Abstract
A capillary viscosimeter was used to measure the kinematic viscosity data of aqueous solutions of ethanol and sucrose at several temperatures (from 20 up to 45°C) and concentrations (from 5% up to 95.11% w/w ethanol concentration with variable sucrose concentrations as a function of its solubility). Two models were proposed for fitting the experimental data (one for water/ethanol and another for water/ethanol/sucrose solutions). These models were based in pre-existing equations like Redlich-Kister and Torok-Rard-Miller (for dependency on concentration), and also Vogel-Fulcher-Tamman (for dependency on temperature). The estimated values by these two models gave low deviations from the experimental data for binary and ternary mixtures.
Keywords:
INTRODUCTION
The knowledge of physical and transport properties is very important for the study and design of industrial processes and also for the analysis of data obtained in laboratory experiments. Specifically, rheological behaviour is dependent on kinematic viscosity value and this property also is important in order to estimate the mass and heat transfer rates. In many cases, knowledge of the kinematic viscosity value is fundamental for determining the overall transfer coefficients value between phases.
Ethanol/water solutions have a great importance in chemical, biochemical, biotechnological, and food systems, and also from the thermodynamics point of view because these solutions exhibit elevated deviations from ideal behavior[Citation1] and anomalies in viscosity.[Citation2] Experimental kinematic viscosity data at 20°C can be found in handbooks[Citation3] and more scarce data at other temperatures were reported by other authors.[Citation2] Ternary ethanol/water/sucrose solutions also show a clear practical interest due to the use in a wide range of concentrations for each component in many processes such as bioethanol production from sugarcane, fermentations in sugar industry,[Citation4] fruit preservations in alcoholic solutions with sugar, systems of extraction, and also, as hypertonic solution usable in osmotic dehydration processes,[Citation5] among others.
The main aim of this article is to determine the kinematic viscosity of binary mixtures of water/ethanol and ternary mixtures of water/ethanol/sucrose at different concentrations of each component and temperatures ranging from 20°C up to 45°C. The other binary system (aqueous sucrose solutions) was already studied by the authors[Citation6] and also other aqueous sugars solutions (glucose,[Citation7] lactose[Citation8]); however, the ternary system water/ethanol/sucrose is more complex in the whole range of concentrations and only scarce data[Citation9] on the tested ternary mixtures were found in the bibliography. Experimental data modelling was achieved by the use of model equations correlating kinematic viscosity of binary and ternary solutions with concentrations and temperature, simultaneously. Redlich-Kister[Citation10,Citation11] and Torok-Rard-Miller[Citation12] type equations for concentrations influence in binary and ternary systems, respectively, and an empirical Vogel-Fulcher-Tamman[Citation13] equation for temperature were selected as the model equations.
MATERIALS AND METHODS
The solutions were prepared by weight, with a Mettler AJ150 balance with a precision of ±0.0001 g using degassed distilled water, commercial ethanol (Panreac, 96% v/v) and, in the case of ternary solutions, sucrose (Merck with purity > 99.5%) previously dried to constant mass in a oven. Kinematic viscosities were determined by using a Schott-Gerate AVS 350 automatic Ubbelohde viscosimeter with the procedure previously described,[Citation14] and applying the following equation:
where ν (m2·s−1) is the kinematic viscosity, K (m2·s−2) is the capillary tube's specific constant, t (s) is the time measured by the equipment, and θ (s) is the Hagenbach correction factor (correction factor values can be found in the capillary tube's instruction booklet).
All measurements were made in five replicates. Temperature control was assured by inserting the capillary viscosimeter into a water thermostatic bath with a Selecta Digiterm heating system (precision of ± 0.1°C). The temperature was varied from 20°C up to 45°C by steps of 5°C. Upper temperature was limited by the presence of volatile compounds (ethanol). During the same experiment, the experimental kinematic viscosities remain constant indicating that solution concentration did not vary.
The capillaries of the viscosimeter were periodically calibrated using tri-distilled water, by determining the kinematic viscosity of water at several temperatures. Each capillary's specific constant (K) was determined by applying EquationEq. (1) using kinematic viscosity values attained by applying EquationEq. (2)[Citation14] obtained in the same range of temperature:
where T is the absolute temperature (K). In terms of concentration, the binary system water/ethanol was studied in the range from 0 to 95.11% (w/w of ethanol), in steps of 5%. The ternary system was studied at several concentrations of ethanol and sucrose (with variable concentrations depending on the different sucrose solubility).
RESULTS
Binary System
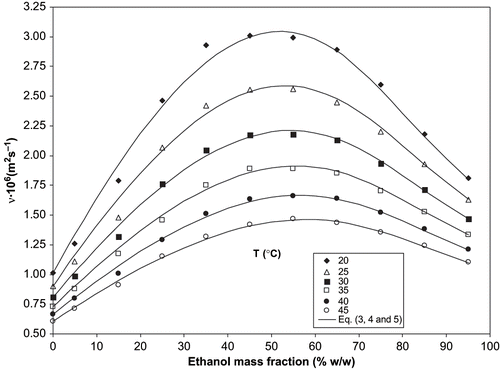
collects the experimental data of the kinematic viscosities of binary ethanol/water solutions, in the range of concentrations and temperatures studied. For all temperatures, a maximum value of kinematic viscosity at ∼45% (w/w) of ethanol concentration is observed. As expected, viscosity values are progressively lower with increasing temperature. Experimental data were compared with data collected from other studies,[Citation2,Citation3] showing minimum deviations.
Table 1 Experimental data of kinematic viscosity of binary solutions ethanol/water
The Redlich-Kister equation,[Citation11] was used to model kinematic viscosity behaviour as a function of binary systems concentration, can be expressed as:
where x i is the molar fraction of component i, x j is the molar fraction of component j, q and k are the number of parameters in EquationEq. (3), A k (m2·s−1) are the parameters, and Δν (m2·s−1) is the excess kinematic viscosity with respect to ideal behaviour given by:
where ν i is the i pure substance's kinematic viscosity. In order to use this equation and determine the values of the fitting parameters for the data sets relative to each temperature, it is necessary to calculate the following items:
-
ν ethanol and ν water (EquationEq. (2)), as pure substances, at each temperature;
-
molar fractions of the components (x i and x j ), for each one of the studied binary systems; and
-
Δν for every kinematic viscosity value determined at each set temperature.
To determine the kinematic viscosity of ethanol, in its pure substance's state, for each set temperature, a linear extrapolation was applied, using experimental kinematic viscosity values relative to higher concentrations employed (75, 85, and 95.11% (w/w)) of ethanol. This fact is due to the absence of reported data of pure ethanol and it was not possible to determine experimentally. These same results are similar to those found by other researchers.[Citation2] The calculations of the molar fractions of each component of the binary systems were made considering water end ethanol molar mass values of 18.016 and 46.07 g·mol−1, respectively.
Excess kinematic viscosity values (Δν) were obtained by EquationEq. (4) using each one of the experimental kinematic viscosity values. Kinematic viscosity value of water in these calculations was attained through EquationEq. (2). For each data set at one of the assayed temperatures, there's a Gaussian behaviour of the excess viscosity (Δν) maximum value with concentration that corresponds to a molar fraction value between 0.174 and 0.242.
Only three parameters of EquationEq. (3) (q = 2) were necessary to achieve a good fit. So, A 0 , A 1 and A 2 values were determined at each temperature, using Δν and molar fraction values and applying EquationEq. (3). Vogel-Fulcher-Tamman function,[Citation13] EquationEq. (5), empirical equation that gives good correlation applied to polar systems, was employed for correlating A i with the temperature.
where a 0i , a 1i and T 0i are constants. Considering T 0i = 0, shows the EquationEq. (5) fitting with experimental data. The values of the parameters are collected in . shows the experimental data (dots) and modelled values of kinematic viscosities (lines) of the several binary systems studied at temperatures ranging from 20°C to 45°C. An average deviation of 1.61% was determined by comparing values attained by estimation through EquationEqs. (3, Equation4, and Equation5) and experimental data.
Figure 1 Linear fit (EquationEq. (5)) of A 0 , A 1 and A 2 parameters of EquationEq. (3) with temperature in the range from 20 to 45°C.
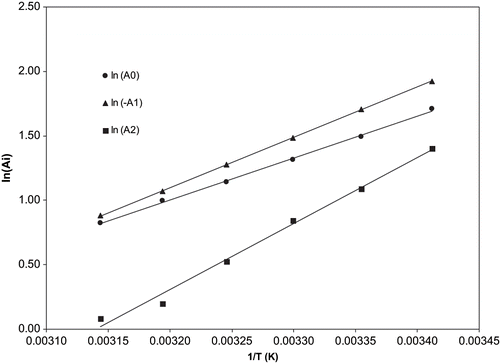
Table 2 Values of coefficients of EquationEq. (5)
Ternary System
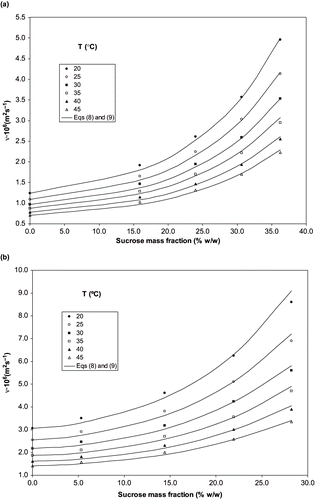
In this case, various ternary systems were studied varying ethanol concentration in the water/ethanol binary solvent (5, 25, 45, 65, and 85% (w/w)) and also system temperature. Certain quantities of sucrose added to the system (depending on sucrose solubility in each binary solvent). collects the experimental results of kinematic viscosities of ternary systems and molar fractions of each system assayed. Kinematic viscosity values increase by adding progressively higher quantities of sucrose in each solvent system. As sucrose solubility is higher in water than ethanol, higher changes are observed in systems with higher water concentrations. In order to model these systems, a Torok-Rard-Miller[Citation12, Citation6] type EquationEq. (6), was applied:
Table 3 Experimental data of kinematic viscosity of ternary solutions ethanol/water/sucrose
where ν solvent is the corresponding kinematic viscosity of ethanol/water solution, x sucrose is the sucrose molar fraction in the ternary solution, T R = T/273.1, and e, f, g and h are fitting parameters.
This original equation must be modified by introducing a correction factor in order to include the significant effect of the interaction between components. In this way, the model employed is given by EquationEq. (7):
where m and n are correction of the fitting parameters. In order to model kinematic viscosity for these ternary systems, the following items must first be determined:
-
ν solvent , for each tested system and system's temperature, by applying EquationEqs. (3, Equation4, and Equation5) with x corresponding to the ternary solution;
-
x sucrose (molar mass 342.30 g·mol−1) and x water , for each system; and
-
ν/ν solvent ratio for each system and system's temperature.
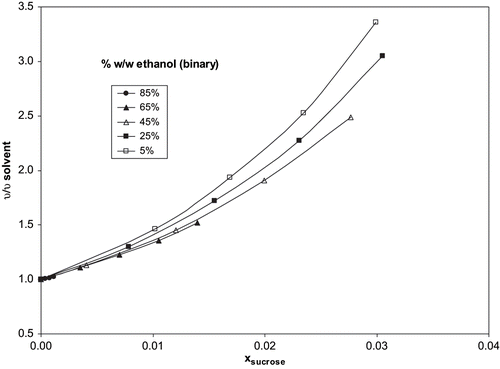
shows, as example at one of the temperatures assayed, the ν/ν solvent ratio as a function of sucrose concentration. As an effect by solvent/solute interaction (due to the ethanol/water ratio varies in the binary solvent system) is observed, the correction factor indicated in the EquationEq. (7) is necessary. The analysis of the different fittings employing EquationEq. (7) of the experimental data at each temperature and concentrations allows obtaining the values of the parameters. Specifically, it was found that only m parameter shows a dependency with the temperature (modelled through a Vogel-Fulcher-Tamman type equation, EquationEq. (5)). Based on this fact, the following equations are proposed:
Figure 3 Values of ν/ν solvent ratio vs sucrose molar fraction, obtained with several ethanol/water binary systems at 35°C.
and
shows, as example, the experimental data (dots) and calculated values (lines) of kinematic viscosity of ternary solutions at two different levels of ethanol concentration, 5 and 45% (w/w). A good agreement between experimental data and calculated values can be observed. The estimated values of kinematic viscosity with the proposed model (EquationEqs. 8 and Equation9) give an average deviation of 3.91% in relation to the corresponding experimental data.
CONCLUSIONS
Kinematic viscosity for Newtonian ternary systems, consisting in a mixture of ethanol, water, and sucrose, was determined using a capillary viscosimeter and satisfactorily modelled within a wide range of concentrations and temperatures ranging from 20°C to 45°C. The binary systems experimental data was similar to previously reported results, and also, their behaviour is far from an ideal system, since the viscosity-concentration profiles show a maximum at ∼ 45% (w/w). Addition of sucrose to the binary solution strongly increases kinematic viscosity values. This was specially noticed in systems with high water content due to higher sucrose solubility. In all cases, the temperature decreases the kinematic viscosity values. The models proposed for binary and ternary solutions satisfactorily fitted the experimental data in spite of the high deviations of the solutions from ideal behaviour. For general application in the food industry, the model presents a good deviation pattern.
In future investigations, it would be interesting to study the application of both models to other kinds of solutes and aqueous solvent systems, so a study on component interactions and the corresponding influence on kinematic viscosity could be performed.
REFERENCES
- Pemberton , R.C. and Mash , C.J. 1978 . Thermodynamics Properties of Aqueous Non-electrolyte Mixtures. 2. Vapor-pressures and Excess Gibbs Energies for Water + Ethanol at 303.15 K to 363.15 K Determined by an Accurate Static Method . J. Chem. Thermodyn. , 10 : 867 – 888 .
- Misra , B.N. and Varshni , Y.P. 1961 . Viscosity Temperature Relation for Solutions . J. Chem. Eng. Data , 6 ( 2 ) : 194 – 196 .
- Weast , R.C. 1983 . CRC Handbook of Chemistry and Physics , 67th , 962 Boca Raton : Chemical Rubber Publishing .
- Decloux , M. , Labatut , F. , Saska , M. and Godshall , M.A. 2001 . Effect of Ethanol on Sucrose Solubility and Molasses Exhaustion . Int. Sugar J. , 103 : 547 – 552 .
- Barbosa-Cánovas , V. and Vega-Mercado , H. 1998 . Dehydration of Foods , 330 Gaithersburg, MD : Aspen Publishers Inc .
- Chenlo , F. , Moreira , R. , Pereira , G. and Ampudia , A. 2002 . Viscosities of Aqueous Solutions of Sucrose and Sodium Chloride of Interest in Osmotic Dehydration Processes . J. Food Eng. , 54 : 347 – 352 .
- Moreira , R. , Chenlo , F. and Pereira , G. 2003 . Viscosities of Ternary Aqueous Solutions with Glucose and Sodium Chloride Employed in Osmotic Dehydration Operation . J. Food Eng. , 57 : 173 – 177 .
- Chenlo , F. , Moreira , R. , Pereira , G. and Bello , P. 2006 . An Equation for Modeling the Kinematic Viscosities of Binary and Ternary Solutions with Sugars and Sodium Chloride as a Function of Concentration and Temperature: Experimental Data of Solutions with Lactose . Int. J. Food Proper. , 9 ( 2 ) : 149 – 156 .
- Dickinson , E. , Thrift , L.J. and Wilson , L. 1980 . Thermal Expansion and Shear Viscosity Coefficients of Water + Ethanol + Sucrose Mixtures . J. Chem. Eng. Data , 25 : 234 – 236 .
- Redlich , O. and Kister , A.T. 1948 . Thermodynamics of Nonelectrolyte Solutions –X-Y-T Relations in a Binary System . Ind. Eng. Chem. , 40 ( 2 ) : 341 – 345 .
- Giro , F. , Gonçalves , M.F. , Ferreira , A.G.M. and Fonseca , I.M.A. 2003 . Viscosity and Density Data of the System Water + n-pentyl acetate + Methanol Calculations with a Modified Redlich-Kwong-Soave Equation of State . Fluid Phase Equil. , 204 : 217 – 232 .
- Torok , T.I. , Rard , J.A. and Miller , D.G. 1993 . Viscosities, Electrolytic Conductivities, and Volumetric Properties of HCl-MgClx-H2O as a function of temperature up to high molar ionic strengths . Fluid Phase Equil. , 88 : 263 – 275 .
- Cocchi , M. , Marchetti , A. , Sanna , G. , Tassi , L. , Ulrichi , A. and Vaccari , G. 1999 . Kinematic Viscosities of Ternary Mixtures Containing Ethane-1,2-diol, 2-Methoxyethanol and Water from −10°C to 80°C . Fluid Phase Equil. , 157 : 317 – 342 .
- Vázquez , G. , Chenlo , F. , Álvarez , E. , Moreira , R. and Pardo , P. 1994 . Viscosities of Solutions of Interest for Studies of Absorption Processes . J. Chem. Eng. Data , 39 : 87 – 89 .