Abstract
Hypoallergenic wheat flour was produced by polished-graded method using a rice polishing machine. Eight fractions (C1–C8) of polished-graded wheat flours were obtained step wise from the outer layer of whole wheat grains by 10% of the total weight, and the distribution of allergenic protein in each fraction was determined. The salt-soluble (albumin/globulin), salt-insoluble (glutenin) and alcohol-soluble (gliadin) proteins obtained from the polished-graded wheat flours were tested for the allergen assay with immunodetection using the sera of wheat allergenic patients. Immunoblotting results confirmed that the innermost fraction (C8) contained a smaller amount of allergenic proteins. Albumin/globulin groups in all fractions (C1–C8) showed different IgE-reactivity patterns, the 60–75 kDa proteins appeared in all of fraction flours. It was higher in C3–C5 fractions. Fractions C3 and C4 contained higher amount of specific wheat allergenic protein including 60–75, 35, 22, and < 20 kDa. IgE-antibody also bound to glutenin (25–37 kDa), especially in C3 and C4, but the binding proteins showed quite faint bands. Gliadin was found in all fractions of wheat flour. We propose that polishing is an appropriate method to obtain hypoallergenic wheat flours, and the fraction C8 may be possible to be consumed by people suffering from wheat allergy.
INTRODUCTION
Wheat is one of the foods which are the most commonly responsible for allergic reactions in children and adults. The occurrence of wheat allergy is increasing in recent years. This phenomenon becomes a serious problem globally, since wheat is the most predominantly consumed crop in the world. Generally, wheat-associated allergy represents the allergic reaction suffered by people who consume various wheat products. The major symptoms are eczema and urticaria that occur shortly after wheat products have been eaten, normally conducing in skin eruption and itching, followed by a severe eruption unless the patients stop eating wheat products.[Citation1,Citation2] Thus, the patients' quality of life (QOL) is significantly affected in developed countries where the custom of consuming wheat products is continued. Therefore, the application of pseudocereal as amaranth grain flour is recommended for breadmaking.[Citation3]
A relationship between protein solubility and various clinical manifestations of wheat allergy has been suggested. According to their solubility, wheat proteins are classified into three fractions: salt-soluble (albumin and globulin), salt-insoluble (glutenin), and alcohol soluble (gliadin).[Citation4] Tsubaki et al.[Citation5] reported that a major wheat allergen occurs with a salt-soluble fraction. Salt-soluble proteins are absorbed in the respiratory tract and therefore suggested to elicit IgE-mediated allergic reactions.[Citation6] On the other hand, Watanabe et al.[Citation7] found that most patients with wheat allergy were sensitive to low molecular weight glutenin, which may be resistant to digestion during passage through the stomach and small intestine. These observations indicate that various wheat proteins are allergenic. Therefore, more specific information on their nature and distribution is needed for the prevention of wheat allergy. Eventually, development of hypoallergenic wheat products can improve QOL for allergic patients.
To eliminate allergenic proteins, several food-processing techniques[Citation8] have been applied to wheat products. It has been reported that the proteinaceous allergen of wheat flour was hydrolyzed and decomposed by digestion with cellulase and actinase.[Citation7,Citation9] In addition, Kobayashi et al.[Citation10] reported that the Japanese soy sauce did not contain the allergenic wheat proteins, and the protease induced by microorganisms in the stage of fermentation of soy sauce degraded the major allergenic wheat proteins.
In the present article, we eliminate allergenic proteins from wheat flours, investigated proteins obtained from various fractions of polished-graded wheat flours, and we examined their distribution in wheat grain by immunoblotting with the sera of patients with wheat-associated allergies. Furthermore, their classification according to the solubility of proteins was determined, and relation of their solubility to the allergy reaction was determined. Based on these results, development of hypoallergenic wheat flours by stepwise polishing was attempted.
MATERIALS AND METHODS
Fractionation of Wheat Grain by Stepwise Polishing
A hard-type wheat grain 1CW (No. 1 Canada Western Red Spring) was used in this study. Fractionation of wheat grain was performed using a rice polisher according to the previous method.[Citation11,Citation12,Citation13] The whole wheat grain was gradually polished to eight fractions (C1–C8) from the outer layer in the increments of 10% of total weight using a modified rice-polisher (Itomen Co., Ltd., Hyogo, Japan), as follows. C1 corresponds to the polished flour from 100–90% of the whole grain; C2, 90–80%; C3, 80–70%; C4, 70–60%; C5, 60–50%; C6, 50–40%; C7, 40–30%, and C8, 30% to the core.
Sera of Wheat-Allergic Patients
Eight serum samples with significant radioimmunosorbent-test (RIST) or radioallegro sorbent-test (RAST) scores against wheat proteins were selected from sera of patients with known clinical histories of allergies. The scores of RIST and RAST were determined using a LUMIWARD immunoassay system kit (Shionogi Pharmaceutical Co., Ltd., Osaka, Japan). One control serum was obtained from a healthy adult volunteer who had no clinical history of allergic diseases. Informed consent was obtained from all donors.
ELISA (Enzyme-linked Immunosorbent Assay)
ELISA was performed from stock solutions of crude wheat proteins. About 10 μL of crude proteins was put in the micro plates, then was solubilized at the same concentration in 150 μL of 0.05 M carbonate buffer pH 9.6 containing 0.1% (w/v) SDS and 0.2% 2-merchaptoethanol, and then coated overnight at 37°C. After coating, the buffer was removed and then washed three times using 150 μL of phosphate-buffered saline (PBS) buffer containing 0.05% (v/v) Tween-20. The well were saturated with 0.5% gelatine (Sigma, G-2500) in PBS-Tween for 1 h at 37°C and then incubation for 2 h at 37°C with patient and control sera diluted 1:250 in PBS/Tween/gelatin. Alkaline phosphatase-labeled goat anti-human IgE diluted 1:2000 were added for 2 h at 37°C. The alkaline phosphatase reactivity was detected by adding the substrate (p-nitrophenylphosphate, Sigma N-2765) in 1 M Tris-HCl buffer (pH 9.8).[Citation14] The absorbance was read at 405 nm with an Iwaki Microplate Reader EZS-ABS (Asahi Techno Glass, Co., Ltd., Tokyo, Japan) after 30 min reaction. Sera were considered to be positive for IgE-binding when the absorbance values were two times higher than those for well containing antigen.
Fractionation of Proteins from Wheat Flour
The fractionation of wheat proteins was performed according to the method of Weiss et al.[Citation15] An aliquot (10 g) of wheat flour was stirred in 40 mL of 50 mM Tris-HCl (pH 8.8) at 4°C for 1 h. The suspension was centrifuged at 20,000 × g for 20 min at 4°C. The resultant supernatant was dialyzed against distilled water at 4°C for 24 h, lyophilized and used as albumin/globulin fraction. The pellet was washed three times with 50 mL of 50 mM Tris-HCl (pH 8.8) and one time with 50 mL of distilled water. Glutenin fraction was extracted from the pellet with 4 mL of 50 mM Tris-HCl (pH 8.8) containing 1% sodium dodecyl sulfate (SDS) and 0.5% dithiothreitol by stirring for 2 h at room temperature. After centrifugation at 20,000 × g for 30 min, the supernatant was dialyzed against distilled water at 4°C for 24 h, lyophilized and used as glutenin fraction. Then, the residual precipitate was extracted three times with 4 mL of 75 % ethanol for 2 h at room temperature and one time with 50 mL of distilled water. After the extract was centrifuged at 20,000 × g for 20 min at 4°C, the resultant supernatant was concentrated using a rotary evaporator at 30°C under vacuum, and used as gliadin fraction. The proteins in samples were measured according to the method of Bradford using a commercial kit (Bradford Protein Assay Reagent, BIO-RAD Laboratories, Hercules, CA). Bovine serum albumin was used as a standard protein.
SDS-Polyacrylamide Gel Electrophoresis (PAGE)
SDS-PAGE was performed according to the modification method of Laemmli.[Citation16] Proteins (10–15 μg) were treated with sample buffer for 3–5 min at 100°C and then run on 13% SDS-polyacrylamide slab gels. After electrophoresis, the gels were stained with Coomassie brilliant blue (CBB) R-250.
Immunoblot Analysis
After SDS-PAGE, the proteins were electrotransferred onto a PVDF membrane (Atto Co., Tokyo, Japan). The membrane was washed three times with TBS containing 0.05% Tween 20 (TBST), and then incubated with 5% skim milk in TBST at 4°C for 12 h for blocking. After washing three times with TBST, the membrane was incubated with sera diluted 1:20 with TBST at room temperature for 4 h.[Citation17] The membrane was washed three times with TBST and incubated with a goat polyclonal anti-human IgE antibody coupled to horseradish peroxidase (Sigma-Aldrich, St. Louis, MO, USA) at a dilution of 1:1000 for 2 h at room temperature. Blots were then washed three times in TTBS before visualization. An ECL kit (Amersham Bioscience, Germany) was used for detection.
Relative Intensity Analysis
Relative intensity was measured using the public domain software Image J for Windows (available at http://rsb.info.nih.gov/ij). All acquired images from one test run were grouped in a stack and subsequently analyzed in an automated procedure using a customized program (macro) written in Java language which integrates in the ImageJ software.
Statistical Methods
All analyses were done three times. Experimental data were performed by using one way analysis variance (ANOVA) (SPSS V.11.0 for Windows software, SPSS, Chicago IL). Significant differences among treatments were identified using the Tukey test at the 95% confidence level.
RESULTS AND DISCUSSION
Protein Composition of Fractionated Wheat Flours
Protein compositions of wheat flour obtained by stepwise polishing are shown in . The inner part of wheat fractions (C7 and C8) contained lower amounts of salt-soluble proteins (albumin and globulin): about 2.16 and 1.99 g/100 g flour, respectively, but it was not significantly different (P < 0.05) compared with C5 and C6. The outer part of wheat fraction (C2) had large amounts of salt-soluble proteins, and it was not significantly different (P < 0.05) compared with C1, C3, and C4. Salt-soluble proteins gradually decreased from outer to inner part of wheat, showing that the accumulation of salt-soluble proteins in the sink organ (seed) was different during mature process. The alcohol soluble (gliadin) wheat protein was not significantly different (P < 0.05) from outer to inner part of wheat (C2–C8), except the surface layer of whole wheat (C1) which was significantly lower (P < 0.05) than the other fractions. Salt-insoluble proteins of wheat fraction were not significantly different (P < 0.05) among each of fraction. The accumulation of alcohol and salt-insoluble proteins in wheat was similar from outer to inner part: may be their relation with the connection among of starch granules. Accumulation of proteins in wheat affected to the quality of bread, showing that the rheological and physicochemical properties of wheat fraction were different, when it was applied to the bread making.[Citation11]
Table 1 Compositions of proteins in polished-graded wheat flours (g/100 g flour)
Immunoreactivity of Wheat-Allergy Patients' Sera to Wheat Flour Protein
To check whether the patient is allergic to wheat, we determined the levels of total allergy IgE or allergy-specific IgE in the patients' sera using either a radioimmunosorbent test (RIST) or a radioallergosorbent test (RAST). Clinical characteristics of wheat-allergy patients are shown in . The level of total allergic IgE was determined by the RIST. The higher score of RIST was found in the sera of patients' number of 4, 7, and 8 as 12,000, 3100, and 5300 IU/ml, respectively. The RAST is the most informative test to be used for determination of the level of allergy-specific serum from allergy positive patient. Especially, patient no. 8 (820 IU/ml) has a higher level of allergy-specific IgE related to wheat allergy, and patient no. 5 has suffered from the respiratory diseases. Patients nos. 3 and 6 have been suffered from eczema, damaged ears, and atopic dermatitis diseases, but their sera contained a lower amount of total allergic IgE.
Table 2 Characteristic of wheat-allergic subjects
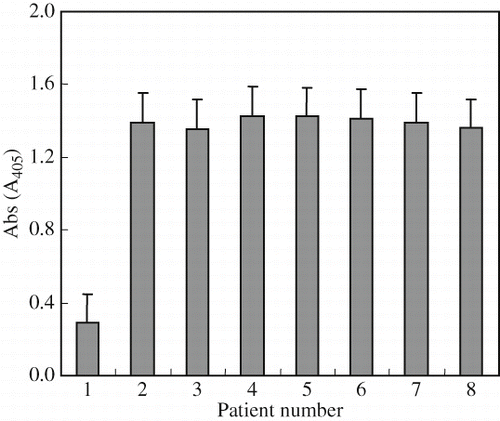
As to enzyme-linked immunosorbent assay, IgE in sera of the allergy positive patients (nos. 2–8) was not significantly different reactions to crude protein of wheat grain (). The patients' sera mostly expressed strong binding reactivity to the crude protein of wheat compared with the control. Generally, ELISA test could not determine the amount of the specific allergenic proteins in crude extract of wheat, because total of wheat-allergy proteins (antigen) bound to antibody of patient sera.
Figure 1 ELISA of control (no. 1) and seven positive (no. 2-8) wheat-allergy patient's sera reacted 3 to crude protein of wheat grain (average ± standard error of the mean, n = 3).
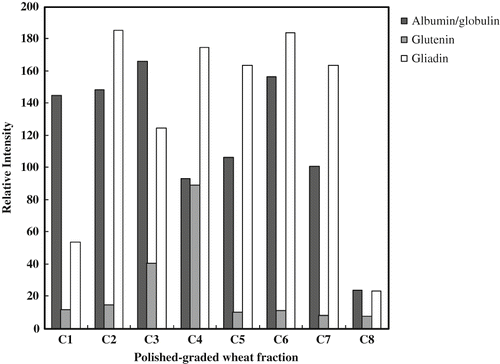
Relative intensity of allergy-specific IgE to wheat proteins is shown in . Four group bands of proteins were found by Western blot analysis, and then the intensity of each fraction was calculated by Image J software. All positive patients' sera had higher intensities than that of the control, and they reacted to high molecular weight protein with higher values more than 30 of relative intensity. However, patient's sera showed different reactions to the band of protein, and three patient's (nos. 3, 4, and 5) sera with higher intensities reacted to >50 kDa of allergenic protein, while they had a low intensity to the <30 kDa protein. One patient's (no. 6) sera also had higher relative intensities with >50 kDa protein, but it was medium intensity on 31–50 kDa and lower intensity on <30 kDa proteins. Two patient's (nos. 2 and 7) was medium intensities on >31 kDa proteins, but they showed a lower intensity to the <20 kDa and did not react to 21–30 kDa proteins. The patient's sera had similar reaction to wheat allergenic proteins by ELISA test; it bound on the different molecular weight of protein, and was shown by intensity of the crude proteins after Western blot analysis ().
Table 3 Reaction of patient sera on wheat-allergic proteins by Western blot analysis.Footnote a
Immunoreactivity of Allergy-Specific Human IgE to Fractionated Wheat Protein
The protein patterns of polished-graded wheat flours were separated by SDS-PAGE (). The higher concentrations of total proteins were found in fractions C1, C2, C7, and C8. The bands of C1 and C2 had many kinds of protein with various ranges of molecular weight. However, fractions C3-C8 showed clear profiles.
Figure 2 Reaction of allergy-specific human IgE to wheat on protein fraction. A, SDS-PAGE of 6 crude samples. B, Salt-soluble albumin/globulin. C, Salt-insoluble protein (glutenin). D, Alcohol-7 soluble protein (gliadin). M, protein marker; C1-C8, polished-graded wheat flours.
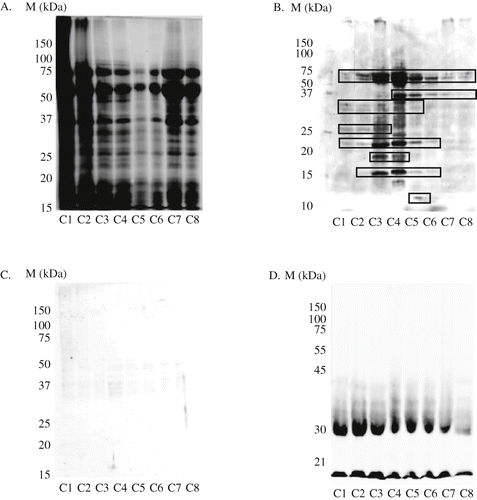
Western blot analysis of three fraction proteins of flours is shown in In immunoblotting, extracted proteins were separated according to their molecular weight by SDS-PAGE, and detected with the patient's sera after transfer to a nitrocellulose membrane. Salt-soluble albumin/globulin with molecular weights 60–75 were found in the all wheat fractions. The 35 kDa of wheat allergenic proteins appeared in C4, C5, C6, C7, and C8, it was gradually disappeared into inner part of wheat (C8 fraction). The 30 kDa proteins were expressed in C1–C5 fractions, but it had very low intensity. The C1–C6 fractions contained the 22 kDa of wheat allergenic proteins; the protein band was lower in C1, C2, and C6, but it was higher in C3 and C4 fractions. The low molecular weight protein <20 kDa was found in the C2–C6 (). In the C3 and C4 fractions, IgE reacted with the 16 and 18 kDa proteins. The C2 and C6 fractions bound to 16 kDa; the C5 fraction reacted with the 11 and 16 kDa proteins. C8 fraction which is the innermost part of wheat grains, contained small amount of allergenic proteins, especially 35 and 60–75 kDa. We suggested that salt-soluble albumin/globulin were the major of wheat allergenic proteins, and they were found in all parts of whole grains of wheat, but they were possible to be eliminated by polishing methods. Similar findings were reported by Mittag et al.[Citation4] and Tsubaki et al.,[Citation5] who found that salt-soluble albumin/globulin are the major of wheat allergenic proteins. IgE-antibody in sera also bound to salt-insoluble proteins (MW = 25–37kDa), especially in C3 and C4, but its binding proteins showed faint bands with quite weak expression (). The allergy-specific IgE-antibody reacted with alcohol-soluble proteins (MW: <20 and 31 kDa) of flour (). Allergenic proteins appeared in all fractions of polished-graded wheat flours although their amounts were lower for C8 than those of other fractions. From these results, polished-grading method was considered to eliminate allergenic proteins of wheat flours successfully.
Allergenic proteins of wheat could be reduced and eliminated by using polished-graded process, because they are not located evenly in all fractions and not found in the inner fractions. Regarding to data about the relative intensity, most fractions of wheat flours contained allergenic-proteins including salt-soluble albumin/globulin, salt-insoluble glutenin and alcohol-soluble gliadin (). As a result, C8 fraction contained lower amounts of these proteins than any other fractions. Therefore, we can infer from this that allergens were eliminated by using the polished-grading method and it is possible for the people suffering from allergic diseases to ingest the innermost fraction of wheat grain.
CONCLUSIONS
Polished-grading method was considered to be appropriate for obtaining the hypoallergenic wheat flours. The polishing method was effectively used to identify the distribution of proteins with various molecular weights in wheat grains and to evaluate or to remove the allergenic proteins. Allergenic proteins (albumin, globulin, gliadin and glutenin) were distributed in all fractions of polished flours, whereas the innermost fraction, C8 showed slight evidence only for the presence of glutenin. Salt-soluble albumin/globulin were found in all fractions with different molecular weights, but the C8 fraction contained lower amount. All fractions contained different amounts of gliadin, and weaker binding was identified especially to allergy-specific human IgE-antibody for the innermost (C8) fractions.
ACKNOWLEDGMENTS
The authors wish to thank the Suzuki Clinic (Kyoto, Japan) for providing sera and Miyake Flour Milling Co., Ltd. (Osaka, Japan) for supplying the wheat flour.
REFERENCES
- Taylor , S.L. , Lemanske , R.F. , Buss , R.K. and Busse , W.W. 1987 . Food Allergens: Structure and Immunologic Properties . Ann. Allergy , 59 : 93 – 99 .
- Martinez , F.D. 1999 . Recognizing Early Asthma . Allergy , 54 : 2 – 28 .
- Ayo , J.A. 2001 . The Effect of Amaranth Grain Flour on the Quality of Bread . Int. J. Food Prop. , 4 : 341 – 351 .
- Mittag , D. , Niggemann , B. , Sander , I. , Reese , I. , Fiedler , E. , Worm , M. , Vieths , S. and Reese , G. 2004 . Immunoglobulin E-reactivity of Wheat-allergic Subjects (Baker's Asthma, Food Allergy, Wheat-dependent, Exercise-induced Anaphylaxis) to Wheat Protein Fractions with Different Solubility ad Digestibility . Mol. Nutr. Food Res. , 48 : 380 – 389 .
- Tsubaki , K. , Takahashi , Y. , Aihara , Y. , Matsuyama , S. , Yokota , S. and Ikezawa , Z. 1995 . Detection of IgE Antibodies to Salt-insoluble Wheat Proteins in Sera of Patients with Atopic Dermatitis by ELISA and Immunobloting Techniques . Allergy , 44 : 134 – 142 . (In Japanese)
- Sutton , R. , Hill , D.J. , Baldo , B.A. and Wrigley , C.W. 1982 . Immunoglobulin E Antibodies to Ingested Cereal Flour Components: Studies with Sera from Subject with Asthma and Eczema . Clin. Allergy , 12 : 63 – 74 .
- Watanabe , M. , Watanabe , J. , Sonomaya , K. and Tanabe , S. 2000 . Novel Method for Producing Hypoallergenic Wheat Flour by Enzymatic Fragmentation of the Constituent Allergens and its Application to Food Processing . Biosci. Biotechnol. Biochem. , 64 : 2663 – 2667 .
- Handoyo , T. and Morita , N. 2006 . Structural and Functional Properties of Fermented Soybean (Tempeh) by Using Rhizopus Oligosporus . Int. J. Food Prop. , 9 : 347 – 355 .
- Tesaki , S. , Watanabe , J. , Tanabe , S. , Sonoyama , K. , Fukushi , E. , Kawabata , J. and Watanabe , M. 2002 . An Active Compound Against Allergen Absorption in Hypoallergenic Wheat Flour Produced by Enzymatic Modification . Biosci. Biotechnol. Biochem. , 66 : 1930 – 1935 .
- Kobayashi , M. , Hashimoto , Y. , Taniuchi , S. and Tanabe , S. 2004 . Degradation of Wheat Allergen in Japanese Soy Sauce . Int. J. Mol. Med. , 13 : 821 – 827 .
- Maeda , T. and Morita , N. 2001 . Effect of Quality of Hard-type Polished-graded Flour on Breadmaking . J. Appl. Glycosci. , 48 : 63 – 70 .
- Tang , H. , Ando , H. , Watanabe , K. , Takeda , Y. and Mitsunaga , T. 2000 . Some Physicochemical Properties of Small-, Medium-, and Large-granule Starches in Fractions of Waxy Barley Grain . Cereal Chem. , 77 : 27 – 31 .
- Tang , H. , Ando , H. , Watanabe , K. , Takeda , Y. and Mitsunaga , T. 2001 . Fine Structures of Amylase and Amylopectin from Large, Medium and Small Waxy Barley Starch Granules . Cereal Chem. , 78 : 111 – 115 .
- Sapats , S. , Gould , G. , Trinidad , L. , Parede , L.H. , David , C. and Ignjatovic , J. 2005 . An ELISA for Detection of Infectious Bursal Disease Virus and Differentiation of Very Virulent Strains Based on Single Chain Recombinant Chicken Antibodies . Avian Pathology , 34 : 449 – 455 .
- Weiss , W. , Vogelmeier , C. and Gorg , A. 1993 . Electrophoretic Characterization of Wheat Grain Allergen from Different Cultivars Involved in Baker's Asthma . Electrophoresis , 14 : 805 – 816 .
- Laemmli , U.K. 1970 . Cleavage of Structural Proteins During the Assembly of the Head of Bacteriophage T4 . Nature , 227 : 680 – 685 .
- Urisu , A. , Kondo , Y. , Morita , Y. , Wada , E. , Tsuruta , M. , Yasaki , T. , Yamada , K , Kuzaya , H , Suzuki , M. , Titani , K. and Kurosawa , K. 1994 . Identification of Major Allergen of Buckwheat Seeds by Immunobloting Methods . Allergy Clin. Immunol. , 6 : 151 – 155 .