 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Safety in use of jamu consumption, as part of traditional medicine from Indonesia, is dependent on the complete and adequate assessment of potential hazards and risks of the botanicals and botanical constituents included. This includes especially hazards and risks related to the presence in jamu of active pharmaceutical ingredients (APIs) as well as of constituents that are genotoxic and carcinogenic. The present review presents an overview of the current state-of-the art on these hazards and risks based on case reports on adulteration, and the actual detection of genotoxic and carcinogenic ingredients of concern in jamu. Based on the overview thus obtained, it appears that drug-adulteration presents important hazards responsible for potential adverse effects, due to overdosing. The potential hazards of exposure to APIs mainly relate to the presence of constituents that may cause liver damage, renal impairment, kidney failure, steroid dependence or genotoxicity and carcinogenicity. For these APIs, a risk characterisation was performed based on comparison of health-based guidance values (HBGVs) and exposure, while for the genotoxic carcinogens the margin of exposure (MOE) approach was used. Results of this risk characterisation should be used by risk managers to impose specification for constituents of health concern to protect consumers. It is concluded that to manage the risks identified and further improve the safety in use of jamu, a collaboration between farmers, manufacturer/producers, academia, government, health professionals, and consumers is indicated.
1. Introduction
Jamu is the collective name for a traditional medicine system from Indonesia. The therapeutic effects are mainly based on empirical data, passed down across generations, while the standardisation of the level of their active ingredients is not required (BPOM-RI Citation2004a). Jamu preparations are generally formulated from selected and mixed botanicals usually from whole, fragmented or cut plants, or parts of plants. In 2019, jamu was recognised by the Indonesian Ministry of Education and Culture as part of Indonesia’s cultural heritage (Suprana Citation2019). Jamu is widespread in Indonesian and global markets, being easily accessible to consumers via supermarkets, drugstores, natural health/food stores, herbal shops, and gyms while there are also ample possibilities to purchase these products via the internet. The easy accessibility of the products without a need for prescription and the trend of consumers to favour natural products also stimulate the use of herbal based jamu. The market demand for jamu keeps growing, and as a result jamu increasingly provides economic and perceived clinical benefits.
The notorious growth in the consumption of jamu has been associated with the increasing demand for alternative therapies, in part due to the mistrust in conventional medicine and pharmaceutical drugs, and building on the perception that herbal products are “safe” and “natural” and thus “healthy”, while at the same time facilitating the tendency for self-medication aiming for increased control over one's own health (Hasler Citation2002; Rietjens et al. Citation2008; Vargas-Murga et al. Citation2011). As a result, there are a lot of jamu manufacturers that keep developing their products and the related potential health claims communicating the products and their potential benefits to consumers (Greger Citation2001). Unfortunately, the increase of consumption has also brought health risks due to the fraudulent adulteration with conventional pharmaceutical drugs, drug analogues, and/or naturally occurring constituents of concern, which can lead to severe side-effects.
To date, no (governmentally supported) coordinated safety assessment program has been implemented for jamu. Lim and Pranata (Citation2020) warned against the insidious threat of jamu and unregulated traditional medicines since there is no solid evidence that the core ingredients of jamu are beneficial and/or safe. The toxicity data and knowledge on the occurrence of adverse reactions as a result of jamu consumption are still limited. As a result, the increasing use of jamu has raised concerns among scientific and regulatory communities especially given the case studies reporting adverse health effects resulting from misuse, misidentification of the botanical species or (deliberate) contamination with extraneous plants or bioactive constituents (Ernst and Pittler Citation2002; Walker Citation2004). Nevertheless, there is also a more recent case, reported in 2018, when there were some unscrupulous manufacturers and distributors who deliberately adulterated jamu through the addition of active pharmaceutical ingredients (APIs)/(Bahan Kimia Obat) (BKO) in order to increase product effectiveness (BPOM-RI Citation2019). Such adulterations have also been reported for other herbal products worldwide, including, for example, herbal dietary supplements and traditional Chinese medicines (TCM), Indian ayurveda, South African traditional herbal remedies, herbal food supplement on the Dutch market, health products in Canada, and herbal drugs in Iran (Snyman et al. Citation2005; Posadzki et al. Citation2013; Reeuwijk et al. Citation2014; Saberi et al. Citation2018; Crighton et al. Citation2019).
Botanicals may contain a wide variety of toxic constituents (see EFSA compendium, https://www.efsa.europa.eu/en/microstrategy/botanical-summary-report) and this may include compounds that are both genotoxic and carcinogenic (EFSA Citation2017). Genotoxic and carcinogenic compounds naturally occurring in botanical preparations may include especially alkenylbenzenes (ABs), pyrrolizidine alkaloids (PAs), and aristolochic acids (AAs) (van Den Berg et al. Citation2011). Our previous risk assessment studies on jamu and herbal products from Indonesia reported the presence of these type of constituents at levels that pose a concern for human health at the proposed levels of daily use of jamu products when considering life-long use, indicating a priority for risk management action of the corresponding herbal products (Suparmi et al. Citation2018, Citation2019, Citation2020).
Although there are also some reported cases on potential microbiological and metal/metalloid contamination in jamu (Limyati and Juniar Citation1998; Ali et al. Citation2005; Paul et al. Citation2005; Noveriza Citation2015; Wasita and Hendrayana Citation2016; Asra et al. Citation2019; Latifa et al. Citation2020), the present review presents an overview of the current state-of-the art related to the hazards and risks of APIs as well as of constituents that are genotoxic and carcinogenic reported to be present in jamu. This overview informs risk managers aiming at enabling prioritising of regulatory actions to support the safety in use of jamu.
2. Methodology
2.1. Data collection of hazards associated with jamu
2.1.1. Adulteration with APIs that are non-genotoxic
Information on adulteration of jamu with APIs that are intentionally added was collected from an online report of BPOM-RI (Citation2006). A literature study was conducted to inform a narrative review on hazards associated with jamu consumption based on the presence of these constituents of concern in jamu. Literature was collected via Pubmed, Scopus, and Google Scholar using the following search terms: “jamu”, “adulteration”, “adulterasi”, “active pharmaceutical ingredients”, “bahan kimia obat”, “alkenylbenzenes”, “pyrrolizidine alkaloids”, and “aristolochic acids”. Data were filtered from 1980 onwards.
2.1.2. Natural occurrence of constituents that are genotoxic and carcinogenic
Hazards of genotoxic carcinogenic constituents which may naturally occur in botanical constituents of jamu were collected based on literature reporting on the occurrence of ABs, PAs, and AAs in jamu.
2.2. Exposure assessment resulting from the drinking of jamu for constituents of concern
Levels of detected compounds and recommended dosages of each of these jamu were retrieved from reported studies (Supplemental Material 1). From these data, the estimated daily intake (EDI) was calculated to quantify the potential exposure to constituents of concern according to EquationEquation (1)(1)
(1) .
(1)
(1)
where EDI values are expressed in µg/kg bw/day. W is the weight of recommended daily use of these samples (Supplemental Material 1) based on the information retrieved from the labels and as presented in the literature expressed in kg jamu/day. L is the detected level of the compounds of interest expressed in µg/kg jamu. BW is the body weight for which the default value used was 54 kg, the average body weight for Indonesian male and female (FAO Citation2017).
2.3. Risk characterisation
2.3.1. Risk characterisation of APIs that are non-genotoxic
Given that the APIs detected were non-genotoxic constituents, to evaluate their risks, a percentage of deviation (Δ) between the EDI as compared to an existing reference dose (RD) was calculated according to EquationEquation (2)(2)
(2) .
(2)
(2)
where the Δ values were expressed in %, EDI values, expressed in µg/kg bw/day, were calculated by EquationEquation (1)
(1)
(1) , and RD values, expressed in µg/kg bw/day, were available RDs like for example an acceptable daily intake (ADI) in situations of repeated daily exposure. For some APIs, including for example those in therapeutic classes of corticoids/glucocorticoids and nonsteroidal anti-inflammatory drugs (NSAIDs) ADI values have actually been defined based on the no-observed-effect levels (NOELs) established by the EFSA CONTAM panel in their opinion on APIs present in food of animal origin (EFSA Citation2018). When an ADI for an API is not available, the EDI for an API can be compared to the known safe therapeutic dose level of the drug as the RD to estimate if an effect can be expected. When the Δ value of the API present in an adulterated jamu exceeds 0 for a representative RD, a potential risk and/or pharmacological effect can no longer be excluded. When the value is below 0 a potential pharmacological or adverse effect is not expected.
2.3.2. Risk characterisation of constituents that are genotoxic and carcinogenic
The risk characterisation of compounds that are both genotoxic and carcinogenic is generally performed by the so-called margin of exposure (MOE) approach (EFSA Citation2005; Barlow et al. Citation2006; Skovgaard Citation2007; Benford et al. Citation2010). The MOE resulting from intake of the jamu samples during a whole lifetime but also for exposure during 2 weeks every year during a lifetime were obtained from our previous risk assessment related to ABs, PAs, and AAs present in jamu (Suparmi et al. Citation2018, Citation2019, Citation2020) (see Supplemental material 2). In these studies, potential exposure to ABs, PAs, and AAs resulting from consuming the jamu was estimated based on the levels of the constituents in individual jamu and the recommended daily use of these samples based on the information provided on the label. The MOE is calculated by dividing the BMDL10, which is the lower confidence limit of the benchmark dose giving 10% extra tumour incidence above background levels for the genotoxic carcinogen of interest, by the EDI. An outcome of the MOE below 10 000 when based on a BMDL10 from an animal study, is considered a priority for risk management while an MOE above 10 000 is considered to be of low priority for risk management action (EFSA Citation2005).
When evaluating the risks of genotoxic carcinogenic constituents detected in jamu by the MOE approach it is also of importance to note that risk assessment by the MOE approach is based on the assumption of daily exposure during a whole lifetimes. To correct exposure estimates for use during shorter periods of time, such as for example only during periods of illness, it has been proposed to use Haber’s rule as a first tier approach to correct for shorter than lifetime exposure (Doull and Rozman Citation2000; Felter et al. Citation2011). Haber’s rule states that the dose times the exposure duration is constant where k is the toxic outcome, C is the concentration (or dose) of the toxic chemical, and T is the duration of exposure. The numbers 1 and 2 indicate two different exposure regimens, for example, lifetime exposure to a dose C1 and 2 weeks yearly during a whole lifetime exposure to a dose C2, respectively). This implies that one could correct for shorter time of exposure in a linear way. Using Haber’s rule, EDIs and resulting MOEs for shorter than daily lifetime exposure were obtained.
3. Results and discussion
3.1. Emerging hazards and risk characterisation of APIs that are non-genotoxic
shows drug-adulterations presenting potential hazards. This includes for example jamu “pegal linu” and jamu “asam urat” that have been reported to be adulterated with NSAIDs and corticoid drugs to improve their efficacy in treating arthritis and related musculoskeletal disorders. In some cases, jamu advertised for slimming, erectile dysfunction, and antidiabetic activity were reported to be adulterated with sibutramine hydrochloride, sildenafil citrate, and glibenclamide, respectively. In the Supplemental Material 1, a more extensive overview of the related APIs including the estimated exposure and relevant RD values is presented. The potential hazards of exposure to APIs mainly relate to the presence of APIs that may cause blood dyscrasias, drug-induced liver injury (DILI), liver damage, gastrointestinal upset, central nervous system toxicity, peptic ulcers, cardiovascular diseases, renal impairment, kidney failure, or steroid dependence.
Table 1. Reported adulteration of jamu with APIs and associated hazards and health risks.
The overview presented in and Supplemental material 1 clearly indicates the potential presence of API adulteration in jamu. Many of the case reports summarised in these tables lack details that would be important for a rigorous hazard and risk assessment. For instance, some fail to mention the exact level of the APIs detected in the samples showing their presence only by qualitative analysis using thin layer chromatography (TLC) (Gitawati Citation2013; Wisnuwardhani et al. Citation2013; Fauziah et al. Citation2015; Mustarichie et al. Citation2017), a spot test (Hanum et al. Citation2017), or a qualitative chemical test (Maesaroh Citation2020). Other issues hampering hazard and/or risk assessment are the simultaneous presence of more than one API (Lau et al. Citation2003; Doshi et al. Citation2009), or the lack of a description of the recommended daily use of these jamu samples to assess the potential exposure to APIs resulting from consuming the jamu.
For the case studies for which quantitative data were available, depicts the percentage of deviation (Δ) between the EDI of the API resulting from use of the jamu samples as compared to an existing RD. In general, it is noted that the EDI of NSAIDs, phenylbutazone, piroxicam, and paracetamol resulting from jamu consumption is lower than the RD for their pharmacological activity. Illegal presence of methampyrone, mefenamic acid, and prednisone in jamu is a public health issue because the EDIs of these API constituents were above their RDs, so that occurrence of a pharmaceutical effect cannot be excluded. Especially, the data for prednisone raise a concern given that the Δ value of 131955.6% indicates that the EDI exceeds the RD 1320.6-fold. The data presented in indicate that at the current levels at which APIs were detected in jamu the adulteration of sibutramine hydrochloride in slimming jamu and sildenafil citrate in jamu for erectile dysfunction are also unlikely to result in pharmacological effects. The use of antidiabetic jamu containing glibenclamide as recommended is not expected to produce toxic effects.
Figure 1. Risk characterisation of adulteration of jamu with APIs based on Δ values obtained by EquationEquation (2)(2)
(2) , using the EDIs and the existing reference doses (RD) (Supplemental material 1). The vertical dashed line represents the RD value of 0 as a threshold for risk evaluation. The inserted graph shows the Δ value for prednisone. Note that there is a different X axis.
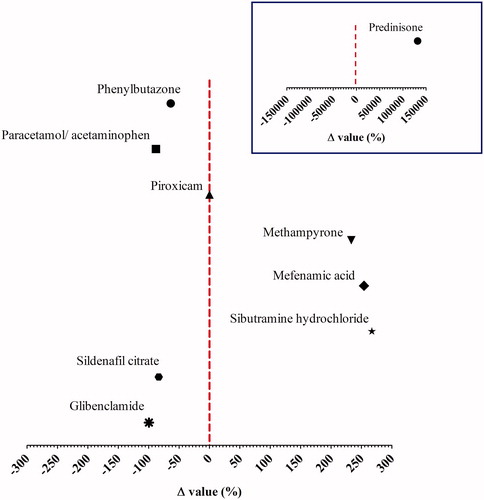
The results presented in and indicate that further investigation of the API adulteration in jamu would be of use to ensure consumer safety. This may hold especially for adulteration of jamu with so-called phosphodiesterase-5 (PDE-5) inhibitors for the treatment of erectile dysfunction, given that this type of adulteration is frequently encountered also in other types of botanical preparations, for which not only adulteration with sildenafil but also with many illegal drug analogues has been reported (Reeuwijk et al. Citation2013; Ahmed et al. Citation2015; Nissan et al. Citation2016; Saberi et al. Citation2018). In case of overdosing, or use of illegal sildenafil analogues with higher potential than the approved drug, there is a risk for especially people using antihypertensives such as nitrates (e.g. nitroglycerine, doxazosin, and terazosin) (Boden et al. Citation2012; EMA Citation2016) and patients suffering from hypotension (Kloner Citation2007) for whom use of PDE-5 inhibitors is contra-indicated.
3.2. Emerging hazards and risk characterisation of constituents that are genotoxic and carcinogenic
With respect to potential chronic adverse health effects, especially the presence of jamu constituents that are genotoxic and carcinogenic raise a concern. ABs, PAs, and AAs occur naturally in some of the plants used for the production of jamu (Supplemental material 2). For these constituents, a safe level of daily exposure cannot be defined because any exposure is assumed to increase the risk of developing cancer.
The AB safrole is categorised in IARC group 2B, probably carcinogenic to humans based on its carcinogenicity in rodent bioassays at high dose levels (Miller et al. Citation1983), and also estragole and methyl eugenol are considered to be genotoxic and carcinogenic (SCF Citation2001a, Citation2001b, Citation2002; Zeller et al. Citation2009). Previously, substantial levels of hepatic methyl eugenol DNA adducts were detected in the liver of human subjects, most likely resulting from regular dietary exposure (Herrmann et al. Citation2013). Twenty-nine out of 30 human liver samples were reported to contain the N2-(trans-methylisoeugenol-3′-yl)-2′-deoxyguanosine adduct at levels up to 36.2 adducts/108 nts (Herrmann et al. Citation2013).
Acute exposure to PAs can cause hepatic veno-occlusive disease (HVOD) with severe liver damage, in some cases with fatal outcome (Mohabbat et al. Citation1976; Tandon et al. Citation1976; Wiedenfeld and Roder Citation1991). Chronic exposure to PA-containing jamu may lead to liver cirrhosis and pulmonary arterial hypertension (EFSA Citation2011). Furthermore, 1,2-unsaturated PAs, including lasiocarpine, monocrotaline, and riddelliine, are considered as being possibly carcinogenic to humans (category 2B) (IARC Citation2012). A possible concern upon chronic exposure to PA-containing jamu is PA-induced liver injury (PA-ILI). Gynura segetum, a PA-producing plant ingredient in jamu (Suparmi et al. Citation2020) is reported as the cause of PA-ILI in 15 patients in China after consumption of Gynura segetum-containing Chinese herbal products for five days up to 2 years (Lin et al. Citation2011).
AAs, including AA-I and AA-II, are nephrotoxic and carcinogenic compounds (Kumar et al. Citation2003). They are classified as group 1 carcinogens and belong to the most potent 2% of known carcinogens (IARC Citation2007; Stiborova et al. Citation2009). Aristolochic acid nephropathy (AAN) is potentially a crucial problem in the Asian area since a lot of people in this region still are convinced that traditional Chinese herbal medicines, which frequently contain AA producing plants, are safer than chemically produced “Western” drugs (Hong et al. Citation2006). AAN was reported in Belgium in 1991, where over 100 young women suffered from end-stage renal disease and in several cases cancer in the kidneys and the upper urinary tract due to the confusing nomenclature, resulting in a replacement of Stephania tetrandra (“Han Fang Ji”) by Aristolochia fangchi (“Guang Fang Ji”) in a Chinese herb-based weight-loss preparation (Gillerot et al. Citation2001). Similar to incidences of AAN, Balkan endemic nephropathy (BEN) occurring in Balkan regions in the 1950s, was ascribed to flour contaminated with Aristolochia clematitis (Arlt et al. Citation2002; Jelaković et al. Citation2014). More cases of AAN were reported in other countries including Spain, Japan, France, Belgium, UK, Taiwan, USA, Germany, China, Korea, Hong Kong, Australia, and Bangladesh (Gillerot et al. Citation2001; Jadot et al. Citation2017). Due to the severity of AAN and the past incidences, the use of AA-containing botanicals, especially Aristolochia sp. (Aristolochiaceae) is banned since 2001 in many countries worldwide (Debelle et al. Citation2008; Heinrich et al. Citation2009), including Indonesia (BPOM-RI Citation2001).
ABs, AAs, and PAs were actually detected in jamu sold on the Indonesian market. The ABs, methyl eugenol, myristicin, safrole, and estragole were detected in 23 out of 25 samples of Indonesian jamu collected by a targeted approach, at levels ranging from 3.8 to 440 μg/g. Methyl eugenol was the major AB detected in most (91.3%) of the samples testing positive for the presence of ABs in Indonesian jamu (Suparmi et al. Citation2018). Later, Suparmi et al. (Citation2019) reported that methyl eugenol at levels amounting to 2.6–443.7 μg/g was detected in 49 out of 114 samples of herbal beverages (including 30 samples of jamu). PAs were detected in 34 out of 35 jamu containing PA-producing botanicals, in the range of 12.3–235.4 μg/kg. A total PA level of 5.9–3.4 μg/kg was found in 17 out of 23 jamu made of non-PA-producing botanicals pointing to contamination with PA-producing plants (Suparmi et al. Citation2020).
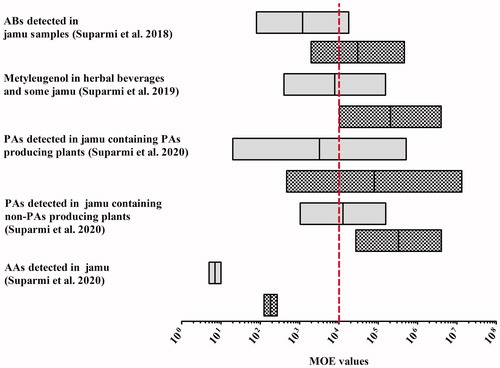
depicts the lowest to highest MOE values obtained for use of the jamu preparations on a daily basis during a lifetime and when assuming use for only 2 weeks every year during a lifetime as reported in our previous studies (Suparmi et al. Citation2018, Citation2019, Citation2020). Overall, the MOE values obtained for these jamu, collected based on a targeted sampling strategy (collecting jamu listing on their label botanicals that could contain the constituents of concern), were generally <10 000, indicating a priority for risk management when assuming daily life-long consumption. Using Haber’s rule, it was estimated that exposure to ABs, including methyl eugenol, to PAs, and to AAs via the majority of products analysed, would be of low concern when the herbal preparations would be consumed for less than 2 weeks per year during a lifetime, although some samples still raised a concern, also when considering such limited use.
Figure 2. Risk characterisation of jamu based on MOE values obtained for the consumption of the preparations based on daily lifetime exposure (grey bars) and 2 weeks a year during a lifetime (shaded bars). The vertical dashed line represents the MOE value of 10 000 as a threshold for risk management action (EFSA Citation2005).
3.3. Future perspectives in supporting the safety of jamu
The emerging hazards and risks upon consumption of jamu assessed in this review indicate that a further risk assessment, as well as risk management actions and related risk communication actions have to be considered by the Indonesian authorities. The challenge for the future is to systematically research this area to minimise the risk. Actions considered should focus on the adverse health effects to the consumers caused by API-adulterated jamu () and risks due to jamu containing genotoxic carcinogens like ABs, PAs, and AAs (). Collaborations between farmers, health professionals (physicians, pharmacists, and nurses), consumers, academia, industry, risk managers, and government are needed to promote the safety of jamu (including generation of analytical data). Some actions to be considered by these stakeholders are presented in Supplemental Material 3. Consumers, health care practitioners, and the manufacturers can contribute to improvement of the pharmacovigilance databases to increase the quantity and quality of adverse reaction reports suspected to be associated with jamu. Pharmacies, hospitals, points of sale can actively contribute to the community-based surveillance to detect cases related to jamu related hazards or risks (Niggemann and Grüber Citation2003; Jordan et al. Citation2010). To tackle the circulation of jamu that may contain constituents of concern in the global market, joint efforts of consumers, industries, and regulatory bodies are required, such as case reporting, audits of jamu manufacturers, and information sharing among regulatory authorities.
Risk managers have to set up specifications including maximum permitted level (MPL) of toxic compounds. BPOM-RI (Citation2016) has stipulated an MPL of 10 mg/kg for estragole and of 0.1 mg/kg for safrole based on Regulation of Head BPOM RI No. 22, while MPLs for methyl eugenol and PAs have not (yet) been established. As discussed previously (Suparmi et al. Citation2019), an MPL value of 0.1–1 mg/kg can be considered to reduce the exposure to methyl eugenol via consuming the herbal products to a level that would no longer raise a concern. In addition, to reduce the exposure to PAs an MPL of 0.1 mg PAs/kg jamu would result in an MOE above 10 000 and thus would no longer raise concern for human health (Suparmi et al. Citation2020).
Furthermore, the high level of PAs detected in a large proportion of especially the Gynura-based jamu, indicates that banning the use of Gynura sp. as botanical constituent in herbal products will increase consumer safety. The level of PAs in Gynura-based jamu was three times higher than the level of PAs detected in dried farfara (Tussilago farfara) flos (Suparmi et al. Citation2020), which was also relatively high. Based on these results, the regulation HK.00.05.23.3644 (BPOM-RI Citation2004b) can be refined by adding Gynura sp. and Tussilago farfara to the list of banned ingredients in Indonesian traditional medicines.
To reduce the exposure to ABs and PAs, also the labelling regulations can be refined by adding restrictions on the recommended daily dose, the duration of consumption, and information on the adverse effects upon prolonged consumption on the label of herbal products to further support consumer safety. The labelling is useful not only for consumers and risk assessors, but also for general practitioners, so they can prescribe the product in the appropriate dose for an appropriate duration. So far, on the label of Indonesian herbal products there is no information about the limitation of consumption duration or use levels.
4. Concluding remarks
Case reports on adulteration or contamination of jamu, and the occurrence of natural constituents of concern like genotoxic and carcinogenic compounds in jamu, as presented in this review, remind us to be more aware of the hazards and risks posed by jamu consumption. Risk management actions including prioritising regulatory actions to reduce potential risks connected to the exposure to genotoxic carcinogens via consumption of herbal products from Indonesia, especially jamu, is urgently required to support the safety in use of jamu. A collaboration between farmers, manufacturers/producers, academia, risk managers, government, health professionals, and consumers may be essential to prevent the risk of potential adverse health effects of these botanical preparations.
| Abbreviations | ||
| AAs | = | Aristolochic acids |
| AAN | = | Aristolochic acid nephropathy |
| ABs | = | Alkenylbenzenes |
| ADI | = | Acceptable daily intake |
| BEN | = | Balkan endemic nephropathy |
| BKO/APIs | = | Bahan Kimia Obat/active pharmaceutical ingredients |
| BMDL10 | = | Lower confidence limit of the benchmark dose resulting in a 10% extra effect |
| BPOM RI | = | Badan Pengawas Obat dan Makanan Republik Indonesia/The National Agency for Drug and Food Control, Republic of Indonesia |
| CPOTB | = | Cara Pembuatan Obat Tradisional yang Baik/Good Manufacturing Practice (GMP) for Traditional Medicine |
| DILI | = | Drug-induced liver injury |
| EDI | = | Estimated daily intake |
| EFSA | = | European Food Safety Authority |
| GACP | = | Good agricultural and collection practices |
| HBGV | = | Health based guidance value |
| HVOD | = | Hepatic veno-occlusive disease |
| MENKES RI | = | Menteri Kesehatan Republik Indonesia/Ministry of Health, Republic of Indonesia |
| MPL | = | Maximum permitted level |
| MOE | = | Margin of exposure |
| NOELs | = | No-observed-effect levels |
| NSAIDs | = | Nonsteroidal anti-inflammatory drugs |
| PAs | = | Pyrrolizidine alkaloids |
| PA-ILI | = | PA-induced liver injury |
| RD | = | Reference dose |
| TCM | = | Traditional Chinese Medicines |
| TLC | = | Thin layer chromatography |
Supplemental Material
Download MS Word (87.5 KB)Acknowledgements
The authors gratefully acknowledge Dr. Roger O. McClellan, Editor-in-Chief of Critical Reviews in Toxicology for critical proofreading of the manuscript in our first submission. The authors also extend a note of appreciation to the reviewers who were selected by the editor. The comments of these anonymous reviewers were very useful to improve the quality of final version of the manuscript.
Declaration of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The author’s affiliation is as shown on the cover page. This manuscript describes the results obtained in the project “Risk and benefit: analysis of herbal products from Indonesia”, that was funded by Indonesian Endowment Fund for Education, Ministry of Finance, The Republic of Indonesia for the financial support through a Beasiswa Pendidikan Indonesia Lembaga Pengelola Dana Pendidikan (BPI LPDP) Doctoral Scholarship for SS [contract number: PRJ-365/LPDP/2016]. SS also received financial support from the Foundation for Research and Innovation in Toxicology (SOIT) for her Postdoc at the Division of Toxicology of Wageningen University and Research, The Netherlands. The funders have no influence on the manuscript’s content. During the past 5 years, none of the authors has been involved in legal or regulatory matters related to the contents of the article.
Supplemental material
Supplemental material for this article is available online here.
References
- Agrawal S, Khazaeni B. 2020. Acetaminophen toxicity. Treasure Island (FL): StatPearls Publishing.
- Ahmed SM, Gadkariem E, Mohamed M. 2015. Adulteration of herbal preparations with sildenafil and tadalafil. The proceeding of The Sixth Annual Post-graduate Studies & Scientific Research Conference. University of Khartoum, Sudan.
- Ali N, Hashim NH, Saad B, Safan K, Nakajima M, Yoshizawa T. 2005. Evaluation of a method to determine the natural occurrence of aflatoxins in commercial traditional herbal medicines from Malaysia and Indonesia. Food Chem Toxicol. 43(12):1763–1772.
- Andrade SE, Martinez C, Walker AM. 1998. Comparative safety evaluation of non-narcotic analgesics. J Clin Epidemiol. 51(12):1357–1365.
- Arlt VM, Stiborova M, Schmeiser HH. 2002. Aristolochic acid as a probable human cancer hazard in herbal remedies: a review. Mutagenesis. 17(4):265–277.
- Aronson JK. 2016. Piroxicam. 16th ed. Oxford: Elsevier; p. 795–798.
- Asra R, Maisitoh M, Rusdi R. 2019. Analysis of metal contents lead and cadmium in uretic acid jamu by using atomic absorption spectrophotometric. J Pharm Sci. 2(1):10–16.
- Baker LR, Cattell WR, Levison DA, Edelman JB. 1984. Mefenamic acid-induced interstitial nephritis and renal failure. Postgrad Med J. 60(699):82–83.
- Barlow S, Renwick AG, Kleiner J, Bridges JW, Busk L, Dybing E, Edler L, Eisenbrand G, Fink-Gremmels J, Knaap A, et al. 2006. Risk assessment of substances that are both genotoxic and carcinogenic. Report of an International Conference organized by EFSA and WHO with support of ILSI Europe. Food Chem Toxicol. 44(10):1636–1650.
- Benford D, DiNovi M, Setzer RW. 2010. Application of the margin-of-exposure (MoE) approach to substances in food that are genotoxic and carcinogenic: example: benzo[a]pyrene and polycyclic aromatic hydrocarbons. Food Chem Toxicol. 48:542–548.
- Berg SJPLvd, Restani P, Boersma MG, Delmulle L, Rietjens IMCM. 2011. Levels of genotoxic and carcinogenic compounds in plant food supplements and associated risk assessment. Food Nutr Sci. 2(9):989–1010.
- Boden WE, Finn AV, Patel D, Peacock WF, Thadani U, Zimmerman FH. 2012. Nitrates as an integral part of optimal medical therapy and cardiac rehabilitation for stable angina: review of current concepts and therapeutics. Clin Cardiol. 35(5):263–271.
- BPOM-RI. 2001. Keputusan Kepala Badan Pengawas Obat dan Makanan Nomor HK.00.05.4.03960 Tentang Larangan Produksi dan Distribusi Obat Tradisional dan Suplemen Makanan yang Mengandung Tanaman Aristolochia Sp.
- BPOM-RI. 2004a. Keputusan Kepala Badan Pengawas Obat dan Makanan Republik Indonesia Nomor HK. 00.05.4.2411 tentang Ketentuan Pokok Pengelompokan dan Penandaan Obat Bahan Alam Indonesia; p. 1–4.
- BPOM-RI. 2004b. Keputusan Kepala Badan Pengawas Obat dan Makanan Nomor Nomor HK.00.05.23.3644 Tentang Ketentuan Pokok Pengawasan Suplemen Makanan; p. 1–12.
- BPOM-RI. 2006. Bahan Kimia Obat (BKO) yang dibubuhkan ke dalam obat tradisional (jamu); [accessed 2020 Nov 17]. https://www.pom.go.id/new/view/more/berita/144/BAHAYA-BAHAN-KIMIA-OBAT–BKO–YANG-DIBUBUHKAN-KEDALAM-OBAT-TRADISIONAL–JAMU-.html
- BPOM-RI. 2016. Peraturan Kepala Badan Pengawas obat dan Makanan Republik Indonesia Nomor 22 Tahun 2016 tentang Penggunaan Bahan Tambahan Perisa. In: Badan Pengawas Obat dan Makanan. http://jdih.pom.go.id/showpdf.php?u=giZCxzW6JpAGRcPnOwhBjW564tWbhWZSLziyNQ6l6oI=.
- BPOM-RI. 2019. Kinerja BPOM Dalam Rangka Triwulan II tahun 2019. https://www.pom.go.id/new/admin/dat/20191212/RTN-TW-2-2019.pdf.
- Ching CK, Lam YH, Chan AYW, Mak TWL. 2012. Adulteration of herbal antidiabetic products with undeclared pharmaceuticals: a case series in Hong Kong. Br J Clin Pharmacol. 73(5):795–800.
- Chong CSY. 2010. Psychosis related to the use of sibutramine disguised as over-the-counter herbal weight loss remedies: a report of two patients. East Asian Arch Psychiatry. 20(4):186–189.
- Crighton E, Coghlan ML, Farrington R, Hoban CL, Power MWP, Nash C, Mullaney I, Byard RW, Trengove R, Musgrave IF, et al. 2019. Toxicological screening and DNA sequencing detects contamination and adulteration in regulated herbal medicines and supplements for diet, weight loss and cardiovascular health. J Pharm Biomed Anal. 176:112834.
- de Simone G, D'Addeo G. 2008. Sibutramine: balancing weight loss benefit and possible cardiovascular risk. Nutr Metab Cardiovasc Dis. 18(5):337–341.
- Debelle FD, Vanherweghem J-L, Nortier JL. 2008. Aristolochic acid nephropathy: a worldwide problem. Kidney Int. 74(2):158–169.
- Doshi HK, Thambiah J, Chan CL, Nga ME, Tambyah PA. 2009. Necrotising fasciitis caused by adulterated traditional Asian medicine: a case report. J Orthop Surg (Hong Kong). 17(2):223–226.
- Doull J, Rozman KK. 2000. Using Haber’s law to define the margin of exposure. Toxicology. 149(1):1–2.
- EFSA. 2005. Opinion of the Scientific Committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA J. 282:1–31.
- EFSA. 2011. Scientific opinion on pyrrolizidine alkaloids in food and feed. EFSA J. 9:2406.
- EFSA. 2017. Compendium of botanicals. https://www.efsa.europa.eu/en/data/compendium-botanicals.
- EFSA. 2018. Update: methodological principles and scientific methods to be taken into account when establishing reference points for action (RPAs) for non-allowed pharmacologically active substances present in food of animal origin. EFSA J. 16:e05332.
- EFSA/EMA. 2013. Joint statement of EFSA and EMA on the presence of residues of phenylbutazone in horse meat. EFSA J. 11:3190.
- EMA. 2016. European Medicines Agency. European Public Assessment Report (EPAR) for Viagra sildenafil; [accessed 2020 Nov 30]. https://www.ema.europa.eu/en/documents/overview/viagra-epar-summary-public_en.pdf
- EMA. 2018a. Assessment report referral under Article 31 of Directive 2001/83/EC metamizole-containing medicinal products. Amsterdam (The Netherlands): European Medicines Agency.
- EMA. 2018b. CHMP assessment report amglidia international non-proprietary name: glibenclamide. London (UK): European Medicines Agency.
- Ernst E, Pittler MH. 2002. Risks associated with herbal medicinal products. Wien Med Wochenschr. 152(7–8):183–189.
- Faich GA. 1987. Risks and indications of phenylbutazone: another look. Pharmacotherapy. 7(1):25–27.
- FAO. 2017. Body weights and heights by countries. http://www.fao.org/docrep/meeting/004/M2846E/M2846E07.htm.
- Fauziah SS, Lestari F, Lukmayani Y, Aprilia WH. 2015. Pengaruh pemberian jamu pegal linu mengandung bahan kimia obat (BKO) terhadap fungsi hati tikus Wistar jantan. In: Ahmadi D, Ilmiah K, Gunawan G, Yulianti Y, Dari S, editors. Prosiding Penelitian SPeSIA Unisba 2015. Bandung: Universitas Islam Bandung; p. 96–103.
- Felter SP, Conolly RB, Bercu JP, Bolger PM, Boobis AR, Bos PMJ, Carthew P, Doerrer NG, Goodman JI, Harrouk WA, et al. 2011. A proposed framework for assessing risk from less-than-lifetime exposures to carcinogens. Crit Rev Toxicol. 41(6):507–544.
- Giam YC, Tham SN, Tan T, Lim A. 1986. Drug eruptions from phenylbutazone in Jamu. Ann Acad Med Singap. 15(1):118–121.
- Gillerot G, Jadoul M, Arlt VM, van Ypersele De Strihou C, Schmeiser HH, But PP, Bieler CA, Cosyns JP. 2001. Aristolochic acid nephropathy in a Chinese patient: time to abandon the term “Chinese herbs nephropathy”? Am J Kidney Dis. 38:E26.
- Gitawati R. 2013. Analysis of adulterated Jamu Pegal Linu obtained from the market in Jakarta. Bul Penelit Sist Kesehat. 16:269–274.
- Greger JL. 2001. Dietary supplement use: consumer characteristics and interests. J Nutr. 131(4):1339S–1343S.
- Hanum F, Sidiq R, Hadi A, Fahdienie F. 2017. Analisis Adulterasi Jamu yang Beredar di Banda Aceh. J Bahana Kesehat Masy. 1:59–65.
- Hasler CM. 2002. Functional foods: benefits, concerns and challenges—a position paper from the American Council on Science and Health. J Nutr. 132(12):3772–3781.
- Heinrich M, Chan J, Wanke S, Neinhuis C, Simmonds MSJ. 2009. Local uses of Aristolochia species and content of nephrotoxic aristolochic acid 1 and 2-A global assessment based on bibliographic sources. J Ethnopharmacol. 125(1):108–144.
- Herrmann K, Schumacher F, Engst W, Appel KE, Klein K, Zanger UM, Glatt H. 2013. Abundance of DNA adducts of methyleugenol, a rodent hepatocarcinogen, in human liver samples. Carcinogenesis. 34(5):1025–1030.
- Holt DW, Johnston A. 2019. Commentary: a herbal treatment for type 2 diabetes – the dangers of adulterated and falsified products. J Diabetes Clin Res. 1(2):37–39.
- Hong Y-T, Fu L-S, Chung L-H, Hung S-C, Huang Y-T, Chi C-S. 2006. Fanconi's syndrome, interstitial fibrosis and renal failure by aristolochic acid in Chinese herbs. Pediatr Nephrol. 21(4):577–579.
- IARC. 2007. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon: IARC Press.
- IARC. 2012. Sampling and sample preparation methods for determining concentrations of mycotoxins in foods and feeds. IARC Sci Publ. 158:39–51.
- Jadot I, Declèves A-E, Nortier J, Caron N. 2017. An integrated view of aristolochic acid nephropathy: update of the literature. Int J Mol Sci. 18(2):297.
- James LP, Mayeux PR, Hinson JA. 2003. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 31(12):1499–1506.
- James WPT, Astrup A, Finer N, Hilsted J, Kopelman P, Rössner S, Saris WHM, Gaal LFV. 2000. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. STORM Study Group. Sibutramine Trial of Obesity Reduction and Maintenance. Lancet (London, England). 356(9248):2119–2125.
- Jelaković B, Nikolić J, Radovanović Z, Nortier J, Cosyns J-P, Grollman AP, Bašić-Jukić N, Belicza M, Bukvić D, Čavaljuga S, et al. 2014. Consensus statement on screening, diagnosis, classification and treatment of endemic (Balkan) nephropathy. Nephrol Dial Transplant. 29(11):2020–2027.
- Jordan SA, Cunningham DG, Marles RJ. 2010. Assessment of herbal medicinal products: challenges, and opportunities to increase the knowledge base for safety assessment. Toxicol Appl Pharmacol. 243(2):198–216.
- Kamour A, Crichton S, Cooper G, Lupton DJ, Eddleston M, Vale JA, Thompson JP, Thomas SHL. 2017. Central nervous system toxicity of mefenamic acid overdose compared with other NSAIDs: an analysis of cases reported to the United Kingdom National Poisons Information Service. Br J Clin Pharmacol. 83(4):855–862.
- Kloner R. 2007. Erectile dysfunction and hypertension. Int J Impot Res. 19(3):296–302.
- Kumar V, Poonam Prasad AK, Parmar VS. 2003. Naturally occurring aristolactams, aristolochic acids and dioxoaporphines and their biological activities. Nat Prod Rep. 20:565–583.
- Latifa R, Wahyono P, Christina L, Fatmawati D, Fauzi A. 2020. The lead content of jamu gendong in Malang traditional market: is it safe to consume? AIP Conf Proc. 2231:30007.
- Lau A-J, Holmes MJ, Woo S-O, Koh H-L. 2003. Analysis of adulterants in a traditional herbal medicinal product using liquid chromatography–mass spectrometry–mass spectrometry. J Pharm Biomed Anal. 31(2):401–406.
- Lees P, Toutain P-L. 2013. Pharmacokinetics, pharmacodynamics, metabolism, toxicology and residues of phenylbutazone in humans and horses. Vet J. 196(3):294–303.
- Levy M, Zylber-Katz E, Rosenkranz B. 1995. Clinical pharmacokinetics of dipyrone and its metabolites. Clin Pharmacokinet. 28(3):216–234.
- Lim MA, Pranata R. 2020. The insidious threat of jamu and unregulated traditional medicines in the COVID-19 era. Diabetes Metab Syndr. 14(5):895–896.
- Limyati DA, Juniar BLL. 1998. Jamu Gendong, a kind of traditional medicine in Indonesia: the microbial contamination of its raw materials and endproduct. J Ethnopharmacol. 63(3):201–208.
- Lin G, Wang JY, Li N, Li M, Gao H, Ji Y, Zhang F, Wang H, Zhou Y, Ye Y, et al. 2011. Hepatic sinusoidal obstruction syndrome associated with consumption of Gynura segetum. J Hepatol. 54(4):666–673.
- Lipscomb GR, Wallis N, Armstrong G, Rees WDW. 1998. Gastrointestinal tolerability of meloxicam and piroxicam: a double-blind placebo-controlled study. Br J Clin Pharmacol. 46(2):133–137.
- Maesaroh I. 2020. Analisis metampiron dalam campuran jamu asam urat. Kesehatan. 6(2):726–732.
- McKillop G, Canning GP. 1987. A case of intravenous and oral mefenamic acid poisoning. Scott Med J. 32(3):81–82.
- McMurray JG, Feldman RA, Auerbach SM, Deriesthal H, Wilson N. 2007. Long-term safety and effectiveness of sildenafil citrate in men with erectile dysfunction. Ther Clin Risk Manag. 3(6):975–981.
- Miller EC, Swanson AB, Phillips DH, et al. 1983. Structure–activity studies of the carcinogenicities in the mouse and rat of some naturally occurring and synthetic alkenylbenzene derivatives related to safrole and estragole. Cancer Res. 43:1124–1134.
- Mohabbat O, Shafiq Younos M, Merzad AA, Srivastava RN, Ghaos Sediq G, Aram GN. 1976. An outbreak of hepatic veno-occlusive disease in North-Western Afghanistan. Lancet. 308(7980):269–271.
- Murray MD, Brater DC. 1993. Renal toxicity of the nonsteroidal anti-inflammatory drugs. Annu Rev Pharmacol Toxicol. 33:435–465.
- Mustarichie R, Ramdhani D, Indriyati W. 2017. Analysis of forbidden pharmaceutical compounds in antirheumatic jamu. Asian J Pharm Clin Res. 10(4):98.
- Niggemann B, Grüber C. 2003. Side-effects of complementary and alternative medicine. Allergy. 58(8):707–716.
- Nissan R, Poperno A, Stein GY, Shapira B, Fuchs S, Berkovitz R, Hess Z, Arieli M. 2016. A case of hepatotoxicity induced by adulterated “Tiger King”, a Chinese herbal medicine containing sildenafil. Curr Drug Saf. 11(2):184–188.
- Noveriza R. 2015. Kontaminasi cendawan dan mikotoksin pada Tumbuhan obat. Perspektif. 7:35–46.
- Paul ME, Jose S, Achar Y, Raghunath BR. 2016. Prednisolone induced Cushing syndrome: a case report. Indian J Pharm Pract. 9(2):141–142.
- Paul J, Duncan JR, Sharp P, Norris A, Siddiq MA, Bacon C, Weighill J. 2005. Agranulocytosis and citrobacter infection associated with jamu, a herbal remedy containing phenylbutazone. Clin Infect Dis. 40(12):1859–1860.
- Posadzki P, Watson L, Ernst E. 2013. Contamination and adulteration of herbal medicinal products (HMPs): an overview of systematic reviews. Eur J Clin Pharmacol. 69(3):295–307.
- Reeuwijk NM, Venhuis BJ, de Kaste D, Hoogenboom LAP, Rietjens IMCM, Martena MJ. 2013. Sildenafil and analogous phosphodiesterase type 5 (PDE-5) inhibitors in herbal food supplements sampled on the Dutch market. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 30(12):2027–2034.
- Reeuwijk NM, Venhuis BJ, de Kaste D, Hoogenboom RLAP, Rietjens IMCM, Martena MJ. 2014. Active pharmaceutical ingredients detected in herbal food supplements for weight loss sampled on the Dutch market. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 31(11):1783–1793.
- Rietjens IMCM, Slob W, Galli C, Silano V. 2008. Risk assessment of botanicals and botanical preparations intended for use in food and food supplements: emerging issues. Toxicol Lett. 180(2):131–136.
- Saberi N, Akhgari M, Bahmanabadi L, Bazmi E, Mousavi Z. 2018. Determination of synthetic pharmaceutical adulterants in herbal weight gain supplements sold in herb shops, Tehran, Iran. Daru. 26(2):117–127.
- SCF. 2001a. Opinion of the scientific committee on food on estragole (1-allyl-4-methoxybenzene). Brussel: Scientific Committee on Food, European Commission.
- SCF. 2001b. Opinion of the scientific committee on food on methyleugenol (4-allyl-1,2-dimethoxybenzene). Brussel: Scientific Committee on Food, European Commission.
- SCF. 2002. Opinion of the scientific committee on food on the safety of the presence of safrole (1-allyl-3,4-methylene dioxybenzene) in flavourings and other food ingredients with flavouring properties. Brussel: Scientific Committee on Food, European Commission.
- Skovgaard N. 2007. Safety evaluation of certain contaminants in food. Int J Food Microbiol. 116(3):420.
- Snyman T, Stewart MJ, Grove A, Steenkamp V. 2005. Adulteration of South African traditional herbal remedies. Ther Drug Monit. 27:86–89.
- Sperling IL. 1969. Adverse reactions with long-term use of phenylbutazone and oxyphenbutazone. Lancet. 2(7619):535–537.
- Stiborova M, Frei E, Arlt VM, Schmeiser HH. 2009. The role of biotransformation enzymes in the development of renal injury and urothelial cancer caused by aristolochic acid: urgent questions and difficult answers. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 153(1):5–12.
- Suparmi S, Mulder PJ, Rietjens IMCM. 2020. Detection of pyrrolizidine alkaloids in jamu available on the Indonesian market and accompanying safety assessment for human consumption. Food Chem Toxicol. 138:111230.
- Suparmi S, Widiastuti D, Wesseling S, Rietjens IMCM. 2018. Natural occurrence of genotoxic and carcinogenic alkenylbenzenes in Indonesian jamu and evaluation of consumer risks. Food Chem Toxicol. 118:53–67.
- Suparmi S, Ginting AJ, Mariyam S, Wesseling S, Rietjens IMCM. 2019. Levels of methyleugenol and eugenol in instant herbal beverages available on the Indonesian market and related risk assessment. Food Chem Toxicol. 125:467–478.
- Suprana J. 2019. Jamu warisan kebudayaan Indonesia; [accessed 2021 Mar 30]. https://www.gelora.co/2019/08/jamu-warisan-kebudayaan-indonesia.html
- Tandon BN, Tandon HD, Tandon RK, Narndranathan M, Joshi YK. 1976. An epidemic of veno-occlusive disease of liver in Central India. Lancet. 308(7980):271–272.
- Ton FN, Gunawardene SC, Lee H, Neer RM. 2004. Effects of low-dose prednisone on bone metabolism. J Bone Miner Res. 20(3):464–470.
- Vargas-Murga L, Garcia-Alvarez A, Roman-Viñas B, Ngo J, Ribas-Barba L, van den Berg SJPL, Williamson G, Serra-Majem L. 2011. Plant food supplement (PFS) market structure in EC Member States, methods and techniques for the assessment of individual PFS intake. Food Funct. 2(12):731–739.
- Walker R. 2004. Criteria for risk assessment of botanical food supplements. Toxicol Lett. 149(1–3):187–195.
- Wasita IKS, Hendrayana IMA. 2016. Identifikasi bakteri Escherichia coli serotipe 0157 dengan media Sorbitol Mac Conkey Agar (SMAC) pada jamu beras kencur dari pedagang jamu gendong di Kota Denpasar. E-J Med Udayana. 5:1–6.
- White KG. 2019. A retrospective analysis of adrenal crisis in steroid-dependent patients: causes, frequency and outcomes. BMC Endocr Disord. 19(1):129.
- Wiedenfeld H, Roder E. 1991. Pyrrolizidine alkaloids from Ageratum conyzoides. Planta Med. 57(6):578–579.
- Wisnuwardhani HA, Fidrianny I, Ibrahim S. 2013. Method development for simultaneous analysis of steroid and non steroid antiinflammatory substances in jamu pegal linu using TLC-spectrophotodensitometry. Int J Pharm Pharm Sci. 5:749–753.
- Ye H, Nelson LJ, Gómez Del Moral M, Martínez-Naves E, Cubero FJ. 2018. Dissecting the molecular pathophysiology of drug-induced liver injury. World J Gastroenterol. 24(13):1373–1385.
- Zeller A, Horst K, Rychlik M. 2009. Study of the metabolism of estragole in humans consuming fennel tea. Chem Res Toxicol. 22(12):1929–1937.
