Abstract
The sorption characteristics of two cassava products were obtained using the gravimetric method at three temperatures, 20, 30, and 40°C. The GAB, BET, and the modified BET models were found to describe the data reasonably well. The heat of sorption, which varied from 39.47 kJ/mol at 1% moisture content to 4.92 kJ.mol−1 at 20% moisture content dry basis, was calculated for the garified product. Similar values for the ungarified product were found to vary from 26.98 kJ/mol at 1% moisture content to 4.97 kJ/mol at 20% moisture content. The shelf-life for both products were estimated from sorption data and recommendations on their packaging and storage conditions given. The garified product was found to have a higher capacity for moisture adsorption and also potentially stores longer than the ungarified product.
Introduction
Cassava is a tropical root crop that can be processed into different types of food products in tropical regions especially in West African countries like Nigeria, Benin, Togo, Ghana, and Cameroon. One of the two products of cassava studied in this paper is obtained by grating the peeled roots, fermenting the mash, dewatering, and garification.[Citation1,Citation2] Garification is a simultaneous process of cooking and drying dewatered cassava to obtain a crisp product called gari and may be milled to size as required. Dewatering could be done simultaneously as the fermentation process. Another product, which is only suitable for animal consumption because it is not cooked, is obtained by spreading the dewatered and fermented product on a suitable surface under the sun for a number of days until dried. In this paper, we refer to the first product as garified and the second as ungarified. While improvements have been made in the processing of these products, a lot more is necessary with regard to their packaging and storage.
The shelf-life of packaged cassava (food) products is influenced by the storage temperature, relative humidity, and moisture content and hence the water activity (aw ) of the product. It is known that the rate of microbial and physico-chemical deterioration is influenced by water activity of stored cassava (food) products.[Citation3–5] Chuzel and Zakhia[Citation6] observed that at high water activities, the moisture content increased with increasing temperature, resulted in a crossing of the isotherms at aw between 0.5 and 0.7. This phenomenon was explained due to changes that occurred in the starch structure and modification of the available water sorption sites.
Oyeniran[Citation7] reported growth of moulds during storage of white and yellow gari at 18.6 and 18.1% initial moisture content, respectively, or dried to 11.2 and 10.8% moisture content, respectively. He also reported that the discoloration and complete deterioration of gari stored at above 18% moisture content in polythene bags after 16 weeks was as a result of the growth of moulds on the products. However gari at less than 12% moisture can be stored safely in polythene bags for 2 months (8 weeks). Ekundayo[Citation8] also observed changes in colour and odour resulting from microbial spoilage of gari stored in polythene bags after 10 weeks. It is thus evident that gari could not be stored well due to its hygroscopic nature.
A moisture sorption isotherm equation is used to describe mathematically the relationship between the activity (aw), and the equilibrium moisture content of a food product. Moisture sorption isotherms are used for a number of purposes in food research.[Citation9] These include calculations for drying time, ingredient mixing predictions, packaging predictions and modeling moisture changes which occur during storage and prediction of shelf-life stability.[Citation10] It also supplies fundamental information about specific interaction between water and the product since it directly relates the thermodynamic potential (or Gibbs free energy) of water in the system to its mass fraction. Related thermodynamic properties such as the enthalpy of sorption as well as information on the structure of the product (e.g. specific surface area, pore volume, and crystallinity in some cases) can be derived.[Citation11]
A number of empirical, semi-empirical, or theoretical models have been derived for the correlation of water sorption in food substances. Among these, the three-parameter Guggenheim-Anderson-de Boer (GAB), Brunauer, Emmet and Teller, (BET) and modified BET equations have been found to be more popular and reliable.[Citation11–14] They are widely used in determining monolayer sorption values and specific surface areas of sorbent material. Whereas the GAB equation is found applicable over a wide range of aw, values, the BET is applicable at low aw (aw < 0.43) and the modified BET gives a good fit at aw less than 0.75.
This paper presents the data for the sorption characteristics of two cassava products and the results of the application of these equations to their sorption isotherms. Theoretical shelf-life is deduced for both materials from their sorption characteristics. Furthermore the binding energy or excess heat of sorption will be calculated using the data obtained in this work.
MATERIALS AND METHOD
The two cassava products were obtained from local markets in Edo State of Nigeria, packaged in sealed polythene bags and transported to Zimbabwe where the author was residing within two days. The composition of the two materials is given in . The samples were then stored at 2–4°C until the sorption isotherms were determined. Before each experimental run, the products were milled into powder, sieved to obtain a size range of +700 μm to −1400 μm that was used for the sorption isotherm determination. Sorption isotherms of these materials were measured at 20, 30, and 40°C using a standard gravimetric method as recommended by COST 90 project with thermally stabilized desiccators.[Citation15] The temperature of the desiccators was maintained by placing them in a thermostatically controlled water bath set at the required temperature. The desiccators contained saturated salt solutions that create known relative humidity in the surrounding atmosphere.[Citation16] The saturated salt solutions used in this study are LiCl, CH3COOK, MgCl2, K2CO3, NaBr, CuCl2, NaCl, (NH4)2SO4, and KNO3 with corresponding water activity of 0.11, 0.23, 0.33, 0.43, 0.57, 0.67,0.75, 0.79, and 0.93, respectively, at temperatures from 20 to 40°C.[Citation17]
Table 1 Initial Composition of the two materials.
The materials were first dried over P2O5 for a period of about 2 weeks to ensure that they were very dry and at the same initial moisture content. About 2 gm portions of the material in triplicates placed on watch glasses were exposed to the different humidity in the desiccators. In order to prevent mould growth at high aw , 0.25% of sodium azide was applied to the samples. The weights of the samples were determined on 2-day intervals until constant weights were obtained. The length of each experimental run was about 2 weeks. The time interval for the removal, weighing, and replacing the sample in the desiccators was less than one minute to minimize any effect on opening and closing on the results. The moisture content of the equilibrated samples was determined by drying each sample in an oven at 110°C for between 16 and 24 hours. The samples are cooled over silica gel before the final weights were taken.
Adsorption Models
The GAB equation is claimed to provide the best equation for the description of food isotherms up to aw 0.9[Citation18] and also adopted by the EEC-COST 90 Group on water activity.[Citation19] The transformed GAB is given as:
The BET sorption isotherm given below is applicable at low aw was also tested.
Moisture-Binding Energy
The water activity of a food substance, which is made up of non ideal mixtures, is known to be a function of temperature; hence it is important to know the effect of temperature on its sorption isotherm. Temperature affects the mobility of water molecules, and the equilibrium between the vapor and adsorbed phases. An increase in temperature, at constant water activity results in a decrease in the amount of adsorbed water.[Citation20] However, for certain sugars and low molecular weight food constituents, which dissolve in water and become more hygroscopic at higher temperatures are exception to this rule.
The level of moisture content at which the differential heat of sorption approaches the heat of vapourisation of pure water is often taken as indicative of the amount of “bound” water existing in the food.[Citation21] Knowledge of the differential heat of sorption is very important for drying process equipment design. This is because heat of vaporization of sorbed water may increase to values above the heat of vapourisation of pure water as food is dehydrated to low moisture levels.[Citation22]
The binding energy is defined as the difference between the isosteric heat of water sorption by the solid substrate and the condensation heat of water vapor at the same temperature. The relationship between activity and temperature can be described by the Clausius-Clapeyron equation:
RESULTS AND DISCUSSION
Adsorption Isotherms
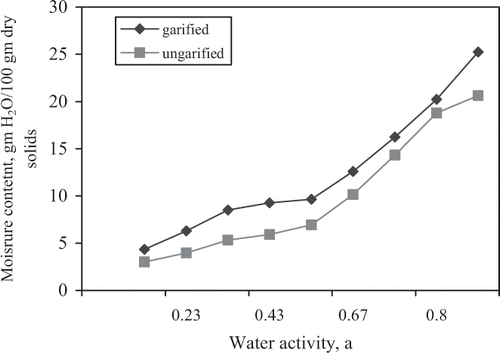
The results of the sorption isotherm measurements are shown in . The sigmoid characteristic curves of the isotherms were obtained for the two products, shown in , have a fairly similar pattern for both materials. The results suggest that the garified product has a stronger affinity for moisture at a given water activity. This implies that the garified product is more hygroscopic compared to the ungarified product. However, this does not suggest at this stage that the ungarified product is more stable and hence can store better. The crossing of adsorption isotherm described by Chuzel and Zakhia[Citation6] was not observed in this study for either products, hence the stability of both cassava-based products could be reduced with increase in storage temperature.
Table 2 Equilibrium moisture contents, g H2O/100 g solids at different water activities for the two materials.
and show the estimated parameters by nonlinear regression for GAB, BET, and Modified BET models for both products. The results show that these equations describe the moisture sorption isotherms of both materials reasonably well as shown by the values of correlation coefficients and the root mean square error (%RMS). The monolayer moisture content (Xm) values obtained from the GAB analysis ( and ) at the three temperatures and for both materials do not appear to be significantly different and they are similar to values obtained for starchy foods.[Citation23] While the monolayer values do not appear to have definite relationship to temperature within the temperature range covered, they appear to depend on the particular model used. It can be observed that the Modified BET tend to correlate the garified products better, whereas the GAB correlates the ungarified product better within the water activity range for which the particular model is said to be valid.
Table 3 Estimated parameters by nonlinear regression for GAB, BET, and Modified BET Models—garified product.
Table 4 Estimated parameters by nonlinear regression for GAB, BET, and Modified BET models—ungarified product.
For the garified product the monolayer values ranged from 5.21 to 5.54 g. H2O/100 g solid for the GAB model, 3.37 to 3.62 g · H2O/100 g solid for the BET model and 11.25 to 11.98 g · H2O/100 g solid for the Modified BET. The corresponding monolayer values for the ungarified product gave the same value of 12.97 g. At the three temperatures for the GAB model, ranged from 5.90 to 8.78 g · H2O/100 g solids for the BET model and from 2.94 to 7.21 g · H2O/100 g solid for the modified BET. These values are similar to those in literature.[Citation6,Citation23–29]
Comparison of these three models indicates that the modifield BET model gave the highest monolayer moisture estimates, followed by the GAB model and then the BET equation for the garified product. On the other hand, the GAB gave the highest monolayer moisture estimates than the other two models. The values of the root mean square error (%RMS) for the garified products suggest that whilst both the GAB and BET gave a rather poor fit, the correlation with the Modified BET is reasonable. However, similar comparison for the ungarified products shows that the GAB model gave smaller values than the other two models. The GAB model can therefore be said to give a better fit compared to the other two models in the water activity range for which the particular model is applicable.
The initial stages (grating, fermentation and dewatering) of production of these products induce a starch damage of about 3–6%.[Citation6] During the process of garification complete gelatinisation does not take place due to the low initial moisture content of about 1 g/g.[Citation30] However, there is a loss of crystallinity and extensive swelling of the starch granules resulting in crisp grain-like products when dry. A complex metastable network is said to be formed that consists of armorhpous regions (containing plasticising water) and hydrated microscrystalline regions which did not dissolve during the partial gelatinisation and serve as junction zones.[Citation31] Chuzel et al.[Citation6] suggest that increase in both temperature and water activity initiates a collapse process, which makes the soluble starch (armorphous fractions and branched segments) to leach out thereby increasing the number of available adsorption sites (glucose residue). The drying of the ungarified products is much slower (days compare to less than 1 hour for the garified products) and also at a much lower temperature (less than 40°C compared to above 100°C for the garified products). Consequently, there is neither partial gelatinization, nor swelling, and the products do not possess the crisp and hard grains like the garified products. The net effect of these is that the ungarified products' capacity for moisture adsorption is lower than that for the garified products.
Moisture-Binding Energy
From EquationEq. (4), plots of ln(aw ) vs (1/T) at different moisture contents can be considered as straight lines, whose slope yield ΔHS / R, which can be calculated by regression analysis.[Citation32] shows the values of excess heat of sorption for both materials obtained by such method of analysis. The values obtained varied from 4.92 kJ/mol at 20% moisture content to 39.47 kJ/mol at 1% moisture content, dry basis for the garified product. The values obtained for the ungarified product varied from 4.79 kJ/mol at 20% moisture content to 26.98 kJ/mol at 1% moisture content, dry basis. Fasina et al.[Citation33] observed that the heat of vaporization of gari reduces with increase in moisture content. The results also show that the process of water sorption by both materials is endothermic. This agrees with Chuzel and Zakhia[Citation6] who observed that when the soluble fraction of gari starch undergoes collapse and leach out, the sorption phenomenon becomes endothermic rather than the usual exothermic behavior found in sorption theory. The monolayer concept is also very relevant to physical and chemical deterioration of dehydrated foods, such as lipid, enzyme activity, non-enzymic browning reactions, aroma retention and textural characteristics.[Citation12,Citation34] Furthermore, Iglesias and Chirife[Citation35] explained that as more water is adsorbed there is a decrease in sorption energy due to reduced activity at the sorption sites. The results also suggest that the garified product has greater interaction energy at the sorption sites compared to the ungarified product. This is supported by the observation that the garified product is more crisp and harder when felt with the fingers.
Table 5 Excess heat of sorption, kJ/mol as a function of moisture content for both materials.
Application of aw to Shelf-Life Estimation
The establishment of the sorption isotherm of a packaged food can aid in the estimation of its shelf-life in given storage conditions. The model of Heiss and Eichner[Citation36] can be used to estimate the potential storage time based on a critical aw for a particular system under given storage conditions. This model was based on the assumption that water sorption vapor is the determining factor, amongst others such as the presence of spoilage bacteria, oxygen, and light that could limit shelf-life. The equation is given as:
The shelf-life of packaged cassava products in 5-kg polyethylene bags (A = 0.266 m−2; KS = 2.28 × 10−6 kg H2O · m−2 · Pa−1 · day−1) was estimated at three temperatures (20, 30, 40°C). For the purpose of estimation, we will assume ambient storage conditions for which the relative humidity is 0.90 (which is reasonable for the tropical conditions that prevail in the countries where these products are a part of the staple food), and a water activity of 0.7.[Citation6] The theoretical shelf-life estimated for different initial moisture contents are shown in and . The results indicate that the garified products will store for longer time compared to the ungarified products as well as having a higher capacity to absorb moisture. This is due to the fact that the garified product was obtained by a more severe heat treatment process resulting in a harder and more crisp substance, and consequently made it less susceptible to mould attack.
Table 6 Estimated shelf-life (days) for safe storage at aw 0.7 at different initial moisture content, (dry basis) for the garified product.
Table 7 Estimated shelf-life (days) for safe storage at aw 0.7 at different initial moisture content, (dry basis) for the ungarified product.
It is possible to suggest that at these conditions for a low cost storage for at least three months shelf-life, one would recommend an initial moisture content of less than 8% for both products at a temperature of less than 30°C using polyethylene materials. This value must be taken with caution, as it is very difficult to dry the products to this low moisture level. Polyethylene material was chosen because of its low cost compared to the more expensive polypropylene, which has a lower permeability to water vapor and oxygen. In this article, attention was directed only to permeability of water vapor to polyethylene, however, it must be mentioned that it is also permeable to oxygen and carbon dioxide, which can cause oxidation and hence deterioration.
CONCLUSION
The sorption isotherms at temperatures of 20, 30 and 40°C for the two cassava-based products were measured using the conventional gravimetric method. The results show that the sigmoid characteristic curves of sorption isotherms were obtained as expected. The data obtained were well described by the three models tested. However, the BET gave a poorer fit as it gave a rather high %RMS values for both products. The monolayer water content values were found not to be a particular function of temperature but were dependent on the particular model used for its estimation. Excess heat of sorption values varied from 4.92 kJ/mol at 20% to 39.47 kJ/mol at 1% moisture content, dry basis for the garified product. For the ungarified products, the values ranged from 4.97 kJ/mol at 20% to 26.58 kJ/mol at 1% moisture content, dry basis. A theoretical estimation of the shelf-life for both materials shows that a low cost storage of at least three months can be obtained using 5 kg polyethylene bags for both products at less than 8% moisture content. It was observed that though the garified product has a higher moisture adsorbing capacity, it also has a longer safe storage period. This was explained to be due to the more severe heat treatment process for the garified product which resulted in a harder, more crisp and hygroscopic material.
REFERENCES
- Audu , T.O.K. and Ikhu-Omoregbe , D.I.O. 1982 . Drying characteristics of fermented ground cassava . Nig. J. of Eng. and Techn. Dev. , 7 ( 2 ) : 12 – 20 .
- Ajibola , O.O. , Makanjuola , G.A. and Almazan , A.M. 1987 . Effects of processing factors on the quality of gari produced by a steam gelatinization technique . J. of Agric Eng Res. , 38 ( 4 ) : 313 – 320 . [CSA] [CROSSREF]
- Sanni , M.O. 1996 . The stability of stored gari . Intl. J. Food Microbiology , 29 ( 1 ) : 119 – 123 . [CSA] [CROSSREF]
- Ukhun , M.E. and Dibie , E.N. 1991 . The ascorbic acid contents of selected marketed foods and influence of water activity (aw) during storage . Food Chemistry , 41 ( 3 ) : 277 – 283 . [CSA] [CROSSREF]
- Ofuya , C.C. and Akpoti , P. 1988 . Post-processing microflora and shelf-life stability of gari marketed in Port Harcourt, Nigeria . Journal of Applied Bacteriology , 64 ( 5 ) : 389 – 394 . [INFOTRIEVE] [CSA]
- Chuzel , G. and Zakhia , N. 1991 . Adsorption isotherms of gari for estimation of packaged shelf-life . Int. Journal of Food Science and Technology , 26 : 583 – 593 . [CSA]
- Oyeniran , J.O. 1981 . “ Mould development in gari during storage in polythene and hessian bags ” . In Annual Report, Nigerian Stored Products Research Institute Vol. 16 , 93 – 99 . Ibadan Nigeria
- Ekundayo , C.A. 1984 . Microbial spoilage of packaged gari in storage . Microbios. Letters , 26 : 145 – 150 . [CSA]
- Labuza , T.P. 1968 . Sorption Phenomena in Foods . Food Technology , 22 : 263 – 272 . [CSA]
- Al-Muhtaseb , A.H. , McMinn , W.A.M. and Magee , T.R.A. 2002 . Moisture sorption isotherm characteristics of food products: A Review. Transactions of The Inst. Of Chemical Engineers, Part C— . Food and Bioproducts Processing , 80 : 118 – 128 . [CSA] [CROSSREF]
- Van der Berg , C. 1984 . “ Desorption of water activity of foods for engineering purposes by means of the GAB model of sorption ” . In Engineering and Foods , Edited by: Mckenna , B.M. Vol. 1 , 311 – 321 . London : Elsevier Applied Science Publishing .
- Iglesias , H.A. and Chirife , J. 1976 . BET monolayer values in dehydrated foods and food components . Lebensm-Wiss. U. Technol. , 9 : 107 – 113 . [CSA]
- Schar , W. and Ruegg , M. 1985 . The evaluation of GAB constants from water vapour sorption data . Lebensm wiss u Technol. , 18 : 225 – 229 . [CSA]
- Kim , H.K. , Song , G.Y. and Yam , K.L. 1991 . Water sorption characteristics of dried red peppers (Caspsium annum L), International . Journal of Food Science and Technology , 29 : 339 – 345 . [CSA]
- Wolf , W. , Speiss , W.E.C. and Jung , G. 1985 . “ Standardization of Isotherm Measurements, (COST Project 90 and 90 BIS) ” . In Properties of Water in Foods , Edited by: Simatos , D. and Multon , J.L. 661 – 677 . Dordrecht, , Netherlands : Martnus Nijhott Publishers .
- Wexler , A. and Hasegawa , S. 1954 . Relative humidity-temperature relationship of some saturated salt solutions in the temperature range 0° to 50° . C. J. Res of Nat. Bureau of St. , 53 : 19 – 26 . [CSA]
- Rockland , L.B. 1960 . Saturated salt solutions for static control of relative humidity between 5° and 40°C . Analytical Chemistry , 32 ( 10 ) : 1375 – 1376 . [CSA] [CROSSREF]
- Van Den Berg , C. 1985 . “ Development of BET like models for sorption of water on foods ” . In Theory and relevance Properties of Water in Foods , Edited by: Simatos , D. and Multon , J.L. Dordrecht, , Netherlands : Martnus Nijhott Publishers .
- Bizot , H. 1983 . “ Using GAB model to construct sorption isotherm ” . In Physical Properties of Food , Edited by: Jowit , R. , Esher , F. , Hallstrom , B. , Meffert , H.F.T. , Spiess , W.E.L. and Vos , G. 43 London : Applied Science Publishers .
- Gustavo , V. and Humberto , V. 1996 . Dehydration of Foods , New York : Chapman and Hall .
- Duckworth , R.B. 1972 . The properties of water around surfaces of food colloids . Proc. Inst. Food Science Technol. , 5 : 60 – 67 . [CSA]
- King , C.J. 1968 . Rate of moisture sorption and desorption in porous, dried foodstuffs . Food Technology , 22 : 509 – 514 . [CSA]
- Rahman , S. 1995 . “ Water activity and sorption properties in food ” . In Food Properties Handbook , 1 – 86 . London : CRC Press .
- Lomauro , C.J. , Bakshi , A.S. and Labuza , T.P. 1985 . Evaluation of food moisture sorption Isotherm equations. Part I: Fruit, Vegetables and Meat products . Leensm-wiss u. Technology , 18 : 111 – 117 . [CSA]
- Ajibola , O.O. 1986 . Desorption isotherms of gari from 40° to 70°C . Journal of Agricultural Engineering Research , 35 ( 3 ) : 207 – 210 . [CSA]
- Wang , N. and Brennan , J.G. 1991 . Moisture sorption characteristics of potatoes at four temperatures . Journal of Food Engineering , 14 : 269 – 287 . [CSA] [CROSSREF]
- Kiranoudis , C.T. , Tsami , E. , Maroulis , Z.B. and Morunos-Kouris , D. 1993 . Equilibrium moisture content and heat of desorption of some vegetables . Journal of Food Engineering , 20 : 55 – 74 . [CSA] [CROSSREF]
- Viollaz , E.P. and Rovedo , O.C. 1999 . Equilibrium sorption isotherms and thermodynamic properties of starch and glutten . Journal of Food Engineering , 40 : 287 – 292 . [CSA] [CROSSREF]
- Al-Muhtaseb , A.H. , McMinn , W.A.M. and Magee , T.R.A. 2001 . “ Moisture Sorption Isotherm Characteristics of Starch Powders at Different Temperatures ” . In Proc. 6th World Congress of Chemical Engineers Melbourne, , Australia
- Gevaudan , A. , Chuzel , G. , Didier , S. and Andrieu , J. 1989 . Thermophysical properties of cassava mash. . International Journal of Food Science and Technology , 24 : 637 – 645 . [CSA]
- Levine , H. and Slade , L. 1988 . Water as a plasticizer; physico-chemical aspects of low moisture polymeric systems . Water Science Reviews , 3 : 79 – 185 . [CSA]
- Soekarto , S.T. and Steinberg , M.P. 1981 . “ Determination of binding energy for three fractions in bound water ” . In “Water Activity:” Influences on Food Quality , Edited by: Rockland , L.B. and Stewart , G.F. 265 New York : Academic Press .
- Fasina , O.O. , Ajibola , O.O. and Tyler , R.T. 1999 . Thermodynamics of moisture sorption in winged been seed and gari . Journal of Food Processing and Preservation , 22 ( 6 ) : 405 – 418 . [CSA]
- Iglesias , H.A. , Chirife , J. and Lombardi , J.L. 1975 . An equation for correlating equilibrium moisture content in foods . Journal of Food Technology , 10 : 289 – 297 . [CSA]
- Iglesias , H.A. and Chirife , J. 1976 . A model for describing the water sorption behaviour of foods . Journal of Food Science , 41 : 984 – 992 . [CSA]
- Heiss , R. and Echner , E. 1971 . Moisture content and shelf life II . Food Manufacture , June : 37 – 42 . [CSA]