Abstract
Thermal processing of honey eliminates the microorganisms responsible for spoilage. Microwave heating, infrared heating, ultrasound processing, and membrane processing have been explored as alternatives to conventional heat processing. Microwave heating provides a rapid method for achieving the desired level of yeast reduction with reduced thermal damage. Infrared heating is not as rapid as microwave heating but desired results are achieved in a relatively shorter duration (3 to 4 minutes) compared to the conventional method. Membrane processing is an athermal process and very effective in the complete removal of yeast cells from honey. Microfiltration and ultrafiltration could be employed to produce enzyme-enriched honey besides clarified honey.
INTRODUCTION
Honey, a natural biological product evolved from nectar and of great benefit to human beings both as medicine and food, is consumed in every country of the world in some form. Honey contains glucose, fructose, and water, in addition to small quantities of proteins, minerals, organic acids, and vitamins.[Citation1] It is liked for its characteristic flavor, sweetness, and texture.
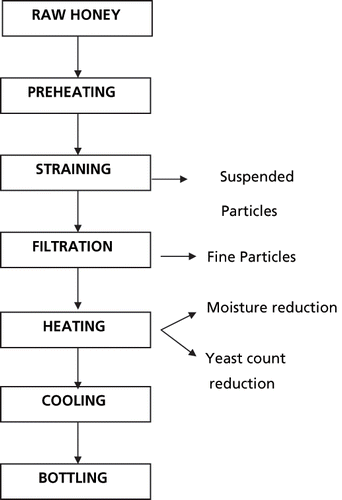
Honey extracted from combs and apiaries contains pollens, beeswax, and other undesirable materials, besides yeast, that are to be removed for better product quality and shelf life. Hence, honey is processed before packing in bottles or other containers. The type of equipment used and steps followed in processing, however, depends upon the scale of operation. Two important stages of honey processing are filtration and heating. The separation of pollens, beeswax, and other materials are normally done through straining and pressure filtration. Heat or thermal processing of honey eliminates the microorganisms responsible for spoilage and reduces the moisture content to a level that retards the fermentation process. The general process flow diagram for conventional honey processing is provided in . The physical separation of undesirable, suspended matter is done before thermally processing honey.
Straining
The straining operation to remove suspended solids (including large wax particles) is carried out either manually or by mechanical means. The method and the equipment used for straining depend on the size of the operation. In small-scale operations, straining is done using cloth or nylon bags, which are frequently cleaned to remove the suspended particles. In large-scale operations, the straining operation is combined with the pre-heating (up to 40°C) operation in a jacketed tank fitted with a stirrer.[Citation2]
Filtration
The strained honey is further processed using pressure filters. Typically a polypropylene micro filter of 80 μm is used as a filter medium. The honey temperature is maintained between 50–55°C, which prevents the melting of the beeswax.[Citation2] Large-scale processors subject honey to coarse filtration, centrifugal clarification, fine filtration, and blending, prior to filling.
THERMAL PROCESSING OF HONEY
The major problem faced by honey producers in tropical countries is its rapid deterioration in quality due to fermentation. Honey generally contains osmophilic (sugar–tolerant) yeast in greater or lesser amount and ferment, if the moisture content is high enough and storage temperature is favorable. Lockhead[Citation3] had reported that a raw honey sample containing more than 20% moisture readily undergoes fermentation irrespective of the initial yeast count. Moisture influences the rate of fermentation, granulation, and honey flavor. Reduction of moisture content below 17% is considered to be a safe level for retarding yeast activity. However, the chances of granulation increase with decrease in moisture content of honey. The unprocessed honey tends to ferment within a few days of storage at ambient temperature because of its high moisture content and yeast count. To prevent fermentation, honey is heat processed before storage. Heat processing of honey eliminates the microorganisms responsible for spoilage and reduces the moisture content to a level that retards the fermentation process.
Honey has high viscosity (1.36 N·s/m2 at 25°C and 21.5% moisture)[Citation4] that pose problems in handling and processing. Viscosity of honey is influenced by several factors. As honey is heated, it initially undergoes a very rapid decrease in viscosity up to 30°C, beyond which the change in viscosity is much slower. Besides temperature and moisture content, variations in viscosity are attributed to the composition of individual sugars and to non-sugar and colloidal material. Mossel et al.[Citation5] successfully described the sugar concentration dependence of the viscosity of honey samples with varying moisture contents with a model that was originally developed to describe the viscosity data of various sugar mixtures. Newton's law of viscosity could adequately describe the flow behavior of honey samples, and the temperature dependency of viscosity was found to follow the Arrehenius model.[Citation6] Yanniotis et al.[Citation7] also reported the Newtonian behavior of honey after studying the effect of moisture content on its viscosity at different temperatures. Generally, honey is a Newtonian fluid but non-Newtonian behaviour has also been reported owing to the presence of colloids (possibly proteins) and the polysaccharide dextran.[Citation8] Recently, Escobedo et al.[Citation9] reported that honey samples at the 12th week of storage showed a strong tendency to behaving as a non-Newtonian fluid owing to the presence of crystals that changed the flow behavior. The authors rightly cautioned that such a change in flow behavior in honey may be due to the nature of the test. Sopade et al.[Citation10] studied the pumping characteristics of Australian honey and proposed an equation for the prediction of pressure loss in a pipeline of typical size at any temperature and flow rate. Bhandari et al.[Citation11] correlated the glass transition temperature (Tg ) with critical viscosity and reported that the critical viscosity is reached at a temperature 10 to 20°C above the Tg .
Crystallization is an undesirable property in handling, processing, and marketing, except for certain purpose such as in the production of creamed honey. Glucose is the principal component that crystallizes in honey as it exists in a supersaturated state. Bhandari et al.[Citation8] summarized some of the methods proposed to stop crystallization of honey: storage at freezing temperature (−40°C), heat treatment to dissolve crystals and crystal nuclei, removal of air bubbles, dust and pollen particles by filtration, filling at higher temperatures (>45°C) to avoid air bubbles incorporation during filling, addition of inhibitors such as isobutyric and sorbic acid, and adjusting the glucose to fructose ratios or the water content. Ultrasound processing has also been reported for preventing crystallization in honey, and the various attempts made are discussed under a separate heading. Heating is a common method to control the crystallization. It helps to melt invisible crystals present in honey. After melting all the crystals and nuclei, even the most crystallisable honey can remain liquid for many months. Presence of air bubbles in the packaging containers can provoke nucleation and crystallization of honey. Filling at higher temperatures eliminates air bubbles and avoids air incorporation during packing due to low viscosity.[Citation8]
Although, honey is thermally processed to eliminate yeast, it could result in product quality deterioration. Uncontrolled heating alters the parameters such as hydroxymethylfurfural (HMF) content and enzyme activity unfavorably. The initial HMF content in different honey types varies drastically and it depends upon the climatic condition of the region besides other factors. Excessive amount of HMF has been considered evidence of overheating, implying a darkening of color and a loss of freshness of honey. The starch–digesting enzyme(s) of honey are also used as an indicator of honey quality because of their sensitivity to heat. The major enzymes present in honey are invertase, amylase (diastase), and glucose oxidase along with minute quantities of catalase and acid phosphatase.[Citation1] The enzyme content in honey is measured as diastase activity and expressed in terms of diastase number (DN). The European Regional Standard for honey[Citation12] specifies a minimum DN of 8 in processed honey.
CONVENTIONAL PROCESSING OF HONEY
The conventional process involves preheating to 40°C, straining, filtering /clarification, and indirect heating of filtered honey at 60–65°C for 25–30 minutes in a tubular heat exchanger followed by rapid cooling in order to protect its natural color, flavor, enzyme content, and other biological substances.[Citation2] Studies have shown that heating honey at 63, 65, and 68°C for 35, 25, and 7.5 minutes, respectively can destroy the yeast cells completely.[Citation13] There is a lack of literature about the application of high-temperature short-time (HTST) heating treatment on honey. Tosi et al.[Citation14] reported that a mild HTST treatment condition, typically heating at 80°C for 60 seconds in the transient stage and 30 seconds in the isothermal stage, destroyed all microorganisms responsible for quality damage without spoiling honey. Temperature beyond 80°C through 140°C, at very short times, did not seem to cause deleterious effects on honey as measured by HMF and diastase activity.
White et al.[Citation15] reported the effect of storage and processing temperatures on honey quality. Samples with high, intermediate and low diastase activity were stored at different temperatures (20–70°C). The half-life time of diastase and invertase activity, estimated using the empirical equations indicated the direct relationship with temperature. Singh and Bath[Citation16] studied the relationship between heating and HMF formation in different types of honey. Heating temperature and time showed significant effect on HMF formation. They observed a large difference in HMF formation between different types of honey and used second order polynomials to effectively predict the HMF formation in different types of honey. Effects of thermal treatment on HMF content in honey was also studied by Tosi et al.[Citation17] and reported that the kinetics of HMF formation did not depend on the initial HMF concentration in honey. They also reported that during thermal processing, the time-temperature combination is very crucial for maintaining the HMF level below the maximum permissible limit. Gupta et al.[Citation18] studied the influence of different treatments and storage conditions on some physicochemical characteristics and sensory qualities of Indian honey. The color of the honey was significantly affected by the storage temperature and period with maximum deterioration at a storage temperature of 40°C. However, granulation was completely eliminated in honey stored at 40°C due to melting. Evaluation of honey samples stored for six months showed comparatively higher overall sensory score for unheated honey stored at 5°C (). Visquert et al.[Citation19] investigated the effect of thermal processing on honey quality during storage. Honey was processed at 35 to 85°C for 1–672 hours, depending on the chosen processing temperature and evaluated with respect to HMF content, acidity, electrical conductivity, and moisture content. Thermal processing increased the HMF content of honey considerably. However, acidity, electrical conductivity, and moisture content were unaffected by thermal processing and during subsequent storage.
Table 1 The effect of different treatment on the sensory characteristics of honey after six months of storage.[Citation18]
Central Bee Research and Training Institute, Pune had developed a honey processing plant to handle Indian varieties of honey, which have very high moisture content.[Citation20] The plant having the capacity to process ∼40 kg/h, could reduce the moisture content by ∼8–10%. The constructional features of the plant include cartridge type micro filters, processing tanks with helical coil heat exchangers, and falling-film evaporator.
In recent times the demand for better quality honey is on the rise as honey is being consumed now for its health benefits. So, efforts are being made to look for alternatives to conventional thermal process, which can produce better quality honey. Application of microwave, ultrasound, and infrared heating of honey has been reported and claims have been made on the improved product quality. Microwave and infrared heating have gained popularity in food processing over conventional heating owing to their inherent advantage of rapidity and better-quality product. Pressure-driven membrane processes are remarkably simple allowing ambient temperature operation and requiring less energy. Membrane technology has the potential in replacing or complementing some of the traditional methods of processing, as well as in developing new products.
MICROWAVE HEAT PROCESSING OF HONEY
The application of microwave heating is well known in the food industry, particularly for tempering, blanching, drying, and pasteurization of food material. Microwave heating is greatly affected by the presence of water in foods, as water is the major absorber of microwave energy in food, consequently, the higher the moisture content, the better the resultant heating. In contrast to conventional heating, microwaves penetrate the material, interact with it, and generate heat leading to its rapid heating. Materials containing polar molecules, such as water, are rapidly heated when exposed to microwave radiation due to molecular friction generated by dipolar rotation in the presence of an alternating electric field. It is also reported that dissolved sugars are the main microwave susceptors in high carbohydrate foods and syrups.[Citation21] Since honey contains a substantial amount of water (18–24%), as well as large amounts of dissolved sugars (70–80%), microwave radiation could be effectively used for heating honey.
The processed honey containing yeast cells could be safely stored at room temperature provided the count is apparently insufficient to initiate fermentation. Ghazali et al.[Citation22] studied the effect of microwave processing of starfruit honey for its storage stability. Their study showed that the fairly short time taken to reach the required processing temperature ensured little change in chemical properties. Honey was heated to 71°C using a microwave oven and stored at two different storage conditions, room temperature (28 ± 2°C) and 4°C for 16 weeks. The physicochemical properties of unheated and heated honey were measured before and during storage (). Spoilage was noticed in unheated honey (control), irrespective of the storage temperature. Heated samples were more resistant to spoilage. The spoilage of honey was attributed to the yeast count in honey, which was much higher (1.02 × 105 cfu/g) in the unheated honey compared to heated honey sample (5.90 × 102 cfu/g). No appreciable variation in yeast count was noticed during storage of heated honey. Granulation was not observed in honey samples that were heated before storing. Storing of unheated honey at room temperature was also free from granulation in agreement with the general observation related to dextrose-to-water ratio and storage temperature. Darkening of honey color was observed during storage in both heated and unheated honey from light golden color to golden brown. However, the honey stored at 4°C was considerably lighter in color than honey stored at room temperature. Heated honey was darker than the unheated honey, whatever the storage temperature. Heating did not alter the moisture content of honey (20.8%). Also, no noticeable change in ash, nitrogen contents, pH, and acidity was observed in microwave heated honey sample. Heat processing of honey led to a 37.5% loss in diastase activity. Storage at 4°C had no effect on subsequent activity. However, room temperature storage led to further loss (33%) in activity. Heating did not show any effect on the sugar contents. However, the concentration of glucose, maltose, and sucrose changed during storage depending on the storage temperature and whether the sample had been heated or not.
Table 2 The physicochemical properties of unheated and microwave heated starfruit honey before and after storage for 16 weeks.[Citation22]
Bath and Singh[Citation23] studied the effect of microwave heating on HMF formation and browning in two types of honey (Helianthus annuus and Eucalyptus lanceolatus). The formation of HMF and browning increased with microwave power levels as well as with heating duration, the former showing greater effect. Both the types of honey differed significantly with respect to HMF formation and browning under similar microwave heating conditions, which was attributed to the difference in chemical composition of honey.
Hebbar et al.[Citation24] conducted studies on microwave heating in a micro-convective oven (2450 MHz, maximum power of 800 W). Experiments were carried out at different power levels (PL) ranging from 10 to 100 (175–800 W) and for different heating periods from 15 to 90 seconds. The extent of change in properties (HMF, diastase activity, moisture content, and yeast count) mainly depended on the power level (power intensity) and duration of heating. The changes in these properties were prominent in samples that were heated at higher power levels and for longer durations. The peak temperature attained by the sample depended on the power level used as well as duration of heating.
The reduction in yeast count was observed to the extent of commercially acceptable level (< 500 cfu/mL) at power levels of 10, 30, and 50 when the samples were heated for more than 45 seconds. At higher power levels of 70 and 100, heating duration of 30 and 15 seconds, respectively, was sufficient to achieve the same level of reduction in yeast count. A semi-log plot of yeast count reduction ratio (ratio of yeast count at any given time to initial count) with time at different power levels is shown in . The reduction in yeast count was rapid, generally during the first 20 to 30 seconds, and the rate of yeast reduction was directly related to the input power intensity. The reduction of yeast count is attributed to the rapid increase in sample temperature due to microwave exposure, leading to the rupture of yeast cell walls.
Figure 2 (a) Reduction in yeast count and (b) Increase in HMF with time at different power levels (PL) of microwave heating.[Citation24]
![Figure 2 (a) Reduction in yeast count and (b) Increase in HMF with time at different power levels (PL) of microwave heating.[Citation24]](/cms/asset/1b7c5a92-ca23-44ce-9b32-bd9eee06caa8/ljfp_a_198071_o_f0002g.gif)
The increase in HMF value was gradual with heating duration at power levels of 10, 30, 50, and 70. But HMF level increased sharply in the samples heated for a longer duration at the maximum power level of 100. However, these values were far below the maximum permissible statutory level of 40 mg/kg of honey.[Citation4] Variation of HMF with duration of heating at different power levels is shown in . The trends in the variation of HMF values of the samples clearly depicted the sensitivity of honey to the period of heating and temperature (power level).
Heating affects the enzyme activity and the diastase activity showed a decline with heating under all conditions employed. The reduction in diastase activity with heating duration at different power levels is depicted in . Long heating periods of 60 to 90 seconds duration at power levels of 30, 50, and 70 reduced the diastase activity of honey by ∼50% of its original value. At a power level of 100, heating above 45 seconds resulted in reduction of the diastase activity to a level lower than the minimum permissible DN of 8.[Citation12]
Figure 3 (a) Reduction in diastase activity and (b) Reduction in moisture content with time at different power levels (PL) of microwave heating.[Citation24]
![Figure 3 (a) Reduction in diastase activity and (b) Reduction in moisture content with time at different power levels (PL) of microwave heating.[Citation24]](/cms/asset/d3102a25-2b19-4bd1-b5e8-773125101eeb/ljfp_a_198071_o_f0003g.gif)
Heating also led to a reduction in moisture content above 9% at power levels of 50, 70, and 100 when the samples were heated for 60 seconds. indicates the reduction in moisture content with heating duration at different power levels. Larger reduction in moisture content was not observed at lower power levels. The final moisture content in most of the samples was in the range of 19.8 to 21.2%, which is below the acceptable level (22%) for commercial processed honey.
Though different combinations of time and microwave power level could be used to achieve the commercially acceptable level of yeast reduction in honey, it is equally important to take the peak temperature attained by the sample into consideration. Heating honey above 90°C results in caramelization of sugar.[Citation25] Also it seems that this has a direct bearing on the increase in HMF value and loss in diastase activity. Therefore, it is beneficial to achieve the desired yeast reduction by choosing any suitable combination of power level and duration that will maintain the temperature of honey well below 85–90°C.
Among the various selected combinations, higher power level and shorter duration seems to be better than lower power level and longer duration. At power level 100 (power intensity 16 W/g), heating for 15 seconds resulted in substantial reduction in yeast count (450 cfu/mL), lower HMF value (3.8 mg/kg) and higher retention of diastase activity (DN 12). Also, the desired reduction in yeast count was achieved with least undesired changes in the sample at a much lower processing temperature (54°C).
INFRARED HEAT PROCESSING OF HONEY
Infrared heating of food is gaining popularity due to its transient response, significant energy savings over other thermal processes and ease of construction of hybrid systems with convective and conductive heating sources.[Citation26] Infrared heaters provide high rates of energy input to the material surface and the radiant heat flux penetrates the material to a depth, which depends on the nature of the material and the wavelength of the incident radiation.[Citation27] Sugar and water are the two major constituents of honey and both have good absorption bands in the thermal radiation region.[Citation26] The above factors could be successfully utilized for efficient processing of honey.
Hebbar et al.[Citation24] conducted studies on infrared heating in a near infrared (NIR) batch oven (locally fabricated) fitted with lamps (1.0 kW, peak wavelength 1.1–1.2 μm). In these experiments, the samples were heated continuously for 2, 3, 4, 5, and 8 minutes. HMF, diastase activity, moisture content and yeast count in these samples were analysed (). In all the cases, heating caused substantial reduction in yeast count. Heating for 5 minutes resulted in a product temperature of 85°C, HMF increase of 220%, and 37% drop in enzyme activity. When the samples were heated for 8 minutes, no viable colony forming units of yeast were noticed. However, the diastase activity drastically reduced in these samples, clearly indicating excessive heating of honey that also showed up in a very high product temperature (110°C). A heating period of 3 to 4 minutes was adequate to obtain a commercially acceptable product, which met all the statutory requirements of quality in terms of HMF (≤40 mg/kg), diastase activity (DN ≥ 8), moisture content, and yeast count.
Table 3 Continuous heating of honey with infrared radiation.[Citation24]
ULTRASOUND PROCESSING FOR LIQUEFYING CRYSTALLIZED HONEY
Granulation or crystallization is a natural tendency of honey and this common phenomenon is a serious problem that affects marketing of honey. Crystallization of honey is a complex process controlled by a number of factors acting simultaneously and sometimes in a contradictory fashion. Concentration and super-saturation of the major constituents (levulose, dextrose, and sucrose) and the minor ones (proteins and dextrins), the presence of colloidal particles (nuclei), and temperature with its varying and contradictory effects are some of the more important factors involved.[Citation28] Traditionally, honey is heated to temperatures of 77°C to kill yeasts and to delay crystallization.[Citation29] Heating affects the delicate flavors of honey, therefore alternate methods by means other than heating such as ultrasonic, freezing, and chemical inhibitor treatments were attempted for preventing crystallization in honey.[Citation28] Ultrasonic waves are sound waves with a higher frequency than the maximum that can be sensed by the human ear. These waves when transmitted through liquid medium cause mechanical and thermal changes in the material through which they pass, and also induce changes in unicellular organisms.[Citation30]
Kaloyereas[Citation31] reported first in 1955 that high frequency sound waves (9 kHz) eliminated the existing crystals and retarded further crystallization in honey. Ultrasound processing destroyed most of the yeast cells that were present in the honey, and those that survived had lost their ability to grow. No crystals were observed in ultrasound treated honey and inhibited granulation for a period (15 months at 16°C) comparable to heating the honey.[Citation28] One disadvantage of this method was that exposure times of 15 to 30 minutes were required with cost implications.
Liebl[Citation29] proposed an improved method for preventing the granulation by exposing the honey to ultrasound waves of a much higher frequency (18 kHz) that drastically reduced the liquefaction time to less than 30 seconds. This patented process was designed to work at lower processing temperature (33°C) facilitating greater retention of aroma and flavor along with huge savings on cost of energy compared to the conventional processing involving heating and cooling steps. Studies were carried out at a considerably higher scale (liquefaction of ∼1500 kg of honey/h) to demonstrate the claims on the cost effectiveness of the process.
Thrasyvoulou et al.[Citation30] studied the effects of ultrasonic waves on the quality of honey focusing on some of the chemical characteristics. Crystallized honey samples (100 g each) were liquefied by ultrasonic waves at 23 kHz and compared with conventionally heated (water bath heating; 60°C for 30 minutes) and untreated samples. The complete liquefaction of honey (5 samples each of blossom honey and honeydew honey) required 18 to 25 minutes of ultrasound processing. Accordingly the energy required for liquefaction varied from 0.1056–0.1466 kWh, and the maximum temperature attained by the samples from 76–82°C. The variation in the time required for liquefaction was attributed to the original granulated condition and the nature of samples.
The combined effect of temperature and processing time resulted in increase in HMF level. The average increase in HMF content was significantly low (86%) in samples liquefied by sonication compared to samples liquefied by heating (129%). Ultrasonic energy negatively affected the distaste activity of samples. The average decrease of diastase activity was 16% after ultrasonic treatment and 23% after heat treatment. The influence of factors other than sonication or heat and typical behavior of individual samples could also affect diastase activity. Moisture content, electrical conductivity, and pH were not significantly affected by the treatments. The ultrasonic and heat treated samples were stored at 25 ± 4°C and there was no significant difference in their recrystallization time. The ultrasound treated samples remained in the liquefied state for 344 ± 39 days and heat treated samples for 282 ± 86 days.[Citation30]
Liquefaction by ultrasonic treatment affect the honey quality to a lesser extent compared to heat treatment. It may be desirable to undertake studies on the effect of ultrasonic treatment on a wide range of frequencies and also by eliminating the associated thermal effect due to temperature rise.
MEMBRANE PROCESSING OF HONEY
Membrane processing is an athermal process and an alternate approach to the conventional process. It is difficult to completely destroy the microorganisms present in honey by traditional thermal processing methods practiced by the industries. Besides, thermal processing results in reduction in enzymatic activity. Anticipated benefits of membrane processing of honey are no cloudiness or sedimentation/granulation in the product, reduced viscosity, commercially sterile product and consistent quality characteristics.[Citation32]
The applications of ultrafiltered honey in gel formulations, cosmetics and pharmaceutical preparations besides its use as sweetener in tea/coffee and fruit beverages have been reviewed by National Honey Board.[Citation32] Itoh et al.[Citation33] observed that poor rising of sponge cakes (castilla) and sediment formation in fruit juices (lemon, grape, and apple), when honey is used as an ingredient, was due to the water-soluble proteins (enzymes) present in the honey. Further, they showed that ultrafiltration (UF) of honey completely eliminated these problems. Besides, UF membrane with a molecular weight cutoff (MWCO) of 10 000 could completely remove the microorganisms present in honey, rendering it as a microbiologically safe ingredient.
But the ultrafiltered honey is devoid of desirable enzymes and proteins, and hence, cannot be regarded for applications related to health foods. The major enzymes present in honey are amylase or diastase (α-amylase), invertase (α-glucosidase), and glucose oxidase. Diastase and invertase are nutritionally important enzymes present in honey. Diastase hydrolyses carbohydrates for easy digestibility while invertase hydrolyses sucrose and maltose.[Citation1] Glucose oxidase is another important enzyme in honey that catalyses glucose to form gluconic acid and hydrogen peroxide.[Citation1] Generally, honey is well known for antimicrobial activity against a number of microorganisms, probably due to the presence of high levels of tetracyclines, phenolic compounds, and hydrogen peroxides.[Citation34] Sato and Miyata[Citation35] attributed the antimicrobial property of honey predominantly due to hydrogen peroxide, which allows its use in the treatment of wounds and gastrointestinal diseases such as dyspepsia, bacterial gastroenteritis, gastric, and duodenal ulcers.
White and Kushnir[Citation36] reported the approximate molecular weights for major honey enzymes as 24 000 Da for amylase and 51 000 Da for α-glucosidase. Bergner and Diemair[Citation37] reported that glucose oxidase exhibits the highest molecular weight (>100 000 Da) among the enzymes present in honey. The enzyme content in honey is measured as diastase activity, which includes only amylases and not other enzymes such as invertase and glucose oxidase. It is also preferable to measure the amylase content owing to its lower molecular weight for the estimation of enzyme retention/rejection by the membranes.
The honey processors practicing membrane technology do not divulge technical information as they are regarded as trade secretes directly linked with their commercial success. There are very few reports available on membrane processing of honey in the literature. Itoh et al.[Citation38] used 7000, 30 000, and 80 000 MWCO membranes in honey processing and reported that bacteria and protein could be eliminated in honey using UF membranes. Itoh et al.[Citation39] also assessed the performance of UF process for honey with various processing parameters in a cross-flow membrane apparatus. The authors showed that the total permeation flux, as well as permeation rate of sugar increased with increase in operating temperature and increased dilution of honey at constant feed flow velocity and applied pressure. Permeate flux also increased with increase in MWCO of the membrane (10 000, 30 000, and 150 000). The authors also measured the water activity at various sugar concentrations in honey for 4 different types of honey, which suggested that the sugar concentration shall be greater than 35.5% to maintain the water activity below 0.94 so as to inhibit multiplication of microorganisms. Protein content in the permeate decreased with decrease in MWCO of the membrane (500 000, 150 000, and 30 000) and the permeate of 30 000 MWCO membrane did not contain any protein.
Barhate et al.[Citation40] examined the rejection of enzymes in honey (50% diluted with water) with various MWCO UF membranes and the effectiveness of these membranes in eliminating yeast cells. Besides, attempts were made using membrane technology to produce a honey that is free of microorganisms and suspended matter, but containing a significant concentration of enzymes.
UF membranes (20 000, 25 000, 50 000, and 100 000 MWCO) completely removed yeasts (). The sugar content in the feed was 220 mg/mL and did not change during the UF process. The conventional heat treatment process used for honey is effective for the inactivation of yeast but not for the other heat resistant organisms. It is reported that microfiltration (MF) membrane with a pore size of 200 nm would remove the viable microorganisms completely and as such could be used for sterilization.[Citation41] Therefore, UF membranes would be useful in eliminating other microorganisms besides effectively removing the yeast cells present in the honey.
Table 4 Rejection and permeate flux of different MF/UF membranes.[Citation40]
There was no diastase activity found in the permeates of UF membranes (). The rejection of lower molecular weight enzymes gave a clear indication that enzymes of higher molecular weights were also rejected by these membranes. These results imply that there are reasons other than pore size of the membrane for the rejection. A secondary layer formed on the upstream side of the membranes seems to be responsible for the complete rejection of enzymes. Secondary layer or in other words dynamic membrane formation is a process in which an active layer is formed on the membrane surface due to adsorption and other deposition phenomenon of the substances contained in the feed being processed. Such layers may also influence the rejection of other solutes present in the feed and can be used advantageously in the process.[Citation42]
Permeate flux is an important factor that determine the economics of the membrane processes. In a situation like this, permeate flux is also influenced by the dynamic active layer besides the flow resistance offered by the porous membrane, which is primarily controlled by the pore size. The dynamic layer offers benefits for the overall processing of honey since the permeate flux can be improved significantly by adopting higher MWCO membranes without making any compromise in the rejection requirements of the process.
As expected MF membranes gave greater permeate flux and lower rejection compared to UF membranes (). The rejection of enzymes was between 26 to 66% depending on the pore size of the membrane. The rejection decreased as the pore size increased.
Enzyme Enriched Honey
The enzymes present in honey, is the special reason for its wide spread use in nutrition, therapeutic, and health related applications.[Citation35] During the UF process, these enzymes are retained in the retentate fraction limiting the use of ultrafiltered honey (with zero diastase activity) in the above applications. A different processing strategy for the production of enzyme enriched honey is possible using a combination of MF and UF membranes, which is outlined in . The use of MF membrane (pore size 100 nm) in the process will ensure elimination of yeast as well as other microorganisms while retaining the nutritional factors in the product stream (permeate) at a higher permeation rate through the system. An appropriate UF membrane (20 000 MWCO) will fractionate the microfiltered honey into two fractions namely enzyme enriched honey and regular ultrafiltered honey. By adopting this method, it is possible to enrich enzymes in honey to the extent of 2.2 fold. Higher enrichment of enzymes can be achieved by altering the volume concentration ratio (VCR) in the membrane process. Such an enriched product with enzymes may find use in very special applications related to health. These studies were conducted in batch mode with stirred membrane cells, and the performance data obtained could form a basis for conducting pilot scale process assessment before commercialization.
Figure 4 Schematic diagram of membrane processing of honey.[Citation40]
![Figure 4 Schematic diagram of membrane processing of honey.[Citation40]](/cms/asset/a91a83ec-d5bb-45df-8d8f-3bc12f7cd463/ljfp_a_198071_o_f0004g.gif)
Concentration of Membrane Processed Honey
Honey is very viscous and is required to dilute with water before membrane processing. In the case of UF and MF, there is no change in the water content during the process. Though water removal is an expensive process step, the added water from the membrane-processed honey is to be removed to obtain the original consistency. Vacuum concentration at low temperatures is desirable to minimize the thermal damage to the product. Despite the loss in the evaporation step (), there is an overall enrichment of enzymes in the UF retentate fraction in the total process ().
Table 5 Quality variations during concentration of membrane processed honey.[Citation40]
CONCLUSIONS
Microwave heating can be effectively used for thermal processing of honey, as it provides a rapid heating to achieve the desired results for long-term storage. Infrared heating is not as rapid as microwave heating but the desired results are obtained in a relatively shorter period of 3 to 4 minutes offering advantages over the conventional method. Further studies in the area of microwave and infrared heating of honey are needed to establish the relationship between various processing conditions and honey quality in continuous flow systems to reach the industrial application level. Ultrasound processing destroys most of the yeast cells present in the honey, besides eliminating the existing crystals and retarding further crystallization in honey. Although this approach was first reported in 1955, projected benefits have not been realized even after 5 decades.
The UF membranes completely reject enzymes and totally eliminate yeast cells in honey. Although scientific data is scanty, UF membranes are in commercial use to produce clarified honey. A combination of MF and UF membranes in the process provides a scope to produce enzyme-enriched honey besides the regular clarified honey.
REFERENCES
- White , J.W. Jr. 1975 . “ Composition of Honey ” . In Honey – A Comprehensive Survey , Edited by: Eva , C. 157 – 206 . London : Heinemann .
- Wakhle , D.M. and Phadke , R.P. 1995 . Design for Honey Processing Unit Part I . Indian Bee J. , 57 : 144 – 146 .
- Lockhead , A.G. 1933 . Factors Concerned with the Fermentation of Honey . Zentbl. Bakt. Parasitkde II Abst. , 88 : 296 – 302 .
- White , J.W. Jr. 1979 . “ Physical Characteristics of Honey ” . In Honey – A Comprehensive Survey , Edited by: Eva , C. 207 – 239 . London : Heinemann .
- Mossel , B. , Bhandari , B. , D' Arcy , B. and Caffin , N. 2003 . Determination of Viscosity of some Australian Honeys Based on Composition . Int. J. Food Prop. , 6 : 87 – 97 .
- Zaitoun , S. , Ghzawi , A.A. , Al-Malah , K.I.M. and Abu-Jdayil , B. 2001 . Rheological Properties of Selected Light Colored Jordanian Honey . Int. J. Food Prop. , 4 : 139 – 148 .
- Yanniotis , S. , Skaltsi , S. and Karaburnioti , S. 2006 . Effect of Moisture Content on the Viscosity of Honey at Different Temperatures . J. Food Eng. , 72 : 372 – 377 .
- Bhandari , B. , D'Arcy , B. and Kelly , C. 1999 . Rheology and Crystallization Kinetics of Honey: Present Status . Int. J. Food Prop. , 2 : 217 – 226 .
- Escobedo , R.M. , Ordóñez , Y.M. , Flores , M.E.J. and Lopez , G.F.G. 2006 . The Composition, Rheological and Thermal Properties of Tajonal (Viguiera Dentata) Mexican Honey . Int. J. Food Prop. , 9 : 299 – 316 .
- Sopade , P.A. , Halley , P.J. , D'Arcy , B. , Bhandari , B. and Caffin , N. 2004 . Friction Factors and Rheological Behavior of Australian Honey in a Straight Pipe . Int. J. Food Prop. , 7 : 393 – 405 .
- Bhandari , B. R. , Datta , N. and Howes , T. 1997 . Problem Associated with Spray Drying of Sugar Rich Foods . Drying Technol. , 15 : 671 – 684 .
- 1969 . Recommended European Regional Standard for honey , Rome : Codex Alimentarius Commission, FAO amd WHO . CAC/RS 12
- Wakhle , D.M. , Phadke , R.P. , Pais , D.V.E. and Shakuntala Nair , K. 1996 . Design for Honey Processing Unit Part II . Indian Bee J. , 58 : 5 – 9 .
- Tosi , E.A. , Re , E. , Lucero , H. and Bulacio , L. 2004 . Effect of Honey High-Temperature Short-Time Heating on Parameters Related to Quality, Crystallisation Phenomena and Fungal Inhibition . Lebensm.-Wiss. U.-Technol. , 37 : 669 – 678 .
- White , J.W. Jr. , Kushnir , I. and Subers , H.M. 1964 . Effect of Storage and Processing Temperature on Honey Quality . Food Technol. , 18 : 555 – 558 .
- Singh , N. and Bath , P.K. 1998 . Relationship Between Heating and Hydroxymethylfurfural Formation in Different Honey Types . J. Food Sci. Technol. , 35 : 154 – 156 .
- Tosi , E.A. , Ciappini , M. , Re , E. and Lucero , H. 2002 . Honey Thermal Treatment Effects on Hydoxymethylfurfural Content . Food Chem. , 77 : 71 – 74 .
- Gupta , J.K. , Kaushik , R. and Joshi , V.K. 1992 . Influence of Different Treatments, Storage Temperature and Period on Some Physico–chemical Characteristics and Sensory Qualities of Indian Honey . J. Food Sci. Technol. , 29 : 84 – 87 .
- Visquert , M. , Escriche , I. , Andres , A. and Fito , P. 2004 . Changes in the Quality Parameters of Honey Caused by Thermal Processes . Alimentacion-Equipos-y-Tecnologia. , 23 : 87 – 92 .
- Wakhle , D.M. and Chaudhary , O.P. 1993 . Processing of Honey: Need of the Hour . Khadigramodyog , August–September : 805 – 808 .
- Tulasidas , T.N. , Raghavan , G.S.V. , Van de Voort , F. and Girard , R. 1995 . Dielectric Properties of Grapes and Sugar Solutions at 2.45 GHz . J. Microwave Power Electromagn. Energy , 30 : 117 – 123 .
- Ghazali , H.M. , Ming , T.C. and Hashim , D.M. 1994 . Effect of Microwave Heating on the Storage and Properties of Starfruit Honey . ASEAN Food J. , 9 : 30 – 35 .
- Bath , P.K. and Singh , N. 2001 . Effect of Microwave Heating on Hydroxymethylfurfural Formation and Browning in Helianthus annuus and Eucalyptus lanceolatus Honey . Food Sci. Technol. , 38 : 366 – 368 .
- Hebbar , U.H. , Nandini , K.E. , Lakshmi , M.C. and Subramanian , R. 2003 . Microwave and Infrared Heat Processing of Honey and its Quality . Food Sci. Technol. Res. , 9 : 49 – 53 .
- Yener , E. , Ungan , S. and Ozilgen , M. 1987 . Drying Behavior of Honey-starch Mixtures . J. Food Sci. , 52 : 1054 – 1058 .
- Sandhu , C. 1986 . Infrared Radiative Drying in Food Engineering: A Process Analysis . Technol. Progress , 2 : 109 – 119 .
- Zbicinski , I. , Jakobsen , A. and Driscoll , J.L. Application of Infrared Radiation for Drying of Particulate Materials . Presented at 8th International Drying Symposium (IDS'92) . August 2–5 1992 , Montreal, Canada. pp. 704 – 711 . New York : Elsevier .
- Kaloyereas , S.A. and Oertel , E. 1958 . Crystallization of Honey as Affected by Ultrawaves, Freezing and Inhibitors . Am. Bee J. , 38 : 442 – 443 .
- Liebl , D.E. Method of Preserving Honey . US Patent 4050952 . Filed 19 November 1976; issued 27 September 1977
- Thrasyvoulou , A. , Manikis , J. and Tselios , D. 1994 . Liquefying Crystallized Honey with Ultrasonic Waves . Apidologie , 25 : 297 – 302 .
- Kaloyereas , S.A. 1955 . Preliminary Reports on the Effect of Ultrasonic Waves on the Crystallization of Honey . Science , 121 : 339 – 340 .
- National Honey Board . 1991 . Ultrafiltered Honey , Longmont, CO : N. H. B. .
- Itoh , S. , Yoshioka , K. , Terakawa , M. , Sekiguchi , Y. , Kokubo , K.I. and Watanabe , A. 1999 . The Use of Ultrafiltration Membrane Treated Honey in Food Processing . J. Jap. Soc. Food Sci. Technol. , 46 : 293 – 302 . (Nippon Shokuhin Kagaku Kogaku Kaishi)
- Mothershaw , A.S. and Jaffer , T. 2004 . Antimicrobial Activity of Foods with Different Physio-Chemical Characteristics . Int. J. Food Prop. , 7 : 629 – 638 .
- Sato , T. and Miyata , G. 2000 . The Neutraceutical Benefits, Part-III: Honey . Nutrition , 16 : 468 – 469 .
- White , J.W. Jr. and Kushnir , I. 1967 . The Enzymes of Honey: Examination by Ion Exchange Chromatography, Gel Filtration and Starch-gel Electrophoresis . J. Apicultural Res. , 6 : 69 – 89 .
- Bergner , K.-G. and Diemair , S. 1975 . Protein in Honey: II. Gel-chromatography, Enzyme Activity and Origin of Honey-proteins . Zeitschrift fur Lebensmittel-Untersuchung und –Forschung , 157 : 7 – 13 .
- Itoh , S. , Watanabe , A. , Terakawa , M. and Yoshioka , K. A Honey Processing Technology with UF Membrane . Proceedings of the International Congress on Membranes and Membrane Processes (ICOM-96) . pp. 867 Yokohama, , Japan : The Membrane Society of Japan .
- Itoh , S. , Terakawa , M. , Masaki , T. , Yamazaki , M. , Kokubo , K. and Watanabe , A. 2000 . Characterization of Honey Ultrafiltration Process . J. Jap. Soc. Food Sci. Technol. , 47 : 431 – 438 . (Nippon Shokuhin Kagaku Kogaku Kaishi)
- Barhate , R.S. , Subramanian , R. , Nandini , K.E. and Umesh Hebbar , H. 2003 . Processing of Honey using Polymeric Microfiltration and Ultrafiltration Membranes . J. Food Eng. , 60 : 49 – 54 .
- Little , B. , Gerchakov , L. and Udey , L. 1987 . A Method for Sterilization of Natural Seawater . J. Microbiol. Methods , 7 : 193 – 200 .
- van Oers , C.W. , Vorstman , M.A.G. and Kerkhof , P.J.A.M. 1995 . Solute Rejection in the Presence of a Deposited Layer During Ultrafiltration . J. Membr. Sci. , 107 : 173 – 192 .