McHale, and Sulpice Citation2022). Furthermore, the Ulva-associated microbial community (i.e., holobiome) can influence algal growth performances and biochemical content; however, this finding warrants further research (Polikovsky et al. Citation2020).
Despite a plethora of data on nutritional content, identifying species selection criteria for different industries has proved challenging for Ulva spp., mainly because the nutritional content is species and site specific. In general, several foliose species like U. fenestrata (formerly U. lactuca), or Ulva compressa have shown high protein content compared to other species, but the protein levels of a species can be doubled by enriching the seaweed with an additional nitrogen source (e.g., Viera et al. Citation2011). In addition, selective pressure in regions where green tides occur result in fast-growing strains with high pigment and protein content, suggesting that these strains may be particularly useful for aquaculture (Fort et al. Citation2020). Therefore, by optimizing cultivation conditions, isolating fast growing strains and combining future improvements in strain selection and selective breeding, Ulva spp. with optimal characteristics for any industry of interest can be produced, as has already been pointed out by Toth et al. (Citation2020). Nevertheless, it will be important to ensure that the highest quality biomass is reserved for food and feed, while lower quality biomass can be used in other industries, biomaterials and biostimulants, in order to avoid any competition between industries.
3. Ulva for human consumption: a candidate for the food industry and new cuisine
In South Asian regions (China, the Republic of Korea, Japan, and Vietnam), seaweeds have been consumed as foods or to alleviate diverse conditions (such as goiter), in some cases, since ancient times (Mouritsen, Rhatigan, and Pérez-Lloréns Citation2019; Blikra et al. Citation2021). The production and consumption of seaweed continues to be critical in the Southeast Asia (China, the Philippines, Indonesia, the Republic of Korea, and Japan), i.e., aonori in Japan or gamtae in Korea. However, commercial products containing Ulva are not commonly found in European supermarkets, except for regions with a tradition of seaweed consumption, for example in some coastal communities, particularly those with Celtic heritage. The interest in Ulva as food has only recently emerged among seaweed producers and the food industry. So far, many products contain dried or fresh whole algae for a small niche market. Fresh or dried Ulva can simply be used as the main ingredient from home cooking to high-end gastronomy, in dishes such as salads, tempura, or pesto, or can replace other green-leaved vegetables in traditional dishes (Turan and Cirik Citation2018). Furthermore, it can replace or reduce salt as seasoning in food preparations (Magnusson et al. Citation2016; Shannon and Abu-Ghannam Citation2019). However, Ulva’s most promising applications are as an enriching ingredient to profit from its versatile potential, better valorize raw biomass in food, and increase its sustainable impact.
Cereal-based products such as bread, pasta, or crackers are mainly produced from refined flour, being poor in fibers and micronutrients. The enrichment of bread with 1–4% Ulva powder or dried flakes can improve the dietary value without impairing the taste (Cofrades, Serdaroǧlu, and Jiménez-Colmenero Citation2013; Kusumawati et al. Citation2022; Menezes et al. Citation2015; Shannon and Abu-Ghannam Citation2019). Incorporation of either 20% fresh (Debbarma et al. Citation2017; Ainsa et al. Citation2022) or 3% dried (Ainsa et al. Citation2022) Ulva in pasta formulations improves the products’ nutritional profiles with higher fiber content while retaining or improving the taste. Furthermore, Ulva can serve as gluten-free ingredient in pasta or bread and and improves the quality when applied in concentrations up to 2%, (Turuk and Banerjee Citation2023; Yesuraj et al. Citation2022).
Ulva is also included in processed meats such as burgers and sausages and meat replacement products. Supplementation with 1–4% Ulva powder in pork patties produces juicier meat with less cooking loss (Jeon and Choi Citation2012). Similar results have been observed for fish burgers with 2% Ulva powder (Kumarathunge, Jayasinghe, and Abeyrathne Citation2016). Similar to other different seaweed species, substituting approximately 40% of meat content in burgers or sausages with whole seaweed can result in a tastier and healthier product with less fat, increased fibers, and reduced CO2 footprint (Cofrades et al. Citation2017; Cox, Abu-Ghannam, and Gupta Citation2010; Mohammed et al. Citation2022; Peñalver et al. Citation2020; Shannon and Abu-Ghannam Citation2019). Additionally, the increasing demand for meat-free analogs opens further opportunities for Ulva (Yesuraj et al. Citation2022). Furthermore, Ulva enriches dairy products such as probiotic milk (del Olmo, Picon, and Nuñez Citation2019), cheese (del Olmo, Picon, and Nuñez Citation2018), seasoned butter and sauces, spreads, and mayonnaise (Yesuraj et al. Citation2022). Additionally, the addition of Ulva biomass or extracts to the manufacturing process can modify the products’ technological properties. Ulva’s antioxidant capacity has been explored to increase the shelf life of processed meat products (Lorenzo et al. Citation2014; Roohinejad et al. Citation2017) or seafood (Jannat-Alipour et al. Citation2019). However, more research is required to develop products that meet customer acceptance. Finally, the characteristic sulfated polysaccharide ulvan has been explored in food processing for its gelling and water-binding properties similar to other known gelling agents, such as carrageenan and agar, rendering ulvan suitable as an alternative stabilizer replacing gelatin in vegan products (Kraan, Citation2012).
3.1. Food safety
The accumulation of contaminants can occur in seaweeds from the environment or during their production, processing and transformation. These elements may pose potential risks to human and animal health (Guo et al. Citation2023). The most common hazards of concern with respect to food safety are linked to chemical hazards, iodine and heavy metal content found in some seaweed species, based on Reports from the FAO/WHO expert meeting (FAO and WHO Citation2022), the Food Safety Authorities of Ireland (FSAI), the Nordic Council of Ministers (NCM – consisting of Norway, Denmark, Sweden, Iceland and The Faroe Island) and New Zealand (NZFS). Additional risks include pathogenic microorganisms (Salmonella, Bacillus, Norovirus), persistent organic pollutants, radionuclides, biotoxins, and microplastics and nanoplastics. Other chemical hazards include environmental pollutants such as PCBs, dioxins and pesticide residues, but these are not exclusive to food made with seaweed. While there is limited data on the occurrence of hazards in seaweed, with an attendant paucity of legislation on the hazards, there is also currently insufficient data available to suggest that physical hazards like microplastics and nanoplastics pose a significant risk to consumers in general through the consumption of seaweed or seaweed-based foods. In addition, there is currently no Codex standard or guidelines that specifically address food safety vis-à-vis seaweed production, processing and utilization. Both FAO and WHO believe that there is a significant global regulatory gap concerning food safety in seaweed (FAO and WHO, Citation2022; Food Safety Authority of Ireland, Citation2020; Nordic Council of Ministers, Citation2023; New Zealand Food Safety, Citation2023).
The maximum level (ML) for iodine varies significantly among countries. In China and the EU there are no established maximum levels (MLs) except in France (2000 mg/kg dry weight), Germany (20 mg/kg dry weight), and Nordic countries (115 mg/kg dry weight). A high intake of iodine in seaweed within a short period may temporarily induce the reversible Wolff-Chaikoff effect (Guo et al., Citation2023). Recently, Jacobsen et al. (Citation2023) reviewed the mean content of iodine in U. intestinalis, which was higher than in U. fenestrata, 12.4 and 3.2 mg/100 g dried weight, respectively, but not detected in U. rigida. Therefore, iodine levels in Ulva spp. do not pose a relevant threat to human health.
Currently, there are no MLs established for heavy metals such as arsenic, cadmium, lead and mercury in food supplements made exclusively or mainly of seaweed. The EU MLs for lead (3.0 mg/kg wet weight) and mercury (0.1 mg/kg wet weight) are set under Regulation (EC) 1881/2006. The MLs for cadmium were recently lowered by Regulation (EU) 1323/2021 to 3 mg/kg wet weight for food supplements. Jacobsen et al. (Citation2023) also found that theamin content of inorganic arsenic, lead, and cadmium may also constitute a risk for health if seaweed, especially Ulva species, were consumed frequently. On the other hand, in a survey of potentially toxic elements in seaweed in Ireland, Norway and Sweden, Ulva fenestrata (formerly U. lactuca) had the lowest levels of potentially toxic elements overall (Jönsson and Nordberg Karlsson Citation2023).
While data are lacking on the occurrence of hazards in seaweed, there are numerous articles in the literature reporting heavy metal concentrations in Ulva spp. In general, the levels are species and site specific, but a thorough review of the literature on this topic is currently in progress by the current authors. Most of the analyses deal with wild-collected seaweed. However, a few studies suggest that aquaculture-grown Ulva spp. may contain lower levels of heavy metals (Roleda et al. Citation2021) and microbial organisms, particularly if they are grown in land-based systems with artificial seawater (authors own unpublished data).
In conclusion, chemical and biological hazards in Ulva spp. may be present, but data occurrence is scarce and limited, especially for toxic forms of heavy metals. The harmonization of analytical methods to identify and quantify chemical hazards is important and crucial to further assess the risk of these contaminants. Standards and guidelines related to seaweed products can vary nationally, and should be unified at the European level. In addition, it is crucial to increase knowledge on how the processing methods may affect the content of hazards, including the possible and unintentional presence of products or substances with adverse health effects. The impacts of post-harvest processing on biomass quality within the context of food safety are discussed in the section below.
3.2. Post-harvest processing
Freshly harvested seaweed quickly deteriorates unless stabilizing processes are used to avoid rapid biodegradation. If the macroalgae are not immediately stabilized, spoilage bacterial counts rapidly exceed upper limits; molds and yeasts also become problematic. Hazard analysis and critical control points (HACCP) or other national guidelines should be used to identify the critical control points and reduce microbial loads at these points (Adams et al. Citation2021). Processing methods such as washing, blanching, drying, freezing, salting, brining, or fermentation, can be performed to ensure food quality and safety (Blikra et al. Citation2021) and are discussed below.
3.2.1. Washing
Harvested macroalgae should be washed to remove fouling, fauna, sand, and other impurities. This is particularly relevant for Ulva biomass harvested from shallow lagoons or at-sea cultivation, whereas for tank-cultivated Ulva grown under controlled conditions this might not be necessary. For practical reasons, seawater should be used for washing, followed by a brief, final rinsing with freshwater if residual sea salt needs to be removed (Zhu et al. Citation2021) which is essential for the use of Ulva as feedstock. However, extended freshwater washing reduces the ash content in Ulva (Stokvis et al. Citation2021) and remarkably influences the overall quality of U. tepida and U. ohnoi biomass (Magnusson et al. Citation2016). The removal of inorganic minerals in the washing process can be explained by leaching due to osmotic changes, which indirectly increase the organic matter proportion, protein content, and caloric energy by 11–24% and 20–50%, respectively, and can leach other valuable compounds from the biomass, such as ulvan (Magnusson et al. Citation2016).
3.2.2. Blanching
Blanching is commonly used in processing seaweed to (i) reduce microbiological hazards caused by harmful bacteria within the seawater or by post-harvest contamination (Quero et al. Citation2015; Blikra et al. Citation2019; Ho and Redan Citation2022; Løvdal et al. Citation2021) (ii) inactivate inherent enzymes that initiate seaweed tissue breakdown (iii) reduce excess iodine levels, mostly relevant for iodine-rich brown algae (FAO and WHO Citation2022; Nielsen et al. Citation2020; Bruhn et al. Citation2019; Stévant et al. Citation2018) but also in Ulva intestinalis (Nitschke and Stengel Citation2016) (iv) potentially improve the profile of beneficial compounds, including a higher ratio of essential amino acids and a higher proportion of omega-3 fatty acids (Nielsen et al. Citation2020) (v) increase value and consumer acceptability by improving seaweed’s organoleptic quality (Akomea-Frempong et al. Citation2021), such as reducing unattractive fishy odors from U. rigida (Thunyawanichnondh et al. Citation2020) and improving seaweed color (Blikra et al. Citation2019).
3.2.3. Drying
Drying is the most applied method for preservation of seaweed ensuring a long shelf life and enabling the most economical solution for storage and transport. Ulva has the advantage of drying quickly due to its thin thallus structure. For large-scale seaweed harvests, either sun-drying or convective drying is usually applied. Although solar drying is the most sustainable solution, its application may be limited to warm and sunny climates. Furthermore, in its simplest form as unprotected open-air drying, the hygienic quality of the biomass for food might be impaired via exposure to possible airborne microbial and other contamination. Well-constructed solar dryers, however, that protect against contamination, e.g., on racks off the ground, with natural ventilation and roofing, can be suitable for food quality. In regions with insufficient solar exposure, convective oven drying is the method of choice for drying seaweed, although this technology requires certain investments and is associated with high energy costs (FAO and WHO Citation2022; Kadam et al. Citation2015; Santiago and Moreira Citation2020). Other drying methods, such as freeze-drying or microwave-assisted drying, better maintain the bioactivities of nutrients (Amorim, Nardelli, and Chow Citation2020) but they are expensive and technically only suitable for low-volume, high-value commercial applications (Badmus, Taggart, and Boyd Citation2019).
The choice of temperature in convective drying modifies the conservation of nutritional values, bioactive compounds, and antioxidant capacity (Rodríguez-Bernaldo de Quirós and López-Hernández Citation2021); it depends on the specific application of the commercial end-product. Rapid drying at higher temperatures can avoid contamination and oxidation, which may occur with prolonged drying at lower temperatures but might deteriorate heat-labile compounds, particularly certain polyphenols (Badmus, Taggart, and Boyd Citation2019). Silva et al. (Citation2019) investigated different oven-drying temperatures (25, 40, and 60 °C) in U. rigida, Gracilaria sp., and Fucus vesiculosus, suggesting 25 °C a favorable temperature for extracting pigments from Ulva, whereas higher temperatures increase ulvan yield. Interestingly, in contrast to Gracilaria and Fucus, higher drying temperatures (60 °C) did not alter the antioxidant activity in Ulva material. In a study with Chilean Ulva spp., Uribe et al. (Citation2019) compared different drying methods (freeze-, vacuum-, solar-, and convective-drying) and showed the best retained physicochemical parameters and antioxidant capacity after convective drying with 70 °C for 120 min. Regarding the seaweed flavor after drying, semi-drying (using a solar-drying system) releases more flavor compounds, such as free amino acids and volatile compounds, resulting in a better taste than fully dried seaweed. Seaweed maturation, also termed curing or ripening, can also improve seaweed flavor and aroma (Stévant et al. Citation2020). Even before processing, the seawater type and quality significantly influence the seaweed flavor (personal observations by Jessica Adams and Laurie Hofmann), and further research is needed to investigate how seaweed can be grown and processed to optimize taste for the European market.
3.2.4. Salting, brining, and fermentation
A low-cost and low-tech stabilization alternative to thermal processing can be dry salting or brining in a salt solution. Compared to thermal drying, osmotic dehydration using salt allows to retain an almost fresh food matrix while maintaining the nutritional values. Pinheiro et al. (Citation2019) investigated the quality and nutritional parameters of Ulva rigida over six months of storage after air drying, brining in salt solution (25%, w/v), and dry-salting (28 and 49%, w/w); the nutritional parameters remained stable in the salt-treated samples while only showing slight alterations in color and texture. Although this traditional preservation method is not common in the Western seaweed industry, this might be an advantage for certain applications and producers.
Fermentation is another traditional way of preserving crops and ensiling Ulva to simultaneously preserve fresh biomass and to improve the quality of feedstock for biorefinery has been demonstrated before (Wu, Li, and Cheng Citation2018). However, seaweed fermentation technology for food purposes is still an underdeveloped yet efficient method for adding nutritional value by enhancing its digestibility and the bioavailability of bioactive compounds (Wu, Li, and Cheng Citation2018; Campbell et al. Citation2020; Reboleira et al. Citation2021; Strauss Citation2023). In the past decade, seaweed lacto-fermentation has been proposed as a promising new sector in the food industry (Uchida and Miyoshi Citation2013), in which beneficial strains such as lactic acid bacteria (LAB) metabolize carbohydrates into lactic acid and CO2. Consequently, the food is preserved by the acidic environment and simultaneously develops distinctive textures and flavors from other organic acids, flavonoids, and free amino acids (Gupta and Abu-Ghannam Citation2012). Importantly, seaweed fermentation improves organoleptic qualities, which has been shown in brown algae (Figueroa, Farfán, and Aguilera Citation2021), but also in Ulva (Hung et al. Citation2023). The unwanted sea smell that often impedes the culinary acceptance of seaweed can be reduced or omitted by lactofermentation (Hung et al. Citation2023; Bruhn et al. Citation2019; Duarte, Bruhn, and Krause-Jensen Citation2021).
However, despite the high total carbohydrate content of approximately 50% in Ulva, only small amounts of these sugars can be directly metabolized by LAB into lactic acid because the majority comprises uncommon, complex polysaccharides (Hwang et al. Citation2011). Therefore, to achieve successful seaweed fermentation, a pretreatment is required, such as heat treatment or biochemical treatment using saccharifying enzymes. This splits the cell wall structure, which otherwise holds valuable compounds for digestion (Gupta, Cox, and Abu-Ghannam Citation2011; Bruhn et al. Citation2019; Maneein et al. Citation2018; Akomea-Frempong et al. Citation2021). Furthermore, the choice of suitable LAB strains is essential for the success of large-scale fermentation. Currently, commercial applications of lactofermentation remain marginal, but these promising results may encourage further research with Ulva and inspire more food applications.
4. Ulva in aquafeed: status quo, challenges, and opportunities
4.1. Status quo
Aquaculture’s further growth, like that of all livestock production industries, depends on the supply of sustainable feed protein and energy resources and optimizing aquafeed production and use will be crucial to support industry growth. Nutrition is the most expensive aspect of producing edible finfish (Naylor et al. Citation2009). The daily aquafeeds consumed account for approximately 75% of production costs (Lamm, Citation2003), mostly from fishmeal and fish oil, although there have been widespread efforts to reduce these costs in past years. However, due to the rapid growth of the entire aquaculture sector, the volumes of these resources must keep up with the demand for sustainability. Conventional feed resources, such as fishmeal, are expensive, and their future availability will be limited by an expected reduction in fisheries. Therefore, significant effort is being directed at reducing the content of the expensive and unsustainable fishmeal and fish oil in formulated feed, replacing them with other more sustainable nutritional ingredients.
Plant products have become common alternatives to replace fishmeal and fish oil in formulated aquafeeds. Increasing regulations on using animal-derived products in feed (e.g., bone meal) and the relatively low cost of plant production favor using plant meals and oils in aquafeeds (Wan et al. Citation2019; Gatlin et al. Citation2007) However, growing demands for land crops for aquafeed production will increase aquaculture’s dependence on the two exhaustible core resources: land and water, both required for plant production, and will compete with the production of human food. For example, China and Norway have been experiencing a shortage of high-quality proteins from soybean (Nair et al. Citation2023; Kim et al. Citation2019; Lindberg et al. Citation2016), and a larger supply of protein crops is recently needed in Europe. The soy industry is associated with ecosystem degradation, resource depletion, and greenhouse gas emissions in some of the world’s most biodiverse regions. Life cycle assessment studies underline that soy as an aquafeed ingredient is the main contributor to the environmental impacts of Norwegian salmon production (Hognes et al. Citation2012; Ólafsdóttir et al. Citation2013; Anmarkrud Citation2023). Furthermore, the high content of anti-nutritional factors (ANFs), low palatability, continued deforestation, and increasing use of nonrenewable fertilizers threaten the potential reliance on plants for aquafeeds. Thus, for more sustainable global aquaculture, alternative protein and lipid sources with lower costs and ecological footprints must be identified.
The application of Ulva in experimental diets for finfish has become an increasingly widespread method since the study by Nakagawa, Kasahara, and Sugiyama (Citation1987) on U. pertusa in the diet of black sea bream (Acanthopagrus schlegeli). Various diets have been developed and tested on species from different nominal trophic levels, including the strict herbivore Nile tilapia (Oreochromis niloticus), omnivores such as the black, red, and gilthead seabream (Acanthopagrus shlegeli, Pagrus major, and Sparus aurata, respectively), and straight carnivores such as the European sea bass (Dicentrarchus labrax), rainbow trout (Oncorhynchus mykiss), and the Atlantic salmon (Salmo salar) ().
Table 4. Summary of the effect of Ulva-supplemented aquafeed on different cultivated seafood.
Many of these species are characterized by high production yields and relatively significant market share in terms of volume, value, or both (FAO Fisheries Division). Similarly, various experiments have been performed on the king prawn (Litopenaeus vannamei), one of the two most produced species by the shrimp aquaculture industry worldwide (Boyd and Jescovitch Citation2020). The examination of Ulva’s nutritional value expanded far beyond any market size or value threshold with trials on fish like goldfish (Carassius auratus), jewfish (Argyrosomus japonicus), croaker (Larimichthys polyactis), nibbler (Girella laevifrons), snapper (Lutjanus stellatus), sole (Solea senegalensis), and others (). Various studies on finfish have shown that regardless of the fish or Ulva species examined, an inclusion rate of 15–20% in the fish diet is not detrimental to fish growth, feed conversion ratio (FCR), or protein intake (). However, the biological trophic level of the fed fish may determine the nutrient utilization efficiency of Ulva. It is likely that aquafeeds with a lower inclusion rate of 5% is more suitable in nutrition of carnivorous fish (e.g., European seabass, rainbow trout). In contrary, a higher ratio of Ulva may be more beneficial for fish of low trophic level like tilapia, carps and catfish (). Moreover, in a trial with Nile tilapia (O. niloticus), 30% of U. intestinalis in the diet did not affect fish growth performances or feed conversion. Similar results were obtained in a 20-week trial with seabream, where 29.1% of U. lactuca in the aquafeed did not harm the fish’s performance even when accompanied by the elimination of fishmeal from the diet and limitation of the fish oil content to only 0.9% (Shpigel et al. Citation2017). In studies of commercially valuable invertebrates, including shrimp, abalone, sea urchins, and sea cucumbers, the results confirmed the potential to increase Ulva content in aquafeeds by over 20% (). Moreover, commercial production of abalone in Europe (Haliotis tuberculata) and South Africa (Haliotis midae) relies on Ulva and other macroalgae or compound feed as a feeding source, contributing to satisfying growth rates. The significant results of the various trials with fish and invertebrates are promising, as the examined Ulva originated from various genotypes (at least eight species), geographical areas, seasons, and environmental conditions, encompassing wild and cultured Ulva. Moreover, they also provided evidence concerning the potential of dietary Ulva in the long-term nutrition of aquatic animals. For example, the positive impact of U. ohnoi in the diet of Senegalese sole (S. senegalensis) on fillet texture and color was evident six months after transferring fish to a commercial Ulva-free diet (Sáez et al. Citation2020).
4.2. Challenges
Large quantities of Ulva biomass are available worldwide, although their quality varies greatly, and biomass valorization is difficult. Natural stocks display a higher uncertainty of biomass yield and quality than cultivated biomass. The levels of protein and essential amino acids can be relatively low and variable depending on seasonal changes, strain variability, and habitat-related forces like the local salinity and depth, as mentioned above. Contrastingly, algaculture appears to be the future for developing Ulva-based products because of the possibility of controlling and optimizing factors related to yield and quality at different stages of production (Calheiros et al. Citation2021). For example, recent studies with large-scale U. fenestrata cultivation in Sweden showed that feasibility and sustainable potential for large-scale offshore cultivation can be achieved by increasing the seedling density in the hatchery, resulting in higher biomass yield (Steinhagen, Enge et al. Citation2021).
The presence of ANFs also presents a challenge to applying Ulva in aquafeeds. Studies that followed the results from trials with sole and Pacific white shrimp revealed that the observed growth deficits were due to the presence of ANFs in the dietary Ulva (Qiu, Neori, et al. Citation2017; Vizcaíno et al. Citation2020). ANFs in Ulva include alkaloids, tannins, saponins, lectins, polyphenolics, phytic acid, and other inhibitors that reduce the bioavailability and digestibility of algal nutrients (Aguilera-Morales et al. Citation2005). Several surveys have been conducted to analyze potential ANFs in wild Ulva, such as those in green tides (Wu et al. Citation2013; Li et al. Citation2018). Calheiros et al. (Citation2021) concluded that the ANFs of Ulva were lower than that of soybean. However, in another study, high levels of trypsin and amylase inhibitors were observed in the winter Ulva-rich biomass beached at Baja California, Mexico (de Oliveira et al. Citation2009). In addition, anti-nutritional tannins, polyphenolics, and phytic acid were observed in the collected biomass. Although data on the harmless inclusion rate of Ulva in aquafeed can be gleaned from previous studies, the number of specific studies on ANFs in Ulva is negligible. Such studies may provide supportive and applicable information on the factors determining the presence and level of ANFs in the biomass, their active mechanism, the threshold level beyond which they become harmful, and methods to neutralize their activity.
4.3. Opportunities
More empirical knowledge on ANFs in Ulva biomass is expected to become available in the coming years due to the rapid increase in Ulva research and development in fish nutrition. The mechanisms by which Ulva ANFs harm fish can be concluded from studies on homologous compounds such as those derived from plants. For example, agglutinin is a common lectin in various plants, which is also found in Ulva (e.g., U. curvata collected in the US; (Bird et al. Citation1993)). Soybean agglutinin in the diet of Atlantic salmon was associated with high mucus secretion in the intestine, limiting the enzymatic and absorptive capacity (Hendriks et al. Citation1990). Few studies have successfully demonstrated the potential active mechanisms of Ulva ANFs in fish diets (Vizcaíno et al. Citation2020; Vizcaíno et al. Citation2019; Sáez et al. Citation2020; Martínez-Antequera et al. Citation2021). Among these, the bioactivity of protease inhibitors in Ulva ohnoi was confirmed in trials introducing digestive proteases from Senegalese sole, gilthead seabream, or European seabass with the U. ohnoi extract. The U. ohnoi extract inhibited fish digestive proteases at a rate of approximately 70% (Vizcaíno et al. Citation2020). However, the study also revealed that heat treatment of this alga significantly reduces the harmful inhibition to only 20%, but is accompanied by damage to amino acids and bioactive molecules (Vizcaíno et al. Citation2020). Another trial revealed that 5% of heat-treated Ulva sp. in the diet improved gilthead sea bream tolerance to hypoxia by enhancing the antioxidant properties of the heat-treated biomass (Magnoni et al. Citation2017). Post-harvest hydrolysis is also considered efficient in improving Ulva’s nutritional value and digestibility. An improved amino acid profile and higher in vitro nitrogen digestibility were observed in an Ulva extract heat-treated with enzymatic hydrolysis (Bikker et al. Citation2016). The inclusion of Ulva hydrolysate in the diet of European bass improved fish protein utilization without detrimentally affecting growth (Fernandes et al. Citation2022). Nevertheless, as in land crops, improving crude biomass often requires high energy investment, thus compromising the cost-effectiveness and sustainability of such processes.
The economic impact of including Ulva in fish diets has not yet been well documented. However, Shpigel et al. (Citation2017) reported the first calculations of the achievable savings in feed, fish production, and operating costs when culturing seabream on a 14.6% protein-rich Ulva diet (30–36% DW protein). The total feed cost was reduced by $0.25 kg−1. A feed conversion ratio of 1.7 resulted in a cost reduction of $0.45 kg−1 of fish produced. Because fish feed can account for more than 60% of the operating costs in intensive aquaculture, approximately 10% savings on feed costs are economically relevant.
In conclusion, Ulva is a new value-added dietary resource in aquafeed. However, seasonal and species-dependent variability in the nutritional content, ANFs and the lack of commercially sufficient quantities of Ulva biomass are limitations that the aquafeed production industry has yet to overcome (Wan et al. Citation2019). Furthermore, sustainable technologies (e.g., IMTA, integrated marine systems) still need to be improved to enable large-scale commercial production of such important aquafeed resources. However, as Ulva spp. can thrive under high stocking densities, they are exceptional candidates for large-scale production for future aquafeed formulations (Al-Hafedh, Alam, and Buschmann Citation2014; Steinhagen, Larsson, et al. Citation2022). Furthermore, before considering Ulva as a biocircular feed ingredient, further measures are needed to monitor their heavy metal content. Therefore, monitoring and selection programs for harvesting Ulva biomass will provide aquafeed manufacturers with high nutritional value and low content of undesired ingredients.
5. Ulva in terrestrial feed
Approximately 70% of protein, including fish meal, for animal feed is imported into Europe. Increasing food-feed-fuel competition for limited natural resources has threatened future economic and supply chain security. Therefore, developing emerging alternative ingredients for animal feed based on locally available resources is being emphasized to reduce feed costs, improve animal feed self-sufficiency, and maximize the land/water/energy space for agricultural production for human consumption across Europe and the world. The expanding global population and greater affluence are expected to increase global demand for animal-derived goods, which will substantially affect animal agribusiness due to the overuse of maize and soybean crops - the two most important traditional livestock feeds. Consequently, more affordable animal feed components are required. Recently, many studies have been conducted using seaweed as a protein source and nutraceuticals in terrestrial animal nutrition. Based on nutrition science, the nutritive value of seaweeds was too poor to be recommended for livestock during the early twentieth century (Evans and Critchley Citation2014). The prevalence of refractory polysaccharides in seaweed cell walls has anti-nutritional consequences in non-ruminants, such as chickens and pigs, and the digestibility of the proteins is inhibited owing to their entrapment in the cellular matrix. Thus, a corresponding reduction in feed breakdown and uptake by retaining vital nutrients (Øverland, Mydland, and Skrede Citation2019) advocates the use of specialized carbohydrate-active enzymes, widely used as feed additives, or fermentation to improve animal digestion and specific growth rate. For example, Bikker et al. (Citation2016) showed that simulated in vitro ileal nitrogen digestibility was increased from 79.9% in intact Ulva lactuca to 84.7% in the extracted fraction, presumably through the release of cell wall-bound or encapsulated protein during pretreatment hydrolysis. Pretreatment hydrolysis and fermentation may also increase protein digestibility by degrading insoluble fiber (Marrion et al. Citation2003). In addition, different studies have highlighted the capacity of the ulvan to stimulate mucin secretion in the intestinal tract (Barcelo et al. Citation2000). The main findings from studies investigating the impact of Ulva as a feed supplement for poligastric and monogastric terrestrial animals and poultry are discussed below.
5.1. Polygastric terrestrial animals (cattle, sheep, and goats)
To date, studies on using marine algae in bovine, caprine, and other ruminant nutrition have mainly concentrated on adding small amounts of various marine algae species to the feed and then evaluating their prebiotic activity for improved animal performance (Morais et al. (Citation2020); the information is scarce). The main drawbacks to using U. lactuca in ruminant diets are frequently cited as their low dry matter and high ash content (Tayyab et al. Citation2016) and low effective degradability (41% for organic matter degradability) values (Arieli, Kissil, and Sklan Citation1993). The high mineral content of seaweeds restricts their net, digestible, and metabolizable energy values and gross energy content. Ulva has been regarded to be nearly equivalent to a low-to-moderate quality forage and ideal to be used with feeds that have a high energy/low protein content like cereal crops (Arieli, Kissil, and Sklan Citation1993).
Samara et al. (Citation2013) reported that 3%–5% (DM) of Ulva lactuca could be safely supplemented to lambs without negatively affecting blood water balance, liver, or kidney functions. However, feeding rams a lamb diet supplemented with intact Ulva lactuca did not positively affect growth performance, thermoregulatory responses, or plasma oxidative status, whereas feeding lambs intact Ulva lactuca negatively affected rams’ seminal and testicular characteristics, which were more pronounced at 5% than at 3%. Male lambs can consume up to 20% U. lactuca in a diet composed primarily of vetch hay or concentrate without negatively affecting the diet’s flavor. It had a moderate energy digestibility (60%) and a low (40%) protein degradability (Arieli, Kissil, and Sklan Citation1993). El-Waziry et al. (Citation2015) reported that U. lactuca supplementation did not affect sheep growth, in vitro gas production, potential degradability, estimated energy, organic matter digestibility, or microbial protein synthesis. Furthermore, Ventura and Castañón’s (Citation1998) concluded that U. lactuca is a high-protein, medium-quality forage for goats.
In a different study (Rey-Crespo, López-Alonso, and Miranda Citation2014), adding a seaweed blend (Ulva rigida, Laminaria ochroleuca, Saccharina latissima, Saccorhiza polyschides, Mastocarpus stellatus, and Sargassum muticum) to the diets of dairy cows at a rate of 100 g/cow/day increased the amount of iodine in the milk. This suggests that incorporating seaweed into the diet is a viable method of increasing iodine levels in milk.
Although various results have been reported with variable effects on rumen fermentation, Ulva has shown promising potential for reducing ruminal methane production in vitro (Dubois et al. Citation2013; Machado et al. Citation2014; Kinley et al. Citation2016). When incubated at 25%, Ulva sp. significantly decreased methanogenesis from 101 mL g−1 DM to 86.2 mL g−1 DM compared to the control; however, the effect on methane production depended on the substrate because all seaweeds decreased methane production when combined with hay, but only Gigartina sp. reduced methane production when incubated with corn silage (Maia et al. Citation2016). Recently, Ulva sp. was the only macroalgae species tested that did not reduce methane production (Mihaila et al. Citation2022), suggesting that more research is needed to reach a more conclusive result regarding the impact of Ulva spp. on methane production.
5.2. Monogastric terrestrial animals (swine, cuniculus)
Recently, seaweeds have been included in low amounts (1–2% for the potential benefits to pig health and meat quality (Mišurcová, Citation2011). A new feed supplement containing Ulva enriched with Zn (II) and Cu (II) as a dietary source of microminerals for pigs showed higher bioavailability than an inorganic salt control when fed to piegs (Michalak, Chojnacka, and Korniewicz Citation2015). Furthermore, an algae–clay-based complex made from Ulva sp., Solieria chordalis, and montmorillonite clay increased the ileal digestibility of energy and essential amino acids when added to the diets of growing pigs (Suarez and Gallissot Citation2016). A study using an in vitro system of porcine intestinal epithelial cells showed that ulvan from Ulva armoricana upregulated the gene expression of cytokines such as IL1, IL6, IL8, and TNF (Berri et al. Citation2017, Bikker et al. Citation2016). The immunomodulatory effect of Ulva armoricana was evaluated in sows by Bussy et al. (Citation2019). The higher dietary level increased anti-Bordetella IgG in the sow’s blood and colostrum, whereas with the middle dietary integration, IgA increased in milk. Thus, based on digestibility, Ulva may be a better feed ingredient for pigs than for poultry (see below), whereas the extracted fraction seems a promising ingredient for further evaluation in both organisms. Based on the essential amino acid content and in vitro nitrogen (85%) and organic matter (90%) digestibility, the extracted fraction seems a promising protein source in diets for monogastric animals with improved characteristics compared to intact Ulva (Bikker et al. Citation2016). Furthermore, a recent study has found that enzymatic supplementation with carbohydrase, such as the recombinant ulvan lyase, may exacerbate the indigestibility effects observed from feeding U. lactuca alone to piglets (Ribeiro et al. Citation2024).
Studies investigating the effect of Ulva-supplemented diets on other terrestrial animals are rare; however, meal supplemented with low amounts (1%) of Ulva have shown positive effects on the growth performance and diet digestibility in rabbits, with no negative hematological or biochemical effects on rabbit health (El-Banna et al. Citation2005).
5.3. Poultry
Several studies have investigated the influence of Ulva spp. as a feed additive on chicken development and/or carcass characteristics (Matshogo, Mnisi, and Mlambo Citation2020; Abudabos et al. Citation2013; Nhlane et al. Citation2021; Thavasi Alagan et al. Citation2020; Ventura, Castañon, and McNab Citation1994). Most studies have shown that Ulva spp. can be used at low inclusion levels (<10%) without any suppression in the growth performance of chickens. Green seaweed meal derived from U. lactuca at 0, 2, 2.5, 3, and 3.5% had no adverse effect on the growth performance, visceral organ size, carcass characteristics, and meat quality of indigenous Boschveld chicken (Nhlane et al. Citation2021). Similarly, the addition of Ulva-based green seaweed meal (0, 20, 25, 30, and 35 g kg−1) had no significant effect on the growth performance of broiler chickens; however, increases in various meat shelf-life indices were observed (Matshogo, Mnisi, and Mlambo 2020). Furthermore, corn can be replaced with 3% U. lactuca without affecting growth performance while improving the dressing percentage and breast muscle yield (Abudabos et al. Citation2013). In addition, broiler diets can be supplemented with U. lactuca without affecting health and growth performance (Nhlane et al. Citation2021). Ventura, Castañon, and McNab (Citation1994) concluded that feeding U. rigida beyond 10% in the diet reduces the feed intake and suppresses the growth performance of broiler chickens, suggesting that Ulva supplementation levels should remain below 10%.
Using Ulva spp. in chicken feed at high concentrations is likely restricted because fiber fractions of indigestible algal cellulose and hemicelluloses (Øverland, Mydland, and Skrede 2019), which primarily consist of gel-forming ulvan and insoluble cellulose, as well as trace amounts of glucuronans and xyloglucan (Lahaye and Robic Citation2007), may impair nutrient digestibility, suppressing growth performance (Kraan, Citation2012). Enriched ash content can also make seaweeds inappropriate for animal diets, especially monogastric animals, at higher concentrations. In contrast, microalgae have been suggested to be a promising alternative feedstock for livestock and poultry (Saadaoui et al. Citation2021). Nevertheless, the scale and cost of production needed and potential competition with human consumption present limitations for microalgae in feed as well. The major benefit of using macroalgae for feed is that biomass from algal blooms which is unsuitable for human consumption could be valorized for feed. Therefore, advanced procedures, which can decrease ash content and improve digestibility are needed to improve the acceptable feed components from Ulva spp.
Digestibility can be improved by enzymatic hydrolysis with appropriate agents for seaweed species since the chemical makeup of seaweeds is different from that of terrestrial plants. Carbohydrate-active enzymes (CAZymes) have emerged as a promising alternative to destroy the Ulva spp. cell wall due to the efficacy of these enzymes in hydrolyzing Ulva spp. material for protein and carbohydrate extractions (Batista et al. Citation2020; Postma et al. Citation2018). Moreover, CAZymes have demonstrated carbohydrate action in microalgae cell walls (Coelho et al. Citation2020). Thus, the degradation of seaweed biomass with feed enzymes would optimize their utilization as feedstuffs to partially replace unsustainable and conventional sources, such as maize and soybean meal (Costa et al. Citation2021). Costa et al. (Citation2022) conducted a trial on broiler chickens to study the influence of U. lactuca (15%) with and without enzyme addition (carbohydrase), indicating that 15% U. lactuca resulted in no harmful effects on growth performance and improved meat quality because of antioxidant influence, mineral, and PUFA (n-3) accumulation. Another study assessed the pretreatment of edible seaweed (Ulva spp.) with a mixture of proteolytic and fibrolytic enzymes on the physical and meat quality characteristics of broiler chickens (Matshogo, Mnisi, and Mlambo Citation2021), demonstrating that seaweed pretreatment with the enzyme mixture did not affect feed consumption, physiological responses, and carcass characteristics of broiler chickens.
In contrast, a co-product of Ulva laetevirens (synonomous with U. australis) exposed to a wide endo-protease supplementation in a broiler’s diet showed no distinct effect on their growth performance, whereas protease pretreatment masked or suppressed the health-promoting bioactive substance of U. laetevirens (Stokvis et al. Citation2022). Ulva laetevirens improved the feed conversion rate of broiler chickens and reduced the digestibility and villus height, whereas protease pretreatment failed to improve growth performance and other health-related traits. It is critical to obtain a better knowledge of how the nutritional components of Ulva spp. behave in in vitro digestibility tests and the digestive tract of broiler chickens to explore the impacts of Ulva inclusion in poultry diets.
Given the protein content of wild Ulva, it can be concluded that wild Ulva is not a good source of protein (compared to any major vegetable protein source used in animal diets) and the presence of structural carbohydrates (usually referred to as fiber) limits the use of Ulva in diets for monogastric animals (poultry and pigs). Nevertheless, Ulva can be used in ruminant (cow, sheep, and goat) diets, however, studies are still underway since the fate of sulfonated carbohydrates is not yet known.
6. Beyond food and feed – Ulva in biomaterials
In 2019, the seaweed hydrocolloid industry was valued at 1.74 billion USD. Nearly 50 years of seaweed bioprospecting have resulted in over 3,000 marine natural products or bioactive molecules from seaweeds (Ferdouse et al. Citation2018). While green seaweeds do not contribute to the hydrocolloid industry and only approximately 8% of the marine natural products or bioactive molecules come from green seaweeds (Ferdouse et al. Citation2018), they still demonstrate significant potential in biomaterials industries, particularly due to the presence of ulvan. Ulvan has attracted increasing attention in the last decade, considering that less than five papers on ulvan were published annually during 2000–2009, but more than 35 papers were published in 2019 (Cindana Mo’o et al. Citation2020). The benefits and potential of ulvan in diverse biotechnical applications have already been thoroughly reviewed by several authors (Kidgell et al. (Citation2019) for review); therefore, ulvan was not the focus of this section. However, we provide insights into the potential use of Ulva in other biomaterials, including packaging, which is comprehensively discussed below.
6.1. Packaging (packaging films and packaging materials)
The new circular economy plan, which is pivotal in the European Green Deal, EC’s agenda, and UNSDGs for sustainable growth, includes designing sustainable products, empowering consumers, and improving reuse in production processes. Some key product value chains include packaging, plastics, textiles, construction, food, water, and nutrients. Thus, to meet these initiatives and those for protecting and restoring the marine environment, new production concepts are required to address the growing demand and provide sufficient quantities of high-quality materials and food in the future (Eroldoğan et al. Citation2022). Over half of the plastics made are only used once (Gross, Citation2017), and over 99% of plastic packaging is produced from petroleum-based sources (Arrieta et al. Citation2017). Rethinking and redesigning sustainable packaging materials using natural resources to replace single-use plastics will be essential to achieving the new circular economy action plan (European Commission Citation2022). Marine resources, including seaweed, provide an enormous pool of yet unexplored and potentially valuable resources for producing diverse biomaterials, including packaging, and interest is growing in the use of macroalgae in the packaging industry. The global seaweed-based packaging market is expected to account for $613.42 million USD by 2029 (Data Bridge Market Research). Diverse types of films for food packaging have recently been developed (Abdul Khalil et al. Citation2017; Amin Citation2021; Gomaa et al. Citation2022; Ganesan, Shanmugam, Palaniappan, et al. Citation2018) from macroalgae-derived sources. Considering Ulva, most packaging biofilms are produced by extracting ulvan and combining it with a plasticizer, such as polyethylene glycol (PEG), sorbitol, or glycerol (Davoodi, Milani, and Farahmandfar Citation2021; Guidara et al. Citation2019, Citation2020). In some cases, ulvan is combined with another natural material, such as cellulose extracted from the same species (Gomaa et al. Citation2022) or red algal polysaccharides (Ganesan, Shanmugam, and Bhat Citation2018; Ganesan et al. Citation2018). In many cases, ulvan addition to the packaging film increased the film’s antioxidant activity (Amin Citation2021; Gomaa et al. Citation2022; Ganesan et al. Citation2018), suggesting that such seaweed-based packaging films may increase the shelf-life of certain packaged products.
Currently, most packaging products are produced from brown or red algae (e.g., products from Evoware (biodegradable, edible seaweed-based packaging), AMAM (agar plasticity), Ooho (edible water packets from seaweed extracts), Loliware (seaweed-based cups and straws), Algopack (brown-algae based bioplastics), Kelpn (kelp-based bioplastic), and Janoodam (seaweed-based bowls and lids)). To our knowledge, the only known Ulva-based packaging products are produced by the company Eranova (https://eranovabioplastics.com/technology/?lang=en), by enriching the content of starch, which is then extracted using an enzymatic cracking technique and then processed into biodegradable or durable bioplastic. However, some of the authors of the current work are presently conducting research to develop a sustainably produced, biodegradable, edible seaweed-based packaging material for the fast-food industry using mixtures of macroalgae biomass. This process requires no extraction, and is essentially waste-free. An initial screening of local seaweed species for packaging functionality led to testing and cultivating several species for seaweed-based packaging. Depending on the type and mixture of seaweed used and the applied preparation technologies (e.g., grinding), different material properties of the produced macroalgal-based packaging can be achieved. For example, the particle size distribution and film-forming abilities of the packaging material vary and have different material characteristics, such as lower or higher porosity, thicker and stronger films, or different material strengths. In external beta testing of the prototype with food products (baked fish and potato salad), 79–91% of customers rated the edible macroalgae packaging as good to very good (Bosse and Hofmann Citation2020). Based on these results, different types of packaging material can be developed for different functional uses, including in industries outside of food, by altering the seaweed species and their respective combinations. To our knowledge, this is the only seaweed-based packaging that uses 100% of the seaweed biomass and requires no extraction method. Further trials are currently underway to test different methods of packaging production from seaweeds (e.g., fiber casting or natural fiber injection molding). Nevertheless, one of the biggest hurdles, as in other industries, remains to be the processing and transport step from raw biomass to packaging production. The properties of Ulva that are conducive to packaging production (e.g., high cellulose and starch content) also make the seaweed a potential candidate for paper production. A recent study has shown that Ulva biomass can be successfully incorporated into filter paper at a concentration up to 4% without weakening the strength of the fiber network, and successfully removed pollutants (Cr, Cu, total Fe and Zn) due to its adsorbant properties (Caprita, Ene, and Cantaragiu Ceoromila Citation2021).
6.2. Plant biostimulants
Biostimulants promote plant processes, including nutrient uptake efficiency, stress tolerance, and crop quality (Regulation (EU) 2019/1009 of the European Parliament), and reduce the demand for inorganic inputs (Shukla et al. Citation2019). Currently, macroalgae are involved in 37% of the biostimulant market (Rouphael and Colla Citation2018) and contain classes of compounds, such as betaines, phenols, and polysaccharides, which are biostimulants (Stengel, Connan, and Popper Citation2011). The main temperate macroalgae-sourced biostimulant products (e.g., Maxicrop) are almost exclusively produced from the brown wild-harvested species Ascophyllum nodosum (www.solabiol.com/en/principles-maxicrop). In addition, Ecklonia maxima is used to produce the biostimulant Kelpak (https://www.kelpak.com/eckloniamaxima.html), which is exported globally. The red, tropical macroalgae Kappaphycus alvarezii has also been extensively studied (Kumar et al. Citation2020; Sahana et al. Citation2022; van Tol de Castro et al. Citation2023; de Araújo Amatuzzi et al. Citation2020) and is also commercially available as a biostimulant (e.g., www.prospersea.com).
Ulva spp. also have biostimulant properties, although there are little studies to date for green macroalgae compared to brown and red macroalgae. Ulva extracts improve salinity stress response (El Boukhari et al. Citation2021; Hussein et al. Citation2021; Latique et al. Citation2021), drought tolerance (Li et al. Citation2020), growth (Castellanos-Barriga et al. Citation2017; Hassan et al. Citation2021; Hussein et al. Citation2021; Mendoza-Morales et al. Citation2019; Michalak et al. Citation2016; Osuna-Ruíz et al. Citation2023), antioxidant activity (Osuna-Ruíz et al. Citation2023; Ennoury et al. Citation2022; Latique et al. Citation2021), and plant flavors (Paulert et al. Citation2021). Using Ulva spp. extracts as a biostimulant source increases crop yield and contributes to global food security while also creating another product route for some biomass produced during green tides (Shefer et al. Citation2022). While first plant trials using biochar produced from Ulva spp. have shown variable effects on plant growth (Kenneth et al. Citation2022; Roberts and de Nys Citation2016), this research field is still in its infancy, and finding solutions to reduce the sodium content in seaweed-based biochar will be a challenge for the future.
6.3. Biorefinery
Biorefineries use a biomass processing approach that facilitates the production of several value-added products from a given biomass feedstock (crops, lignocellulosic biomass, seaweed, microalgae, and insects) and ensure the full usage of resources, leading to zero waste and minimal greenhouse gas emissions (Barragán-Ocaña et al. Citation2023). By producing multiple products from the same raw materials, biorefineries increase the revenue per mass of feedstock, and diversify applications, making the whole system economically resilient (Golberg et al. Citation2020). The co-production of multiple products usually requires multiple subsequent processing steps produces one or several different products (Golberg et al. Citation2020). In an Ulva biorefinery, the separation of salt, cellulose, ulvan, starch, proteins, lipids, simple monosaccharides, and peptides has been reported in various process configurations (). High protein yields from Ulva can be obtained after extracting salt and ulvan from the seaweed (Magnusson et al. Citation2019), and extracting protein and water-soluble molecules in the first step increases the content and purity of residual water-soluble polysaccharides such as ulvan (Golberg et al. Citation2020). This is critical because achieving a certain level of concentration and purity is a major economic and technological hurdle for biorefineries. In addition, certain processes, such as crushing, milling, and other cell and tissue disruptions, usually benefit most subsequent processes by improving the biomass contact surface and the accessibility of intracellular components without intermediate drying. Therefore, the energy and cost of such processes benefit the entire processing chain rather than a single product (Zollmann et al. Citation2019). Although thermochemical approaches using high temperatures and solvents continue to be used at industrial scales to obtain different seaweed-derived products, more environment-friendly techniques to better preserve the product’s quality and functionality are emerging, including enzyme-, microwave-, or ultrasound-assisted extraction, supercritical fluid extraction, pressurized solvent extraction, pulsed electric fields, or ohmic heating (Matos et al. Citation2021).
Table 5. Ulva biorefinery. Co-production of various ingredients from Ulva and their transformation to additional products.
6.4. Knowledge gaps and future challenges
Currently, biorefinery design is a major challenge, i.e., choosing the processes and equipment and integrating them into one optimized process flow (Golberg et al. Citation2020) because biomass processes and equipment are usually designed to solely produce one product and not preserve the byproducts for subsequent processing targeting zero waste. Currently, there is no widespread and successful standard for industrial seaweed biorefinery design. Polysaccharides have been identified as the most common potential product from Chlorophyta (mainly Ulva spp.), accounting for 40% of the applications. Protein was second (21%), with lipids and pigments considered less frequently (Joniver et al. Citation2021). Future steps for the development of an Ulva biorefinery should include the demonstration of using common food and chemical industry methods for processing and equipment that can be scaled. These efforts will require mass and energy balances for each step. The subsequent steps should include process simulation using common industry tools, such as AspenPlus (https://www.aspentech.com/en/products/engineering/aspen-plus) or SuperPro (https://www.intelligen.com/) process design software, enabling economic and life cycle analysis of processes and plants. Further steps should include an integrated process demonstration for assessing product safety, stability, final applications, and complete economic and environmental analysis. This step will require the collaboration of biomass producers, process engineers, and environmental economists.
7. Patents of Ulva-based products and technologies
The diversification of macroalgal applications, including Ulva species, in increasingly sophisticated products, is accelerating the emerging macroalgal biotechnology patent market. The rate of patent registrations for seaweed-related products has been steadily increasing annually by approximately 11% (Mazarrasa et al. Citation2014). Similarly, increased Ulva production and demand for natural products have promoted research and development in Ulva biotechnology. Consequently, patents for processes and applications of Ulva have rapidly grown. Since 2014, 405 Ulva-related patents have been registered, representing 5.4% of seaweed-related registered patents (Mazarrasa et al. Citation2014). A more recent search using the keyword “Ulva” on the European Patent Office (EPO) website (https://www.epo.org/searching-for-patents.html) resulted in 2,954 patents related to Ulva-based products, which are classified under nine different codes according to the International Patent Classification (IPC) System (Supplementary Table 2). Of the classified patents, Ulva-related patents are distributed into categories relating to medicine (31%), cosmetics (31%), feed (19%) and modifications of nutritive qualities of food and dietic products (10%), revealing that Ulva-related patents in the food industry are lagging behind the medicine, cosmetics and feed industries.
8. Future research directions
Several technical and environmental challenges must be overcome to fully capture and realize the potential of Ulva biomass in the European circular economy. In fact, this is one of the main goals of the recently established COST Action CA20106 SeaWheat, which has resulted in the establishment of the network of authors who have collaborated on this manuscript. For successful large-scale production of Ulva spp. for the food, feed, and biomaterials industry, several challenges must be overcome through research, innovation, knowledge transfer and collaboration.
Green seaweed species misidentification inhibits production data analysis, making a thorough overview of national and global productions difficult. Ulva species must be correctly identified owing to their popular application in commercial projects and industrial product labeling. Consequently, an integrative systematics approach is required to accurately identify Ulva spp., considering morphological characters, DNA sequencing of different markers, and species biogeography. Such scientific advances will contribute to a better understanding of the Ulva biomass produced, allowing the development of a management plan and policy framework adapted to the specificity of this species’ clades. Nevertheless, it is unrealistic to expect that existing and future producers of Ulva biomass will have the time and resources to double-check the identity of their product using genetic sequencing methods. In order for the Ulva-based industry in Europe to grow, new methods need to be developed that simplify this task, such as the sequencing-free assay for foliose Ulva species identification developed by Fort et al. (Citation2021). Such technological advances will provide quicker and cheaper tools that will enable producers to guarantee the identity of the products they are selling.
Likewise, not all Ulva strains and species with economic potential have been identified, and further research into fine-tuned selection for specific applications is required. Additionally, population, strain, and environment-induced chemical variations complicate the development of chemically consistent biomass, which is key for developing an industry striving for nutritionally valuable ingredients or bioactive components for high-end uses. Correct identification of Ulva spp. would also contribute to a better understanding of the effect of genetic and environmental parameters on their composition, growth, and reproduction in their natural and controlled environment. Future research must therefore focus on harnessing the benefits of both genetic and environmental factors in order to support growth of the Ulva cultivation industry. This includes identifying robust strains (e.g., green tide strains Fort, et al. (Citation2020)) with exceptional growth rates and/or high temperature tolerance, and optimizing the cultivation conditions in order to produce biomass with the desired traits (e.g., high proteins or high lipids). Future monitoring and selection programs are necessary to support these efforts and provide food and feed manufacturers with high quality biomass. As a first step toward closing these knowledge gaps, the SeaWheat COST Action has initiated a European-wide collection campaign to measure the nutritional content and microbiome of genetically barcoded Ulva species throughout Europe using standardized methods. These data will provide the first coordinated, European-wide effort to assess the species diversity and the assosiated biochemical and nutritional profiles and epibiontic microbial interactions of Ulva spp. throughout Europe.
Additionally, the effect of associated microbiota on Ulva must be further investigated. The close association between bacteria and the development and morphology of Ulva biomass has been demonstrated; however, further interactions within the seaweed holobiont could provide clues for manipulating desired traits in the seaweed. For example, current research by the authors is showing that protoplast development in Ulva spp. can occur naturally. While we do not yet know what triggers the natural production of protoplasts, if we could induce the production by manipulating the microbiome or other environmental factors, then this would provide a cheap and simple method to vegetatively produce large amounts of biomass, without relying on the expensive enzymes that are currently required to induce protoplast development in Ulva spp.
Sustainable algal biomass production should include safe approaches, ensuring no harm to natural environments and ecosystems, including wild seaweed populations. Recently, three major challenges were identified for the expansion of seaweed cultivation in temperate environments: global climate change, limited carrying capacity in areas where large-scale cultivation already occurs, and the increasing cost and age of the seaweed labor force (Rieve, Citation2023). In order to overcome these challenges, the availability and suitability of areas for sea-based cultivation must be accounted for in marine spatial planning and form a beneficial component in coastal conservation, though restrictions on infrastructure may occur. The land-based cultivation approaches must be energy- and cost-efficient, based on full life-cycle assessment and implementation. Furthermore, significant investments must be made in the preservation of seaweed strains for preserving biodiversity, strain selection and selective breeding campaigns for the development of robust strains. Several initiatives have begun to support these efforts among European (SeaStrains), American (SugarKelpBase) global (Global Seaweed Coalition), and Asian networks.
The general principles and requirements of seaweed food safety in the EU are subject to the EU-enforced Regulation (EC) number 852/2004 on food hygiene. In many countries, the food manufacturing process is subject to the HACCP assessment, a system adopted by the WHO and the Codex Alimentarius Commission as recommended international code of practice for general principles of food hygiene. The regulation (EC) 68/2013 defines the use of algae as accepted feed materials. Green seaweeds are solely mentioned in the case of seaweed meals. No specific mentions are made for Ulva species. Whether offered live or processed (chilled, frozen, or dried), the compulsory declarations are the contents of crude protein, crude fat, crude ash, and iodine, which should be <100 ppm. However, considering the new market trends and processing technologies of seaweed feed and food products, guidelines and legislation on specific seaweed feed and food products remain lacking. Furthermore, whether legislation from one part of the world can be transferred to other areas without considering the biological (seaweed and microbial flora) and environmental (climatic) factors is doubtful.
Although societal acceptance of algal products has increased in Europe recently, further progress is needed to replace conventional biomass or nutritional constituent sources with plant and algae-based ingredients. Clear regulations on quality control relating to environmental contaminants, such as heavy metals and potential pathogens, must be implemented while ensuring the satisfaction of regulatory bodies, consumer safety, and medium- and large-scale practicability for industries. Quality control of strain/biomass origin and chemical composition must be assured based on standardized methodologies. Currently, the European Committee for Electrotechnical Standardization (CEN) is developing standards for algae and algae-based products, representing a major step toward realizing this potential. EU regulatory bodies, including EFSA, must review current restrictions on algae-derived products to support the effective integration of Ulva (or other seaweed) utilization in the European circular economy. Therefore, the risks related to feed and food safety and the possible environmental contamination from aquaculture, among other potential sources of pollution, represent important issues that require special attention from scientists and policymakers concerning the safe use of Ulva spp. in food and feed products.
Ulva species produced in land-based systems under a controlled environment would present easily manageable food and feed safety risks. The EU has also listed organic regulations (Council Regulation 834/2007) that classify farmed or wild-collected seaweeds, including Ulva species, as organic. However, when produced under IMTA, more data and scientific-based evidence would be needed relating to the risk posed by the production of low-level trophic organisms using different waste streams and processes. Such information would also help in developing the lacking regulations appropriate for IMTA products. Further, the wild-harvested biomass should be closely monitored because heavy metals, associated pathogens, and other persistent environmental or anthropogenic pollutants can pose risks stemming from seaweed consumption.
Using Ulva spp. and its derivative bioactive compounds in feed and food can be challenging as many of the compound’s bioactivity remains unrecognized. Furthermore, high polysaccharide content and non-protein nitrogen are problematic and reduce digestibility. Non-starch polysaccharides (e.g., ulvan) also trap about 5% of the amino acid in the biomass. Solutions such as fermentation to improve digestibility, extended fresh water washing to reduce ash content, and biorefinery approaches for producing bioactive extracts for feed additives have all shown promising results. Nevertheless, further research is required to provide supportive and applicable information regarding the factors determining the presence and level of ANFs in the biomass, their active mechanism, the threshold level at which they are harmful, and methods for neutralizing their activity. Such research will contribute to defining the safe yet bioactive level of inclusion of Ulva ingredients in feed and food formulation. Further, when used as food or feed ingredients, the interactions between Ulva or its derivative ingredients with the matrix should be researched to ensure its safe utilization or conservation of the bioactive characteristics in the formulated feed or food. Furthermore, studies addressing the effect of culinary treatments on the levels of contaminants and bioavailability of health-promoting compounds are also needed.
The major challenge that currently hinders the widespread integration of Ulva into feed and food is competition with soybean. As presented above, differences in lipid and protein content separate the nutritional profile of Ulva most strongly from soy. Soybean has about 36.5% protein (USDA FoodData Central), compared to harvested Ulva, which ranges from about 4–27%. Only enriched Ulva grown with supplemental nitrogen can reach protein levels comparable to soybean. Additionally, the cost of Ulva production cannot currently compete with the cheap production of soybean. According to the US Department of Agriculture, soybean production costs about $162/acre. Assuming an average yield of 50 bushels/acre and a conversion rate of 40 bushels/ton, soybean production on a weight basis is approximately $130/ton. In comparison, estimates of production costs for seaweed farms range from $225 – $10,000/dry ton, depending on scale (Kite-Powell et al. Citation2022). Therefore, even the largest seaweed farm with the lowest production costs is still 1.7 times more expensive to produce than soybean, and most seaweed farms have even higher production costs. Unfortunately, there are no known published production costs for Ulva cultivation, but life cycle assessments of land-based production are currently underway. Another major limitation for integrating Ulva into the feed industry, both terrestrial and aquafeed, is the scale of production. Currently, the scale of Ulva production is catering mainly to human consumption, while biomass from green tides is used for animal feed research. Clearly it is important to save high quality biomass from aquaculture for human food, and the feed and biomaterials industries should not compete with human food for Ulva biomass, but the unpredictability of the availability of biomass from year to year continues to be a difficult challenge for industries relying on wild biomass. In both the food and feed industries, digestibility, taste, palatability, and flavors must be investigated further to better understand the potential attractiveness of Ulva products to animals or consumers. Customer acceptance and willingness to pay must be examined to determine the marketability and price of Ulva-based products. Currently, some producers are approaching well-known chefs to include seaweeds in their recipes, or to provide cooking courses. Such activities will help raise awareness about how to use seaweeds in general and Ulva specifically in food, and make European consumers more comfortable with incorporating Ulva into their diet.
Although Ulva is naturally available and culture techniques exist, several key issues exist regarding the market, technology, and product development (i.e., increased supply of Ulva biomass, product innovation, and processing). The effects of processing on chemical composition and potential modifications in bioactive profiles must be identified by companies and adjusted for end-user applications. To capitalize on the potential for increased profitability, the existing Ulva sector must migrate into the identified opportunity areas. The nutraceutical, pharmaceutical, and cosmetics industries represent greater profit opportunities than the agri-products and horticultural products sector alone (Barbier et al. Citation2019). To achieve this, the sector must identify specific market opportunities, innovate, and introduce greater automation, including new processing and packaging technology.
Effective Ulva biomass utilization in a cascading biorefinery concept will still need to demonstrate its potential to avoid compound loss and waste. Because improving crude biomass often requires high energy investments, the cost-effectiveness and environmental sustainability of such processes must be investigated. Life cycle assessment of current production systems will provide key insights into the sustainability and cost-effectiveness of Ulva production.
Therefore, the Ulva industry has been assigned an ambitious target. Greater value in the Ulva sector can only be achieved by industry activities in association with funding agencies and research providers.
Author contributions
The author order reflects the first, second, third, and last (senior) authors. Authors four through nine contributed significantly to the writing as section leaders. All other authors are in alphabetical order and the order does not reflect their contribution. The author contributions are summarized as follows: LCH: conceptualization, writing of the original draft, visualization, review & editing, supervision, resources, investigation, methodology, project administration; SS, AJM-M: conceptualization, writing of the original draft, review & editing, methodology, project administration, supervision; JMMA, UA, KB, AGB, JJB, RD, ÖD, OTE, AF, NG, AG, LG, FM, SS, DBS, ES, GT, SZ-S, ZZ-S: writing of the original draft, review & editing. CR: writing of the original draft, review and editing, conceptualization; MS: funding acquisition, conceptualization, review & editing, project administration, supervision. ÖD, LCH, KIK: data visualization, MM: sample analysis. The following authors made major contributions to the writing as section leaders: LCH, AJM-M, KB, GZ, SS, LG, NG, GT, CR.
Supplemental Material
Download MS Word (71.1 KB)Acknowledgements
The authors would like to thank the European Cooperation in Science and Technology (COST) for making this work possible, as well as two anonymous reviewers whose constructive comments greatly improved the manuscript.
Disclosure statement
Antonio Jesús Meléndez-Martínez carries out consultancy work for diverse companies. Alexander Golberg has interests in Genesea Advansed Technologies which commercializes Ulva protein process.
Additional information
Funding
References
- Abd El-Baky, H. H., F. K. El-Baz, and G. S. El-Baroty. 2009. Natural preservative ingredient from marine alga Ulva lactuca L. International Journal of Food Science & Technology 44 (9):1688–95. doi: 10.1111/j.1365-2621.2009.01926.x.
- Abdel-Warith, A.-W. A., E.-S. Younis, and N. A. Al-Asgah. 2016. Potential use of green macroalgae Ulva lactuca as a feed supplement in diets on growth performance, feed utilization and body composition of the African Catfish, Clarias gariepinus. Saudi Journal of Biological Sciences 23 (3):404–9. doi: 10.1016/j.sjbs.2015.11.010.
- Abdul Khalil, H. P. S., C. K. Saurabh, Y. Y. Tye, T. K. Lai, A. M. Easa, E. Rosamah, M. R. N. Fazita, M. I. Syakir, A. S. Adnan, H. M. Fizree, et al. 2017. Seaweed based sustainable films and composites for food and pharmaceutical applications: A review. Renewable and Sustainable Energy Reviews 77:353–62. doi: 10.1016/j.rser.2017.04.025.
- Abudabos, A. M., A. B. Okab, R. S. Aljumaah, E. M. Samara, K. A. Abdoun, and A. A. Al-Haidary. 2013. Nutritional value of green seaweed (Ulva lactuca) for broiler chickens. Italian Journal of Animal Science 12 (2):e28. doi: 10.4081/ijas.2013.e28.
- Adams, J., M. M. S. M. Morris, L. Steege, J. Robinson, and C. Bavington. 2021. Food-grade biorefinery processing of macroalgae at scale: Considerations, observations and recommendations. Journal of Marine Science and Engineering 9 (10):1082. doi: 10.3390/jmse9101082.
- Aguilera-Morales, M., M. Casas-Valdez, S. Carrillo-Domínguez, B. González-Acosta, and F. Pérez-Gil. 2005. Chemical composition and microbiological assays of marine algae Enteromorpha spp. as a potential food source. Journal of Food Composition and Analysis 18 (1):79–88. doi: 10.1016/j.jfca.2003.12.012.
- Ainsa, A., A. Honrado, P. Marquina, J. A. Beltrán, and J. Calanche. 2022. Influence of seaweeds on the quality of pasta as a plant-based innovative food. Foods 11 (16):2525. doi: 10.3390/foods11162525.
- Akomea-Frempong, S., D. I. Skonberg, M. E. Camire, and J. J. Perry. 2021. Impact of blanching, freezing, and fermentation on physicochemical, microbial, and sensory quality of sugar kelp (Saccharina latissima). Foods 10 (10):2258. doi: 10.3390/foods10102258.
- Al-Hafedh, Y. S., A. Alam, and A. H. Buschmann. 2014. Bioremediation potential, growth and biomass yield of the green seaweed, Ulva lactuca in an integrated marine aquaculture system at the Red Sea Coast of Saudi Arabia at different stocking densities and effluent flow rates. Reviews in Aquaculture 7 (3):161–71. doi: 10.1111/raq.12060.
- Amin, H. H. 2021. Safe Ulvan silver nanoparticles composite films for active food packaging. American Journal of Biochemistry and Biotechnology 17 (1):28–39. doi: 10.3844/ajbbsp.2021.28.39.
- Amorim, A. M., A. E. Nardelli, and F. Chow. 2020. Effects of drying processes on antioxidant properties and chemical constituents of four tropical macroalgae suitable as functional bioproducts. Journal of Applied Phycology 32 (2):1495–509. doi: 10.1007/s10811-020-02059-7.
- Andrade, C., P. L. Martins, L. C. Duarte, A. C. Oliveira, and F. Carvalheiro. 2022. Development of an innovative macroalgae biorefinery: Oligosaccharides as pivotal compounds. Fuel 320 (July):123780. doi: 10.1016/j.fuel.2022.123780.
- Angell, A. R., L. Mata, R. de Nys, and N. A. Paul. 2016. The protein content of seaweeds: A universal nitrogen-to-protein conversion factor of five. Journal of Applied Phycology 28 (1)February 30): :511–24. doi: 10.1007/s10811-015-0650-1.
- Anisuzzaman, M., U.-C. Jeong, F. Jin, K. Kabery, J.-K. Choi, D.-I. Lee, H. S. Yu, S.-J. Kang, and K. Seok-Joong. 2018. Effects of Ulva lactuca and Laminaria japonica algae in prepared feeds on growth, survival, fatty acid compositions and interleukin (IL)-10 production of sea cucumber Apostichopus japonicus. International Journal of Fisheries and Aquatic Studies 6 (2):387–95. www.fisheriesjournal.com.
- Anmarkrud, M. K. 2023. The use of soy in Norwegian fish farming–an industry perspective on sustainability in the food supply chain. MSc Thesis, University of Oslo.
- Anon. 2024. USDA food data central. Accessed February 22. https://fdc.nal.usda.gov.
- Ansary, M. W. R., S. Il Baek, H. S. Jeong, K. W. Lee, S. H. Cho, H. S. Kim, and M.-S. Jwa. 2019. Substitution effect of the combined fouling macroalgae Ulva australis and Sargassum horneri for Undaria pinnatifida in formulated diets on growth and body composition of juvenile abalone (Haliotis discus, Reeve 1846) subjected to air exposure stressor. Journal of Applied Phycology 31 (5):3245–54. doi: 10.1007/s10811-019-01812-x.
- Araújo, G. S., T. Morais, J. Cotas, S. García-Poza, J. W. A. Silva, A. M. M. Gonçalves, and L. Pereira. 2022. A road to the sustainable seaweed aquaculture. In Sustainable global resources of seaweeds, ed. A. R.Rao, and G. A. Ravishankar, vol. 1, 63–73. Cham: Springer International Publishing.
- Araújo, R., F. Vázquez Calderón, J. Sánchez López, I. C. Azevedo, A. Bruhn, S. Fluch, M. Garcia Tasende, et al. 2021. Current status of the algae production industry in Europe: An emerging sector of the blue bioeconomy 7. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85100760000&doi = 10.3389%2Ffmars.2020.626389&partnerID = 40&md5 = 8a2dba06b754e79b8b55009ddd44de96.
- Arieli, A., G. Kissil, and D. Sklan. 1993. A note on the nutritive value of Ulva lactuca for ruminants. Animal Science 57 (2):329–31. https://www.cambridge.org/core/product/51D4AD1E81AD61BF9AC9B3FE7BC0C7AF. doi: 10.1017/S0003356100006978.
- Arrieta, M. P., L. Peponi, D. López, J. López, and J. M. Kenny. 2017. An overview of nanoparticles role in the improvement of barrier properties of bioplastics for food packaging applications. In Food packaging, ed. A. M. Grumezescu, 391–424. London, UK: Elsevier.
- Arul Manikandan, N., and P. N. L. Lens. 2023. Sustainable biorefining and bioprocessing of green seaweed (Ulva spp.) for the production of edible (Ulvan) and non-edible (polyhydroxyalkanoate) biopolymeric films. Preprint Article SSRN. https://papers.ssrn.com/abstract=4377216.
- Asino, H., Q. Ai, and K. Mai. 2011. Evaluation of Enteromorpha prolifera as a feed component in large yellow croaker (Pseudosciaena crocea, Richardson, 1846) diets. Aquaculture Research 42 (4):525–33. doi: 10.1111/j.1365-2109.2010.02648.x.
- Azaza, M. S., F. Mensi, J. Ksouri, M. N. Dhraief, B. Brini, A. Abdelmouleh, and M. M. Kraïem. 2008. Growth of Nile Tilapia (Oreochromis niloticus L.) fed with diets containing graded levels of green algae Ulva meal (Ulva rigida) reared in geothermal waters of Southern Tunisia. Journal of Applied Ichthyology 24 (2):202–7. doi: 10.1111/j.1439-0426.2007.01017.x.
- Badmus, U. O., M. A. Taggart, and K. G. Boyd. 2019. The effect of different drying methods on certain nutritionally important chemical constituents in edible brown seaweeds. Journal of Applied Phycology 31 (6):3883–97. doi: 10.1007/s10811-019-01846-1.
- Bakan, M., B. Peksezer, N. S. Börekçi, M. T. Alp, and D. Ayas, Mersin University. 2021. Effect of regional differences on fatty acid profiles of Ulva linza (Linnaeus 1753), Enteromorpha flexuosa (Agardh, 1883) and Taonia atomaria (Agardh, 1848). Acta Natura et Scientia 2 (1):76–85. doi: 10.29329/actanatsci.2021.314.12.
- Bansemer, M. S., J. G. Qin, J. O. Harris, D. N. Duong, K.-L. Currie, G. S. Howarth, and D. A. J. Stone. 2016. Dietary inclusions of dried macroalgae meal in formulated diets improve the growth of Greenlip Abalone (Haliotis laevigata). Journal of Applied Phycology 28 (6):3645–58. doi: 10.1007/s10811-016-0829-0.
- Bansemer, M. S., J. G. Qin, J. O. Harris, D. N. Duong, T. H. Hoang, G. S. Howarth, and D. A. J. Stone. 2016. Growth and feed utilisation of Greenlip Abalone (Haliotis laevigata) fed nutrient enriched macroalgae. Aquaculture 452 (February):62–8. doi: 10.1016/j.aquaculture.2015.10.025.
- Bao, M., J. S. Park, Q. Xing, P. He, J. Zhang, C. Yarish, H. I. Yoo, and J. K. Kim. 2022. Comparative analysis of physiological responses in two Ulva prolifera strains revealed the effect of eutrophication on high temperature and copper stress tolerance. Frontiers in Marine Science 9:863918. doi: 10.3389/fmars.2022.863918.
- Barbier, M., B. Charrier, R. Araujo, S. L. Holdt, B. Jacquemin, and C. Rebours. 2019. PEGASUS – PHYCOMORPH European guidelines for a sustainable aquaculture of seaweeds. Applied Phycology. doi: 10.21411/2c3w-yc73.
- Barcelo, A., J. Claustre, F. Moro, J.-A. Chayvialle, J.-C. Cuber, and P. Plaisancié. 2000. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut 46 (2):218. http://gut.bmj.com/content/46/2/218.abstract. doi: 10.1136/gut.46.2.218.
- Barragán-Ocaña, A., H. Merritt, O. E. Sánchez-Estrada, J. L. Méndez-Becerril, and M. Del Pilar Longar-Blanco. 2023. Biorefinery and sustainability for the production of biofuels and value-added products: A trends analysis based on network and patent analysis. PLOS One. 18 (1):e0279659–e0279659. https://pubmed.ncbi.nlm.nih.gov/36634105. doi: 10.1371/journal.pone.0279659.
- Bartolo, A. G., G. Zammit, A. F. Peters, and F. C. Küpper. 2020. The current state of DNA barcoding of macroalgae in the Mediterranean Sea: Presently lacking but urgently required. Botanica Marina 63 (3):253–72. doi: 10.1515/bot-2019-0041.
- Barzkar, N., S. Tamadoni Jahromi, H. B. Poorsaheli, and F. Vianello. 2019. Metabolites from marine microorganisms, micro, and macroalgae: Immense scope for pharmacology. Marine Drugs 17 (8):464. https://pubmed.ncbi.nlm.nih.gov/31398953. doi: 10.3390/md17080464.
- Batista, S., M. Pintado, A. Marques, H. Abreu, J. L. Silva, F. Jessen, F. Tulli, and L. M. P. Valente. 2020. Use of technological processing of seaweed and microalgae as strategy to improve their apparent digestibility coefficients in European Seabass (Dicentrarchus labrax) juveniles. Journal of Applied Phycology 32 (5):3429–46. doi: 10.1007/s10811-020-02185-2.
- Becker, E. W., and L. V. Venkataraman. 1984. Production and utilization of the blue-green alga Spirulina in India. Biomass 4 (2):105–25. doi: 10.1016/0144-4565(84)90060-X.
- Ben-Ari, T., A. Neori, D. Ben-Ezra, L. Shauli, V. Odintsov, and M. Shpigel. 2014. Management of Ulva lactuca as a biofilter of mariculture effluents in IMTA system. Aquaculture 434:493–8. doi: 10.1016/j.aquaculture.2014.08.034.
- Beril, N., and E. C. Çankırılıgil. 2019. The elemental composition of green seaweed (Ulva rigida) collected from Çanakkale, Turkey. Aquatic Sciences and Engineering 34 (3):74–9. doi: 10.26650/ASE2019557380.
- Bermejo, R., A. H. Buschmann, E., Capuzzo, E. J. Cottier, Cook, A. Fricke, I. Hernández, L. C. Hofmann, R. Pereira, and S. W. K. van den Burg. 2022. State of knowledge regarding the potential of macroalgae cultivation in providing climate-related and other ecosystem services: A report of the Eklipse Expert Working Group on Macroalgae cultivation and Ecosystem Services No. 01/2022. Eklipse, 2022. https://eklipse.eu/wp-content/uploads/website_db/Request/Macro-Algae/EKLIPSE_DG-Mare-Report-PrintVersion_final.pdf
- Berri, M., M. Olivier, S. Holbert, J. Dupont, H. Demais, M. Le Goff, and P. N. Collen. 2017. Ulvan from Ulva armoricana (Chlorophyta) activates the PI3K/Akt signalling pathway via TLR4 to induce intestinal cytokine production. Algal Research 28:39–47. doi: 10.1016/j.algal.2017.10.008.
- Berri, M., C. Slugocki, M. Olivier, E. Helloin, I. Jacques, H. Salmon, H. Demais, M. Le Goff, and P. N. Collen. 2016. Marine-sulfated polysaccharides extract of Ulva armoricana green algae exhibits an antimicrobial activity and stimulates cytokine expression by intestinal epithelial cells. Journal of Applied Phycology 28 (5):2999–3008. doi: 10.1007/s10811-016-0822-7.
- Bikker, P., M. M. van Krimpen, P. van Wikselaar, B. Houweling-Tan, N. Scaccia, J. W. van Hal, W. J. J. J. Huijgen, J. W. Cone, and A. M. López-Contreras. 2016. Biorefinery of the green seaweed Ulva lactuca to produce animal feed, chemicals and biofuels. Journal of Applied Phycology 28 (6):3511–25. doi: 10.1007/s10811-016-0842-3.
- Bird, K. T., T. C. Chiles, R. E. Longley, A. F. Kendrick, and M. D. Kinkema. 1993. Agglutinins from marine macroalgae of the Southeastern United States. Journal of Applied Phycology 5 (2):213–8. doi: 10.1007/BF00004020.
- Blikra, M. J., T. Løvdal, M. R. Vaka, I. S. Roiha, B. T. Lunestad, C. Lindseth, and D. Skipnes. 2019. Assessment of food quality and microbial safety of brown macroalgae (Alaria esculenta and Saccharina latissima). Journal of the Science of Food and Agriculture 99 (3):1198–206. doi: 10.1002/jsfa.9289.
- Blikra, M. J., T. Altintzoglou, T. Løvdal, G. Rognså, D. Skipnes, T. Skåra, M. Sivertsvik, and E. N. Fernández. 2021. Seaweed products for the future: Using current tools to develop a sustainable food industry. Trends in Food Science & Technology 118:765–76. doi: 10.1016/j.tifs.2021.11.002.
- Blomster, J., C. A. Maggs, and M. J. Stanhope. 1999. Extensive intraspecific morphology variation in Enteromorpha muscoides (Chlorophyta) revealed by molecular analysis. Journal of Phycology 35 (3):575–86. doi: 10.1046/j.1529-8817.1999.3530575.x.
- Blomme, J., T. Wichard, T. B. Jacobs, and O. De Clerck. 2023. Ulva: An emerging green seaweed model for systems biology. Journal of Phycology 59 (3):433–40. doi: 10.1111/jpy.13341.
- Bolton, J. J., D. V. Robertson-Andersson, D. Shuuluka, and L. Kandjengo. 2009. Growing Ulva (Chlorophyta) in integrated systems as a commercial crop for abalone feed in South Africa: A Swot analysis. Journal of Applied Phycology 21 (5):575–83. doi: 10.1007/s10811-008-9385-6.
- Bolton, J. J. 2020. The problem of naming commercial seaweeds. Journal of Applied Phycology 32 (2):751–8. doi: 10.1007/s10811-019-01928-0.
- Bolton, J. J., M. D. Cyrus, M. J. Brand, M. Joubert, and B. M. Macey. 2016. Why grow Ulva? Its potential role in the future of aquaculture. Perspectives in Phycology 3 (3):113–20. doi: 10.1127/pip/2016/0058.
- Bosse, R., and L. C. Hofmann. 2020. Verpackung Aus Algen. Bundesministerium Für Ernährung Und Landwirtschaft. https://www.innovationstage-digital.de/fachsektionen/ressourcenschonende-lebensmittelherstellung/verpackung-aus-algen/.
- Boyd, C. E., and L. N. Jescovitch. 2020. Penaeid shrimp aquaculture. Fisheries and Aquaculture. New York: Oxford University Press.
- Bruhn, A., G. Brynning, A. Johansen, M. S. Lindegaard, H. H. Sveigaard, B. Aarup, L. Fonager, L. L. Andersen, M. B. Rasmussen, M. M. Larsen, et al. 2019. Fermentation of sugar kelp (Saccharina latissima)—effects on sensory properties, and content of minerals and metals. Journal of Applied Phycology 31 (5):3175–87. doi: 10.1007/s10811-019-01827-4.
- Bussy, F., L. G. Matthieu, H. Salmon, J. Delaval, M. Berri, and N. C. Pi. 2019. Immunomodulating effect of a seaweed extract from Ulva armoricana in pig: Specific IgG and total IgA in colostrum, milk, and blood. Veterinary and Animal Science 7:100051. https://www.sciencedirect.com/science/article/pii/S2451943X18302308. doi: 10.1016/j.vas.2019.100051.
- Cai, J. 2021. Global status of seaweed production, trade and utilization,Seaweed innovation forum Belize, https://www.competecaribbean.org/wp-content/uploads/2021/05/Global-status-of-seaweed-production-trade-and-utilization-Junning-Cai-FAO.pdf
- Calheiros, A. C., L. P. M. Sales, A. D. Pereira Netto, D. N. Cavalcanti, B. Castelar, and R. P. Reis. 2021. Commercial raw materials from algaculture and natural stocks of Ulva Spp. 33 (3):1805–18. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85102278681&doi=10.1007/s10811-021-02413-3&partnerID=40&md5=5feee57ca303872342c1e21985a823f4.
- Campbell, M., J. Ortuño, L. Ford, D. R. Davies, A. Koidis, P. J. Walsh, and K. Theodoridou. 2020. The effect of ensiling on the nutritional composition and fermentation characteristics of brown seaweeds as a ruminant feed ingredient. Animals 10 (6):1019. doi: 10.3390/ani10061019.
- Caprita, F.-C., A. Ene, and A. Cantaragiu Ceoromila. 2021. Valorification of Ulva rigida algae in pulp and paper industry for improved paper characteristics and wastewater heavy metal filtration. Sustainability 13 (19):10763. doi: 10.3390/su131910763.
- Cardoso, C., A. Ripol, C. Afonso, M. Freire, J. Varela, H. Quental-Ferreira, P. Pousão-Ferreira, and N. Bandarra. 2017. Fatty acid profiles of the main lipid classes of green seaweeds from fish pond aquaculture. Food Science & Nutrition 5 (6):1186–94. doi: 10.1002/fsn3.511.
- Cardoso, I., A. Meiβner, A. Sawicki, I. Bartsch, K.-U. Valentin, S. Steinhagen, B. H. Buck, and L. C. Hofmann. 2023. Salinity as a tool for strain selection in recirculating land-based production of Ulva spp. from germlings to adults. Journal of Applied Phycology 35 (5):1971–86. doi: 10.1007/s10811-023-02960-x.
- Castellanos-Barriga, L. G., F. Santacruz-Ruvalcaba, G. Hernández-Carmona, E. Ramírez-Briones, and R. M. Hernández-Herrera. 2017. Effect of seaweed liquid extracts from Ulva lactuca on seedling growth of mung bean (Vigna radiata). Journal of Applied Phycology 29 (5):2479–88. doi: 10.1007/s10811-017-1082-x.
- Cerezo, I. M., M. Fumanal, S. T. Tapia-Paniagua, R. Bautista, V. Anguís, C. Fernández-Díaz, F. J. Alarcón, M. A. Moriñigo, and M. C. Balebona. 2022. Solea senegalensis bacterial intestinal microbiota is affected by low dietary inclusion of Ulva ohnoi. Frontiers in Microbiology 12:801744. https://pubmed.ncbi.nlm.nih.gov/35211100. doi: 10.3389/fmicb.2021.801744.
- Chapman, V. J., and D. J. Chapman. 1980. Mariculture of seaweeds. In Seaweeds and their uses, 241–52. Dordrecht: Springer Netherlands.
- Charlier, R. H., P. Morand, C. W. Finkl, and A. Thys. 2006. Green tides on the Brittany Coasts. In 2006 IEEE US/EU Baltic International Symposium, 1–13, IEEE.
- Charlier, R. H., P. Morand, and C. W. Finkl. 2008. How Brittany and Florida coasts cope with green tides. International Journal of Environmental Studies 65 (2):191–208. doi: 10.1080/00207230701791448.
- Chopin, T. 2021. Seaweeds are finally getting their moment. How do we translate it into a momentum beyond the present hype? International Aquafeed 24 (9):12–3
- Cindana Mo’o, F. R., G. Wilar, H. P. Devkota, and N. Wathoni. 2020. Ulvan, a polysaccharide from macroalga Ulva sp.: A review of chemistry. Biological activities and potential for food and biomedical applications. Applied Sciences 10 (16):5488.
- Coelho, D., P. A. Lopes, V. Cardoso, P. Ponte, J. Brás, M. S. Madeira, C. M. Alfaia, N. M. Bandarra, C. M. G. A. Fontes, and J. A. M. Prates. 2020. A two-enzyme constituted mixture to improve the degradation of Arthrospira platensis microalga cell wall for monogastric diets. Journal of Animal Physiology and Animal Nutrition 104 (1):310–21. doi: 10.1111/jpn.13239.
- Cofrades, S., J. Benedí, A. Garcimartin, F. J. Sánchez-Muniz, and F. Jimenez-Colmenero. 2017. A comprehensive approach to formulation of seaweed-enriched meat products: From technological development to assessment of healthy properties. Food Research International 99:1084–94. doi: 10.1016/j.foodres.2016.06.029.
- Cofrades, S., M. Serdaroǧlu, and F. Jiménez-Colmenero. 2013. Design of healthier foods and beverages containing whole algae. Functional Ingredients from Algae for Foods and Nutraceuticals :609–33.
- Colombo, M. L., P. Risè, F. Giavarini, L. De Angelis, C. Galli, and C. L. Bolis. 2006. Marine macroalgae as sources of polyunsaturated fatty acids. Plant Foods for Human Nutrition 61 (2):64–9. doi: 10.1007/s11130-006-0015-7.
- Costa, M., C. Cardoso, C. Afonso, N. M. Bandarra, and J. A. M. Prates. 2021. Current knowledge and future perspectives of the use of seaweeds for livestock production and meat quality: A systematic review. Journal of Animal Physiology and Animal Nutrition 105 (6):1075–102. doi: 10.1111/jpn.13509.
- Costa, M. M., J. M. Pestana, P. Carvalho, C. M. Alfaia, C. F. Martins, D. Carvalho, M. Mourato, S. Gueifão, I. Delgado, I. Coelho, et al. 2022. Effect on broiler production performance and meat quality of feeding Ulva lactuca supplemented with carbohydrases. Animals 12 (13):1720. doi: 10.3390/ani12131720.
- Cottier-Cook, E. J., N. Nagabhatla, Y. Badis, M. Campbell, T. Chopin, W. Dai, J. Fang, et al. 2016. Safeguarding the future of the global seaweed aquaculture industry. United Nations University (INWEH) and Scottish Association for Marine Science Policy Brief. https://inweh.unu.edu/wp-content/uploads/2016/09/unu-seaweed-aquaculture-policy.pdf.
- Cox, S., N. Abu-Ghannam, and S. Gupta. 2010. An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. International Food Research Journal 17 (1):205–20.
- Creed, J. C., V. Vieira, T. A. Norton, and D. Caetano. 2019. A meta-analysis shows that seaweeds surpass plants, setting life-on-earth’s limit for biomass packing. BMC Ecology 19 (1):6. doi: 10.1186/s12898-019-0218-z.
- Cruz, C. 2019. A diet based on Ulva lactuca flour improves growth fingerlings sea chub Girella laevifrons (Pisces: Kyphosidae). Scientia Agropecuaria 10 (2):191–7. doi: 10.17268/sci.agropecu.2019.02.04.
- Cyrus, M. D., J. J. Bolton, R. Scholtz, and B. M. Macey. 2015. The advantages of Ulva (Chlorophyta) as an additive in sea urchin formulated feeds: Effects on palatability. Consumption and Digestibility. Aquaculture Nutrition 21 (5): 578–591.
- Davoodi, M. N., J. M. Milani, and R. Farahmandfar. 2021. Preparation and characterization of a novel biodegradable film based on sulfated polysaccharide extracted from seaweed Ulva intestinalis. Food Science and Nutrition 9 (8):4108–16. doi: 10.1002/fsn3.2370.
- Debbarma, J., P. Viji, B. M. Rao, and M. M. Prasad. 2017. Nutritional and physical characteristics of noodles incorporated with green seaweed (Ulva reticulata) and fish (Pangasianodon hypophthalmus) mince. Indian Journal of Fisheries 64 (2). doi: 10.21077/ijf.2017.64.2.58918-14.
- de Araújo Amatuzzi, J. C., L. do Nascimento Vieira, B. F. Sant’Anna-Santos, M. D. Noseda, and H. Pacheco de Freitas Fraga. 2020. Improved in vitro development of Epidendrum secundum (Orchidaceae) by using aqueous extract of the seaweed Kappaphycus alvarezii (Rhodophyta, Solieriaceae). Acta Physiologiae Plantarum 42 (8):136. doi: 10.1007/s11738-020-03129-6.
- de Clerck, O., S.-M. Kao, K. A. Bogaert, J. Blomme, F. Foflonker, M. Kwantes, E. Vancaester, L. Vanderstraeten, E. Aydogdu, J. Boesger, et al. 2018. Insights into the evolution of multicellularity from the sea lettuce genome. Current Biology 28 (18):2921–33.e5. doi: 10.1016/j.cub.2018.08.015.
- de Oliveira, M. N., A. L. P. Freitas, A. F. U. Carvalho, T. M. T. Sampaio, D. F. Farias, D. I. Alves Teixeira, S. T. Gouveia, J. G. Pereira, M. M, and d C. C. d Sena. 2009. Nutritive and non-nutritive attributes of washed-up seaweeds from the Coast of Ceará, Brazil. Food Chemistry 115 (1):254–9. doi: 10.1016/j.foodchem.2008.12.004.
- del Olmo, A., A. Picon, and M. Nuñez. 2018. Cheese supplementation with five species of edible seaweeds: Effect on microbiota, antioxidant activity, colour, texture and sensory characteristics. International Dairy Journal 84:36–45. doi: 10.1016/j.idairyj.2018.04.004.
- del Olmo, A., A. Picon, and M. Nuñez. 2019. Probiotic dynamics during the fermentation of milk supplemented with seaweed extracts: The effect of milk constituents. LWT 107:249–55. doi: 10.1016/j.lwt.2019.03.006.
- De Viçose, G. C., M. P. Viera, S. Huchette, and M. S. Izquierdo. 2012. Larval settlement, early growth and survival of Haliotis tuberculata coccinea using several algal cues. Journal of Shellfish Research 31 (4):1189–98. doi: 10.2983/035.031.0430.
- Dhargalkar, V. K., and P. Neelam. 2005. Seaweed: Promising plant of the millennium. Science and Culture :60–6.
- Diamahesa, W. A., T. Masumoto, D. Jusadi, and M. Setiawati. 2017. Growth and protein content of Ulva prolifera maintained at different flow rates in integrated aquaculture system. Jurnal Ilmu dan Teknologi Kelautan Tropis 9 (2):429–41. doi: 10.29244/jitkt.v9i2.19257.
- Diler, I., A. A. Tekinay, B. Guroy, and D. Guroy. 2007. Effects of Ulva rigida on the growth, feed intake and body composition of common carp, Cyprinus carpio L. Journal of Biological Sciences 7 (2):305–8. doi: 10.3923/jbs.2007.305.308.
- Dominguez, H., and E. P. Loret. 2019. Ulva lactuca, a source of troubles and potential riches. Marine Drugs 17 (6):357. doi: 10.3390/md17060357.
- Du, X., Y. Xu, Z. Jiang, Y. Zhu, Z. Li, H. Ni, and F. Chen. 2021. Removal of the fishy malodor from Bangia fusco-purpurea via fermentation of Saccharomyces cerevisiae, Acetobacter pasteurianus, and Lactobacillus plantarum. Journal of Food Biochemistry 45 (5):e13728. doi: 10.1111/jfbc.13728.
- Duarte, C. M., A. Bruhn, and D. Krause-Jensen. 2021. A seaweed aquaculture imperative to meet global sustainability targets. Nature Sustainability 5 (3):185–93. doi: 10.1038/s41893-021-00773-9.
- Duarte, C. M., M. Holmer, Y. Olsen, D. Soto, N. Marbà, J. Guiu, K. Black, and I. Karakassis. 2009. Will the oceans help feed humanity? BioScience 59 (11):967–76. doi: 10.1525/bio.2009.59.11.8.
- Duarte, C. M., N. Marbá, and M. Holmer. 2007. Rapid domestication of marine species. Science 316 (5823):382–3. doi: 10.1126/science.1138042.
- Dubois, B., N. W. Tomkins, R. D. Kinley, M. Bai, S. Seymour, N. A. Paul, and R. de Nys. 2013. Effect of tropical algae as additives on rumen in vitro gas production and fermentation characteristics. American Journal of Plant Sciences 04 (12):34–43. doi: 10.4236/ajps.2013.412A2005.
- El-Banna, S. G., A. A. Hassan, A. B. Okab, A. A. Koriem, and M. A. Ayoub. 2005. Effect of Feeding diets supplemented with seaweed on growth performance and some blood hematological and biochemical characteristics of male Baladi rabbits. In 4th International Conference on Rabbit Production in Hot Climate. Sharm El-Sheikh, Egypt.
- El Boukhari, M. E. M., M. Barakate, N. Choumani, Y. Bouhia, and K. Lyamlouli. 2021. Ulva lactuca extract and fractions as seed priming agents mitigate salinity stress in tomato seedlings. Plants 10 (6):1104. https://pubmed.ncbi.nlm.nih.gov/34070914. doi: 10.3390/plants10061104.
- Elizondo-González, R., E. Quiroz-Guzmán, C. Escobedo-Fregoso, P. Magallón-Servín, and A. Peña-Rodríguez. 2018. Use of seaweed Ulva lactuca for water bioremediation and as feed additive for white shrimp Litopenaeus vannamei. PeerJ. doi: 10.7717/peerj.4459.
- El-Tawil, N. E. 2010. Effects of green seaweeds (Ulva sp.) as feed supplements in Red Tilapia (Oreochromis sp.) Diet on growth performance, feed utilization and body composition. Journal of the Arabian Aquaculture Society 5 (2).
- EL-Waziry, A., A. Al-Haidary, A. Okab, E. Samara, and K. Abdoun. 2015. Effect of dietary seaweed (Ulva lactuca) supplementation on growth performance of sheep and on in vitro gas production kinetics. Turkish Journal of Veterinary and Animal Sciences 39:81–6. doi: 10.3906/vet-1403-82.
- Emre, Y., S. Ergun, A. Kurtoglu, B. Guroy, and D. Guroy. 2013. Effects of Ulva meal on growth performance of gilthead seabream (Sparus aurata) at different levels of dietary lipid. Turkish Journal of Fisheries and Aquatic Sciences 13 (5). doi: 10.4194/1303-2712-v13_5_08.
- Ennoury, A., R. BenMrid, N. Nhhala, Z. Roussi, S. Latique, Z. Zouaoui, and M. Nhiri. 2022. River’s Ulva intestinalis L. extract protects common bean plants (Phaseolus vulgaris L.) against salt stress. South African Journal of Botany 150 (November 1):334–41. https://www.sciencedirect.com/science/article/abs/pii/S0254629922004069. doi: 10.1016/j.sajb.2022.07.035.
- Ergün, S., M. Soyutürk, B. Güroy, D. Güroy, and D. Merrifield. 2009. Influence of Ulva meal on growth, feed utilization, and body composition of juvenile Nile Tilapia (Oreochromis niloticus) at two levels of dietary lipid. Aquaculture International 17 (4):355–61. doi: 10.1007/s10499-008-9207-5.
- Eroldoğan, O. T., B. Glencross, L. Novoveska, S. P. Gaudêncio, B. Rinkevich, G. C. Varese, M. de Fátima Carvalho, D. Tasdemir, I. Safarik, S. L. Nielsen, et al. 2022. From the sea to aquafeed: A perspective overview. Reviews in Aquaculture 15 (3):1028–57. doi: 10.1111/raq.12740.
- Evans, F. D., and A. T. Critchley. 2014. Seaweeds for animal production use. Journal of Applied Phycology 26 (2):891–9. doi: 10.1007/s10811-013-0162-9.
- European Commission. 2013. Commission Regulation No 68/2013 of 16 January 2013 on the catalogue of feed materials. Official Journal of the European Union 29 (1):1–64.
- European Commission. 2022. Communication from the commission to the European Parliament, the council, the European Economic and Social Committee and the Committee of the Regions. Towards a strong and sustainable EU algae sector. https://oceans-and-fisheries.ec.europa.eu/system/files/2022-11/COM-2022-592_en.pdf.
- European Council. 2007. Council Regulation (EC) No 834/2007 of 28 June 2007 on organic production and labelling of organic products and repealing Regulation (EEC) No 2092/91. Official Journal of the European Union, L 189 (1):1–23.
- European Parliament and the Council of the European Union. 2002. Directive 2002/32/EC of 7 May 2002 on undesirable substances in animal feed – council statement. OJ L 140, 30.5.2002, 10–22. http://data.europa.eu/eli/dir/2002/32/oj.
- European Parliament and the Council of the European Union. 2002. EC Regulation 178:2002. Official Journal of the European Communities, L31:1–24.
- FAO. 2021. FAOSTAT. http://www.fao.org/faostat/en/#data.
- FAO. 2022. The state of world fisheries and aquaculture 2022. Towards blue transformation. Rome: FAO.
- FAO and WHO. 2022. Report of the expert meeting on food safety for seaweed – current status and future perspectives. FAO and WHO.
- Faber, I., K. Henn, M. Brugarolas, F. J, and A. Perez‐Cueto. 2021. Relevant characteristics of food products based on alternative proteins according to european consumers. Journal of the Science of Food and Agriculture 102 (12):5034–43. doi: 10.1002/jsfa.11178.
- Ferdouse, F., S. L. Holdt, R. Smith, P. Murúa, and Z. Yang. 2018. The global status of seaweed production, trade and utilization. FAO Globefish. http://www.fao.org/publications/card/en/c/CA1121EN.
- Fernandes, H., N. Martins, L. Vieira, J. M. Salgado, C. Castro, A. Oliva-Teles, I. Belo, and H. Peres. 2022. Pre-treatment of Ulva rigida improves its nutritional value for European Seabass (Dicentrarchus Labrax) juveniles. Algal Research 66:102803. doi: 10.1016/j.algal.2022.102803.
- Ferreira, M., C. Teixeira, H. Abreu, J. Silva, B. Costas, V. Kiron, and L. M. P. Valente. 2021. Nutritional value, antimicrobial and antioxidant activities of micro- and macroalgae, single or blended, unravel their potential use for aquafeeds. Journal of Applied Phycology 33 (6):3507–18. doi: 10.1007/s10811-021-02549-2.
- Figueroa, V., M. Farfán, and J. M. Aguilera. 2021. Seaweeds as novel foods and source of culinary flavors. Food Reviews International 39 (1):1–26. doi: 10.1080/87559129.2021.1892749.
- Fleurence, J., G. Gutbier, S. Mabeau, and C. Leray. 1994. Fatty acids from 11 marine macroalgae of the French Brittany Coast. Journal of Applied Phycology 6 (5–6):527–32. doi: 10.1007/BF02182406.
- Floreto, E. A. T., S.-I. Teshima, and M. Ishikawa. 1996. The effects of seaweed diets on the growth, lipid and fatty acids of juveniles of the white sea urchin Tripneustes gratilla. Fisheries Science 62 (4):589–93. doi: 10.2331/fishsci.62.589.
- Food Safety Authority of Ireland. 2020. Report of the Scientific Committee of the Food Safety Authority of Ireland – safety considerations of seaweed and seaweed-derived foods available on the Irish market.
- Forster, J., and R. Radulovich. 2015. Seaweed and food security. Seaweed Sustainability 1:289–313. https://www.sciencedirect.com/science/article/pii/B9780124186972000118.
- Fort, A., M. Lebrault, M. Allaire, A. A. Esteves-Ferreira, M. McHale, F. Lopez, J. M. Fariñas-Franco, S. Alseekh, A. R. Fernie, and R. Sulpice. 2019. Extensive variations in diurnal growth patterns and metabolism among Ulva spp. strains. Plant Physiology 180 (1):109–23. doi: 10.1104/pp.18.01513.
- Fort, A., C. Linderhof, I. Coca-Tagarro, M. Inaba, M. McHale, K. Cascella, P. Potin, M. D. Guiry, and R. Sulpice. 2021. A sequencing-free assay for foliose Ulva species identification, hybrid detection and bulk biomass characterisation. Algal Research 55:102280. https://www.sciencedirect.com/science/article/pii/S2211926421000990. doi: 10.1016/j.algal.2021.102280.
- Fort, A., C. Mannion, J. M. Fariñas-Franco, and R. Sulpice. 2020. Green tides select for fast expanding Ulva strains. Science of the Total Environment 698:134337. https://www.sciencedirect.com/science/article/pii/S0048969719343281. doi: 10.1016/j.scitotenv.2019.134337.
- Fort, A., M. McHale, K. Cascella, P. Potin, M.-M. Perrineau, P. D. Kerrison, E. da Costa, et al. 2022. Exhaustive reanalysis of barcode sequences from public repositories highlights ongoing misidentifications and impacts taxa diversity and distribution. Molecular Ecology Resources 22 (1):86–101. doi: 10.1111/1755-0998.13453.
- Fort, A., M. McHale, K. Cascella, P. Potin, B. Usadel, M. D. Guiry, and R. Sulpice. 2021. Foliose Ulva species show considerable inter-specific genetic diversity, low intra-specific genetic variation, and the rare occurrence of inter-specific hybrids in the wild. Journal of Phycology 57 (1):219–33. doi: 10.1111/jpy.13079.
- Gajaria, T. K., P. Suthar, R. S. Baghel, N. B. Balar, P. Sharnagat, V. A. Mantri, and C. R. K. Reddy. 2017. Integration of protein extraction with a stream of byproducts from marine macroalgae: A model forms the basis for marine bioeconomy. Bioresource Technology 243 (November):867–73. doi: 10.1016/j.biortech.2017.06.149.
- Ganesan, A. R., M. Shanmugam, and R. Bhat. 2018. Producing novel edible films from semi refined Carrageenan (SRC) and Ulvan polysaccharides for potential food applications. International Journal of Biological Macromolecules 112:1164–70. doi: 10.1016/j.ijbiomac.2018.02.089.
- Ganesan, A. R., M. Shanmugam, S. Palaniappan, and G. Rajauria. 2018. Development of edible film from Acanthophora Spicifera: Structural, rheological and functional properties. Food Bioscience 23:121–8. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85040015261&doi=10.1016/j.fbio.2017.12.009&partnerID=40&md5=47f2bd40604bdeabde148f6147175de2. doi: 10.1016/j.fbio.2017.12.009.
- Gao, K., and K. R. McKinley. 1994. Use of macroalgae for marine biomass production and CO2 remediation: A review. Journal of Applied Phycology 6 (1):45–60. doi: 10.1007/BF02185904.
- Gao, S., X. Chen, Q. Yi, G. Wang, G. Pan, A. Lin, and G. Peng. 2010. A strategy for the proliferation of Ulva prolifera, main causative species of green tides, with formation of sporangia by fragmentation. PLOS One. 5 (1):e8571. doi: 10.1371/journal.pone.0008571.
- Gao, Z., D. Xu, C. Meng, X. Zhang, Y. Wang, D. Li, J. Zou, Z. Zhuang, and N. Ye. 2013. The green tide-forming macroalga Ulva linza outcompetes the red macroalga Gracilaria lemaneiformis via allelopathy and fast nutrients uptake. Aquatic Ecology 48 (1):53–62. doi: 10.1007/s10452-013-9465-9.
- Gatlin, D. M., F. T. Barrows, P. Brown, K. Dabrowski, T. G. Gaylord, R. W. Hardy, E. Herman, G. Hu, Å. Krogdahl, R. Nelson, et al. 2007. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquaculture Research 38 (6):551–79. doi: 10.1111/j.1365-2109.2007.01704.x.
- Ghaderiardakani, F., J. C. Coates, and T. Wichard. 2017. Bacteria-induced morphogenesis of Ulva intestinalis and Ulva mutabilis (Chlorophyta): A contribution to the lottery theory. FEMS Microbiology Ecology 93 (8). doi: 10.1093/femsec/fix094.
- Ghaderiardakani, F., L. Langhans, V. B. Kurbel, S. Fenizia, and T. Wichard. 2022. Metabolite profiling reveals insights into the species-dependent cold stress response of the green seaweed holobiont Ulva (Chlorophyta). Environmental and Experimental Botany 200:104913. doi: 10.1016/j.envexpbot.2022.104913.
- Ghosh, S., S. Greiserman, A. Chemodanov, P. M. Slegers, B. Belgorodsky, M. Epstein, A. Kribus, M. Gozin, G.-Q. Chen, and A. Golberg. 2021. Polyhydroxyalkanoates and biochar from green macroalgal Ulva sp. biomass subcritical hydrolysates: Process optimization and a priori economic and greenhouse emissions break-even analysis. Science of the Total Environment 770 (May):145281. doi: 10.1016/j.scitotenv.2021.145281.
- Glasson, C. R. K., I. M. Sims, S. M. Carnachan, R. de Nys, and M. Magnusson. 2017. A cascading biorefinery process targeting sulfated polysaccharides (Ulvan) from Ulva ohnoi. Algal Research 27 (November):383–91. doi: 10.1016/j.algal.2017.07.001.
- Golberg, A., A. N. Robin, M. Zollmann, H. Traugott, R. R. Palatnik, and A. Israel. 2020. Macroalgal biorefineries for the Blue Economy World Scientific.
- Gomaa, M., A. A. Al-Badaani, A. F. Hifney, and M. S. Adam. 2022. Utilization of cellulose and Ulvan from the green seaweed Ulva lactuca in the development of composite edible films with natural antioxidant properties. Journal of Applied Phycology 34 (5):2615–26. doi: 10.1007/s10811-022-02786-z.
- Gross, M. 2017. Our planet wrapped in plastic. Current Biology 27 (16):R785–R788. doi: 10.1016/j.cub.2017.08.007.
- Guerreiro, I., R. Magalhães, F. Coutinho, A. Couto, S. Sousa, C. Delerue-Matos, V. F. Domingues, A. Oliva-Teles, and H. Peres. 2019. Evaluation of the seaweeds Chondrus crispus and Ulva lactuca as functional ingredients in Gilthead Seabream (Sparus aurata). Journal of Applied Phycology 31 (3):2115–24. doi: 10.1007/s10811-018-1708-7.
- Guidara, M., H. Yaich, S. Benelhadj, Y. D. Adjouman, A. Richel, C. Blecker, M. Sindic, S. Boufi, H. Attia, and H. Garna. 2020. Smart Ulvan films responsive to stimuli of plasticizer and extraction condition in physico-chemical, optical, barrier and mechanical properties. International Journal of Biological Macromolecules 150:714–26. doi: 10.1016/j.ijbiomac.2020.02.111.
- Guidara, M., H. Yaich, A. Richel, C. Blecker, S. Boufi, H. Attia, and H. Garna. 2019. Effects of extraction procedures and plasticizer concentration on the optical, thermal, structural and antioxidant properties of novel Ulvan films. International Journal of Biological Macromolecules 135:647–58. doi: 10.1016/j.ijbiomac.2019.05.196.
- Guo, Y., A.-K. Lundebye, N. Li, Å. Ergon, S. Pang, Y. Jiang, W. Zhu, Y. Zhao, X. Li, L. Yao, et al. 2023. Comparative assessment of food safety regulations and standards for arsenic, cadmium, lead, mercury and iodine in macroalgae used as food and feed in China and Europe. Trends in Food Science & Technology 141 (November):104204. doi: 10.1016/j.tifs.2023.104204.
- Gupta, S., and N. Abu-Ghannam. 2012. Probiotic fermentation of plant based products: Possibilities and opportunities. Critical Reviews in Food Science and Nutrition 52 (2):183–99. doi: 10.1080/10408398.2010.499779.
- Gupta, S., S. Cox, and N. Abu-Ghannam. 2011. Effect of different drying temperatures on the moisture and phytochemical constituents of edible Irish brown seaweed. LWT - Food Science and Technology 44 (5):1266–72. https://www.sciencedirect.com/science/article/pii/S0023643810004469. doi: 10.1016/j.lwt.2010.12.022.
- Güroy, D., B. Güroy, D. L. Merrifield, S. Ergün, A. A. Tekinay, and M. Yiğit. 2011. Effect of dietary Ulva and Spirulina on weight loss and body composition of rainbow trout, Oncorhynchus mykiss (Walbaum), during a starvation period. Journal of Animal Physiology and Animal Nutrition 95 (3):320–7. doi: 10.1111/j.1439-0396.2010.01057.x.
- Hassan, S. M., M. Ashour, N. Sakai, L. Zhang, H. A. Hassanien, A. Gaber, and G. Ammar. 2021. Impact of seaweed liquid extract biostimulant on growth, yield, and chemical composition of cucumber (Cucumis sativus). Agriculture 11 (4):320. doi: 10.3390/agriculture11040320.
- Hasselström, L., J.-B. Thomas, J. Nordström, G. Cervin, G. M. Nylund, H. Pavia, and F. Gröndahl. 2020. Socioeconomic prospects of a seaweed bioeconomy in Sweden. Scientific Reports 10 (1) doi: 10.1038/s41598-020-58389-6.
- Hasselström, L., and J.-B. E. Thomas. 2022. A critical review of the life cycle climate impact in seaweed value chains to support carbon accounting and blue carbon financing. Cleaner Environmental Systems 6:100093. doi: 10.1016/j.cesys.2022.100093.
- Hayden, H. S., J. Blomster, C. A. Maggs, P. C. Silva, M. J. Stanhope, and J. R. Waaland. 2003. Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera. European Journal of Phycology 38 (3):277–94. doi: 10.1080/1364253031000136321.
- Hendriks, H., T. Van den Ingh, Å. Krogdahl, J. Olli, and J. F. J. G. Koninkx. 1990. Binding of soybean agglutinin to small intestinal brush border membranes and brush border membrane enzyme activities in Atlantic salmon (Salmo salar). Aquaculture 91 (1–2):163–70. https://www.sciencedirect.com/science/article/pii/004484869090185P. doi: 10.1016/0044-8486(90)90185-P.
- Ho, K. K. H. Y., and B. W. Redan. 2022. Impact of thermal processing on the nutrients, phytochemicals, and metal contaminants in edible algae. Critical Reviews in Food Science and Nutrition 62 (2):508–26. doi: 10.1080/10408398.2020.1821598.
- Hofmann, L. C., J. C. Nettleton, C. D. Neefus, and A. C. Mathieson. 2010. 2010. Cryptic diversity of Ulva (Ulvales, Chlorophyta) in the Great Bay Estuarine System (Atlantic USA): Introduced and indigenous distromatic species. European Journal of Phycology 45 (3):230–9. doi: 10.1080/09670261003746201.
- Hognes, E. S., K. Nilsson, V. Sund, and F. Ziegler. 2012. 2014 LCA of Norwegian salmon production. https://hdl.handle.net/11250/2458163.
- Holdt, S. L., and S. Kraan. 2011. Bioactive compounds in seaweed: Functional food applications and legislation. Journal of Applied Phycology 23 (3):543–97. doi: 10.1007/s10811-010-9632-5.
- Hughey, J. R., P. W. Gabrielson, C. A. Maggs, and F. Mineur. 2021. Genomic analysis of the lectotype specimens of European Ulva rigida and Ulva lacinulata (Ulvaceae, Chlorophyta) reveals the ongoing misapplication of names. European Journal of Phycology 57 (2):143–53. doi: 10.1080/09670262.2021.1914862.
- Hughey, J. R., P. W. Gabrielson, C. A. Maggs, F. Mineur, and K. A. Miller. 2020. Taxonomic revisions based on genetic analysis of type specimens of Ulva conglobata, U. laetevirens, U. pertusa and U. spathulata (Ulvales, Chlorophyta). Phycological Research 69 (2):148–53. doi: 10.1111/pre.12450.
- Hughey, J. R., C. A. Maggs, F. Mineur, C. Jarvis, K. A. Miller, S. H. Shabaka, and P. W. Gabrielson. 2019. Genetic analysis of the Linnaean Ulva lactuca (Ulvales, Chlorophyta) holotype and related type specimens reveals name misapplications, unexpected origins, and new synonymies. Journal of Phycology 55 (3):503–8. doi: 10.1111/jpy.12860.
- Hung, Y.-H. R., C.-Y. Peng, M.-Y. Huang, W.-J. Lu, H.-J. Lin, C.-L. Hsu, M.-C. Fang, and H.-T. V. Lin. 2023. Monitoring the aroma compound profiles in the microbial fermentation of seaweeds and their effects on sensory perception. Fermentation 9 (2):135. doi: 10.3390/fermentation9020135.
- Hussein, M. H., E. Eltanahy, A. F. Al Bakry, N. Elsafty, and M. M. Elshamy. 2021. Seaweed extracts as prospective plant growth bio-stimulant and salinity stress alleviator for Vigna sinensis and Zea mays. Journal of Applied Phycology 33 (2):1273–91. doi: 10.1007/s10811-020-02330-x.
- Hwang, H. J., S. Y. Lee, S. M. Kim, and S. B. Lee. 2011. Fermentation of seaweed sugars by Lactobacillus species and the potential of seaweed as a biomass feedstock. Biotechnology and Bioprocess Engineering 16 (6):1231–9. doi: 10.1007/s12257-011-0278-1.
- Irfan, I., S. Raj, A. Jaya-Ram, and S. P. Woo. 2022. Preliminary evaluation of seaweed of Ulva lactuca as supplemental diet for sea cucumber, Holothuria scabra, in aquaculture. Journal of Survey in Fisheries Sciences 9 (1):27–32. doi: 10.18331/SFS2022.9.1.3.
- Ismail, B. P., L. Senaratne-Lenagala, A. Stube, and A. Brackenridge. 2020. Protein demand: Review of plant and animal proteins used in alternative protein product development and production. Animal Frontiers 10 (4:53–63. doi: 10.1093/af/vfaa040.
- Ismail, M. M., and E. S. Mohamed. 2017. Differentiation between some Ulva Spp. by morphological, genetic and biochemical analyses. Vavilov Journal of Genetics and Breeding 21 (3):360–7. doi: 10.18699/VJ17.253.
- Ivanova, V., M. Stancheva, and D. Petrova. 2013. Fatty acid composition of Black Sea Ulva rigida and Cystoseira crinita. Bulgarian Journal of Agricultural Science 19:42–7.
- Jacobsen, M., M. Bianchi, J. P. Trigo, I. Undeland, E. Hallström, and S. Bryngelsson. 2023. Nutritional and toxicological characteristics of Saccharina latissima, Ulva fenestrata, Ulva intestinalis, and Ulva rigida: A review. International Journal of Food Properties 26 (1):2349–78. doi: 10.1080/10942912.2023.2246677.
- Jannat-Alipour, H., M. Rezaei, B. Shabanpour, M. Tabarsa, and F. Rafipour. 2019. Addition of seaweed powder and sulphated polysaccharide on shelf-life extension of functional fish Surimi restructured product. Journal of Food Science and Technology 56 (8):3777–89. doi: 10.1007/s13197-019-03846-y.
- Jansen, H. M., M. S. Bernard, M. A. J. Nederlof, I. M. van der Meer, and A. van der Werf. 2022. Seasonal variation in productivity, chemical composition and nutrient uptake of Ulva spp. (Chlorophyta) strains. Journal of Applied Phycology 34 (3):1649–60. doi: 10.1007/s10811-022-02708-z.
- Jeon, M.-R., and S.-H. Choi. 2012. Quality characteristics of pork patties added with seaweed powder. Korean Journal for Food Science of Animal Resources 32 (1):77–83. doi: 10.5851/kosfa.2012.32.1.71.
- Jiang, R., Y. Linzon, E. Vitkin, Z. Yakhini, A. Chudnovsky, and A. Golberg. 2016. Thermochemical hydrolysis of macroalgae Ulva for biorefinery: Taguchi robust design method. Scientific Reports 6 (1):27761. doi: 10.1038/srep27761.
- Joaquina Ibarra-Arana, M., Z.-H. Liao, H.-Y. Chen, and F.-H. Nan. 2018. The effects of dietary supplmented Ulva lactuca on the feeding preference and growth of sea cucumber Apostichopus japonicus (Selenka, 1867). Journal of the Fisheries Societyof Taiwan 45 (3):201–8.
- Joniver, C. F. H., A. Photiades, P. J. Moore, A. L. Winters, A. Woolmer, and J. M. M. Adams. 2021. The global problem of nuisance macroalgal blooms and pathways to its use in the circular economy. Algal Research 58:102407. https://www.sciencedirect.com/science/article/pii/S2211926421002265?via%3Dihub. doi: 10.1016/j.algal.2021.102407.
- Jönsson, M., and E. Nordberg Karlsson. 2023. Chemical food safety of seaweed: Species, spatial and thallus dependent variation of potentially toxic elements (PTEs) and techniques for their removal. Journal of Applied Phycology. doi: 10.1007/s10811-023-03131-8.
- Kadam, S. U., C. Álvarez, B. K. Tiwari, and C. P. O’Donnell. 2015. Processing of seaweeds. In Seaweed sustainability, 61–78. Elsevier.
- Kendel, M., G. Wielgosz-Collin, S. Bertrand, C. Roussakis, N. Bourgougnon, and G. Bedoux. 2015. Lipid composition, fatty acids and sterols in the seaweeds Ulva armoricana, and Solieria chordalis from Brittany (France): An analysis from nutritional, chemotaxonomic, and antiproliferative activity perspectives. Marine Drugs 13 (9):5606–28. doi: 10.3390/md13095606.
- Kenneth, F., C. F. H. Joniver, W. Meredith, and J. M. M. Adams. 2022. The productivity effects of macroalgal biochar from Ulva Linnaeus bloom species on Arabidopsis thaliana Linnaeus seedlings. European Journal of Phycology 58 (3):284–99. doi: 10.1080/09670262.2022.2103739.
- Khairy, H. M., and S. M. El-Shafay. 2013. Seasonal variations in the biochemical composition of some common seaweed species from the Coast of Abu Qir Bay, Alexandria, Egypt. Oceanologia 55 (2):435–52. doi: 10.5697/oc.55-2.435.
- Khairy, H. M., and M. A. El-Sheikh. 2015. Antioxidant activity and mineral composition of three Mediterranean common seaweeds from Abu-Qir Bay, Egypt. Saudi Journal of Biological Sciences 22 (5):623–30. https://pubmed.ncbi.nlm.nih.gov/26288568. doi: 10.1016/j.sjbs.2015.01.010.
- Kidgell, J. T., M. Magnusson, R. de Nys, and C. R. K. Glasson. 2019. Ulvan: A systematic review of extraction, composition and function. Algal Research 39 (May):101422. doi: 10.1016/j.algal.2019.101422.
- Kim, S. W., J. F. Less, L. Wang, T. Yan, V. Kiron, S. J. Kaushik, and X. G. Lei. 2019. Meeting global feed protein demand: challenge, opportunity, and strategy. Annual Review of Animal Biosciences 7 (1):221–43. doi: 10.1146/annurev-animal-030117-014838.
- Kinley, R. D., M. J. Vucko, L. Machado, and N. W. Tomkins. 2016. In vitro evaluation of the antimethanogenic potency and effects on fermentation of individual and combinations of marine macroalgae. American Journal of Plant Sciences 07 (14):2038–54. doi: 10.4236/ajps.2016.714184.
- Kirst, G. O. 1990. Salinity tolerance of eukaryotic marine algae. Annual Review of Plant Physiology and Plant Molecular Biology 41 (1):21–53. doi: 10.1146/annurev.pp.41.060190.000321.
- Kite-Powell, H. L., E. Ask, S. Augyte, D. Bailey, J. Decker, C. A. Goudey, G. Grebe, Y. Li, S. Lindell, D. Manganelli, et al. 2022. Estimating Production Cost for Large-Scale Seaweed Farms. Applied Phycology 3 (1):435–45. December 31): doi: 10.1080/26388081.2022.2111271.
- Koeman, R. P. T., and C. van den Hoek. 1981. The taxonomy of Ulva (Chlorophyceae) in the Netherlands. British Phycological Journal 16 (1):9–53. doi: 10.1080/00071618100650031.
- Kraan, S. 2012. Algal polysaccharides, novel applications and outlook. In Carbohydrates-comprehensive studies on glycobiology and glycotechnology. IntechOpen.
- Kraft, L. G. K., G. T. Kraft, and R. F. Waller. 2010. Investigations into Southern Australian Ulva (Ulvophyceae, Chlorophyta) taxonomy and molecular phylogeny indicate both cosmopolitanism and endemic cryptic species. Journal of Phycology 46 (6):1257–77. doi: 10.1111/j.1529-8817.2010.00909.x.
- Kumar, R., K. Trivedi, K. G. V. Anand, and A. Ghosh. 2020. Science behind biostimulant action of seaweed extract on growth and crop yield: Insights into transcriptional changes in roots of maize treated with Kappaphycus alvarezii seaweed extract under soil moisture stressed conditions. Journal of Applied Phycology 32 (1):599–613. doi: 10.1007/s10811-019-01938-y.
- Kumarathunge, N. C., J. M. P. Jayasinghe, and E. D. N. S. Abeyrathne. 2016. Development of sea lettuce (Ulva lactuca) and Catla (Catla catla) incorporated protein and fiber rich fish burger. International Journal of Research in Agricultural Sciences 3:2348–3997.
- Kusumawati, R., E. Sinurat, D. Fransiska, A. H. Purnomo, B. S. B. Utomo, and J. Basmal. 2022. Utilization of Ulva spp. in biscuit formulation: Feasibility studies at the household scale. In IOP Conference Series: Earth and Environmental Science, vol. 978, 12036. IOP Publishing.
- Kut Güroy, B., Cirik, D. Güroy, F. Sanver, and A. A. Tekinay. Ş 2007. Effects of Ulva rigida and Cystoseira barbata meals as a feed additive on growth performance, feed utilization, and body composition of Nile Tilapia, Oreochromis niloticus. Turkish Journal of Veterinary and Animal Sciences 31 (2):91–7.
- Lahaye, M., and A. Robic. 2007. Structure and function properties of Ulvan, a polysaccharide from green seaweeds. Biomacromolecules 8 (6):1765–74. doi: 10.1021/bm061185q.
- Lähteenmäki-Uutela, A., M. Rahikainen, M. T. Camarena-Gómez, J. Piiparinen, K. Spilling, and B. Yang. 2021. European Union legislation on macroalgae products. Aquaculture International 29 (2):487–509. doi: 10.1007/s10499-020-00633-x.
- Lamm, R. 2003. Governance barriers to sustainability. World Futures 59 (3–4):275–85. doi: 10.1080/02604020310119.
- Lanfer-Marquez, U. M., R. M. C. Barros, and P. Sinnecker. 2005. Antioxidant activity of chlorophylls and their derivatives. Food Research International 38 (8–9):885–91. doi: 10.1016/j.foodres.2005.02.012.
- Lange, L., K. O. Connor, S. Arason, U. Bundgård-Jørgensen, A. Canalis, D. Carrez, J. Gallagher, et al. 2021. Developing a sustainable and circular bio-based economy in EU: By partnering across sectors, upscaling and using new knowledge faster, and for the benefit of climate, environment and biodiversity, and people and business. Frontiers in Bioengineering and Biotechnology 8. doi: 10.3389/fbioe.2020.619066.
- Laramore, S., R. Baptiste, P. S. Wills, and M. D. Hanisak. 2018. Utilization of IMTA-produced Ulva lactuca to supplement or partially replace pelleted diets in shrimp (Litopenaeus vannamei) reared in a clear water production system. Journal of Applied Phycology 30 (6):3603–10. doi: 10.1007/s10811-018-1485-3.
- Latique, S., R. Ben Mrid, I. Kabach, A. Kchikich, H. Sammama, A. Yasri, M. Nhiri, M. El Kaoua, A. Douira, and K. Selmaoui. 2021. Foliar application of Ulva rigida water extracts improves salinity tolerance in wheat (Triticum durum L.). Agronomy 11 (2):265. doi: 10.3390/agronomy11020265.
- Lauber, K., R. Ralston, M. Mialon, A. Carriedo, and A. B. Gilmore. 2020. Non-communicable disease governance in the era of the sustainable development goals: A qualitative analysis of food industry framing in WHO consultations. Globalization and Health 16 (1):76. https://pubmed.ncbi.nlm.nih.gov/32847604. doi: 10.1186/s12992-020-00611-1.
- Lawton, R. J., L. Mata, R. de Nys, and N. A. Paul. 2013. Algal bioremediation of waste waters from land-based aquaculture using Ulva: Selecting target species and strains. PLOS One. 8 (10):e77344. doi: 10.1371/journal.pone.0077344.
- Lawton, R. J., J. E. Sutherland, C. R. K. Glasson, and M. E. Magnusson. 2021. Selection of temperate Ulva species and cultivars for land-based cultivation and biomass applications. Algal Research 56:102320. https://www.sciencedirect.com/science/article/abs/pii/S2211926421001399. doi: 10.1016/j.algal.2021.102320.
- Lee, S. Y., J. H. Chang, and S. B. Lee. 2014. Chemical composition, saccharification yield, and the potential of the green seaweed Ulva pertusa. Biotechnology and Bioprocess Engineering 19 (6):1022–33. doi: 10.1007/s12257-014-0654-8.
- Li, F., S. Zuo, Y. Chi, C. Du, Z. Shen, X. Han, X. Wang, and P. Wang. 2020. Alleviation of drought stress in wheat using exogenous Ulva prolifera extract produced by enzymatic hydrolysis. Journal of Renewable Materials 8 (11):1519–29. https://www.techscience.com/jrm/v8n11/40269. doi: 10.32604/jrm.2020.011453.
- Li, J.-Y., F. Yang, L. Jin, Q. Wang, J. Yin, P. He, and Y. Chen. 2018. Safety and quality of the green tide algal species Ulva prolifera for option of human consumption: A nutrition and contamination study. Chemosphere 210:1021–8. doi: 10.1016/j.chemosphere.2018.07.076.
- Lindberg, J., G. Lindberg, J. Teräs, G. Poulsen, S. Solberg, K. Tybirk, J. Przedrzymirska, et al. 2016. Nordic alternative protein potentials mapping of regional bioeconomy opportunities. Nordic Council of Ministers.
- Lomartire, S., and A. M. M. Gonçalves. 2022. An overview of potential seaweed-derived bioactive compounds for pharmaceutical applications. Marine Drugs 20 (2):141. https://pubmed.ncbi.nlm.nih.gov/35200670. doi: 10.3390/md20020141.
- Lopes, D., T. Melo, F. Rey, J. Meneses, F. L. Monteiro, L. A. Helguero, M. H. Abreu, A. I. Lillebø, R. Calado, and M. R. Domingues. 2020. Valuing bioactive lipids from green, red and brown macroalgae from aquaculture, to foster functionality and biotechnological applications. Molecules 25 (17):3883. https://www.mdpi.com/1420-3049/25/17/3883. doi: 10.3390/molecules25173883.
- Lopes, D., A. S. P. Moreira, F. Rey, E. da Costa, T. Melo, E. Maciel, A. Rego, M. H. Abreu, P. Domingues, R. Calado, et al. 2019. Lipidomic signature of the green macroalgae Ulva rigida farmed in a sustainable integrated multi-trophic aquaculture. Journal of Applied Phycology 31 (2):1369–81. doi: 10.1007/s10811-018-1644-6.
- Lorenzo, J. M., J. Sineiro, I. R. Amado, and D. Franco. 2014. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Science 96 (1):526–34. https://www.sciencedirect.com/science/article/pii/S0309174013004919. doi: 10.1016/j.meatsci.2013.08.007.
- Loureiro, R., C. M. M. Gachon, and C. Rebours. 2015. Seaweed cultivation: Potential and challenges of crop domestication at an unprecedented pace. New Phytologist 206 (2):489–92. doi: 10.1111/nph.13278.
- Løvdal, T., B. T. Lunestad, M. Myrmel, J. T. Rosnes, and D. Skipnes. 2021. Microbiological food safety of seaweeds. Foods 10 (11):2719. doi: 10.3390/foods10112719.
- Macey, B. M., M. J. Brand, M. Brink-Hull, M. D. Cyrus, and J. J. Bolton. 2021. Effluent grown Ulva as a functional ingredient for farmed abalone: Impacts on growth, physiology and microbiome. In Aquaculture.
- Machado, L., M. Magnusson, N. A. Paul, R. de Nys, and N. Tomkins. 2014. Effects of marine and freshwater macroalgae on in vitro total gas and methane production. PLOS One. 9 (1):e85289. doi: 10.1371/journal.pone.0085289.
- Madibana, M. J., V. Mlambo, B. Lewis, and C. Fouché. 2017. Effect of graded levels of dietary seaweed (Ulva sp.) on growth, hematological and serum biochemical parameters in dusky kob, Argyrosomus japonicus, Sciaenidae. Egyptian Journal of Aquatic Research 43 (3):249–54. doi: 10.1016/j.ejar.2017.09.003.
- Maehre, H. K., M. K. Malde, K.-E. Eilertsen, and E. O. Elvevoll. 2014. Characterization of protein, lipid and mineral contents in common Norwegian seaweeds and evaluation of their potential as food and feed. Journal of the Science of Food and Agriculture 94 (15):3281–90. doi: 10.1002/jsfa.6681.
- Magnoni, L. J., J. A. Martos-Sitcha, A. Queiroz, J. A. Calduch-Giner, J. F. M. Gonçalves, C. M. R. Rocha, H. T. Abreu, J. W. Schrama, R. O. A. Ozorio, and J. Pérez-Sánchez. 2017. Dietary supplementation of heat-treated Gracilaria and Ulva seaweeds enhanced acute hypoxia tolerance in gilthead sea bream (Sparus aurata). Biology Open 6 (6):897–908. https://pubmed.ncbi.nlm.nih.gov/28495962. doi: 10.1242/bio.024299.
- Magnusson, M., C. R. K. Glasson, M. J. Vucko, A. Angell, T. L. Neoh, and R. de Nys. 2019. Enrichment processes for the production of high-protein feed from the green seaweed Ulva ohnoi. Algal Research 41:101555. doi: 10.1016/j.algal.2019.101555.
- Magnusson, M., C. Carl, L. Mata, R. de Nys, and N. A. Paul. 2016. Seaweed salt from Ulva: A novel first step in a cascading biorefinery model. Algal Research 16:308–16. doi: 10.1016/j.algal.2016.03.018.
- Maia, M., R. G. A. J. M. Fonseca, H. M. Oliveira, C. Mendonça, and A. R. J. Cabrita. 2016. the potential role of seaweeds in the natural manipulation of rumen fermentation and methane production. Scientific Reports 6 (1):32321. doi: 10.1038/srep32321.
- Mamede, R., F. Ricardo, M. H. Abreu, E. F. da Silva, C. Patinha, and R. Calado. 2021. Spatial variability of elemental fingerprints of sea lettuce (Ulva spp.) and its potential use to trace geographic origin. Algal Research 59:102451. doi: 10.1016/j.algal.2021.102451.
- Maneein, S., J. J. Milledge, B. V. Nielsen, and P. J. Harvey. 2018. A review of seaweed pre-treatment methods for enhanced biofuel production by anaerobic digestion or fermentation. Fermentation 4 (4):100. doi: 10.3390/fermentation4040100.
- Mapelli-Brahm, P., F. J. Barba, F. Remize, C. Garcia, A. Fessard, A. Mousavi Khaneghah, A. S. Sant’Ana, J. M. Lorenzo, D. Montesano, and A. J. Meléndez-Martínez. 2020. The impact of fermentation processes on the production, retention and bioavailability of carotenoids: An overview. Trends in Food Science & Technology 99:389–401. doi: 10.1016/j.tifs.2020.03.013.
- Marinho, G., C. Nunes, I. Sousa-Pinto, R. Pereira, P. Rema, and L. M. P. Valente. 2013. The IMTA-cultivated Chlorophyta Ulva spp. as a sustainable ingredient in Nile Tilapia (Oreochromis niloticus) diets. Journal of Applied Phycology 25 (5):1359–67. doi: 10.1007/s10811-012-9965-3.
- Marrion, O., A. Schwertz, J. Fleurence, J. L. Guéant, and C. Villaume. 2003. Improvement of the digestibility of the proteins of the red alga Palmaria palmata by physical processes and fermentation. Nahrung [Food] 47 (5):339–44. doi: 10.1002/food.200390078.
- Martin, N., and H. Maes. 1979. Multivariate analysis. London, UK: Academic.
- Martínez-Antequera, F. P., J. A. Martos-Sitcha, J. M. Reyna, and F. J. Moyano. 2021. Evaluation of the inclusion of the green seaweed Ulva ohnoi as an ingredient in feeds for gilthead sea bream (Sparus aurata) and European sea bass (Dicentrarchus labrax). Animals 11 (6):1684. doi: 10.3390/ani11061684.
- Masasa, M., A. Kushmaro, E. Kramarsky-Winter, M. Shpigel, R. Barkan, A. Golberg, A. Kribus, N. Shashar, and L. Guttman. 2021. Mono-specific algal diets shape microbial networking in the gut of the sea urchin Tripneustes gratilla elatensis. Animal Microbiome 3 (1):79. doi: 10.1186/s42523-021-00140-1.
- Mata, L., M. Magnusson, N. A. Paul, and R. de Nys. 2016. The intensive land-based production of the green seaweeds Derbesia tenuissima and Ulva ohnoi: Biomass and bioproducts. Journal of Applied Phycology 28 (1):365–75. doi: 10.1007/s10811-015-0561-1.
- Mata, L., A. Schuenhoff, and R. Santos. 2010. A direct comparison of the performance of the seaweed biofilters, Asparagopsis armata and Ulva rigida. Journal of Applied Phycology 22 (5):639–44. doi: 10.1007/s10811-010-9504-z.
- Matos, G. S., S. G. Pereira, Z. A. Genisheva, A. M. Gomes, J. A. Teixeira, and C. M. R. Rocha. 2021. Advances in extraction methods to recover added-value compounds from seaweeds: Sustainability and functionality. Foods 10 (3):516. doi: 10.3390/foods10030516.
- Matshogo, T. B., C. M. Mnisi, and V. Mlambo. 2020. Dietary green seaweed compromises overall feed conversion efficiency but not blood parameters and meat quality and stability in broiler chickens. Agriculture 10 (11):547. doi: 10.3390/agriculture10110547.
- Matshogo, T. B., C. M. Mnisi, and V. Mlambo. 2021. Effect of pre-treating dietary green seaweed with proteolytic and fibrolytic enzymes on physiological and meat quality parameters of broiler chickens. Foods 10 (8):1862. doi: 10.3390/foods10081862.
- Mazarrasa, I., Y. S. Olsen, E. Mayol, N. Marbà, and C. M. Duarte. 2014. Global unbalance in seaweed production, research effort and biotechnology markets. Biotechnology Advances 32 (5):1028–36. doi: 10.1016/j.biotechadv.2014.05.002.
- Meléndez-Martínez, A. J., A. I. Mandić, F. Bantis, V. Böhm, G. I. A. Borge, M. Brnčić, A. Bysted, et al. 2021. A comprehensive review on carotenoids in foods and feeds: Status Quo, applications, patents, and research needs. Critical Reviews in Food Science and Nutrition 62 (8):1999–2049. doi: 10.1080/10408398.2020.1867959.
- Mendoza-Morales, L. T., A. C. Mendoza-González, L. E. Mateo Cid, and A. Rodríguez-Dorantes. 2019. Análisis Del Efecto de Extractos de Sargassum vulgare y Ulva fasciata Como Bioestimulantes Del Crecimiento de Lens esculenta. Mexican Journal of Biotechnology 4 (4):15–28. https://docs.wixstatic.com/ugd/38ce56_8b3325c087b84dd0a2901e56d8f3f940.pdf. doi: 10.29267/mxjb.2019.4.4.15.
- Menezes, B. S., M. S. Coelho, S. L. R. Meza, M. Salas-Mellado, and M. Souza. 2015. Macroalgal biomass as an additional ingredient of bread. International Food Research Journal 22 (2).
- Mhatre, A., S. Gore, A. Mhatre, N. Trivedi, M. Sharma, R. Pandit, A. Anil, and A. Lali. 2019. Effect of multiple product extractions on bio-methane potential of marine macrophytic green alga Ulva lactuca. Renewable Energy. 132 (March):742–51. doi: 10.1016/j.renene.2018.08.012.
- Michalak, I., K. Chojnacka, and D. Korniewicz. 2015. New feed supplement from macroalgae as the dietary source of microelements for pigs. Open Chemistry 13 (1). doi: 10.1515/chem-2015-0149.
- Michalak, I., B. Górka, P. P. Wieczorek, E. Rój, J. Lipok, B. Łęska, B. Messyasz, R. Wilk, G. Schroeder, A. Dobrzyńska-Inger, et al. 2016. Supercritical fluid extraction of algae enhances levels of biologically active compounds promoting plant growth. European Journal of Phycology 51 (3):243–52. doi: 10.1080/09670262.2015.1134813.
- Mihaila, A. A., C. R. K. Glasson, R. Lawton, S. Muetzel, G. Molano, and M. Magnusson. 2022. New temperate seaweed targets for mitigation of ruminant methane emissions: an in vitro assessment. Applied Phycology 3 (1):274–84. doi: 10.1080/26388081.2022.2059700.
- Mišurcová, L. 2011. Chemical composition of seaweeds. In Handbook of marine macroalgae, 171–92. Wiley.
- Mohammed, H. O., M. N. O’Grady, M. G. O’Sullivan, R. M. Hamill, K. N. Kilcawley, and J. P. Kerry. 2022. Acceptable inclusion levels for selected brown and red Irish seaweed species in pork sausages. Foods11(10):1522. doi: 10.3390/foods11101522.
- Morais, T., A. Inácio, T. Coutinho, M. Ministro, J. Cotas, L. Pereira, and K. Bahcevandziev. 2020. Seaweed potential in the animal feed: A review. Journal of Marine Science and Engineering 8 (8):559. doi: 10.3390/jmse8080559.
- Moreira, A., S. Cruz, R. Marques, and P. Cartaxana. 2022. The underexplored potential of green macroalgae in aquaculture. Reviews in Aquaculture 14 (1):5–26. doi: 10.1111/raq.12580.
- Mouritsen, O. G., P. Rhatigan, and J. L. Pérez-Lloréns. 2019. The rise of seaweed gastronomy: Phycogastronomy. Botanica Marina 62 (3):195–209. doi: 10.1515/bot-2018-0041.
- Mustafa, M. G., S. Wakamatsu, T. Takeda, T. Umino, and H. Nakagawa. 1995. Effect of algae as a feed additive on growth performance in red sea bream, Pagrus major. Trace Nutrients Research 12:67–72.
- Nair, R. M., V. N. Boddepalli, M.-R. Yan, V. Kumar, B. Gill, R. S. Pan, C. Wang, G. L. Hartman, R. Silva e Souza, and P. Somta. 2023. Global status of vegetable soybean. Plants 12 (3):609. doi: 10.3390/plants12030609.
- Nakagawa, H., S. Kasahara, and T. Sugiyama. 1987. Effect of Ulva meal supplementation on lipid metabolism of black sea bream, Acanthopagrus schlegeli (Bleeker). Aquaculture 62 (2):109–21. 10.1016/0044-8486(87)90315-2.
- Natify, W., M. Droussi, N. Berday, A. Araba, and M. Benabid. 2015. Effect of the seaweed Ulva lactuca as a feed additive on growth performance, feed utilization and body composition of Nile Tilapia (Oreochromis niloticus L.). International Journal of Agriculture and Agricultural Research 7:85–92.
- Naylor, R. L., R. W. Hardy, D. P. Bureau, A. Chiu, M. Elliott, A. P. Farrell, I. Forster, et al. 2009. Feeding aquaculture in an era of finite resources. Proceedings of the National Academy of Sciences of the United States of America 106 (36):15103–10. https://pubmed.ncbi.nlm.nih.gov/19805247. doi: 10.1073/pnas.0905235106.
- Neto, R., C. Marçal, A. Queirós, H. Abreu, A. Silva, and S. Cardoso. 2018. Screening of Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima as functional ingredients. International Journal of Molecular Sciences 19 (10): 2987. doi: 10.3390/ijms19102987.
- New Zealand Food Safety. 2023. Evaluation of food safety risks associated with seaweed and seaweed products. New Zealand Food Safety Technical Paper No: 2023/01. Palmerston North: New Zealand Food Safety Science & Research Centre.
- Nhlane, L. T., C. M. Mnisi, V. Mlambo, and M. J. Madibana. 2021. Effect of seaweed-containing diets on visceral organ sizes, carcass characteristics, and meat quality and stability of Boschveld indigenous hens. Poultry Science 100 (2):949–56. https://www.sciencedirect.com/science/article/pii/S0032579120308907. doi: 10.1016/j.psj.2020.11.038.
- Nielsen, C. W., S. L. Holdt, J. J. Sloth, G. S. Marinho, M. Sæther, J. Funderud, and T. Rustad. 2020. Reducing the high iodine content of Saccharina latissima and improving the profile of other valuable compounds by water blanching. Foods 9 (5):569. doi: 10.3390/foods9050569.
- Nitschke, U., and D. B. Stengel. 2016. Quantification of iodine loss in edible Irish seaweeds during processing. Journal of Applied Phycology 28 (6):3527–33. doi: 10.1007/s10811-016-0868-6.
- Nordic Council of Ministers. 2023. A Nordic approach to food safety risk management of seaweed for use as food. https://pub.norden.org/temanord2022-564.
- Ólafsdóttir, G., G. Viera, E. Larsen, T. Nielsen, G. M. Ingólfsdóttir, E. Yngvadóttir, and S. Bogason. 2013. D.1.1, Key environmental challenges for food groups and regions representing the variation within the EU, Ch.3 Salmon Aquaculture Supply Chain. SENSE: Harmonised Environmental Sustainability in the European food and drink chain. http://www.ascs.is/wp-content/uploads/2010/04/SENSE_D1_1_Aquaculture_review.pdf
- Olsson, J., S. Raikova, J. J. Mayers, S. Steinhagen, C. J. Chuck, G. M. Nylund, and E. Albers. 2020. Effects of geographical location on potentially valuable components in Ulva intestinalis sampled along the Swedish Coast. Applied Phycology 1 (1):80–92. doi: 10.1080/26388081.2020.1827454.
- Olsson, J., G. B. Toth, A. Oerbekke, S. Cvijetinovic, N. Wahlström, H. Harrysson, S. Steinhagen, A. Kinnby, J. White, U. Edlund, et al. 2020. Cultivation conditions affect the monosaccharide composition in Ulva fenestrata. Journal of Applied Phycology 32 (5):3255–63. doi: 10.1007/s10811-020-02138-9.
- Ortiz, J., N. Romero, P. Robert, J. Araya, J. Lopez-Hernández, C. Bozzo, E. Navarrete, A. Osorio, and A. Rios. 2006. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chemistry 99 (1):98–104. doi: 10.1016/j.foodchem.2005.07.027.
- Osuna-Ruíz, I., A. K. D. Ledezma, E. Martínez-Montaño, J. A. Salazar-Leyva, V. A. R. Tirado, and I. B. García. 2023. Enhancement of in-vitro antioxidant properties and growth of amaranth seed sprouts treated with seaweed extracts. Journal of Applied Phycology 35 (1):471–81. doi: 10.1007/s10811-022-02872-2.
- Øverland, M., L. T. Mydland, and A. Skrede. 2019. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. Journal of the Science of Food and Agriculture 99 (1):13–24. doi: 10.1002/jsfa.9143.
- Paiva, L., E. Lima, A. I. Neto, M. Marcone, and J. Baptista. 2016. Health-promoting ingredients from four selected Azorean macroalgae. Food Research International 89 (November):432–8. doi: 10.1016/j.foodres.2016.08.007.
- Patrick, R., and J. Kingsley. 2017. Health promotion and sustainability programmes in Australia: Barriers and enablers to evaluation. Global Health Promotion 26 (2):82–92. doi: 10.1177/1757975917715038.
- Paulert, R., R. Ascrizzi, S. Malatesta, P. Berni, M. D. Noseda, M. Mazetto de Carvalho, I. Marchioni, et al. 2021. Ulva intestinalis extract acts as biostimulant and modulates metabolites and hormone balance in basil (Ocimum Basilicum L.) and parsley (Petroselinum Crispum L.). Plants 10 (7):1391. doi: 10.3390/plants10071391.
- Peñalver, R., J. M. Lorenzo, G. Ros, R. Amarowicz, M. Pateiro, and G. Nieto. 2020. Seaweeds as a functional ingredient for a healthy diet. Marine Drugs 18 (6):301. doi: 10.3390/md18060301.
- Peña-Rodríguez, A., T. P. Mawhinney, D. Ricque-Marie, and L. E. Cruz-Suárez. 2011. Chemical composition of cultivated seaweed Ulva clathrata (Roth) C. Agardh. Food Chemistry 129 (2)November): :491–8. doi: 10.1016/j.foodchem.2011.04.104.
- Pezoa-Conte, R., A. Leyton, I. Anugwom, S. von Schoultz, J. Paranko, P. Mäki-Arvela, S. Willför, M. Muszyński, J. Nowicki, M. E. Lienqueo, et al. 2015. Deconstruction of the green alga Ulva rigida in ionic liquids: Closing the mass balance. Algal Research 12 (November):262–73. doi: 10.1016/j.algal.2015.09.011.
- Pinheiro, V. F., C. Marçal, H. Abreu, J. A. Lopes da Silva, A. M. S. Silva, and S. M. Cardoso. 2019. Physicochemical changes of air-dried and salt-processed Ulva rigida over storage time. Molecules 24 (16):2955. doi: 10.3390/molecules24162955.
- Pirian, K., K. Piri, J. Sohrabipour, S. T. Jahromi, and J. Blomster. 2016. Nutritional and phytochemical evaluation of the common green algae, Ulva spp. (Ulvophyceae), from the Persian Gulf. Fundamental and Applied Limnology 188 (4):315–27. doi: 10.1127/fal/2016/0947.
- Polikovsky, M., G. Califano, N. Dunger, T. Wichard, and A. Golberg. 2020. Engineering bacteria-seaweed symbioses for modulating the photosynthate content of Ulva (Chlorophyta): Significant for the feedstock of bioethanol production. Algal Research 49:101945. doi: 10.1016/j.algal.2020.101945.
- Population Reference Bureau. 2023. World population data sheet. https://www.prb.org/wp-content/uploads/2023/12/2023-World-Population-Data-Sheet-Booklet.pdf
- Postma, P. R., O. Cerezo-Chinarro, R. J. Akkerman, G. Olivieri, R. H. Wijffels, W. A. Brandenburg, and M. H. M. Eppink. 2018. Biorefinery of the macroalgae Ulva lactuca: Extraction of proteins and carbohydrates by mild disintegration. Journal of Applied Phycology 30 (2):1281–93. doi: 10.1007/s10811-017-1319-8.
- Prabhu, M., A. Chemodanov, R. Gottlieb, M. Kazir, O. Nahor, M. Gozin, A. Israel, Y. D. Livney, and A. Golberg. 2019. Starch from the sea: The green macroalga Ulva ohnoi as a potential source for sustainable starch production in the marine biorefinery. Algal Research 37:215–27. doi: 10.1016/j.algal.2018.11.007.
- Prato, E., G. Fanelli, A. Angioni, F. Biandolino, I. Parlapiano, L. Papa, G. Denti, M. Secci, M. Chiantore, M. S. Kelly, et al. 2018. Influence of a prepared diet and a macroalga (Ulva sp.) on the growth, nutritional and sensory qualities of gonads of the sea urchin Paracentrotus lividus. Aquaculture 493 (August):240–50. doi: 10.1016/j.aquaculture.2018.05.010.
- Qi, H., T. Zhao, Q. Zhang, Z. Li, Z. Zhao, and R. Xing. 2005. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). Journal of Applied Phycology 17 (6):527–34. doi: 10.1007/s10811-005-9003-9.
- Qiu, S., S. Ge, P. Champagne, and R. M. Robertson. 2017. Potential of Ulva lactuca for municipal wastewater bioremediation and fly food. Desalination and Water Treatment 91:23–30. doi: 10.5004/dwt.2017.20767.
- Qiu, X., A. Neori, J. K. Kim, C. Yarish, M. Shpigel, L. Guttman, D. Ben Ezra, V. Odintsov, and D. A. Davis. 2017. Evaluation of green seaweed Ulva sp. as a replacement of fish meal in plant-based practical diets for Pacific white shrimp, Litopenaeus vannamei. Journal of Applied Phycology 30 (2):1305–16. doi: 10.1007/s10811-017-1278-0.
- Queirós, A. S., A. R. Circuncisão, E. Pereira, M. Válega, M. H. Abreu, A. M. S. Silva, and S. M. Cardoso. 2021. Valuable nutrients from Ulva rigida: Modulation by seasonal and cultivation factors. Applied Sciences 11 (13):6137. doi: 10.3390/app11136137.
- Quero, G. M., L. Fasolato, C. Vignaroli, and G. M. Luna. 2015. Understanding the association of Escherichia coli with diverse macroalgae in the Lagoon of Venice. Scientific Reports 5 (1):1–11. doi: 10.1038/srep10969.
- Radulovich, R., A. Neori, D. Valderrama, C. R. K. Reddy, H. Cronin, and J. Forster. 2015. Farming of seaweeds. Seaweed Sustainability 1:27–59. https://www.sciencedirect.com/science/article/abs/pii/B9780124186972000039.
- Rajapakse, N., and S. K. Kim. 2011. Nutritional and digestive health benefits of seaweed. Advances in food and nutrition research 64:17–28
- Rama Nisha, P., A. Elezabeth Mary, M. Uthayasiva, and S. Arularasan. 2014. Seaweed Ulva Reticulata a potential feed supplement for growth, colouration and disease resistance in fresh water ornamental gold fish, Carassius auratus. Journal of Aquaculture Research and Development 5 (5):1000254.
- Rashad, S., G. El-Chaghaby, E. C. Lima, and G. Simoes dos Reis. 2023. Optimizing the ultrasonic-assisted extraction of antioxidants from Ulva lactuca algal biomass using factorial design. Biomass Conversion and Biorefinery 13 (7):5681–90. doi: 10.1007/s13399-021-01516-8.
- Ratana-Arporn, P., and A. Chirapart. 2006. Nutritional evaluation of tropical green seaweeds Caulerpa lentillifera and Ulva reticulata. Agriculture and Natural Resources 40 (6):75–83. https://li01.tci-thaijo.org/index.php/anres/article/view/244017.
- Reboleira, J., S. Silva, A. Chatzifragkou, K. Niranjan, and M. F. L. Lemos. 2021. Seaweed fermentation within the fields of food and natural products. Trends in Food Science & Technology 116 (October):1056–73. doi: 10.1016/j.tifs.2021.08.018.
- Rey-Crespo, F., M. López-Alonso, and M. Miranda. 2014. The use of seaweed from the Galician Coast as a mineral supplement in organic dairy cattle. Animal 8 (4):580–6. https://www.cambridge.org/core/product/BC62F1F0022E5EB29716B5CD5D721F3D. doi: 10.1017/S1751731113002474.
- Ribeiro, D. M., D. Coelho, M. Costa, D. F. P. Carvalho, C. C. Leclercq, J. Renaut, J. P. B. Freire, A. M. Almeida, and J. A. Mestre Prates. 2024. Integrated transcriptomics and proteomics analysis reveals muscle metabolism effects of dietary Ulva lactuca and ulvan lyase supplementation in weaned piglets. Scientific Reports 14 (1):4589. doi: 10.1038/s41598-024-55462-2.
- Rieve, K. 2023. Are investors in the seaweed sector looking in the wrong place? The Fish Site. https://thefishsite.com/articles/are-investors-in-the-seaweed-sector-looking-in-the-wrong-place-hatch-seaweed-insights#:∼:text=Our%20extensive%20research%20suggests%20that,growth%20of%20the%20seaweed%20sector.
- Roberts, D. A., and R. de Nys. 2016. The effects of feedstock pre-treatment and pyrolysis temperature on the production of biochar from the green seaweed Ulva. Journal of Environmental Management 169:253–60. doi: 10.1016/j.jenvman.2015.12.023.
- Robic, A., C. Rondeau-Mouro, J.-F. Sassi, Y. Lerat, and M. Lahaye. 2009. Structure and interactions of Ulvan in the cell wall of the marine green algae Ulva rotundata (Ulvales, Chlorophyceae). Carbohydrate Polymers 77 (2):206–16. doi: 10.1016/j.carbpol.2008.12.023.
- Robin, A., P. Chavel, A. Chemodanov, A. Israel, and A. Golberg. 2017. Diversity of monosaccharides in marine macroalgae from the Eastern Mediterranean Sea. Algal Research 28 (December):118–27. doi: 10.1016/j.algal.2017.10.005.
- Robin, A., M. Kazir, M. Sack, A. Israel, W. Frey, G. Mueller, Y. D. Livney, and A. Golberg. 2018. Functional protein concentrates extracted from the green marine macroalga Ulva sp., by high voltage pulsed electric fields and mechanical press. ACS Sustainable Chemistry & Engineering 6 (11):13696–705. doi: 10.1021/acssuschemeng.8b01089.
- Rodríguez-Bernaldo de Quirós, A., and J. López-Hernández. 2021. An overview on effects of processing on the nutritional content and bioactive compounds in seaweeds. Foods 10 (9):2168. doi: 10.3390/foods10092168.
- Roleda, M. Y., and S. Heesch. 2021. Chemical profiling of Ulva species for food applications: What is in a name? Food Chemistry 361:130084. doi: 10.1016/j.foodchem.2021.130084.
- Roleda, M. Y., S. Lage, D. F. Aluwini, C. Rebours, M. B. Brurberg, U. Nitschke, and F. G. Gentili. 2021. Chemical profiling of the Arctic Sea lettuce Ulva lactuca (Chlorophyta) mass-cultivated on land under controlled conditions for food applications. Food Chemistry 341:127999.
- Roohinejad, S., M. Koubaa, F. J. Barba, S. Saljoughian, M. Amid, and R. Greiner. 2017. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Research International 99:1066–83. doi: 10.1016/j.foodres.2016.08.016.
- Rosa, J., M. F. L. Lemos, D. Crespo, M. Nunes, A. Freitas, F. Ramos, M. Â. Pardal, and S. Leston. 2020. Integrated multitrophic aquaculture systems – potential risks for food safety. Trends in Food Science & Technology 96:79–90. doi: 10.1016/j.tifs.2019.12.008.
- Rosa, J., S. Leston, A. Freitas, A. S. Vila Pouca, J. Barbosa, M. F. L. Lemos, M. A. Pardal, and F. Ramos. 2019. Oxytetracycline accumulation in the macroalgae Ulva: Potential risks for IMTA systems. Chemosphere 226 (July):60–6. doi: 10.1016/j.chemosphere.2019.03.112.
- Rothman, M. D., R. J. Anderson, L. Kandjengo, and J. J. Bolton. 2020. Trends in seaweed resource use and aquaculture in South Africa and Namibia over the last 30 years. Botanica Marina 63 (4):315–25. doi: 10.1515/bot-2019-0074.
- Rouphael, Y., and G. Colla. 2018. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Frontiers in Plant Science 9:1655. https://www.frontiersin.org/article/10.3389/fpls.2018.01655/full. doi: 10.3389/fpls.2018.01655.
- Ruangrit, K., S. Chaipoot, R. Phongphisutthinant, K. Duangjan, K. Phinyo, I. Jeerapan, J. Pekkoh, and S. Srinuanpan. 2023. A successful biorefinery approach of macroalgal biomass as a promising sustainable source to produce bioactive nutraceutical and biodiesel. Biomass Conversion and Biorefinery 13 (2):1089–99. doi: 10.1007/s13399-021-01310-6.
- Saadaoui, I., R. Rasheed, A. Aguilar, M. Cherif, H. Al Jabri, S. Sayadi, and S. R. Manning. 2021. Microalgal-based feed: Promising alternative feedstocks for livestock and poultry production. Journal of Animal Science and Biotechnology 12 (1):76. doi: 10.1186/s40104-021-00593-z.
- Sáez, M. I., A. Vizcaíno, A. Galafat, V. Anguís, C. Fernández-Díaz, M. C. Balebona, F. J. Alarcón, and T. F. Martínez. 2020. Assessment of long-term effects of the macroalgae Ulva ohnoi included in diets on Senegalese Sole (Solea senegalensis) fillet quality. Algal Research 47:101885. doi: 10.1016/j.algal.2020.101885.
- Sahana, B. N., M. K. PrasannaKumar, H. B. Mahesh, P. Buela Parivallal, M. E. Puneeth, C. Gautam, T. R. Girish, S. Nori, and S. Suryanarayan. 2022. Biostimulants derived from red seaweed stimulate the plant defence mechanism in rice against Magnaporthe oryzae. Journal of Applied Phycology 34 (1):659–65. doi: 10.1007/s10811-021-02627-5.
- Samara, E. M., A. B. Okab, K. A. Abdoun, A. M. El-Waziry, and A. A. Al-Haidary. 2013. Subsequent influences of feeding intact green seaweed Ulva lactuca to growing lambs on the seminal and testicular characteristics in rams. Journal of Animal Science 91 (12):5654–67. doi: 10.2527/jas.2013-6719.
- Santiago, A., and R. Moreira. 2020. Drying of edible seaweeds. In Sustainable seaweed technologies, 131–54. Amsterdam: Elsevier.
- Santizo-Taan, R., M. Bautista-Teruel, and J. R. H. Maquirang. 2020. Enriched Ulva Pertusa as partial replacement of the combined fish and soybean meals in juvenile abalone Haliotis asinina (Linnaeus) diet. Journal of Applied Phycology 32 (1):741–9. doi: 10.1007/s10811-019-01977-5.
- Sauvageau, C. 1920. Utilisation des algues marines. Encyclopédie scientifique. Paris, France: Librairie Octave Doin.
- Sebök, S., W. B. Herppich, and D. Hanelt. 2019. Outdoor cultivation of Ulva lactuca in a recently developed ring-shaped photobioreactor: Effects of elevated CO2 concentration on growth and photosynthetic performance. Botanica Marina 62 (2):179–90. http://www.degruyter.com/view/j/botm.2019.62.issue-2/bot-2018-0016/bot-2018-0016.xml. doi: 10.1515/bot-2018-0016.
- Shahar, B., and L. Guttman. 2020. An integrated, two-step biofiltration system with Ulva fasciata for sequenced removal of ammonia and nitrate in mariculture effluents. Algal Research 52:102120. doi: 10.1016/j.algal.2020.102120.
- Shahar, B., M. Shpigel, R. Barkan, M. Masasa, A. Neori, H. Chernov, E. Salomon, M. Kiflawi, and L. Guttman. 2020. Changes in metabolism, growth and nutrient uptake of Ulva fasciata (Chlorophyta) in response to nitrogen source. Algal Research 46:101781. doi: 10.1016/j.algal.2019.101781.
- Shannon, E., and N. Abu-Ghannam. 2019. Seaweeds as nutraceuticals for health and nutrition. Phycologia 58 (5):563–77. doi: 10.1080/00318884.2019.1640533.
- Sharmiladevi, N., A. Swetha, and K. P. Gopinath. 2023. Processing of Gracilaria edulis and Ulva lactuca for bioethanol and bio-oil production: An integrated approach via fermentation and hydrothermal liquefaction. Biomass Conversion and Biorefinery 13 (12):11099–107. doi: 10.1007/s13399-021-01925-9.
- Shefer, S., M. Lebendiker, A. Finkelshtein, D. A. Chamovitz, and A. Golberg. 2022. Ulvan crude extract’s chemical and biophysical profile and its effect as a biostimulant on Arabidopsis thaliana. Algal Research 62:102609. https://www.sciencedirect.com/science/article/abs/pii/S2211926421004288. doi: 10.1016/j.algal.2021.102609.
- Shpigel, M., L. Guttman, L. Shauli, V. Odintsov, D. Ben-Ezra, and S. Harpaz. 2017. Ulva lactuca from an integrated multi-trophic aquaculture (IMTA) biofilter system as a protein supplement in gilthead seabream (Sparus aurata) diet. Aquaculture 481:112–8. doi: 10.1016/j.aquaculture.2017.08.006.
- Shpigel, M., L. Shauli, V. Odintsov, N. Ashkenazi, and D. Ben-Ezra. 2018. Ulva lactuca biofilter from a land-based integrated multi trophic aquaculture (IMTA) system as a sole food source for the tropical sea urchin Tripneustes gratilla elatensis. Aquaculture 496:221–31. doi: 10.1016/j.aquaculture.2018.06.038.
- Shukla, P. S., E. G. Mantin, M. Adil, S. Bajpai, A. T. Critchley, and B. Prithiviraj. 2019. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Frontiers in Plant Science 10:655. https://www.frontiersin.org/article/10.3389/fpls.2019.00655/full. doi: 10.3389/fpls.2019.00655.
- Shuuluka, D., J. J. Bolton, and R. J. Anderson. 2013. Protein content, amino acid composition and nitrogen-to-protein conversion factors of Ulva rigida and Ulva capensis from natural populations and Ulva lactuca from an aquaculture system, in South Africa. Journal of Applied Phycology 25 (2):677–85. doi: 10.1007/s10811-012-9902-5.
- Siddik, M. A. B., and N. T. N. Anh. 2015. Preliminary assessment of the gut weed Ulva intestinalis as food for herbivorous fish. International Aquatic Research 7 (1):41–6. doi: 10.1007/s40071-014-0091-5.
- Silva, A. F. R., H. Abreu, A. M. S. Silva, and S. M. Cardoso. 2019. Effect of oven-drying on the recovery of valuable compounds from Ulva rigida, Gracilaria sp. and Fucus vesiculosus. Marine Drugs 17 (2):90. doi: 10.3390/md17020090.
- Silva, D. M., L. M. P. Valente, I. Sousa-Pinto, R. Pereira, M. A. Pires, F. Seixas, and P. Rema. 2015. Evaluation of IMTA-produced seaweeds (Gracilaria, Porphyra, and Ulva) as dietary ingredients in Nile Tilapia, Oreochromis niloticus L., juveniles. Effects on growth performance and gut histology. Journal of Applied Phycology 27 (4):1671–80. doi: 10.1007/s10811-014-0453-9.
- Simon, C., M. McHale, and R. Sulpice. 2022. Applications of Ulva biomass and strategies to improve its yield and composition: A perspective for Ulva aquaculture. Biology 11 (11):1593. https://pubmed.ncbi.nlm.nih.gov/36358294. doi: 10.3390/biology11111593.
- Smetacek, V., and A. Zingone. 2013. Green and golden seaweed tides on the rise. Nature 504 (7478):84–8. doi: 10.1038/nature12860.
- Stedt, K., O. Gustavsson, B. Kollander, I. Undeland, G. B. Toth, and H. Pavia. 2022. Cultivation of Ulva fenestrata using herring production process waters increases biomass yield and protein content. Frontiers in Marine Science 9:988523. doi: 10.3389/fmars.2022.988523.
- Stedt, K., G. B. Toth, J. Davegård, H. Pavia, and S. Steinhagen. 2022. Determination of nitrogen content in Ulva fenestrata by color image analysis – a rapid and cost-efficient method to estimate nitrogen content in seaweeds. Frontiers in Marine Science 9:2510. https://www.frontiersin.org/articles/10.3389/fmars.2022.1081870/full. doi: 10.3389/fmars.2022.1081870.
- Stedt, K., J. P. Trigo, S. Steinhagen, G. M. Nylund, B. Forghani, H. Pavia, and I. Undeland. 2022. Cultivation of seaweeds in food production process waters: Evaluation of growth and crude protein content. Algal Research 63:102647. doi: 10.1016/j.algal.2022.102647.
- Steinbruch, E., D. Drabik, M. Epstein, S. Ghosh, M. S. Prabhu, M. Gozin, A. Kribus, and A. Golberg. 2020. Hydrothermal processing of a green seaweed Ulva sp. for the production of monosaccharides, polyhydroxyalkanoates, and hydrochar. Bioresource Technology 318 (December):124263. doi: 10.1016/j.biortech.2020.124263.
- Steinhagen, S., F. Weinberger, and R. Karez. 2019. Molecular analysis of Ulva compressa (Chlorophyta, Ulvales) reveals its morphological plasticity, distribution and potential invasiveness on German North Sea and Baltic Sea Coasts. European Journal of Phycology 54 (1):102–14. doi: 10.1080/09670262.2018.1513167.
- Steinhagen, S., S. Enge, G. Cervin, K. Larsson, U. Edlund, A. E. M. Schmidt, N. Wahlström, B. Kollander, H. Pavia, I. Undeland, et al. 2022. harvest time can affect the optimal yield and quality of sea lettuce (Ulva fenestrata) in a sustainable sea-based cultivation. Frontiers in Marine Science 9:816890. doi: 10.3389/fmars.2022.816890.
- Steinhagen, S., S. Enge, K. Larsson, J. Olsson, G. M. Nylund, E. Albers, H. Pavia, I. Undeland, and G. B. Toth. 2021. Sustainable large-scale aquaculture of the northern hemisphere sea lettuce, Ulva fenestrata, in an off-shore seafarm. Journal of Marine Science and Engineering 9 (6):615. doi: 10.3390/jmse9060615.
- Steinhagen, S., S. Hoffmann, H. Pavia, and G. B. Toth. 2023. Molecular identification of the ubiquitous green algae Ulva Reveals high biodiversity, crypticity, and invasive species in the Atlantic-Baltic sea region. Algal Research 73:103132. https://www.sciencedirect.com/science/article/pii/S2211926423001650. doi: 10.1016/j.algal.2023.103132.
- Steinhagen, S., L. Kramár, and G. B. Toth. 2022. The unheeded existence of the tubular greens: Molecular analyses reveal the distribution of a new Ulva species (Ulvophyceae, Chlorophyta), Ulva Capillata sp. Nov. in the Atlantic-Baltic Sea transect. Journal of Applied Phycology 35 (1):509–22. doi: 10.1007/s10811-022-02886-w.
- Steinhagen, S., K. Larsson, J. Olsson, E. Albers, I. Undeland, H. Pavia, and G. B. Toth. 2022. Closed life-cycle aquaculture of sea lettuce (Ulva fenestrata): Performance and biochemical profile differ in early developmental stages. Frontiers in Marine Science 9:942679. doi: 10.3389/fmars.2022.942679.
- Stengel, D. B., S. Connan, and Z. A. Popper. 2011. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnology Advances 29 (5):483–501. https://linkinghub.elsevier.com/retrieve/pii/S0734975011000711. doi: 10.1016/j.biotechadv.2011.05.016.
- Stévant, P., H. Marfaing, A. Duinker, J. Fleurence, T. Rustad, I. Sandbakken, and A. Chapman. 2018. Biomass soaking treatments to reduce potentially undesirable compounds in the edible seaweeds sugar kelp (Saccharina latissima) and winged kelp (Alaria esculenta) and health risk estimation for human consumption. Journal of Applied Phycology 30 (3):2047–60. doi: 10.1007/s10811-017-1343-8.
- Stévant, P., A. Ólafsdóttir, P. Déléris, J. Dumay, J. Fleurence, B. Ingadóttir, R. Jónsdóttir, É. Ragueneau, C. Rebours, and T. Rustad. 2020. Semi-dry storage as a maturation process for improving the sensory characteristics of the edible red seaweed dulse (Palmaria palmata). Algal Research 51 (October):102048. https://linkinghub.elsevier.com/retrieve/pii/S2211926419310938. doi: 10.1016/j.algal.2020.102048.
- Stévant, P., C. Rebours, and A. Chapman. 2017. Seaweed aquaculture in Norway: Recent industrial developments and future perspectives. Aquaculture International 25 (4):1373–90. doi: 10.1007/s10499-017-0120-7.
- Stokvis, L., M. M. van Krimpen, R. P. Kwakkel, and P. Bikker. 2021. Evaluation of the nutritional value of seaweed products for broiler chickens’ nutrition. Animal Feed Science and Technology 280 (October):115061. doi: 10.1016/j.anifeedsci.2021.115061.
- Stokvis, L., C. Rayner, M. M. van Krimpen, J. Kals, W. H. Hendriks, and R. P. Kwakkel. 2022. A proteolytic enzyme treatment to improve Ulva laetevirens and Solieria chordalis seaweed co-product digestibility, performance, and health in broilers. Poultry Science 101 (5):101777. doi: 10.1016/j.psj.2022.101777.
- Strauss, S. 2023. Seaweed fermentation. In Functional ingredients from algae for foods and nutraceuticals. Cambridge: Elsevier.
- Suarez, M. G., and M. Gallissot. 2016. Effects of an algae-clay based biocatalyst on ileal digestibility performance of growing pigs, 15. Vienna: BOKU-Symposium Tierernährung.
- Suckling, C. C., M. D. Zavell, A. L. Byczynski, and B. T. Takeda. 2022. Assessing the potential of the unexploited Atlantic Purple sea urchin, Arbacia punctulata, for the edible market. Frontiers in Marine Science 9:895061. doi: 10.3389/fmars.2022.895061.
- Sudhakar, K., R. Mamat, M. Samykano, W. H. Azmi, W. F. W. Ishak, and T. Yusaf. 2018. An overview of marine macroalgae as bioresource. Renewable and Sustainable Energy Reviews 91:165–79. doi: 10.1016/j.rser.2018.03.100.
- Sudhakar, M. P., B. R. Kumar, T. Mathimani, and K. Arunkumar. 2019. a review on bioenergy and bioactive compounds from microalgae and macroalgae-sustainable energy perspective. Journal of Cleaner Production 228:1320–33. doi: 10.1016/j.jclepro.2019.04.287.
- Tabarsa, M., M. Rezaei, Z. Ramezanpour, and J. R. Waaland. 2012. Chemical compositions of the marine algae Gracilaria salicornia (Rhodophyta) and Ulva lactuca (Chlorophyta) as a potential food source. Journal of the Science of Food and Agriculture 92 (12):2500–6. doi: 10.1002/jsfa.5659.
- Taboada, C., R. Millán, and I. MÃguez. 2009. Composition, nutritional aspects and effect on serum parameters of marine algae Ulva rigida. Journal of the Science of Food and Agriculture 90 (3):445–449. doi: 10.1002/jsfa.3836.
- Tayyab, U., M. Novoa-Garrido, M. Y. Roleda, V. Lind, and M. R. Weisbjerg. 2016. Ruminal and intestinal protein degradability of various seaweed species measured in situ in dairy cows. Animal Feed Science and Technology 213:44–54. doi: 10.1016/j.anifeedsci.2016.01.003.
- Thavasi Alagan, V., R. Nakulan Vatsala, I. Sagadevan, V. Subbiah, and V. Ragothaman. 2020. Effect of dietary supplementation of seaweed (Ulva lactuca) and azolla on growth performance, haematological and serum biochemical parameters of aseel chicken. Beni-Suef University Journal of Basic and Applied Sciences 9 (1):58. doi: 10.1186/s43088-020-00087-3.
- Thompson, S. E., and J. C. Coates. 2017. Surface sensing and stress-signalling in Ulva and fouling diatoms–potential targets for antifouling: A review. Biofouling 33 (5):410–32. doi: 10.1080/08927014.2017.1319473.
- Thunyawanichnondh, J., N. Suebsiri, S. Leartamonchaikul, W. Pimolsri, W. Jittanit, and S. Charoensiddhi. 2020. Potential of green seaweed Ulva rigida in Thailand for healthy snacks. Journal of Fisheries and Environment 44 (1):29–39.
- Toth, G. B., H. Harrysson, N. Wahlström, J. Olsson, A. Oerbekke, S. Steinhagen, A. Kinnby, J. White, E. Albers, U. Edlund, et al. 2020. Effects of irradiance, temperature, nutrients, and pco2 on the growth and biochemical composition of cultivated Ulva fenestrata. Journal of Applied Phycology 32 (5):3243–54. doi: 10.1007/s10811-020-02155-8.
- Tran, L.-A. T., C. Vieira, S. Steinhagen, C. A. Maggs, M. Hiraoka, S. Shimada, T. Van Nguyen, O. De Clerck, and F. Leliaert. 2022. An appraisal of Ulva (Ulvophyceae, Chlorophyta) taxonomy. Journal of Applied Phycology 34 (5):2689–703. doi: 10.1007/s10811-022-02815-x.
- Trigo, J. P., N. Engström, S. Steinhagen, L. Juul, H. Harrysson, G. B. Toth, H. Pavia, N. Scheers, and I. Undeland. 2021. In vitro digestibility and Caco-2 cell bioavailability of sea lettuce (Ulva fenestrata) proteins extracted using PH-shift processing. Food Chemistry 356:129683. doi: 10.1016/j.foodchem.2021.129683.
- Trivedi, J., M. Aila, D. P. Bangwal, S. Kaul, and M. O. Garg. 2015. Algae based biorefinery – How to make sense? Renewable and Sustainable Energy Reviews 47:295–307. doi: 10.1016/j.rser.2015.03.052.
- Troell, M., P. J. G. Henriksson, A. H. Buschmann, T. Chopin, and S. Quahe. 2022. Farming the ocean – seaweeds as a quick fix for the climate? Reviews in Fisheries Science & Aquaculture 31 (3):285–95. doi: 10.1080/23308249.2022.2048792.
- Turan, G., and S. Cırık. 2018. Sea vegetables. In Vegetables – importance of quality vegetables to human health, ed. Md. Asaduzzaman and T. Asao, 85–102. London: Intechopen.
- Turland, N., J. Wiersema, F. Barrie, W. Greuter, D. Hawksworth, P. Herendeen, S. Knapp, et al., Eds. 2018. International code of nomenclature for algae, fungi, and plants, vol. 159. Glashütten: Koeltz Botanical Books.
- Turuk, A. S., and K. Banerjee. 2023. Blending seaweed into bakery products. Journal of Applied Phycology 35 (4):1893–909. doi: 10.1007/s10811-023-02982-5.
- Uchida, M., and T. Miyoshi. 2013. Algal fermentation – the seed for a new fermentation industry of foods and related products. Japan Agricultural Research Quarterly 47 (1):53–63. doi: 10.6090/jarq.47.53.
- United Nations. 2015. Transforming our world: The 2030 agenda for sustainable development, A/RES/70/1, 17th Session of the United Nations General Assembly.
- Uribe, E., A. Vega-Gálvez, V. García, A. Pastén, J. López, and G. Goñi. 2019. Effect of different drying methods on phytochemical content and amino acid and fatty acid profiles of the green seaweed, Ulva spp. Journal of Applied Phycology 31 (3):1967–79. doi: 10.1007/s10811-018-1686-9.
- Valente, L. M. P., A. Gouveia, P. Rema, J. Matos, E. F. Gomes, and I. S. Pinto. 2006. Evaluation of three seaweeds Gracilaria bursa-pastoris, Ulva rigida and Gracilaria cornea as dietary ingredients in European Sea Bass (Dicentrarchus labrax) juveniles. Aquaculture 252 (1):85–91. doi: 10.1016/j.aquaculture.2005.11.052.
- van Ginneken, V. J. T., J. P. Helsper, W. de Visser, H. van Keulen, and W. A. Brandenburg. 2011. Polyunsaturated fatty acids in various macroalgal species from north Atlantic and tropical seas. Lipids in Health and Disease 10 (1):104. doi: 10.1186/1476-511X-10-104.
- van Tol de Castro, T. A., O. C. H. Tavares, D. F. de Oliveira Torchia, H. F. Oliveira da Silva, O. V. T. de Moura, R. E. Cantarino, S. de Abreu Lopes, C. V. Viêgas, A. L. do Amaral Vendramini, L. A. Santos, et al. 2023. Organic fragments of K-carrageenan, lipids and peptides plus K-rich inorganic fraction in Kappaphycus alvarezii biomass are responsible for growth stimulus in rice plant when applied both foliar and root pathway. Algal Research 71 (April):103040. doi: 10.1016/j.algal.2023.103040.
- Vazquez Calderon, F., and J. Sanchez Lopez. 2022. An overview of the algae industry in Europe , eds. J. Guillen Garcia and M. Avraamides, Luxembourg: Publications office of the European Union, JRC130107.
- Venables, W. R. B. 1997. Modern applied statistics with S-Plus, 2nd ed. New York: Springer-Verlag.
- Ventura, M. R., and J. I. R. Castañón. 1998. The nutritive value of seaweed (Ulva lactuca) for goats. Small Ruminant Research 29 (3):325–7. https://www.sciencedirect.com/science/article/pii/S092144889700134X. doi: 10.1016/S0921-4488(97)00134-X.
- Ventura, M. R., J. I. R. Castañon, and J. M. McNab. 1994. Nutritional value of seaweed (Ulva rigida) for poultry. Animal Feed Science and Technology 49 (1–2):87–92. doi: 10.1016/0377-8401(94)90083-3.
- Viera, M. P., G. C. de Vicose, J. L. Gómez-Pinchetti, A. Bilbao, H. Fernandez-Palacios, and M. S. Izquierdo. 2011. Comparative performances of juvenile abalone (Haliotis tuberculata coccinea Reeve) fed enriched vs. non-enriched macroalgae: Effect on growth and body composition. Aquaculture 319 (3–4):423–429. doi: 10.1016/j.aquaculture.2011.07.024.
- Vizcaíno, A. J., M. Fumanal, M. I. Sáez, T. F. Martínez, M. A. Moriñigo, C. Fernández-Díaz, V. Anguis, M. C. Balebona, and F. J. Alarcón. 2019. Evaluation of Ulva ohnoi as functional dietary ingredient in juvenile Senegalese Sole (Solea senegalensis): Effects on the structure and functionality of the intestinal mucosa. Algal Research 42:101608. doi: 10.1016/j.algal.2019.101608.
- Vizcaíno, A. J., A. Galafat, M. I. Sáez, T. F. Martínez, and F. J. Alarcón. 2020. Partial characterization of protease inhibitors of Ulva ohnoi and their effect on digestive proteases of marine fish. Marine Drugs 18 (6):319. https://pubmed.ncbi.nlm.nih.gov/32570719. doi: 10.3390/md18060319.
- Vizcaíno, A. J., S. I. Mendes, J. L. Varela, I. Ruiz-Jarabo, R. Rico, F. L. Figueroa, R. Abdala, M. Á. Moriñigo, J. M. Mancera, and F. J. Alarcón. 2016. Growth, tissue metabolites and digestive functionality in Sparus aurata juveniles fed different levels of macroalgae, Gracilaria cornea and Ulva rigida. Aquaculture Research 47 (10):3224–38. doi: 10.1111/are.12774.
- van der Wal, H., B. L. H. M. Sperber, B. Houweling-Tan, R. R. C. Bakker, W. Brandenburg, and A. M. López-Contreras. 2013. Production of acetone, butanol, and ethanol from biomass of the green seaweed Ulva lactuca. Bioresource Technology 128 (January):431–7. doi: 10.1016/j.biortech.2012.10.094.
- van Ginneken, V. J., J. P. Helsper, W. de Visser, H. van Keulen, and W. A. Brandenburg. 2011. Polyunsaturated fatty acids in various macroalgal species from North Atlantic and tropical seas. Lipids in Health and Disease 10 (1):104. doi: 10.1186/1476-511X-10-104.
- Wan, A. H. L., S. J. Davies, A. Soler-Vila, R. Fitzgerald, and M. P. Johnson. 2019. Macroalgae as a sustainable aquafeed ingredient. Reviews in Aquaculture 11 (3):458–92. doi: 10.1111/raq.12241.
- Wassef, E. A. 2005. Alternative protein sources for fish feeds in Egypt. In Mediterranean Fish Nutrition, ed. D. Montero, B. Basurco, I. Nengas, M. Alexis, and M. Izquierdo, Zaragoza: CIHEAM.
- Wassef, E. A., A.-F. M. El-Sayed, and E. M. Sakr. 2013. Pterocladia (Rhodophyta) and Ulva (Chlorophyta) as feed supplements for European Seabass, Dicentrarchus labrax L., fry. Journal of Applied Phycology 25 (5):1369–76. doi: 10.1007/s10811-013-9995-5.
- Wassef, E. A., M. H. El Masry, and F. R. Mikhail. 2001. Growth enhancement and muscle structure of striped mullet, Mugil cephalus L., fingerlings by feeding algal meal-based diets. Aquaculture Research 32 (1):315–322. https://onlinelibrary.wiley.com/doi/full/10.1046/j.1355-557x.2001.00043.x. doi: 10.1046/j.1355-557x.2001.00043.x.
- Wichard, T. 2023. From model organism to application: Bacteria-induced growth and development of the green seaweed Ulva and the potential of microbe leveraging in algal aquaculture. Seminars in Cell & Developmental Biology 134:69–78. doi: 10.1016/j.semcdb.2022.04.007.
- Wichard, T., B. Charrier, F. Mineur, J. H. Bothwell, O. De Clerck, and J. C. Coates. 2015. The green seaweed Ulva: A model system to study morphogenesis. Frontiers in Plant Science 6:72. https://pubmed.ncbi.nlm.nih.gov/25745427. doi: 10.3389/fpls.2015.00072.
- Willett, W., J. Rockström, B. Loken, M. Springmann, T. Lang, S. Vermeulen, T. Garnett, et al. 2019. Food in the anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. The Lancet 393 (10170):447–92. doi: 10.1016/s0140-6736. (18)31788-4.
- Wolf, M. A., K. Sciuto, C. Andreoli, and I. Moro. 2012. Ulva (Chlorophyta, Ulvales) biodiversity in the North Adriatic Sea (Mediterranean, Italy): Cryptic species and new introductions. Journal of Phycology 48 (6):1510–21. doi: 10.1111/jpy.12005.
- Wu, C., J. Ma, S. Gao, M. Ju, X. Hu, J. Yang, R. Xu, and S. Ye. 2013. Nutrition analysis and food safety evaluation of green tide algae in 2010. Journal of Fisheries of China 37 (1):141. doi: 10.3724/SP.J.1231.2013.38344.
- Wu, J. 2022. Emerging sources and applications of alternative proteins: An introduction. Advances in Food and Nutrition Research 101:1–15. http://europepmc.org/abstract/MED/35940701.
- Wu, Z.-Z., D.-Y. Li, and Y.-S. Cheng. 2018. Application of ensilage as a green approach for simultaneous preservation and pretreatment of macroalgae Ulva lactuca for fermentable sugar production. Clean Technologies and Environmental Policy 20 (9):2057–65. doi: 10.1007/s10098-018-1574-7.
- Yaich, H., H. Garna, S. Besbes, M. Paquot, C. Blecker, and H. Attia. 2011. Chemical composition and functional properties of Ulva lactuca seaweed collected in Tunisia. Food Chemistry 128 (4):895–901. doi: 10.1016/j.foodchem.2011.03.114.
- Yesuraj, D., C. Deepika, G. A. Ravishankar, and A. R. Rao. 2022. Seaweed-based recipes for food, health-food applications, and innovative products including meat and meat analogs. In Sustainable global resources of seaweeds, ed. A. R. Rao and G. A. Ravishankar, Vol. 2, 267–92. Cham: Springer International Publishing.
- Yildirim, Ö., S. Ergün, S. Yaman, and A. Türker. 2009. Effects of two seaweeds (Ulva lactuca and Enteromorpha linza) as a feed additive in diets on growth performance, feed utilization, and body composition of rainbow trout (Oncorhynchus mykiss) | Rasyonlarda Yem Katkı Maddesi Olarak İki Deniz Yosununun. Ulv. Kafkas Universitesi Veteriner Fakultesi Dergisi 15:455–60.
- Yong, W. T. L., V. Y. Thien, R. Rupert, and K. F. Rodrigues. 2022. Seaweed: A potential climate change solution. Renewable and Sustainable Energy Reviews 159:112222. doi: 10.1016/j.rser.2022.112222.
- Zertuche-González, J. A., J. M. Sandoval-Gil, L. K. Rangel-Mendoza, A. I. Gálvez-Palazuelos, J. M. Guzmán-Calderón, and C. Yarish. 2021. Seasonal and interannual production of sea lettuce (Ulva sp.) in outdoor cultures based on commercial size ponds. Journal of the World Aquaculture Society 52 (5):1047–58. doi: 10.1111/jwas.12773.
- Zhu, D., X. Wen, X. Xuan, S. Li, and Y. Li. 2016. The green alga Ulva lactuca as a potential ingredient in diets for juvenile white spotted snapper Lutjanus stellatus akazaki. Journal of Applied Phycology 28 (1):703–11. doi: 10.1007/s10811-015-0545-1.
- Zhu, X., L. Healy, Z. Zhang, J. Maguire, D. Sun, and B. K. Tiwari. 2021. Novel postharvest processing strategies for value-added applications of marine algae. Journal of the Science of Food and Agriculture 101 (11):4444–55. doi: 10.1002/jsfa.11166.
- Zollmann, M., A. Robin, M. Prabhu, M. Polikovsky, A. Gillis, S. Greiserman, and A. Golberg. 2019. Green technology in green macroalgal biorefineries. Phycologia 58 (5):516–34. doi: 10.1080/00318884.2019.1640516.
