Abstract
A surge of public interest in NMN supplementation has been observed in recent years. However, whether NMN supplements are effective in improving metabolic health remains unclear. The objective of the review was to assess the effects of NMN supplementation on fasting glucose, triglycerides, total cholesterol, LDL-C, and HDL-C in adults. Studies were located by searching four databases (PubMed, Embase, Cochrane, and Web of Science). Two reviewers independently conducted title/abstract and full-text screening, data extraction, and risk-of-bias assessment. Of the 4049 records reviewed, 12 studies with a total of 513 participants met the criteria for analysis. Random-effects meta-analyses found an overall significant effect of NMN supplementation in elevating blood NAD levels. However, most of the clinically relevant outcomes were not significantly different between NMN supplementation and control group. Risk-of-bias assessment using RoB2 showed some concerns in seven studies and high risk of bias in the other five studies. Together, our findings suggest that an exaggeration of the benefits of NMN supplementation may exist in the field. Although the limited number of eligible studies was sufficiently powered to detect changes in the abovementioned primary outcomes, more studies are needed to conclude about the exact effects of NMN supplementation.
Keywords:
Introduction
The process of aging involves a gradual decline in physiological integrity (López-Otín et al. Citation2013). This age-related deterioration is the primary risk factor for major human diseases, such as cancer, diabetes, cerebro-cardiovascular diseases, and neurodegenerative diseases (Blagosklonny Citation2008; Hung et al. Citation2010; López-Otín et al. Citation2013). Apart from that, loss of muscle mass and disrupted muscle metabolism have also been strongly implicated during the aging process, which lead to an increased risk of frailty, mitochondrial dysfunction, oxidative stress, metabolic inflexibility, and decreased insulin sensitivity.(Amati et al. Citation2009; Distefano et al. Citation2018; Fried et al. Citation2001; Riera and Dillin Citation2015; Romani et al. Citation2021). Of particular note is the depletion with age in nicotinamide adenine dinucleotide [NAD] metabolites whose muscle abundance has been proved to be positively correlated with muscle and mitochondrial health parameters in older adults (Janssens et al. Citation2022).
There are, in general, three main roles in which NAD plays in order to maintain integrity in lives. It is, first of all, described as the cell’s hydrogen carrier for redox reactions (Warburg and Christian Citation1936). Secondly, NAD serves as the substrate for signaling of numerous enzymes, including the sirtuins [SIRT1˗7] and poly(adenosine diphosphate-ribose) polymerases [PARP] (Chambon, Weill, and Mandel Citation1963). Thirdly, NAD directly regulates protein-protein interactions (Li et al. Citation2017). Due to the profound effects of NAD on health and survival in lives, approaches to restore NAD levels against its natural decline are speculated to have the potent to prevent and treat age-related loss of physiological integrity, thereby restoring vigor and increasing health-span in late life (Rajman, Chwalek, and Sinclair Citation2018). These therapeutic approaches may include exercise, balanced diet or administration of NAD precursors such as nicotinamide [NAM], nicotinamide riboside [NR], nicotinamide mononucleotide [NMN] (Rajman, Chwalek, and Sinclair Citation2018).
In fact, the beneficial effects of NAD-boosting molecules on health-related parameters have been extensively investigated in rodent models. Waves of evidence have suggested that administration with NAD precursors is related to promotion in cognitive and sensory function (Gong et al. Citation2013; Hou et al. Citation2018; Long et al. Citation2015; Sorrentino et al. Citation2017; Wang et al. Citation2016), immunity (Fang et al. Citation2016; Kaneko et al. Citation2006), gluconeogenesis in liver (Cantó et al. Citation2012; Yoshino et al. Citation2011), endothelial cell proliferation (de Picciotto et al. Citation2016), insulin sensitivity (Mills et al. Citation2016), and lipogenesis in adipose tissue (Mills et al. Citation2016). In addition, NMN supplementation has been found to ameliorate postprandial hyperglycemia in mice fed with high-fat diet (Nagahisa et al. Citation2022) and attenuate the increase in urinary albumin excretion in diabetic mice (Yasuda et al. Citation2021). However, whether these observed benefits from mice models can be translated into human beings remains unclear. To the best of our knowledge, only two review articles in the current body of literature systematically examined and critically assessed the effects of NAD-boosting molecules supplementation in humans (Damgaard and Treebak Citation2023; Gindri et al. Citation2023). Gindri et al. (Citation2023) evaluated the safety and effectiveness of different kinds of NAD supplementation (i.e., NADH, NR, NA, NAM, and NMN) under various clinical conditions and claimed that NAD supplementation overall showed low incidence of side effects and increased the general quality of life and health. On the contrary, Damgaard and Treebak (Citation2023) summarized the currently available researches on NR supplementation in humans and found that its supplementation had only few clinically relevant effects. Additionally, they revealed that the unfavorable effects of NR such as increase in insulin resistance and body weight were either glossed over or attributed to the effect of time. Therefore, evidence for NAD-boosting molecules as a promising supplementation strategy to treat age-related conditions in humans is still equivocal. Meanwhile, despite possibilities that different types of NAD-boosting molecules are likely to cause varied physiological responses that affect metabolism in humans, most of the articles combined the findings using the general term “NAD precursors” (Covarrubias et al. Citation2021; Gindri et al. Citation2023; Rajman, Chwalek, and Sinclair Citation2018), whereas individual examination into each type of them is scarce (Damgaard and Treebak Citation2023).
Collectively, this systematic review with meta-analysis aimed to summarize the currently available randomized controlled trials in humans using NMN supplementation only and to quantitatively evaluate the effects of its supplementation on glucose and lipid metabolism, blood NAD levels, and physical performance in comparison with the placebo control. Results from this meta-analysis would be crucial to provide insights into the actual future potential for NMN supplementation for the field of aging research.
Methods
This review conformed to the Preferred Reporting Items for Systematic Review and Meta-Analysis. The review protocol was registered in PROSPERO international prospective register of systematic reviews prior to data extraction (CRD42023482534).
Eligibility criteria and information sources
The eligible studies had to meet all of the following criteria:
Types of studies: We included all published randomized controlled trials (RCTs). The language was restricted to English. We did not exclude studies in the basis of the date of publication.
Types of participants: Adults (≥18 years of age) of either gender.
Types of interventions: Oral NMN supplementation at any dose with a placebo-controlled comparator. The supplementation period should be at least 14 days.
Types of outcome measures: Primary outcomes included fasting glucose (FG), triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C). Secondary outcomes included levels of NAD concentration, levels of NMN concentration, glycated hemoglobin (HbA1c), fasting insulin levels, homeostatic model assessment for insulin resistance (HOMA-IR), body weight, body mass index (BMI), and physical performance.
We conducted electronic searches for eligible studies within each of the following databases:
Embase (OvidSP) (1910 to May 04 2024);
PubMed (1965 to May 04 2024);
Science Citation Index Expanded and Social Science Citation Index (Web of Science) (1956 to May 04 2024); and
Cochrane Central Register for Controlled Trials (CENTRAL) (1898 to May 04 2024).
The full search strategy for each database could be found in the Appendix 1. In addition, we searched the reference lists of all eligible study reports to identify further eligible studies or study reports (up to May 10, 2024).
Data collection and analysis
Selection of studies
Three independent researchers (ZJ, EP, SW) reviewed titles and abstracts of the first 150 records and discussed inconsistencies until consensus was obtained. Then, ZJ independently screened titles and abstracts of all articles retrieved. EP and SW checked a random sample of screened records. In case of disagreement, consensus on which articles to screen full-text was reached by discussion. Next, ZJ and EP independently screened full-text articles for inclusion. In case of disagreement, consensus was reached on inclusion or exclusion by discussion and if necessary, the third researcher (SW) was consulted.
Data extraction process
We designed a data extraction form based that used by Allida et al. (Citation2023) and Wells et al. (Citation2024), which ZJ used to extract data form eligible studies. Extracted data was checked independently by EP with any discrepancies being solved through discussion.
We collected data on:
the study: author, year, country, funding sources, and declaration of interest;
the research design: treatment assignment mechanism and analysis;
the participants: number of participants in each intervention arm, sex ratio, age, inclusion and exclusion criteria;
the intervention: dose, duration, timing, mode of delivery, and reporting of adverse events;
the primary and secondary outcomes.
If data were available in a trial at multiple time points, we only included data at the end of intervention. And to prevent selective inclusion of data based on the results, we used the following a priori defined decision rules to select data from trials.
Where both final values and change from baseline values were reported for the same outcome, we included final values.
When both unadjusted and adjusted values were reported for the same outcome, we included unadjusted values.
When data analyzed based on the intention-to-treat (ITT) sample and per-protocol sample were reported, we included ITT-analyzed data.
Where trials had more than one measure of levels of NAD concentration, we included data for analysis based on the following a priori defined list:
Levels of NAD concentration in whole blood;
Levels of NAD concentration in serum;
Levels of NAD concentration in PBMCs.
Where trials had more than one measure of physical performance, we included data for analysis based on the following a priori defined list:
Handgrip strength;
Six-minute walk test;
Assessment of risk of bias
We assessed risk of bias in the included studies using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) (Sterne et al. Citation2019), which addresses five specific domains: (1) bias arising from the randomization process; (2) bias due to deviations from intended interventions; (3) bias due to missing outcome data; (4) bias in measurement of the outcome; and (5) bias in selection of the reported result. ZJ independently applied the tool to each included study and recorded supporting information and justifications for judgements of risk of bias for each domain (low, some concerns, and high). Judgements of risk of bias and supporting information were checked independently by EP with any discrepancies resolved by discussion to reach consensus. If consensus could not be reached, a third review author (SW) acted as an arbiter. Following guidance given for RoB 2 (Sections 4–8) (Sterne et al. Citation2019), we derived a risk-of-bias judgment for each domain, whereas the overall bias for the study was determined by the highest risk-of-bias judgment in any of the domains that were assessed.
Measures of treatment effect
For continuous outcomes, data were pooled for meta-analysis when four or more studies measured the same outcome. When single outcome measure was used, mean difference (MD) with 95% confidence interval (CI) was calculated as effect size. When different outcome measures were used, standardized mean difference (SMD) with 95% CI was calculated as effect size (Hedge’s g).
Unit of analysis issues
When trials included two or more intervention arms and only one placebo-controlled arm, we compared data form each treatment arm with data from the total number of participants in the control arm divided by the number of intervention arms. Comparisons are presented as separate trials.
Dealing with missing data
For missing data, an attempt was made to contact the corresponding author of the study to obtain the unavailable or ambiguous data. If the study author was unresponsive and the data remained unavailable, the available graph data were extracted using WebPlotDigitizer (Rohatgi Citation2017).
Assessment of heterogeneity
Methodological heterogeneity was assessed by examining the study characteristics. I2 statistics was used to measure heterogeneity amongst the trials in each analysis and interpreted as follows: 0 − 40%, low heterogeneity; 30 − 60%, moderate heterogeneity; 50 − 90%, substantial heterogeneity; 75 − 100%, considerable heterogeneity (Borenstein et al. Citation2011).
Sensitivity analysis was conducted using GOSH (graphical display of study heterogeneity) plot and leave-one-out method to identify influential cases. In addition, when I2 reached 40%, repeat analysis was conducted after removing the possible outliers (Viechtbauer and Cheung Citation2010).
Assessment of reporting bias
The funnel plot was used to examine small study bias for outcomes where there were 10 or more trials contributing data to the analysis (Higgins et al. Citation2023).
Data synthesis
Statistical analyses were conducted using the meta package in the statistical software R (V.4.3.2) (Harrer et al. Citation2021). As we anticipated considerable heterogeneity, a random-effects model using restricted maximum likelihood (REML) variance estimator and inverse-variance method were used to pool effect size. 95% CI for the summary effect was calculated using Knapp-Hartung adjustment (Knapp and Hartung Citation2003). Random-effects 95% prediction intervals (PI) were calculated to aid in their interpretation by quantifying expected treatment effects in a future randomized controlled trial. Between study heterogeneity was assessed by inspecting forest plots and by calculating the I2 statistics. The 95% CI around I2 was also calculated to judge our confidence about these metrics. Statistical significance was set at p < 0.05.
Subgroup analysis and investigation of heterogeneity
Given that the effects of NMN supplementation might vary by different lengths of treatment and different doses of daily intake, we conducted subgroup analyses estimating effect sizes for supplementation ≤30 days and >30 days and daily NMN dose ≤600 mg/d and >600 mg/d, respectively, when there were more than eight reports for the pooled analysis and at least three reports in each subgroup.
Furthermore, we suspected that the effects of NMN supplementation might differ based on participants’ age, BMI, and fasting blood glucose. Therefore, sub-group analyses estimating effect sizes for participants ≤55 years of age and >55 years of age, BMI <25.0 kg/m2 and ≥25.0 kg/m2, and fasting blood glucose ≤100 mg/dL and >100 mg/dL were conducted when there were more than eight reports for the pooled analysis and at least three reports in each subgroup.
Results
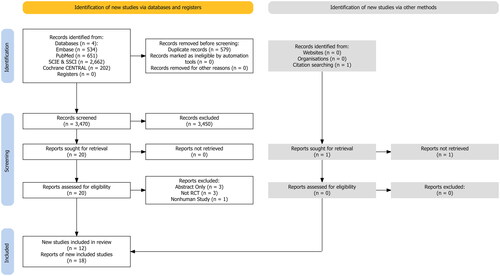
Combined searches generated a total of 4049 articles. After removal of duplicates and exclusion of articles based on abstract and title, 20 full-text articles remained for screening against inclusion and exclusion criteria. One study was identified via backwards and forward citation searching but the full report was not able to be retrieved (Zhao et al. Citation2022). Among the 20 full-text articles, three studies with abstract only (Akasaka et al. Citation2022; Barker et al. Citation2022; Naito et al. Citation2023), one study using the animal model (Turner et al. Citation2021), and three studies without placebo control group (Kimura et al. Citation2022; Yamaguchi et al. Citation2024; Yamane et al. Citation2023) were excluded. Two studies tested three different daily doses of NMN supplementation against the same placebo control group (Liao et al. Citation2021; Yi et al. Citation2023) and another two studies tested two supplementation protocols with two placebo control groups following either of the supplementation protocol (Kim et al. Citation2022; Pencina, Lavu, et al. Citation2023). In addition, two studies used data from the same cohort of participants, and therefore were considered as only one report (Kuerec et al. Citation2024). Hence, after full-text screening, a total of 18 reports from 12 studies met the stated inclusion criteria (Akasaka et al. Citation2023; Fukamizu et al. Citation2022; Huang Citation2022; Igarashi et al. Citation2022; Katayoshi et al. Citation2023; Kim et al. Citation2022; Liao et al. Citation2021; Okabe et al. Citation2022; Pencina, Lavu, et al. Citation2023; Pencina, Valderrabano, et al. Citation2023; Yi et al. Citation2023; Yoshino et al. Citation2021). The flow of papers through the search and inclusion process is presented in .
Study, participant, and intervention characteristics
Participant and intervention details of included randomized controlled trials are provided in and Supplementary Appendix 2. Total participants numbered 513 (NMN supplementation: n = 304; placebo control: n = 209). Seven studies (Akasaka et al. Citation2023; Fukamizu et al. Citation2022; Igarashi et al. Citation2022; Katayoshi et al. Citation2023; Kim et al. Citation2022; Liao et al. Citation2021; Okabe et al. Citation2022) of 284 participants were normal weight (BMI <25 kg/m2), while the remaining five studies (Huang Citation2022; Pencina, Lavu, et al. Citation2023; Pencina, Valderrabano, et al. Citation2023; Yi et al. Citation2023; Yoshino et al. Citation2021) of 229 participants were overweight/obese. Eight studies (Fukamizu et al. Citation2022; Huang Citation2022; Igarashi et al. Citation2022; Katayoshi et al. Citation2023; Okabe et al. Citation2022; Pencina, Lavu, et al. Citation2023; Pencina, Valderrabano, et al. Citation2023; Yi et al. Citation2023) of 318 participants were within normal range of fasting glucose (≤100 mg/dL), two studies (Akasaka et al. Citation2023; Yoshino et al. Citation2021) of 39 participants were in prediabetic or diabetic conditions, and the remaining two studies (Kim et al. Citation2022; Liao et al. Citation2021) of 156 participants were not indicated of their glucose status. Participants’ mean age ranged from 35 to 81 years of age, daily NMN doses ranged from 250 to 1250 mg/d NMN, and the intervention duration lasted from 2 to 24 wk. Both NMN supplementation and placebo control groups were instructed not to change their existing dietary habits.
Table 1. Characteristics of included studies in comparison with preclinical studies using rodent animals.
Comparative outcome measures
Summary on the effects of NMN supplementation per synthesis is presented in forest plot () and summary statistics per outcome measure for each meta-analysis and sub-group analysis are reported in . Sensitivity analyses are reported in figures by presenting the recalculated pooled effects with one study omitted each time (Supplementary Appendix 3, Figures S1–13).
Figure 2. Pooled analysis on the effect of NMN supplementation on fasting glucose.Pencina 2023b(1): NMN intake once daily; Pencina 2023b(2): NMN intake twice daily.
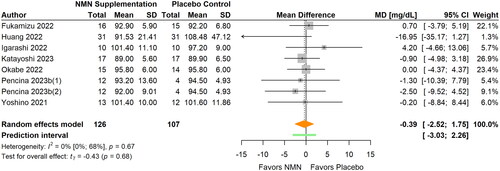
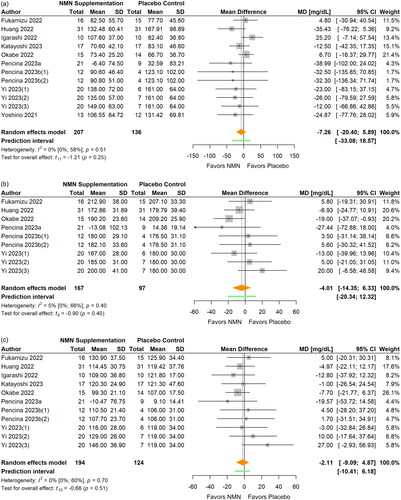
Figure 3. Pooled analysis on the effect of NMN supplementation on lipids: (a) TG; (b) TC; (c) LDL-C; (d) HDL-C.Pencina 2023b(1): NMN intake once daily; Pencina 2023b(2): NMN intake twice daily; Yi Citation2023(1): NMN intake 300 mg/d; Yi Citation2023(2): NMN intake 600 mg/d; Yi Citation2023(3): NMN intake 900 mg/d.
Figure 4. Pooled analysis on the effect of NMN supplementation on blood NAD concentrations.Pencina 2023b(1): NMN intake once daily; Pencina 2023b(2): NMN intake twice daily; Yi Citation2023(1): NMN intake 300 mg/d; Yi Citation2023(2): NMN intake 600 mg/d; Yi Citation2023(3): NMN intake 900 mg/d.
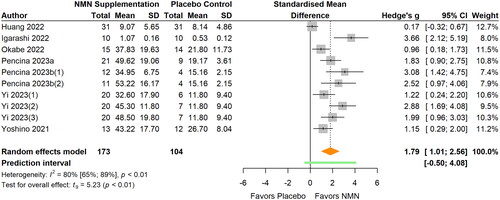
Figure 5. Pooled analysis on the effect of NMN supplementation on blood NMN concentrations.Pencina 2023b(1): NMN intake once daily; Pencina 2023b(2): NMN intake twice daily.
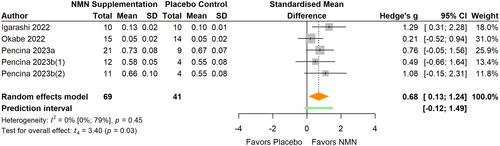
Figure 6. Pooled analysis on the effect of NMN supplementation on glucose metabolism: (a) insulin; (b) HOMA-IR; (c) HbA1c.Pencina 2023b(1): NMN intake once daily; Pencina 2023b(2): NMN intake twice daily; Yi Citation2023(1): NMN intake 300 mg/d; Yi Citation2023(2): NMN intake 600 mg/d; Yi Citation2023(3): NMN intake 900 mg/d.
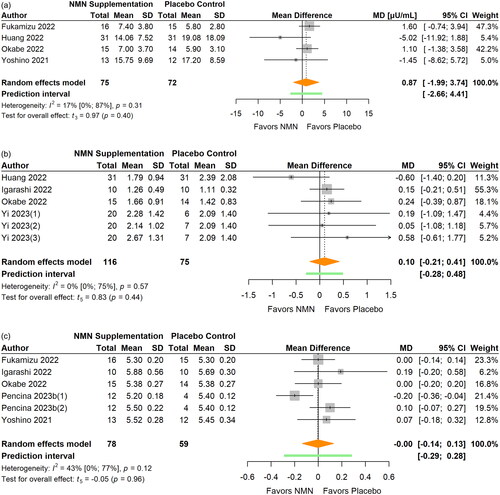
Figure 7. Pooled analysis on the effect of NMN supplementation on body composition: (a) body weight; (b) BMI.Liao Citation2021(1): NMN intake 300 mg/d; Liao Citation2021(2): NMN intake 600 mg/d; Liao Citation2021(3): NMN intake 1200 mg/d.
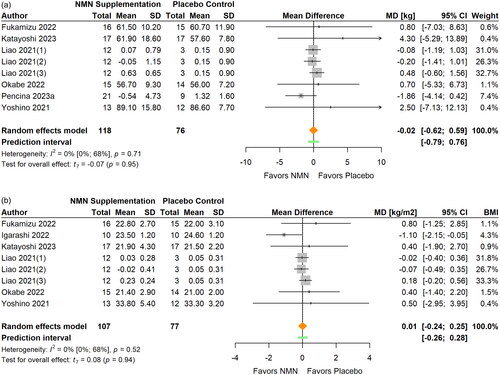
Figure 8. Pooled analysis on the effect of NMN supplementation on physical performance.Igarashi Citation2022(1): Grip strength right; Igarashi Citation2022(2): Grip strength left; Kim Citation2022(1): NMN intake once daily ante-meridian; Kim Citation2022(2): NMN intake once daily post-meridian; Liao Citation2021(1): NMN intake 300 mg/d; Liao Citation2021(2): NMN intake 600 mg/d; Liao Citation2021(3): NMN intake 1200 mg/d; Yi Citation2023(1): NMN intake 300 mg/d; Yi Citation2023(2): NMN intake 600 mg/d; Yi Citation2023(3): NMN intake 900 mg/d.
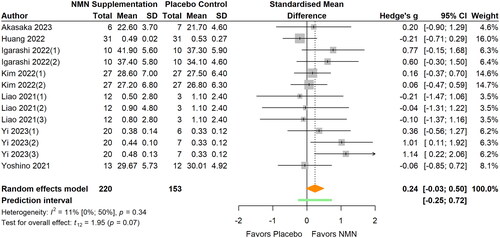
Figure 9. Risk of bias assessment for included studies. Kim et al. Citation2022(1): NMN intake once daily ante-meridian; Kim et al. Citation2022(2): NMN intake once daily post-meridian; Liao et al. Citation2021(1): NMN intake 300 mg/d; Liao et al. Citation2021(2): NMN intake 600 mg/d; Liao et al. Citation2021(3): NMN intake 1200 mg/d; Pencina, Lavu, et al. Citation2023b(1): NMN intake once daily; Pencina, Lavu, et al. Citation2023b(2): NMN intake twice daily; Yi et al. Citation2023(1): NMN intake 300 mg/d; Yi et al. Citation2023(2): NMN intake 600 mg/d; Yi et al. Citation2023(3): NMN intake 900 mg/d.
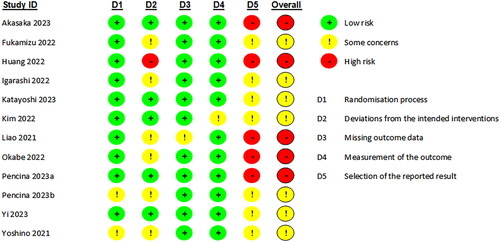
Table 2. Comparative outcome measures: Summary statistics of the effect of NMN supplementation per outcome.
Fasting glucose
Seven included studies (Fukamizu et al. Citation2022; Huang Citation2022; Igarashi et al. Citation2022; Katayoshi et al. Citation2023; Okabe et al. Citation2022; Pencina, Lavu, et al. Citation2023; Yoshino et al. Citation2021) assessed the effects of NMN supplementation on FG (, ). The pooled analysis showed no significant effect on fasting glucose (MD −0.39 mg/dL; 95% CI −2.52 to 1.75; p = 0.683; I2=0%) after NMN supplementation. The statistical heterogeneity was low and no outlier was identified. The magnitude of the pooled effect remained relatively stable in sensitivity analyses. There were no significant differences between subgroups in terms of participants’ age, BMI, daily NMN dose, and intervention duration (all p > 0.05).
Lipids
TG was examined in nine studies with 12 data sets available for meta-analysis (Fukamizu et al. Citation2022; Huang Citation2022; Igarashi et al. Citation2022; Katayoshi et al. Citation2023; Okabe et al. Citation2022; Pencina, Lavu, et al. Citation2023; Pencina, Valderrabano, et al. Citation2023; Yi et al. Citation2023; Yoshino et al. Citation2021) (, ). NMN supplementation did not significantly change TG levels (MD −7.26 mg/dL; 95% CI −20.40 to 5.89; p = 0.250; I2=0%) when all data were pooled together. The statistical heterogeneity was low and no outlier was identified. The magnitude of the pooled effect remained relatively stable in sensitivity analyses. Subgroup analyses showed a greater reduction in TG in overweight/obese participants compared to normal weight participants (MD −27.80 versus 5.57 mg/dL, respectively; p < 0.001). However, no significant differences were found between subgroups in terms of participants’ age, daily NMN dose, and intervention duration (all p > 0.05).
TC was reported in six studies with nine available data sets (Fukamizu et al. Citation2022; Huang Citation2022; Okabe et al. Citation2022; Pencina, Lavu, et al. Citation2023; Pencina, Valderrabano, et al. Citation2023; Yi et al. Citation2023) (, ). The results of meta-analysis revealed that no significant differences were observed for TC (MD −4.01 mg/dL; 95% CI −14.35 to 6.33; p = 0.397, I2=5%) between NMN supplementation and placebo control. The statistical heterogeneity was low and no outlier was identified. Re-calculated pooled effect was smaller (MD −0.43 mg/dL) after sensitivity analyses that removed influential cases. Subgroup analyses showed a greater reduction in TC when daily dose was ≤600 mg/d compared to >600 mg/d (MD −9.99 versus 5.64 mg/dL, respectively; p = 0.049). However, no significant differences were found between subgroups in terms of participants’ age and intervention duration (all p > 0.05).
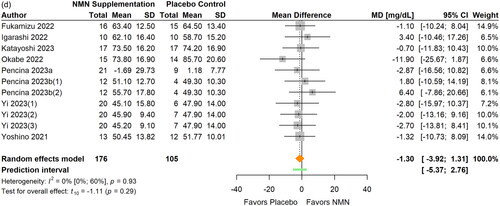
LDL-C was measured in eight studies (Fukamizu et al. Citation2022; Huang Citation2022; Igarashi et al. Citation2022; Katayoshi et al. Citation2023; Okabe et al. Citation2022; Pencina, Lavu, et al. Citation2023; Pencina, Valderrabano, et al. Citation2023; Yi et al. Citation2023) (, ) and HDL-C in eight studies (Fukamizu et al. Citation2022; Igarashi et al. Citation2022; Katayoshi et al. Citation2023; Okabe et al. Citation2022; Pencina, Lavu, et al. Citation2023; Pencina, Valderrabano, et al. Citation2023; Yi et al. Citation2023; M. Yoshino et al. Citation2021) (, ). There were no significant differences in LDL-C (MD −2.11 mg/dL; 95% CI −9.09 to 4.87; p = 0.515; I2=0%) or HDL-C (MD −1.30 mg/dL; 95% CI −3.92 to 1.31; p = 0.293, I2=0%) between NMN supplementation and the placebo control. The statistical heterogeneity was low and no outlier was identified in both analyses. The magnitude of the pooled effect for both LDL-C and HDL-C remained relatively stable in sensitivity analyses. Furthermore, no significant differences in LDL-C or HDL-C were found between subgroups in terms of participants’ age, BMI, daily NMN dose, and intervention duration (all p > 0.05).
Blood NAD levels
A total of ten reports from seven studies were included in the meta-analysis (Huang Citation2022; Igarashi et al. Citation2022; Okabe et al. Citation2022; Pencina, Lavu, et al. Citation2023; Pencina, Valderrabano, et al. Citation2023; Yi et al. Citation2023; Yoshino et al. Citation2021) (, ). Three studies examined NAD levels in whole blood (Igarashi et al. Citation2022; Pencina, Lavu, et al. Citation2023; Pencina, Valderrabano, et al. Citation2023), two studies in serum (Huang Citation2022; Katayoshi et al. Citation2023; Yi et al. Citation2023), and one study in PBMCs (Yoshino et al. Citation2021). In comparison with the placebo control, NMN supplementation significantly elevated blood NAD concentrations (SMD 1.79; 95% CI 1.01 to 2.56; p < 0.001; I2=80%). Sensitivity analyses that removed outliers showed consistent results with the primary meta-analyses (SMD 1.97). No significant differences were found between subgroups in terms of participants’ age, daily NMN dose, and intervention duration (all p > 0.05).
Blood NMN levels
Blood NMN levels were reported in four studies (Igarashi et al. Citation2022; Okabe et al. Citation2022; Pencina, Lavu, et al. Citation2023; Pencina, Valderrabano, et al. Citation2023) (, ). The pooled analysis showed that NMN supplementation significantly increased blood NMN concentrations (SMD 0.69; 95% CI 0.13 to 1.24; p = 0.027; I2=0%). The statistical heterogeneity was low. Sensitivity analyses that removed influential cases showed consistent results with the primary meta-analyses (SMD 0.74).
Glucose metabolism
The summary statistics for other glucose metabolic markers are showed in . Four studies examined fasting insulin (Fukamizu et al. Citation2022; Huang Citation2022; Okabe et al. Citation2022; Yoshino et al. Citation2021) (, ), four studies examined HOMA-IR (Huang Citation2022; Igarashi et al. Citation2022; Okabe et al. Citation2022; Yi et al. Citation2023) (, ), and five studies examined HbA1c (Fukamizu et al. Citation2022; Igarashi et al. Citation2022; Okabe et al. Citation2022; Pencina, Lavu, et al. Citation2023; Yoshino et al. Citation2021) (, ). Overall, no significant effects on insulin (MD 0.87 μU/mL; 95% CI −1.99 to 3.74; p = 0.403; I2=17%) (), HOMA-IR (MD 0.10; 95% CI −0.21 to 0.41; p = 0.444; I2=0%), or HbA1c (MD 0.00%, 95% CI −0.14 to 0.13; p = 0.962, I2=43%) were observed after NMN supplementation. The statistical heterogeneity was low for insulin and HOMA-IR. However, sensitivity analyses that removed influential cases showed inconsistent results with the primary meta-analyses for insulin (MD −0.68 μU/mL) and HbA1c (MD 0.04%).
Body composition
Six studies reported data on body weight (Fukamizu et al. Citation2022; Katayoshi et al. Citation2023; Liao et al. Citation2021; Okabe et al. Citation2022; Pencina, Valderrabano, et al. Citation2023; Yoshino et al. Citation2021) (, ) and six studies on BMI (Fukamizu et al. Citation2022; Igarashi et al. Citation2022; Katayoshi et al. Citation2023; Liao et al. Citation2021; Okabe et al. Citation2022; Yoshino et al. Citation2021) (, ). Pooled analyses revealed no significant effects on body weight (MD −0.02 kg; 95% CI −0.62 to 0.59; p = 0.948; I2=0%) or BMI (MD 0.01, 95% CI −0.24 to 0.25; p = 0.938, I2=0%) after NMN supplementation. The statistical heterogeneity was low and no outliers were identified. Re-calculated pooled effect was larger for weight (MD −0.08 kg) and BMI (MD −0.26) after sensitivity analyses that removed influential cases. Subgroup analyses were not conducted due to the insufficient number of reports in subgroups.
Physical performance
Physical performance was examined in seven studies with 13 reports available for meta-analysis (Akasaka et al. Citation2023; Huang Citation2022; Igarashi et al. Citation2022; Katayoshi et al. Citation2023; Liao et al. Citation2021; Yi et al. Citation2023; Yoshino et al. Citation2021) (, ). No significant effects of NMN supplementation on physical performance were observed from meta-analysis (SMD 0.24; 95% CI −0.03 to 0.50; p = 0.074, I2=11%). The statistical heterogeneity was low and no outliers were identified. The magnitude of the pooled effect remained relatively stable in sensitivity analyses. No significant differences were found between subgroups in terms of participants’ age and BMI (all p > 0.05).
Risk of bias and publication bias
RoB 2 was employed to assess each publication’s risk of bias. In summary, seven studies exhibited some concerns and the other five studies exhibited high risk of bias. Most of the risk of bias was attributable to deviations from intended interventions and selection of the reported result (). Justifications were provided in Supplementary Appendix 2 for each of the five individual domains of the “Risk of bias” assessment.
To examine small study and publication bias, contour-enhanced funnel plots of the effect sizes were plotted against their standard errors for TG, LDL-C, HDL-C, blood NAD levels, and physical performance, because there were 10 or more trials contributing data to the analysis (). Visual inspection of the contour enhanced funnel plots revealed an absence of negative effects of NMN supplementation on TG. Egger regression tests quantitatively confirmed this visual impression, providing evidence of possible publication bias (t = −2.57; df = 10; p = 0.028).
Discussion
The primary purpose of this systematic review and meta-analysis was to evaluate the effects of NMN supplementation on glucose and lipid metabolism in adults. While animal models have provided strong cases for the health benefits from NMN administration, only 12 acceptable human clinical trials have examined NMN supplementation in adults in comparison with a placebo control. Meta-analysis results of the 12 randomized controlled trials with sufficient data revealed statistically significant effects of NMN supplementation in elevating blood NAD and NMN concentrations and decreasing TG levels in participants with overweight/obesity. In addition, a trend for improving physical performance was also observed. However, most of the clinically relevant effects and body composition outcome were not found to be significantly improved by NMN supplementation. There is, unfortunately, a tendency in the current body of literature for exaggerating its significance and benefits. Nevertheless, given that many of the conducted studies have been focused on the safety and efficacy of its supplementation, examined only clinically relevant blood testing results, and had generally healthy, albeit in some cases overweight/obese or prediabetic/diabetic, participants, whether NMN has a geroprotective effect in humans remains to be examined from a more mechanistic perspective such as gene expression and epigenomic regulation. In addition, data on the NAD-boosting effect of NMN in samples other than blood is limited to muscle biopsy at this time.
NAD, a coenzyme found in every living cell, plays a critical role in various cellular processes including energy production and more than 500 enzymatic reactions (Ansari and Raghava Citation2010; Harden and Young Citation1906). Dynamic changes in the synthesis, degradation and recycling of NAD take place within nearly all major organelles such as cytoplasm, nucleus, Golgi, and peroxisomes (Anderson et al. Citation2003). Generally, there are two canonical pathways to synthesize NAD, the de novo pathway and the salvage pathway. While the former utilizes NAD from the essential amino acid Tryptophan (Ikeda et al. Citation1965), the latter recycles NAD intermediates such as NAM, NA, NMN, and NR (Magni et al. Citation2004; Magni et al. Citation1999). Among those intermediates, NAM is primarily produced within cells by NAD-consuming enzymes (i.e., sirtuins and PARPs), and the others could be uptaked from the daily food intake (Bogan and Brenner Citation2008; Trammell, Yu, et al. Citation2016). Though being possible to replenish NAD via daily diet, its natural content in food is relatively low (e.g., 7.96 mg NAD intermediates in per liter cow milk) (Trammell, Yu, et al. Citation2016), making the approach hard to be achieved. Therefore, supplementation with NAD intermediates to boost NAD levels has aroused much more interest among researchers in recent years. In fact, accumulating evidence has asserted that constitutive upregulation in NAD levels could be achieved via NAM (Kaneko et al. Citation2006; Majamaa et al. Citation1996) and NR (Cantó et al. Citation2012; Trammell, Yu, et al. Citation2016) supplementation in mammals. Consistently, our meta-analysis results showed a significant increase in blood NAD levels with NMN supplementation. Although another two studies did not meet the inclusion criteria for meta-analysis due to the single-arm study design, they also reported a significant increase in plasma and PBMCs NAD levels (Yamaguchi et al. Citation2024; Yamane et al. Citation2023). However, the route of delivery after NMN intake is currently under debate. Ratajczak et al. (Citation2016) proposed that the uptake of NMN would undergo extracellular degradation and then recomposition inside the cell, while Mills et al. (Citation2016) reported a rapid absorption and conversion to NAD in liver and muscle, suggesting a direct uptake of NMN by a transporter. A newly identified gene, Slc12a8, which is highly expressed in the small intestine, has been proposed as a potentially promising NMN transporter (Grozio et al. Citation2019). Additional research will be necessary to further validate this finding.
A renaissance interest in NAD over the past decade came with the discovery of the sirtuins, a family of protein deacylases whose activity is regulated by the abundance of NAD (Araki, Sasaki, and Milbrandt Citation2004; Cantó et al. Citation2009). Studies have shown that increased NAD levels by activating NAD biosynthetic enzyme NMN adenylyltransferase [NMNAT] 1 (Araki, Sasaki, and Milbrandt Citation2004), as well as increased NAD+/NADH ratio by activating AMP-activated protein kinase [AMPK] (Cantó et al. Citation2009), promoted SIRT1 activity. Another study using transgenic mouse model has shown that high levels of Sirt1 overexpression increased metabolic activity, reduced blood lipid levels, and improved glucose metabolism (Bordone et al. Citation2007). More recently, it has been reported that NMN administration increased expression of Sirt1 in diabetic mouse model and subsequently attenuated diabetic albuminuria via epigenetic modulation in podocytes (Hasegawa et al. Citation2013; Yasuda et al. Citation2021). However, to our dismay, none of the included studies in our meta-analysis examined the sirtuins levels and we did not observe the acclaimed metabolic benefits in humans with NMN supplementation despite an evident increase in NAD levels. Consistent with our findings, NR supplementation, another NAD-boosting molecule, was also found to be unable to improve the metabolic health and display few clinically relevant effects (Damgaard and Treebak Citation2023). It should be acknowledged that we are facing multiple challenges when translating basic research into human clinical practice (Smoliga, Vang, and Baur Citation2012). For example, trends of fasting blood glucose over the lifespan differ between mice and humans and the associated mortality risk (Palliyaguru et al. Citation2021). Decline in NAD levels associated with aging and metabolic diseases may also be different in diverse organs between mice and humans. However, contrary to animal studies that have examined NAD levels in multiple tissues such as liver, gastrocnemius muscle, adipose tissue, and artery (See summary of major findings from preclinical studies using rodent animals in ), studies that examined NAD levels in human organs are limited to blood and muscle biopsy of quadriceps at this time. Thus, even with the extensive research and promising results from basic science groups, more effort is needed to establish a detailed molecular understanding on the mechanism of action of NMN and whether the sirtuins can be activated by increased NAD levels via NMN intake in humans to provide a strong justification for its therapeutic potential.
The outcome measures analyzed in this meta-analysis are closely relevant for monitoring the aging progress. Aging is a continuum of risk factor for developing diabetes and cardiovascular diseases [CVD] (Savji et al. Citation2013). Diabetes mellitus, in turn, accelerates aging and is a strong risk factor for mortality (Chia, Egan, and Ferrucci Citation2018). During human lifespan, fasting blood glucose increases gradually from adulthood into old age and the precipitous decline in glucose tolerance was most evident between 60 and 92 years (Chia, Egan, and Ferrucci Citation2018). The defects in glucose homeostasis are also associated with a wide range of metabolic dysfunction in older adults such as visceral fat accumulation, sarcopenia, and rise of proinflammatory cytokines (Barzilai and Ferrucci Citation2012). Nevertheless, the results of this meta-analysis revealed no statistically significant changes in all outcome measures related to glucose metabolism. This is in sharp contrast with what has been found in animal models that NMN administration is related with improvement in insulin tolerance in diabetic female mice (J. Yoshino et al. Citation2011), insulin sensitivity in the adipocyte-specific Nampt knock-out mice (Stromsdorfer et al. Citation2016) and aged wild-type C57/6J mice (Mills et al. Citation2016), and plasma glucose concentration during glucose tolerance test in high-fat diet fed C57/6J female mice (Uddin et al. Citation2016). We speculate that the disparity in the dosage of NMN and the way of NMN administration may be responsible for the inconsistency of findings. Mice are typically administered at a dose of 500 mg NMN/kg body weight/day, whereas in humans, the maximum dose observed is 1250 mg NMN/day, which is only equivalent to 25 mg NMN/kg body weight/day for a 50 kg individual, indicating a significantly lower dosage in humans. NMN is administered either more directly via intraperitoneal injection or via drinking water that spreads over one day in mice, while it is supplemented via oral intake once or twice daily in humans, which requires further gastrointestinal uptake and translocation. Recent findings from animal models shed light on the effects of NMN administration on postprandial glycemic control by increasing early-phase insulin secretion via GLP-1, an intestinal hormone (Nagahisa et al. Citation2022). Future studies are encouraged to examine the effects of NMN supplementation on GLP-1 production in humans.
Lipids play a key role in atherosclerotic plaque formation and lead to the development of CVD (Anitschkow Citation1913; Windaus Citation1910). According to Anitschkow (Citation1913) and Windaus (Citation1910), there is a direct causal relationship between cholesterol and pathogenesis of atherosclerosis. Large epidemiological studies, for example, the Framingham Study (Kannel et al. Citation1961) and the Seven Countries Studies (Keys et al. Citation1986), also revealed a strong association between high blood cholesterol levels and cardiovascular events. However, we were only able to observe a statistically significant decrease in TG levels in participants with overweight/obesity (MD −27.80 mg/dL). The overall effects of NMN supplementation on TG, TC, LDL-C, and HDL-C were not significant. Nevertheless, this finding may still have some clinical implication, given that the relative risk per 1 mmol/L (equal to 88.5 mg/dL) of TG for CVD is 1.32 in men and 1.76 in women according to one population-based meta-analysis that included 16 prospective studies from the United States, Scandinavia, and other European countries (Hokanson and Austin Citation1996).
In terms of adverse events related to long-term NMN supplementation, most reported side effects belong to gastrointestinal disorders such as diarrhea and abdominal pain. Other minor issues include skin problem and mouth ulcer. The side effects reported are mild to moderate, which is consistent with the findings from a previous systemic review evaluating the safety of NAD in different clinical conditions that NAD has a low incidence of side effects (Gindri et al. Citation2023). In addition, no significant changes in liver enzymes from baseline value are observed, suggesting that a dose up to 1250 mg NMN/d can be tolerated in humans.
In conclusion, results of this meta-analysis reveal that NMN supplementation effectively boosts blood NAD levels and provides a moderate improvement in TG levels in overweight/obese participants. Nonetheless, most of the clinically relevant outcomes and body composition were not proven effective in humans receiving NMN supplementation. However, it should be noted that the clinical laboratory test results of the majority of participants had been already within healthy normal range before the trial began, thus leaving little space for a statistically significant improvement to be evident and possibly undermining the therapeutic effects of NMN. Future studies are encouraged to develop a biological age algorithm using big data and machine learning techniques to combine results of different metabolic markers altogether for a more comprehensive analysis and apply the biological age as a supplementary outcome. It is also possible that sex differences might cause disparities in efficacy. Recent evidence from animal models has shown greater improvement in metabolic parameters after NMN treatment in female mice than in male mice with diet-induced diabetes (J. Yoshino et al. Citation2011). Finally, the small number of included studies precluded the statistical power of our analysis and disallowed us from furthering conducting subgroup analyses for some outcome measures. Nonetheless, it must also be emphasized that although NMN is proposed as a calorie-restriction and exercise mimetics (Bonkowski and Sinclair Citation2016), it should not be taken as an excuse for gluttony and a substitute for physical activity. Perhaps currently the most potent and practical approach to combat age-related decline in physiological integrity remain still regular exercise and balanced diet (Ekmekcioglu Citation2020; Grevendonk et al. Citation2021). Although the limited number of eligible studies was powered to detect changes in the primary outcomes, a significant number of studies exhibited with moderate to high risk-of-bias. Therefore, future randomized controlled trials with more rigid study design are needed to conclude about the exact effects of NMN supplementation.
Author contributions
ZJ and SW designed the project; ZJ and EP conducted literature search, study selection, and data extraction; ZJQ and EP performed data analysis, interpreted the results, and drafted the manuscript. All authors acknowledge full responsibility for the analyses and interpretation of the report.
Supplementary_Material_CLEAN.docx
Download MS Word (1.4 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Akasaka, H., H. Nakagami, K. Sugimoto, Y. Yasunobe, T. Minami, T. Fujimoto, M. Kanou, K. Yamana, S. I. Imai, and H. Rakugi. 2022. Impact of nicotinamide mononucleotide (NMN) for older diabetic patients with impaired physical performance: A prospective, placebo-controlled, doubleblind study. European Geriatric Medicine 13 (Supplement 1):S271. doi: 10.1007/s41999-022-00711-8.
- Akasaka, H., H. Nakagami, K. Sugimoto, Y. Yasunobe, T. Minami, T. Fujimoto, K. Yamamoto, C. Hara, A. Shiraki, K. Nishida, et al. 2023. Effects of nicotinamide mononucleotide on older patients with diabetes and impaired physical performance: A prospective, placebo-controlled, double-blind study. Geriatrics & Gerontology International 23 (1):38–43. doi: 10.1111/ggi.14513.
- Allida, S. M., C. F. Hsieh, K. L. Cox, K. Patel, A. Rouncefield-Swales, C. E. Lightbody, A. House, and M. L. Hackett. 2023. Pharmacological, non‐invasive brain stimulation and psychological interventions, and their combination, for treating depression after stroke. The Cochrane Database of Systematic Reviews 7 (7):CD003437. doi: 10.1002/14651858.CD003437.pub5.
- Amati, F., J. J. Dubé, P. M. Coen, M. Stefanovic-Racic, F. G. S. Toledo, and B. H. Goodpaster. 2009. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care 32 (8):1547–9. doi: 10.2337/dc09-0267.
- Anderson, R. M., K. J. Bitterman, J. G. Wood, O. Medvedik, and D. A. Sinclair. 2003. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature 423 (6936):181–5. doi: 10.1038/nature01578.
- Anitschkow, N. 1913. Ueber experimentelle Cholesterinsteatose und ihre Bedeutehung einiger pathologischer Prozesse. Centrbl Allg Pathol Pathol Anat 24:1–9.
- Ansari, H. R., and G. P. S. Raghava. 2010. Identification of NAD interacting residues in proteins. BMC Bioinformatics 11 (1):160. doi: 10.1186/1471-2105-11-160.
- Araki, T., Y. Sasaki, and J. Milbrandt. 2004. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305 (5686):1010–3. doi: 10.1126/science.1098014.
- Barker, F., A. Hart, A. Sayer, and M. Witham. 2022. Effects of nicotinamide adenine dinucleotide precursors on physical function and frailty: A systematic review. European Geriatric Medicine 13 (Supplement 1):S89. doi: 10.1007/s41999-022-00711-8.
- Barzilai, N., and L. Ferrucci. 2012. Insulin resistance and aging: A cause or a protective response? The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 67 (12):1329–31. doi: 10.1093/gerona/gls145.
- Blagosklonny, M. V. 2008. Aging: ROS or TOR. Cell Cycle 7 (21):3344–54. doi: 10.4161/cc.7.21.6965.
- Bogan, K. L., and C. Brenner. 2008. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD + precursor vitamins in human nutrition. Annual Review of Nutrition 28 (1):115–30. doi: 10.1146/annurev.nutr.28.061807.155443.
- Bonkowski, M. S., and D. A. Sinclair. 2016. Slowing ageing by design: The rise of NAD(+) and sirtuin-activating compounds. Nature Reviews-Molecular Cell Biology 17 (11):679–90. doi: 10.1038/nrm.2016.93.
- Bordone, L., D. Cohen, A. Robinson, M. C. Motta, E. van Veen, A. Czopik, A. D. Steele, H. Crowe, S. Marmor, J. Luo, et al. 2007. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 6 (6):759–67. doi: 10.1111/j.1474-9726.2007.00335.x.
- Borenstein, M., L. V. Hedges, J. P. T. Higgins, and H. R. Rothstein. 2011. Introduction to meta-analysis. New Jersey: Wiley.
- Cantó, C., Z. Gerhart-Hines, J. N. Feige, M. Lagouge, L. Noriega, J. C. Milne, P. J. Elliott, P. Puigserver, and J. Auwerx. 2009. AMPK regulates energy expenditure by modulating NAD + metabolism and SIRT1 activity. Nature 458 (7241):1056–60. doi: 10.1038/nature07813.
- Cantó, C., R. H. Houtkooper, E. Pirinen, D. Y. Youn, M. H. Oosterveer, Y. Cen, P. J. Fernandez-Marcos, H. Yamamoto, P. A. Andreux, P. Cettour-Rose, et al. 2012. The NAD + precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metabolism 15 (6):838–47. doi: 10.1016/j.cmet.2012.04.022.
- Chambon, P., J. Weill, and P. Mandel. 1963. Nicotinamide mononucleotide activation of a new DNA-dependent polyadenylic acid synthesizing nuclear enzyme. Biochemical and Biophysical Research Communications 11 (1):39–43. doi: 10.1016/0006-291x(63)90024-x.
- Chia, C. W., J. M. Egan, and L. Ferrucci. 2018. Age-related changes in glucose metabolism, hyperglycemia, and cardiovascular risk. Circulation Research 123 (7):886–904. doi: 10.1161/circresaha.118.312806.
- Covarrubias, A. J., R. Perrone, A. Grozio, and E. Verdin. 2021. NAD + metabolism and its roles in cellular processes during ageing. Nature Reviews-Molecular Cell Biology 22 (2):119–41. doi: 10.1038/s41580-020-00313-x.
- Damgaard, M. V., and J. T. Treebak. 2023. What is really known about the effects of nicotinamide riboside supplementation in humans. Science Advances 9 (29):eadi4862. doi: 10.1126/sciadv.adi4862.
- de Picciotto, N. E., L. B. Gano, L. C. Johnson, C. R. Martens, A. L. Sindler, K. F. Mills, S. i Imai, and D. R. Seals. 2016. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15 (3):522–30. doi: 10.1111/acel.12461.
- Distefano, G., R. A. Standley, X. Zhang, E. A. Carnero, F. Yi, H. H. Cornnell, and P. M. Coen. 2018. Physical activity unveils the relationship between mitochondrial energetics, muscle quality, and physical function in older adults. Journal of Cachexia, Sarcopenia and Muscle 9 (2):279–94. doi: 10.1002/jcsm.12272.
- Ekmekcioglu, C. 2020. Nutrition and longevity – from mechanisms to uncertainties. Critical Reviews in Food Science and Nutrition 60 (18):3063–82. doi: 10.1080/10408398.2019.1676698.
- Fang, E. F., H. Kassahun, D. L. Croteau, M. Scheibye-Knudsen, K. Marosi, H. Lu, R. A. Shamanna, S. Kalyanasundaram, R. C. Bollineni, M. A. Wilson, et al. 2016. NAD + replenishment improves lifespan and healthspan in ataxia telangiectasia models via mitophagy and DNA repair. Cell Metabolism 24 (4):566–81. doi: 10.1016/j.cmet.2016.09.004.
- Fried, L. P., C. M. Tangen, J. Walston, A. B. Newman, C. Hirsch, J. Gottdiener, T. Seeman, R. Tracy, W. J. Kop, G. Burke, et al. 2001. Frailty in older adults: Evidence for a phenotype. The Journals of Gerontology-Series A, Biological Sciences and Medical Sciences 56 (3):M146–M156. doi: 10.1093/gerona/56.3.M146.
- Fukamizu, Y., Y. Uchida, A. Shigekawa, T. Sato, H. Kosaka, and T. Sakurai. 2022. Safety evaluation of beta-nicotinamide mononucleotide oral administration in healthy adult men and women. Scientific Reports 12 (1):14442. doi: 10.1038/s41598-022-18272-y.
- Gindri, I. M., G. Ferrari, L. P. S. Pinto, J. Bicca, I. K. Dos Santos, D. Dallacosta, and C. R. M. Roesler. 2023. Evaluation of safety and effectiveness of NAD in different clinical conditions: A systematic review. American Journal of Physiology-Endocrinology and Metabolism 326 (4):E417–E427. doi: 10.1152/ajpendo.00242.2023.
- Gomes, A. P., N. L. Price, A. J. Y. Ling, J. J. Moslehi, M. K. Montgomery, L. Rajman, J. P. White, J. S. Teodoro, C. D. Wrann, B. P. Hubbard, et al. 2013. Declining NAD + induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155 (7):1624–38. doi: 10.1016/j.cell.2013.11.037.
- Gong, B., Y. Pan, P. Vempati, W. Zhao, L. Knable, L. Ho, J. Wang, M. Sastre, K. Ono, A. A. Sauve, et al. 2013. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiology of Aging 34 (6):1581–8. doi: 10.1016/j.neurobiolaging.2012.12.005.
- Grevendonk, L., N. J. Connell, C. McCrum, C. E. Fealy, L. Bilet, Y. M. H. Bruls, J. Mevenkamp, V. B. Schrauwen-Hinderling, J. A. Jörgensen, E. Moonen-Kornips, et al. 2021. Impact of aging and exercise on skeletal muscle mitochondrial capacity, energy metabolism, and physical function. Nature Communications 12 (1):4773. doi: 10.1038/s41467-021-24956-2.
- Grozio, A., K. F. Mills, J. Yoshino, S. Bruzzone, G. Sociali, K. Tokizane, H. C. Lei, R. Cunningham, Y. Sasaki, M. E. Migaud, et al. 2019. Slc12a8 is a nicotinamide mononucleotide transporter. Nature Metabolism 1 (1):47–57. doi: 10.1038/s42255-018-0009-4.
- Harden, A., and W. J. Young. 1906. The alcoholic ferment of yeast-juice. Part II.–the conferment of yeast-juice. Proceedings of the Royal Society of London Series B, 78: 369–75. https://ui.adsabs.harvard.edu/abs/1906RSPSB.78.369H.
- Harrer, M., P. Cuijpers, T. A. Furukawa, and D. D. Ebert. 2021. Doing meta-analysis with R: A hands-on guide. 1st ed. Boca Raton, FL and London: Chapman & Hall/CRC Press.
- Hasegawa, K., S. Wakino, P. Simic, Y. Sakamaki, H. Minakuchi, K. Fujimura, K. Hosoya, M. Komatsu, Y. Kaneko, T. Kanda, et al. 2013. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nature Medicine 19 (11):1496–504. doi: 10.1038/nm.3363.
- Higgins J. P. T., J. Thomas, J., Chandler, M. Cumpston, T. Li, Page M. J., and V. A. Welch (editors). Cochrane handbook for systematic reviews of interventions version 6.4 (updated August 2023). Cochrane, 2023. www.training.cochrane.org/handbook.
- Hokanson, J. E., and M. A. Austin. 1996. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies. Journal of Cardiovascular Risk 3 (2):213–9. doi: 10.1097/00043798-199604000-00014.
- Hou, Y., S. Lautrup, S. Cordonnier, Y. Wang, D. L. Croteau, E. Zavala, Y. Zhang, K. Moritoh, J. F. O’Connell, B. A. Baptiste, et al. 2018. NAD + supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proceedings of the National Academy of Sciences of the United States of America 115 (8):E1876–E1885. doi: 10.1073/pnas.1718819115.
- Huang, H. 2022. A multicentre, randomised, double blind, parallel design, placebo controlled study to evaluate the efficacy and safety of Uthever (NMN supplement), an orally administered supplementation in middle aged and older adults. Frontiers in Aging 3:851698. doi: 10.3389/fragi.2022.851698.
- Hung, C.-W., Y.-C. Chen, W.-L. Hsieh, S.-H. Chiou, and C.-L. Kao. 2010. Ageing and neurodegenerative diseases. Ageing Research Reviews 9 (Suppl 1):S36–S46. doi: 10.1016/j.arr.2010.08.006.
- Igarashi, M., Y. Nakagawa-Nagahama, M. Miura, K. Kashiwabara, K. Yaku, M. Sawada, R. Sekine, Y. Fukamizu, T. Sato, T. Sakurai, et al. 2022. Chronic nicotinamide mononucleotide supplementation elevates blood nicotinamide adenine dinucleotide levels and alters muscle function in healthy older men. NPJ Aging 8 (1):5. doi: 10.1038/s41514-022-00084-z.
- Ikeda, M., H. Tsuji, S. Nakamura, A. Ichiyama, Y. Nishizuka, and O. Hayaishi. 1965. Studies on the biosynthesis of nicotinamide adenine dinucleotide: II. A role of picolinic carboxylase in the biosynthesis of nicotinamide adenine dinucleotide from tryptophan in mammals. Journal of Biological Chemistry 240 (3):1395–401. doi: 10.1016/S0021-9258(18)97589-7.
- Janssens, G. E., L. Grevendonk, R. Z. Perez, B. V. Schomakers, J. de Vogel-van den Bosch, J. M. W. Geurts, M. van Weeghel, P. Schrauwen, R. H. Houtkooper, and J. Hoeks. 2022. Healthy aging and muscle function are positively associated with NAD + abundance in humans. Nature Aging 2 (3):254–63. doi: 10.1038/s43587-022-00174-3.
- Kaneko, S., J. Wang, M. Kaneko, G. Yiu, J. M. Hurrell, T. Chitnis, S. J. Khoury, and Z. He. 2006. Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. The Journal of Neuroscience 26 (38):9794–804. doi: 10.1523/JNEUROSCI.2116-06.2006.
- Kannel, W. B., T. R. Dawber, A. Kagan, N. Revotskie, and J. Stokes, III, 1961. Factors of risk in the development of coronary heart disease—six-year follow-up experience: The Framingham Study. Annals of Internal Medicine 55 (1):33–50. doi: 10.7326/0003-4819-55-1-33.
- Katayoshi, T., S. Uehata, N. Nakashima, T. Nakajo, N. Kitajima, M. Kageyama, and K. Tsuji-Naito. 2023. Nicotinamide adenine dinucleotide metabolism and arterial stiffness after long-term nicotinamide mononucleotide supplementation: A randomized, double-blind, placebo-controlled trial. Scientific Reports 13 (1):2786. doi: 10.1038/s41598-023-29787-3.
- Keys, A., A. Menotti, M. J. Karvonen, C. Aravanis, H. Blackburn, R. Buzina, B. S. Djordjevic, A. S. Dontas, F. Fidanza, and M. H. Keys. 1986. The diet and 15-year death rate in the seven countries study. American Journal of Epidemiology 124 (6):903–15. doi: 10.1093/oxfordjournals.aje.a114480.
- Kim, M., J. Seol, T. Sato, Y. Fukamizu, T. Sakurai, and T. Okura. 2022. Effect of 12-week intake of nicotinamide mononucleotide on sleep quality, fatigue, and physical performance in older japanese adults: A randomized, double-blind placebo-controlled study. Nutrients 14 (4):755. doi: 10.3390/nu14040755.
- Kimura, S., M. Ichikawa, S. Sugawara, T. Katagiri, Y. Hirasawa, T. Ishikawa, W. Matsunaga, and A. Gotoh. 2022. Nicotinamide mononucleotide is safely metabolized and significantly reduces blood triglyceride levels in healthy individuals. Cureus 14 (9):e28812. doi: 10.7759/cureus.28812.
- Knapp, G., and J. Hartung. 2003. Improved tests for a random effects meta-regression with a single covariate. Statistics in Medicine 22 (17):2693–710. doi: 10.1002/sim.1482.
- Kuerec, A. H., W. Wang, L. Yi, R. Tao, Z. Lin, A. Vaidya, S. Pendse, S. Thasma, N. Andhalkar, G. Avhad, et al. 2024. Towards personalized nicotinamide mononucleotide (NMN) supplementation: Nicotinamide adenine dinucleotide (NAD) concentration. Mechanisms of Ageing and Development 218:111917. doi: 10.1016/j.mad.2024.111917.
- Li, J., M. S. Bonkowski, S. Moniot, D. Zhang, B. P. Hubbard, A. J. Y. Ling, L. A. Rajman, B. Qin, Z. Lou, V. Gorbunova, et al. 2017. A conserved NAD + binding pocket that regulates protein-protein interactions during aging. Science 355 (6331):1312–7. doi: 10.1126/science.aad8242.
- Liao, B., Y. Zhao, D. Wang, X. Zhang, X. Hao, and M. Hu. 2021. Nicotinamide mononucleotide supplementation enhances aerobic capacity in amateur runners: A randomized, double-blind study. Journal of the International Society of Sports Nutrition 18 (1):54. doi: 10.1186/s12970-021-00442-4.
- Long, A. N., K. Owens, A. E. Schlappal, T. Kristian, P. S. Fishman, and R. A. Schuh. 2015. Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer’s disease-relevant murine model. BMC Neurology 15 (1):19. doi: 10.1186/s12883-015-0272-x.
- López-Otín, C., M. A. Blasco, L. Partridge, M. Serrano, and G. Kroemer. 2013. The hallmarks of aging. Cell 153 (6):1194–217. doi: 10.1016/j.cell.2013.05.039.
- Magni, G., A. Amici, M. Emanuelli, G. Orsomando, N. Raffaelli, and S. Ruggieri. 2004. Enzymology of NAD + homeostasis in man. Cellular and Molecular Life Sciences 61 (1):19–34. doi: 10.1007/s00018-003-3161-1.
- Magni, G., A. Amici, M. Emanuelli, N. Raffaelli, and S. Ruggieri. 1999. Enzymology of NAD + synthesis. Advances in Enzymology and Related Areas of Molecular Biology 73:135–82, xi. doi: 10.1002/9780470123195.ch5.
- Majamaa, K., H. Rusanen, A. M. Remes, J. Pyhtinen, and I. E. Hassinen. 1996. Increase of blood NAD + and attenuation of lactacidemia during nicotinamide treatment of a patient with the MELAS syndrome. Life Sciences 58 (8):691–9. doi: 10.1016/s0024-3205(96)80008-7.
- Mills, K. F., S. Yoshida, L. R. Stein, A. Grozio, S. Kubota, Y. Sasaki, P. Redpath, M. E. Migaud, R. S. Apte, K. Uchida, et al. 2016. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metabolism 24 (6):795–806. doi: 10.1016/j.cmet.2016.09.013.
- Nagahisa, T., S. Yamaguchi, S. Kosugi, K. Homma, K. Miyashita, J. Irie, J. Yoshino, and H. Itoh. 2022. Intestinal epithelial NAD + biosynthesis regulates GLP-1 production and postprandial glucose metabolism in mice. Endocrinology 163 (4):bqac023. doi: 10.1210/endocr/bqac023.
- Naito, K., T. Katayoshi, S. Uehata, N. Nakashima, T. Nakajo, N. Kitajima, and M. Kageyama. 2023. A randomized controlled trial of long-term nicotinamide mononucleotide supplementation in healthy adults. Annals of Nutrition and Metabolism 79 (Supplement 1):493. doi: 10.1159/000530786.
- Okabe, K., K. Yaku, Y. Uchida, Y. Fukamizu, T. Sato, T. Sakurai, K. Tobe, and T. Nakagawa. 2022. Oral administration of nicotinamide mononucleotide is safe and efficiently increases blood nicotinamide adenine dinucleotide levels in healthy subjects. Frontiers in Nutrition 9:868640. doi: 10.3389/fnut.2022.868640.
- Palliyaguru, D. L., E. J. Shiroma, J. K. Nam, E. Duregon, C. Vieira Ligo Teixeira, N. L. Price, M. Bernier, S. Camandola, K. L. Vaughan, R. J. Colman, et al. 2021. Fasting blood glucose as a predictor of mortality: Lost in translation. Cell Metabolism 33 (11):2189–200.e2183. doi: 10.1016/j.cmet.2021.08.013.
- Pencina, K. M., S. Lavu, M. Dos Santos, Y. M. Beleva, M. Cheng, D. Livingston, and S. Bhasin. 2023. MIB-626, an oral formulation of a microcrystalline unique polymorph of beta-nicotinamide mononucleotide, increases circulating nicotinamide adenine dinucleotide and its metabolome in middle-aged and older adults. The Journals of Gerontology: Series A 78 (1):90–6. doi: 10.1093/gerona/glac049.
- Pencina, K. M., R. Valderrabano, B. Wipper, A. R. Orkaby, K. F. Reid, T. Storer, A. P. Lin, S. Merugumala, L. Wilson, N. Latham, et al. 2023. Nicotinamide adenine dinucleotide augmentation in overweight or obese middle-aged and older adults: A physiologic study. The Journal of Clinical Endocrinology and Metabolism 108 (8):1968–80. doi: 10.1210/clinem/dgad027.
- Rajman, L., K. Chwalek, and D. A. Sinclair. 2018. Therapeutic potential of NAD-boosting molecules: The in vivo evidence. Cell Metabolism 27 (3):529–47. doi: 10.1016/j.cmet.2018.02.011.
- Ratajczak, J., M. Joffraud, S. A. J. Trammell, R. Ras, N. Canela, M. Boutant, S. S. Kulkarni, M. Rodrigues, P. Redpath, M. E. Migaud, et al. 2016. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nature Communications 7 (1):13103. doi: 10.1038/ncomms13103.
- Riera, C. E., and A. Dillin. 2015. Tipping the metabolic scales towards increased longevity in mammals. Nature Cell Biology 17 (3):196–203. doi: 10.1038/ncb3107.
- Rohatgi, A. 2017. WebPlotDigitizer. https://apps.automeris.io/wpd/.
- Romani, M., V. Sorrentino, C.-M. Oh, H. Li, T. I. de Lima, H. Zhang, M. Shong, and J. Auwerx. 2021. NAD + boosting reduces age-associated amyloidosis and restores mitochondrial homeostasis in muscle. Cell Reports 34 (3):108660. doi: 10.1016/j.celrep.2020.108660.
- Savji, N., C. B. Rockman, A. H. Skolnick, Y. Guo, M. A. Adelman, T. Riles, and J. S. Berger. 2013. Association between advanced age and vascular disease in different arterial territories: A population database of over 3.6 million subjects. Journal of the American College of Cardiology 61 (16):1736–43. doi: 10.1016/j.jacc.2013.01.054.
- Smoliga, J. M., O. Vang, and J. A. Baur. 2012. Challenges of translating basic research into therapeutics: Resveratrol as an example. The Journals of Gerontology-Series A, Biological Sciences and Medical Sciences 67 (2):158–67. doi: 10.1093/gerona/glr062.
- Sorrentino, V., M. Romani, L. Mouchiroud, J. S. Beck, H. Zhang, D. D’Amico, N. Moullan, F. Potenza, A. W. Schmid, S. Rietsch, et al. 2017. Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature 552 (7684):187–93. doi: 10.1038/nature25143.
- Sterne, J. A. C., J. Savović, M. J. Page, R. G. Elbers, N. S. Blencowe, I. Boutron, C. J. Cates, H.-Y. Cheng, M. S. Corbett, S. M. Eldridge, et al. 2019. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366: L 4898. doi: 10.1136/bmj.l4898.
- Stromsdorfer, K. L., S. Yamaguchi, M. J. Yoon, A. C. Moseley, M. P. Franczyk, S. C. Kelly, N. Qi, S-i Imai, and J. Yoshino. 2016. NAMPT-mediated NAD + biosynthesis in adipocytes regulates adipose tissue function and multi-organ insulin sensitivity in mice. Cell Reports 16 (7):1851–60. doi: 10.1016/j.celrep.2016.07.027.
- Tarantini, S., M. N. Valcarcel-Ares, P. Toth, A. Yabluchanskiy, Z. Tucsek, T. Kiss, P. Hertelendy, M. Kinter, P. Ballabh, Z. Süle, et al. 2019. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biology 24:101192. doi: 10.1016/j.redox.2019.101192.
- Trammell, S. A., L. Yu, P. Redpath, M. E. Migaud, and C. Brenner. 2016. Nicotinamide riboside is a major NAD + precursor vitamin in cow milk. The Journal of Nutrition 146 (5):957–63. doi: 10.3945/jn.116.230078.
- Trammell, S. A. J., M. S. Schmidt, B. J. Weidemann, P. Redpath, F. Jaksch, R. W. Dellinger, Z. Li, E. D. Abel, M. E. Migaud, and C. Brenner. 2016. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nature Communications 7 (1):12948. doi: 10.1038/ncomms12948.
- Turner, J., A. Licollari, E. Mihalcea, and A. M. Tan. 2021. Safety evaluation for Restorin® NMN, a NAD plus precursor. Frontiers in Pharmacology 12:749727. doi: 10.3389/fphar.2021.749727.
- Uddin, G. M., N. A. Youngson, D. A. Sinclair, and M. J. Morris. 2016. Head to head comparison of short-term treatment with the NAD(+) precursor nicotinamide mononucleotide (NMN) and 6 weeks of exercise in obese female mice. Frontiers in Pharmacology 7:258. doi: 10.3389/fphar.2016.00258.
- Viechtbauer, W., and M. W. Cheung. 2010. Outlier and influence diagnostics for meta-analysis. Research Synthesis Methods 1 (2):112–25. doi: 10.1002/jrsm.11.
- Wang, X., X. Hu, Y. Yang, T. Takata, and T. Sakurai. 2016. Nicotinamide mononucleotide protects against β-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Research 1643:1–9. doi: 10.1016/j.brainres.2016.04.060.
- Warburg, O., and W. Christian. 1936. Pyridin, the hydrogen-transferring component of the fermentation enzymes (pyridine nucleotide). Biochemistry Z 287 (1):180–212.
- Wells, G. A., S. C. Hsieh, J. Peterson, C. Zheng, S. E. Kelly, B. Shea, and P. Tugwell. 2024. Etidronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. The Cochrane Database of Systematic Reviews 4 (4):CD003376. doi: 10.1002/14651858.CD003376.pub4.
- Windaus, A. 1910. Über den Gehalt normaler und atheromatöser Aorten an Cholesterin und Cholesterinestern. Biological Chemistry 67 (2):174–176. doi: 10.1515/bchm2.1910.67.2.174.
- Yamaguchi, S., J. Irie, M. Mitsuishi, Y. Uchino, H. Nakaya, R. Takemura, E. Inagaki, S. Kosugi, H. Okano, M. Yasui, et al. 2024. Safety and efficacy of long-term nicotinamide mononucleotide supplementation on metabolism, sleep, and nicotinamide adenine dinucleotide biosynthesis in healthy, middle-aged Japanese men. Endocrine Journal 71 (2):153–69. doi: 10.1507/endocrj.EJ23-0431.
- Yamane, T., M. Imai, T. Bamba, and S. Uchiyama. 2023. Nicotinamide mononucleotide (NMN) intake increases plasma NMN and insulin levels in healthy subjects. Clinical Nutrition ESPEN 56:83–6. doi: 10.1016/j.clnesp.2023.04.031.
- Yasuda, I., K. Hasegawa, Y. Sakamaki, H. Muraoka, T. Kawaguchi, E. Kusahana, T. Ono, T. Kanda, H. Tokuyama, S. Wakino, et al. 2021. Pre-emptive short-term nicotinamide mononucleotide treatment in a mouse model of diabetic nephropathy. Journal of the American Society of Nephrology 32 (6):1355–70. doi: 10.1681/asn.2020081188.
- Yi, L., A. B. Maier, R. Tao, Z. Lin, A. Vaidya, S. Pendse, S. Thasma, N. Andhalkar, G. Avhad, and V. Kumbhar. 2023. The efficacy and safety of β-nicotinamide mononucleotide (NMN) supplementation in healthy middle-aged adults: A randomized, multicenter, double-blind, placebo-controlled, parallel-group, dose-dependent clinical trial. GeroScience 45 (1):29–43. doi: 10.1007/s11357-022-00705-1.
- Yoshino, J., K. F. Mills, M. J. Yoon, and S-i Imai. 2011. Nicotinamide mononucleotide, a key NAD + intermediate, treats the pathophysiology of diet-and age-induced diabetes in mice. Cell Metabolism 14 (4):528–36. doi: 10.1016/j.cmet.2011.08.014.
- Yoshino, M., J. Yoshino, B. D. Kayser, G. J. Patti, M. P. Franczyk, K. F. Mills, M. Sindelar, T. Pietka, B. W. Patterson, S. I. Imai, et al. 2021. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science 372 (6547):1224–9. doi: 10.1126/science.abe9985.
- Zhao, B., C. Liu, L. Qiang, J. Liu, Z. Qiu, Z. Zhang, J. Zhang, Y. Li, and M. Zhang. 2022. Clinical observation of the effect of nicotinamide mononucleotide on the improvement of insomnia in middle-aged and old adults. American Journal of Translational Medicine 6 (4):167–76. https://ajtm.journals.publicknowledgeproject.org/index.php/ajtm/article/view/2535.