Abstract
Fifty-one samples of Omani honey were analyzed for their contents of glucose, fructose, maltose, and sucrose. The Fructose/glucose ratio and its role in honey crystallization were also studied, as well as the protein content of the honey samples. This study has shown that the mean percentage of reducing sugars lie within the limits set by local Omani legislation. Fructose and glucose represented the largest portion. Maltose and sucrose levels were low 3.62 kg/100 kg and 1.29 kg/100 kg, respectively. Most honey samples did not crystallize after 18 months of storage. Protein content in all samples was low with an average of 2 kg/100 kg. Fructose, glucose, and sucrose levels were higher in summer honey samples (69.7 kg/100 kg) than the winter ones (60.6 kg/100 kg).
INTRODUCTION
Commercial beekeeping in Oman is relatively underdeveloped, resulting in low production of honey that is limited to local consumption and hardly keeps up with the demand leading to imported honey, some of which is sold as “local.” This growing demand indicates that honey production in Oman is likely to increase if potential honey producing areas are developed.[Citation1] Traditionally, Omani honey has been evaluated by sensory tests such as aroma, taste, colour, and texture, which often fail to detect the quality of the honey accurately unless melissopalynological studies are carried out. To date, negligible melissopalynological or biochemical studies have been published on the native Omani honey. Our current article focuses on the biochemical analysis of honey samples obtained from the Muscat and Batinah regions, which represent an important agricultural region and is the center of farming in Oman.[Citation2,Citation3] It is also one of the potential honey production regions.[Citation1] The aims of this study were; (i) Determining the biochemical properties of honey, such as colour, sugar profile, fructose/glucose ratio and protein content; (ii) comparing the characteristics of summer and winter, unifloral and multifloral honeys; and (iii) comparing the characteristics of Apis mellifera and Apis florea honeys.
In addition to pollen, the forage of honeybees consists of nectar, which is the basic raw material for honey and normally contains an aqueous solution of sugars that serve as source of energy for the honeybees during their routine work.[Citation4] Honey is a highly variable natural product, particularly in its sensory properties, water content, ash, pH value, and sugar composition. These depend highly on the types of flower nectar used by the bees, as well as climatic conditions. Chemically, honey comprises carbohydrates (70–80 kg/100 kg honey), water (17–20 kg/100 kg), and other substances such as organic acids, and vitamins.[Citation5] Sugars represent the largest portion of its composition (95–99 kg/100 kg of the honey solids). Fructose and glucose are the most abundant sugars found, but others are usually mentioned, namely sucrose and maltose, and so on.[Citation6] The colouring matters in honey include chlorophyll, xanthophylls, anthocyanin, tannin, carotene, and minerals. Dark honey contains more mineral salts than lighter ones. Sulphur and chlorine content in honey promote degree of pigmentation.[Citation7] Honey colour varies with botanical origin, age and storage condition, but its transparency or clarity depends on the amount of suspended material such as pollen.[Citation8] Relatively little attention has been given to honey protein for many years due to its low and variable amount. It is thought that protein in honey results from pollen, nectar and royal jelly contamination.[Citation8] Reports indicate that honey contains 4 to 7 proteins in addition to 17 free amino acids.[Citation9,Citation10] The main proteins in honey are invertase, diastase, acid phosphatase, catalase, and glucose oxidase.[Citation7,Citation11]
MATERIALS AND METHODS
Honey Sample Collection
Fifty-one samples of liquid honey from Apis mellifera or Apis florea colonies were obtained during 1999–2002; of these, 14 were from Muscat and 37 were from the Batinah region. These honeys are grouped as unifloral (32 samples) and multifloral honeys (19 samples). According to the season, they can also be grouped as 33 summer and 18 winter honey samples. Samples were marked H1–H51. The pressed honeys were collected from A. florea and traditional A. mellifera combs (locally known as tubl). While the extracted honeys were obtained from A. mellifera frame hives. Authenticated pure honey samples were obtained directly from honey Apiaries to ensure that the samples were not mixed with pollen stores in the comb. It was also ensured that beekeepers did not feed their bees with external pollen pellets. Honey samples were subjected to colour estimation, qualitative and quantitative pollen, and biochemical (sugars and protein) analysis; then classified into three categories according to their type (unifloral or multifloral), season (summer or winter), and collecting honeybee species (Apis mellifera or Apis florea).
Sugar Analysis in Honey
HPLC was used to identify and quantify the main sugar profile (fructose, glucose, sucrose and maltose) in honey samples, following standard methods.[Citation12,Citation13] Four sugars in the pure phase and HPLC grade (Sigma chemical Co.) were used as standards, viz., fructose, glucose sucrose, and maltose. For each sugar a standard 4.0 gl−1 solution was prepared using deionized water. Honey samples (0.25 g) were dissolved in 5 ml of deionized water. Three replicates were prepared for each honey sample. Samples were cleaned by passing them through a Sep-Pak plus®C18 cartridge (Waters Corporation) and a 0.2 μm filter (Satorius-Minister® NML). The cartridge was activated by methanol and rinsed with water before use. Two ml were used to wash the cartridge materials. The samples were freshly prepared and immediately analysed.
HPLC (Waters Corporation, USA), equipped with a Binary HPLC pump with an injector (Waters 2410), a refractive index detector (RI, Waters 2410), and a software monitor with Breeze program. The column used was LC-NH2 (SUPELCOIL™ LC- NH2, 250 × 4.6 mm, 5 μm, Supelco). The refractive index detector was used to monitor the column effluent. The temperature of the column and the refractometer was adjusted at 40°C. Acetonitrile solvent was HPLC grade from Sigma Chemical Co., USA. Several sugar standard solutions (20 μl) were injected with a glass syringe (50 μl, Waters Corporation) into the HPLC column several times at different mobile phase compositions and flow rates. The best separation was achieved with a mobile phase acetonitrile/water (85: 15) solvent system at a flow rate of 1.5 ml/min. Sugar standard was injected into HPLC prior to any honey sample (20 μl) injections. The sugar profile in the sample was identified and quantified by the software program Breeze (Waters Corporation, Milford, MA, USA) by using the amount and peak area of standards and peak area of the sugar sample, the amount of each sugar in a honey sample was estimated. The fructose/glucose ratio was calculated. The average amount of each sugar in all honey samples was obtained and compared with the Omani standards.
Proteins in the Honey
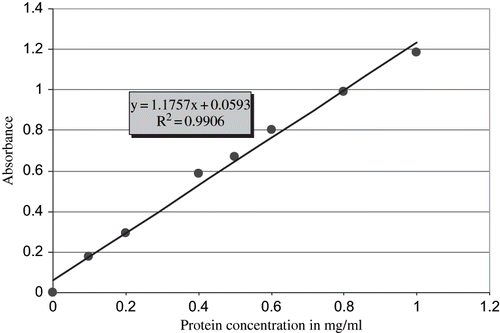
Proteins in honey samples were estimated using the Lowry (Folin-Ciocalteu) assay.[Citation14] This was carried out as follows: egg albumin stock solution (1.0 mgml−1) was used and dilutions of this solution were made. Eight concentrations were prepared and used to construct a standard curve for the estimation of proteins (). A total of 37 honey samples were analyzed. Each sample (0.2 g) dissolved in 10 ml of deionized water. Three replicates were prepared for each sample. Solutions were centrifuged (Eppendorf high speed 5402) at 4000 rpm for 5 min at 25°C to sediment the pollen and any undissolved material. 1 ml of the supernatant was used for the analysis of the protein content. Average content of protein in all honey samples was obtained and compared with the universal standards.
Statistical Analysis
Mean percentage, standard deviation (SD) and range were calculated. Analysis of variance (ANOVA) and t-test were carried out using MS Excel. Single factor ANOVA was performed to determine significant differences at 5% level between the amounts of individual sugars and the total content of sugars in each honey sample. Tukey test from SPSS 9.0 was also used to compare the quantity of sugars. Paired t-test was applied to determine the significant differences between fructose and glucose in each sample (P < 0.05).
RESULTS AND DISCUSSION
Sugars in Honey
Fructose, glucose, sucrose, and maltose are the most important sugars analyzed in the honey samples. They are related to those present in the raw material (nectar) foraged by bees to make honey in such a way that identification of source is possible.[Citation16] Four sugars were analysed by HPLC in 51 honey samples—these are fructose, glucose, sucrose, and maltose. Their peaks, retention times, areas and amounts are recorded on the chromatogram (). Mean sugar content with the corresponding standard deviation, fructose/glucose ratio (F/G) and the crystallized honeys (C) are shown in . The three reducing sugars fructose, glucose, and maltose were detected in all honey samples. Sucrose, however, was not detected in 11 samples (). These 4 sugars account for 67.41 kg/100 kg of the dry matter of the samples analysed. Fructose and glucose represented the largest portion of honey (62.5 kg/100 kg). Sucrose formed the lowest portion (1.29 kg/100 kg), while maltose was 3.62 kg/100 kg (). The mean percentage of reducing sugars was found to be 66.12 kg/100 kg, which complies with the honey requirements (65 kg/100 kg) of Codex Alimentarius & Standardization and Metrology Organization for GCC Countries as adopted by the Ministry of Commerce and Industry, Sultanate of Oman. Sucrose mean percentage was also within their limits of ≤10 kg/100 kg.[Citation15,Citation17,Citation18] Total sugar levels in the 51 honey samples were not significantly different.
Figure 2 HPLC chromatogram of four sugars in a honey sample. FRU (Fructose), GLU (Glucose), SUC (Sucrose), and MAL (Maltose).
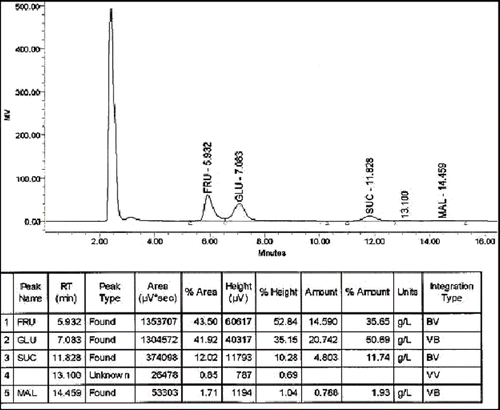
Table 1 Biochemical data of honey samples of their sugar composition, fructose/glucose (F/G) ratio, crystallization, and protein amount
Fresh nectar obtained from flower normally contains only fructose, glucose and sucrose. The proportion of fructose to glucose fluctuates considerably but there is always more fructose than glucose.[Citation4] The results of this study agree with these findings. Reducing sugars, mainly fructose and glucose, stand for the largest portion of honey composition, while sucrose content was the lowest in all honey samples. These results are in conformity with the standard requirements,[Citation17,Citation18] this indicates adequate honey processing, good maturity, energy value, and high viscosity of these samples.[Citation11,Citation19,Citation20,Citation21] Absence of significant difference between the honey samples from Muscat and Batinah may indicate similar bee sources in these regions. While Mateo and Bosch-Reig[Citation22] found significant differences among seven Spanish honey types, and thus were able to divide them to several groups. The sugar profile of honey depends on the type of plants in the geographical area and also climatic conditions.[Citation7]
Fructose
Fructose formed 32.89 kg/100 kg of the honey dry matter, but its levels were significantly different in the honey samples. This sugar had the highest average (49.13 kg/100 kg, P < 0.05) of the 4 sugars analysed. Its mean level varied from 19.40 kg/100 kg to 40.75 kg/100 kg. The lowest was in sample H15 and the highest in sample H38. This shows broad range with high variation (SD 4.98). Compared with glucose, fructose levels were significantly higher in all samples except for sample H2 () indicating the innate, purity, and the high quality of these honeys being levorotatory, comprise mainly of fructose in contrast to honeydew and some adulterated honeys, which are usually dextrorotatory, comprise mainly of glucose.[Citation7,Citation23] Fructose is responsible for most of the physical and nutritional characteristics of honey.[Citation8] At the temperature of the honeycombs (30°C), the solubility of glucose in a solution of fructose increases abruptly if fructose concentration is raised above 1.5 g per gram of water. Besides the fructose, a gram of water can hold 1.25 g of glucose. Glucose is then oxidized to gluconic acid and hydrogen peroxide by the enzyme glucose oxidase.[Citation11] These activities may explain the dominance of fructose in the honey. Mendes et al.[Citation24] found fructose to be the largest portion of 50 Portuguese honey samples evaluated. Golob and Plestenjak[Citation5] found that the differences in fructose and glucose mass fraction among different types of honey are significant. Similar result was found in the present study.
The mean value of fructose (32.94 kg/100 kg) is within the range of Mohamed et al.,[Citation25] and Mendes et al.,[Citation24] for Libyan and Portuguese honey, respectively. However, Gomez Barez et al.[Citation26] reported higher mean value (36 kg/100 kg) of fructose of some honey samples. The current maximum value of fructose (40.75 kg/100 kg) is similar to maximum values of (40.1 kg/100 kg and 40.6 kg/100 kg) in two honey samples from Spain evaluated by Mateo and Bosch-Reig.[Citation22] Minimum percentage value (19.40 kg/100 kg) was not reported by other investigators. This value is too low when compared with other studies; this may be due to the nature of the nectar of arid countries, which is highly dependent on weather conditions.[Citation26] Fructose range was widely distributed, indicating the variety of floral sources from which the honey samples originated.
Glucose
Glucose is the second important sugar in honey after fructose. In most cases its levels were more or less the same or lower than that of fructose. Glucose formed 29.61 kg/100 kg of the honey dry matter () and represented 44 kg/100 kg of the total sugars analysed in the honey samples. Its levels were significantly different in honey samples (SD 5.87) ranging from 17.14 kg/100 kg–44.77 kg/100 kg (). Sample H15 contained lowest amount and sample H2 contained highest amount of glucose (). Although glucose amount appears to be close to that of fructose, it was significantly lower. Its inverse occurrence in sample H2 may be due to the sugar composition of the nectars involved in honey production.[Citation10,Citation7] Horvath and Molnar-Perl.[Citation28] found 37.5 kg/100 kg of the samples had higher glucose content than fructose from a total of eight honey samples studied. The glucose mean value (29.8 kg/100 kg) of the present study matches with the results reported by Golob and Plestenjak.[Citation5] of 29.4 kg/100 kg and Gomez Barez et al.,[Citation26] of 29.2 kg/100 kg for Slovene and Spanish honeys, respectively. However, the latter authors reported lower value of glucose of 25.6 kg/100 kg in another study and Mohamed et al.[Citation25] reported 25 kg/100 kg for glucose in Libyan honey. The range of glucose in this study was broad and comparable with the range values[Citation11] for Russia, USA, Romania, and Australia (20.4 kg/100 kg–44.4 kg/100 kg).
Sucrose
Sucrose was not detectable in eleven of the 51 honey samples studied. Other samples contained significantly small amount of sucrose in their dry matter with average amount of 1.29 kg/100 kg. This is the lowest percentage (1.24 kg/100 kg) in the total sugars analysed (). The distribution of sucrose levels in the honey samples studied was broad, ranging from 0 to 12.29 kg/100 kg in sample H30 (). Sucrose content was the lowest in the 4 sugars studied in honey samples. This is as a result of the enzyme invertase action, which breaks down the disaccharide molecule of sucrose in the nectar into the monosaccharides, glucose and fructose during the process of ripening of honey; this is because sucrose is not highly soluble in honey's water.[Citation11,Citation29,Citation30] Although this sugar has minor importance, its presence can provide information about adulteration and botanical origin of the honey.[Citation8] The low amount of sucrose in most samples could indicate the unadulterated state of the samples.[Citation4] The mean percentage of sucrose in the present study is 1.3 kg/100 kg, which is similar to that found by Perez-Arquillue et al.,[Citation23] and Golob and Plestenjak,[Citation5] in Spanish and Slovakian honeys, respectively, but it is lower than the 3.3 kg/100 kg and 3.0 kg/100 kg sucrose reported by Abu-Tarboush et al.,[Citation30] and Yilmaz and Yavuz,[Citation32] in Saudi and Turkish honeys, respectively. Perez-Arquillue et al.[Citation23] reported sucrose content below 1 kg/100 kg in several Spanish honey samples of different botanical origins. Absence of sucrose in 11 samples may be due to these honeys being obtained from nectars, which contain just fructose and glucose as a result of total conversion of sucrose into these monosaccharides before their secretion nectars.[Citation10,Citation33] White et al.[Citation34] and Agwu et al.[Citation4] detected only fructose and glucose in some of their analysed honey samples from Indonesia and Nigeria, respectively. They referred this to the nectar quality.
The broad range of sucrose (0 to 12.3 kg/100 kg) and its relatively high amounts in samples H26, H36, H37, and H47 from 5.15 kg/100 kg to 7.5 kg/100 kg could be due to a sucrose-rich nectar[Citation15,Citation33] or to feeding of bees with concentrated sucrose solution in the apiaries. This was reported by the beekeepers who supplied some samples for the present investigation. Feeding with high concentration of sugar diverts the bee foragers from collecting nectar and causes them to collect pollen instead.[Citation11] Aparna and Rajalakshmi from India[Citation7] and Golob and Plestenjak from Slovakia[Citation5] reported that the maximum content of sucrose in some honeys could reach 8 kg/100 kg and 10.1 kg/100 kg, respectively. Although sucrose content is important to detect heavy sugar feeding of the bees or adulteration by direct addition of sucrose,[Citation24] this relatively high amount of sucrose does not signify the adulteration because direct adulteration with sucrose may give a value of 30 kg/100 kg or more.[Citation11] The high level of sucrose (12.29 kg/100 kg) in sample H30 may be because of unripe honey sample as it was collected from uncapped honeycomb.[Citation4,Citation11] Similar results have been reported[Citation16] and were attributed to the high moisture content of honey associated with high values of sucrose. Further, this sample may have been collected during the heavy nectar flow period (February), during which the honeybees convert the nectar into honey very quickly with less of the enzyme action that inverts sucrose to fructose and glucose.[Citation35]
Maltose
Maltose formed 3.62 kg/100 kg of the honey dry matter and represented 5.40 kg/100 kg of the total sugar content of the honey samples (). It was detected in all honey samples in significantly smaller amounts in comparison with fructose and glucose. Significant differences were found in maltose levels in different samples. Highest maltose level (7.19 kg/100 kg) was found in sample H3 and lowest level (1.51 kg/100 kg) was in sample H28 (). The low level of maltose in honey may be due to its low content in its origin.[Citation36] Low et al.[Citation37,Citation38] reported that maltose found in honey does not originate in the nectar but arise from of transglycosylation activity of α- and β-glucosidases enzymes contributed by honeybees during the honey process. Although maltose is quantitatively of minor importance, its presence can provide information about the botanical origin of honey.[Citation8] Few studies carried out on the analysis of maltose in honey and no specific standards were found for this sugar because it is one of the rare sugars in nectar.[Citation11] This sugar was found in every sample of the present study, whereas Agwu et al.,[Citation4] reported its absence in some Nigerian honeys. Horvath and Molnar-Perl[Citation28] reported 2 values of maltose in 2 samples of Hungarian mixed flower honeys, which agree with the mean value (3.72 kg/100 kg) of this sugar in the present results. Gomez Barez et al.[Citation26] reported lower mean value of 1.65 kg/100 kg of maltose in Spanish honeys, whereas Perez-Arquillue et al.[Citation23] reported higher mean value of maltose of 7.2 kg/100 kg. Maltose range in this study agrees with its range (2.7 kg/100 kg–16 kg/100 kg) in USA honeys.[Citation11] It is also not far from the range (0.9 kg/100 kg–6.2 kg/100 kg) for Spanish honey as reported by Mateo and Bosch-Reig's.[Citation16]
Fructose to Glucose (F/G) Ratio and Crystallization
Fructose to glucose ratio (F/G) represents an indication of the ability of honey to crystallize. Honey sample H2 with low F/G ratio (0.90) crystallized after its collection and storage for three months at room temperature. shows that eleven honeys were crystallized; their F/G ratio ranged between 0.90–1.17. The other 40 samples did not show crystallization even after eighteen months of collection. The highest F/G ratio was observed in sample H11 of 1.59 and the lowest was observed in sample H2 of 0.90 (). The ratio of fructose/glucose is used to typify honey samples from different origins; moreover it indicates the tendency of crystallization; a high or low ratio signifies liquid or crystallized honey, respectively.[Citation31] Crystallization of sample H2 appears to be due to excess glucose over fructose because glucose is a relatively insoluble sugar in honey, and its amount largely determines the tendency of the honey to crystallize or to remain liquid.[Citation11] Similar results were found with Litchi honeys analyzed by Suryanarayana et al.[Citation39] In other crystallized samples both amounts were close to each other, which explains the fast crystallization. Forty samples did not show crystallization, even though some of their F/G ratio was approximately 1. This may due to their high viscosity, which slows down the rate of crystallization by reducing the rate at which molecules of sugar migrate through the fluid to be deposited upon the growing crystals.[Citation10,Citation20,Citation21,47] The viscosity of honey can be examined by its texture. It is expected that such honeys (e.g., H9 and H11) with high F/G ratio may not crystallize even during prolonged storage.[Citation10,Citation39,Citation21,47] The wide range of F/G ratio (0.90 kg/100 kg–1.59 kg/100 kg) in this study may be indicative of the variety of floral sources from which the honey samples originated. Mateo and Bosch-Reig[Citation22] obtained a similar range of F/G ratio for Spanish honey samples (0.99 kg/100 kg–1.40 kg/100 kg). While Perez-Arquillue et al.[Citation23] reported narrower range (1.06 kg/100 kg-1.13 kg/100 kg) rosemary honey, which displayed a remarkable low variation among unifloral samples.
Unifloral and Multifloral Honeys
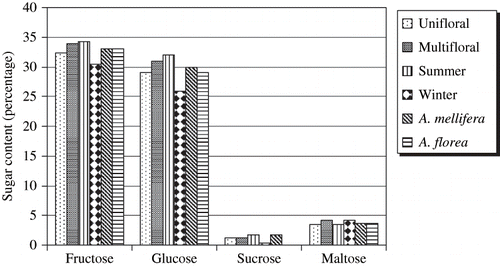
When sugars are compared between unifloral and multifloral honeys, no significant difference was found between the average content of the four sugars of 32 unifloral and 19 multifloral honeys. The mean percentage of reducing sugars in multifloral honeys (69.05 kg/100 kg) was higher than the unifloral honeys (64.86 kg/100 kg). While sucrose amount was approximately equal in both honey types (). The quantitative data of both honeys are shown in . No significant difference between the sugars of both unifloral and multifloral honeys may indicate similar concentrations of sugars in the composition of nectars from Muscat and Batinah plants. Perhaps this is because of similar factors affecting the quality of the nectar such as humidity, temperature, solar energy, and rainfall.[Citation11,Citation40] Horvath and Molnar-Perl[Citation28] found that multifloral honeys contain significantly higher amounts of monosaccharides than those of unifloral honeys and no significant differences between sucrose and maltose levels in both types of honeys. This shows that the composition of the honey depends highly on the types of flowers used by the bees, as well as regional and climatic conditions.[Citation6]
Summer and Winter Honeys
shows the comparison between the mean values of the four sugars that were analysed in 33 summer honeys and 18 winter honeys. Fructose levels as mean percentages were significantly different in both honey types. This was also found with the mean percentages of each of glucose and sucrose. All the sugars were higher in summer honeys, while maltose was lower in summer than in winter honeys (), but it was not significantly different in both types of honey. The mean percentage of reducing sugars in summer honeys was of 69.68 kg/100 kg, while it was 60.63 kg/100 kg in winter honeys. The sucrose mean percentage of summer honeys was of 1.76 kg/100 kg and it was of 0.42 kg/100 kg in winter honeys ().
Higher level of fructose, glucose and sucrose in summer honeys indicate the rich blooming flora in Muscat and Batinah during spring after the rainy season in January and February, while the lower level of these sugars in winter honeys may be due to the restricted number of bee plants that bloom in autumn[Citation41,Citation42] and the dearth of pollen and nectar in June-August,[Citation43] the period of winter honey formation. At that time the forage of bees is of fairly poor quality, and the nectar is low in sugar content due to absorption of water at high humidity. Also at high temperatures, water stress can occur in plants reducing the transport of sugar through the phloem to the nectarines and, more importantly, its photosynthesis is affected, thereby nectar secretion is reduced.[Citation11,Citation44] Although, the clovers and crucifers are blooming at this time having higher sugar concentration in their nectars,[Citation10] but they are not distributed in most forage areas of Muscat and Batinah. Absence of significant differences between the amount of maltose in the summer and winter honeys may be due to the fact that this sugar is not affected by the climatic condition due to its origin from the action of transglycosylation enzymes produced by honeybees. It does not form in the nectar.[Citation38]
Apis Mellifera and Apis Florea Honeys
and compare sugar content of 39 A. mellifera honey samples with 12 A. florea honey samples. Reducing sugars and sucrose in both honey types are within the limits set by the legislation.[Citation15] The mean and range of fructose, glucose, and maltose percentage were close to each other in honeys collected from both bee species. The mean value for sucrose was significantly lower in A. florea honeys. Also, the range of sucrose was narrower in Apis florea honeys than A. mellifera honeys. Similar means and ranges of reducing sugars in both types of honeys indicate their similar floral origin. This is shown in Whitcombe's[Citation40] investigation in Khabura, who found that both bee species were exploiting the same source of nectar of Acacia tortilis and Prosopis cineraria almost exclusively. It has been established that there are common “nectar plants,” which are visited by both bee species.[Citation45] However, the higher mean of sucrose in A. mellifera honeys compared to A. florea honeys may be due to heavy sucrose feeding of the A. mellifera bees. Omani consumers seem to prefer A. florea honey mainly because of its low content of sucrose. They also believe that this honey is naturally produced without any addition of sugar by traders and without being exposed to pesticides. It was observed that Apis florea honey did not crystallize.[Citation43] In this study, the range of fructose was close to each other in both honey types. This trend was also found for glucose and maltose. Higher levels of fructose and glucose for both types of honeys were also reported,[Citation11] so is the case for sucrose range in A. florea honeys (0–2 kg/100 kg) but a higher range of sucrose in A. mellifera honeys.[Citation11] The present mean content (62.5 kg/100 kg) of fructose + glucose of A. mellifera honeys fall within the range determined by Vit et al.[Citation46] for honey samples from Venezuela where fructose and glucose account for 58 kg/100 kg–95 kg/100 kg.
Different factors such as mineral contents and protein amounts affect the colour of honey. One of the factors that make honey dark is related to a high content of amino acids (especially tyrosine and tryptophan), although not entirely.[Citation8,Citation11] This may explain the significant variation of the protein amounts in the six different coloured honey samples of this study.
Proteins in Honey
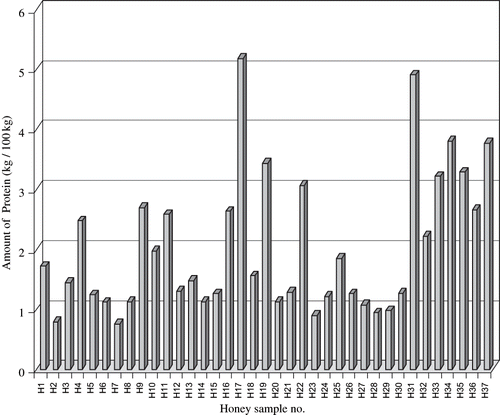
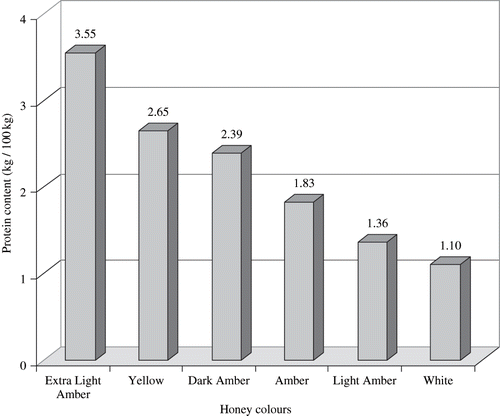
Protein content was highly variable in the 37 samples studied; the average protein content was about 2 kg/100 kg ± 1 kg/100 kg. The maximum content was recorded in honey sample H17 (5.18 kg/100 kg) and the minimum was recorded in honey sample H7 (0.75 kg/100 kg). Fourteen honey samples contained >2 kg/100 kg protein, while the majority, 23 samples, contained <2 kg/100 kg protein ( and ). All standard colours were found in these 37 honey samples except extra white. Samples were found to be significantly different in their protein content. shows that the highest mean of protein (3.55 kg/100 kg) was found in extra light amber honeys and lowest mean (1.10 kg/100 kg) was found in water white honey. The dark amber honeys contain moderate protein (2.39 kg/100 kg). No significant differences were found between proteins in unifloral and multifloral honeys as well as between summer and winter honeys and Apis mellifera and Apis florea honeys. The protein concentration ranges in each of these groups were in close proximity to each other ().
The present study recorded very low mean amount (2 kg/100 kg) of protein in comparison with the sugars mean amount (67.41 kg/100 kg) in the honey samples. Although the protein concentration of the majority of the honey samples was <2 kg/100 kg, the range of protein, which was (0.75 kg/100 kg–5.18 kg/100 kg) is broad in comparison with most studies that reported protein range (0.06 kg/100 kg–2 kg/100 kg), where the norm is around 0.2 kg/100 kg.[Citation8,Citation11] This may be due to certain conditions such as the floral preference of the honeybee, from which the proteins and colloids are derived. Honeys with unusual amount of protein are thixotropic because these high molecular weight proteins give the honeys a viscosity much above normal.[Citation11] Ziziphus spina-christi and Prosopis juliflora honeys showed high amount of protein, may be because these plants produce large amounts of pollen and nectar, which as contaminants, are thought to supply the honeys with protein. Similar amount of protein in most honey samples, in spite of their types, seasons and producer bees, possibly refer to the analogous forage plant flora.[Citation11]
CONCLUSION
In Oman, honey production needs to be elaborated and quality controlled to recognize adulterated or non-Omani samples. There is a need to establish geographical limits of production with the aim to protect production zones. Excess amounts of glucose cause honey to crystallize, other factors including temperature and storage conditions can also accelerate crystallization. There was a wide range of F/G ratio suggesting perhaps a variety of floral sources. Omani honey shows low protein content of about 2 kg/100 kg.
Notes
2. Anonymous. Geography of Oman. Ministry of Information. 1995. Oman 1995. Sultanate of Oman, Muscat.
3. Anonymous. Geography of Oman. Ministry of Information. 2000. Oman 2000. Sultanate of Oman, Muscat.
15. Anonymous. Omani Standard, Methods of Test for Honey, No. 49; Ministry of Commerce and Industry. Directorate General for specifications and measurements: Muscat, Sultanate of Oman, 1984.
18. Anonymous. Standardization & Metrology Organization for G. C. C. Countries. Honeybee honey, No. 243; Bahrain, 1993.
45. Sajwani. A.M.; Farook, S.A.; Eltayeb, E.A.; Patzelt, A. (Unpublished results, submitted to Palynology) In Press, 31, 2007.
REFERENCES
- Anderson , D. 1990 . “ A consultancy on Beekeeping in the Sultanate of Oman. Report commissioned by FAO and UN from 25/10/1989 to 8/11/1989 ” . In Black Mountain Laboratories. CSIRO Collingwood, , Australia
- 2. Anonymous. Geography of Oman. Ministry of Information. 1995. Oman 1995. Sultanate of Oman, Muscat.
- 3. Anonymous. Geography of Oman. Ministry of Information. 2000. Oman 2000. Sultanate of Oman, Muscat.
- Agwu , C. , Obuekwe , A. and Iwu , M. 1989 . Pollen Analytical and Thin-Layer Chromatographic Examination of Nsukka (Nigeria) Honey. Pollen Et Spores. , 31 : 29 – 43 .
- Golob , T. and Plestenjak , A. 1999 . Quality of Slovene Honey . Food Technology Biotechnology , 37 : 197 – 201 .
- LaGrange , V. and Sanders , W. 1988 . Honey in Cereal-based New Food Products . Cereal Food World. , 33 : 833 – 838 .
- Aparna , A. and Rajalakshmi , D. 1999 . Honey-its Characteristics, Sensory Aspects, and Application . Food Review International. , 4 : 455 – 471 .
- Krell , R. 1996 . Value-added Products from Beekeeping , Rome, , Italy : FAO .
- White , J. Jr. and Rudyj , O. 1978 . The Protein Content of Honey . Journal of Apicultural Research , 17 : 234 – 238 .
- Hooper , T. 1983 . Guide to Bees and Honey , London : Blandford, Press .
- Crane , E. 1990 . Bees and Beekeeping Science, Practice and World Resources , London : Heinemann Newness .
- Bogdanov , S. and Baumann , E. 1988 . Determination of Honey Sugars with HPLC . Mitteilungen aus dem Gebiete der Lebensmitteluntersuchung und Hygiene , 79 : 198 – 206 .
- Myhara , R. , Shahidi , F. and Naczk , M. 1990 . Effect of Processing on the Soluble Sugars of Brassica Seeds . Journal of Food Science , 55 : 5 – 10 .
- Boyer , R. 1986 . Modern Experimental Biochemistry , San Francisco, CA : The Benjamin/Cummings Publishing Company, Inc .
- 15. Anonymous. Omani Standard, Methods of Test for Honey, No. 49; Ministry of Commerce and Industry. Directorate General for specifications and measurements: Muscat, Sultanate of Oman, 1984.
- Mateo , R. and Bosch-Reig , F. 1997 . Sugar Profiles of Spanish Unifloral Honeys . Food Chemistry , 60 : 33 – 41 .
- Anonymous . 1984 . “ Codex Alimentarius ” . In Codex Standard for Honey , 11 Rome, , Italy : FAO .
- 18. Anonymous. Standardization & Metrology Organization for G. C. C. Countries. Honeybee honey, No. 243; Bahrain, 1993.
- Mora , M. and Marioli , J. 2001 . Honey Carbohydrate Analysis by HPLC, with Electrochemical Detection, Using a Ni-cralloy Electrode . J. Liq. Chrom & Rel. Technol. , 24 : 711 – 720 .
- Conforti , P.A. , Lupano , C.E. , Malacalza , N.H. , Arias , V. and Castells , C.B. 2006 . Crystallization of Honey at −20°C. . International Journal of Food Properties , 9 ( 1 ) : 99 – 107 .
- Mossel , B. , Bhandari , B. , D'Arcy , B. and Caffin , N. Determination of Viscosity of Some Australian Honeys Based on Composition . International Journal of Food Properties 2003 , 6 ( 1 ) 87 – 97 .
- Mateo , R. and Bosch-Reig , F. 1998 . Classification of Spanish Unifloral Honeys by Discriminant Analysis of Electrical Conductivity. Colour, water content, sugars and pH . Journal of Agriculture & Food Chemistry. , 46 : 393 – 400 .
- Perez-Arqillue , C. , Conchello , P. , Arino , A. , Juan , T. and Herrera , A. 1994 . Quality Evaluation of Spanish Rosemary (Rosmarinus Officinalis) Honey . Food Chemistry , 51 : 207 – 210 .
- Mendes , E. , Brojo Proenca , E. , Ferreira , I. and Ferreira , M. 1998 . Quality Evaluation of Portuguese Honey . Carbohydrate Polymers. , 37 : 219 – 223 .
- Mohamed , A. , Ahmed , A. and Mazid , M. 1982 . Studies on Libyan Honeys . Journal of Food Quality , 4 : 182 – 201 .
- Gomez Barez , J. , Garcia-Villanova , R. , Elvira Garcia , S. , Rivas Pala , T. , Gonzalez Paramas , A. and Sanchez Sanchez , J. 2000 . Geographical Discrimination of Honeys Through the Employment of Sugar Patterns and Common Chemical Quality Parameters . Europe Food Research & Technology , 210 : 437 – 444 .
- Morse , R. 1986 . The Complete Guide to Beekeeping , New York : E.P. Dutton .
- Horvath , K. and Molnar-Perl , I. 1997 . Simultaneous Quantitation of Mono-, Di-, and Trisaccharides by GC-MS of their TMS ether Oxime Derivative: II . In Honey. Chromatographia , 45 : 328 – 335 .
- Harborne , B. 1984 . Phytochemical Methods , London : Chapmen & Hall .
- Trump , R. 1987 . Bees and Their Keepers , Iowa : Iowa State University Press .
- Abu-Tarboush , H. , Al-Kahtani , H. and El-Sarrage , M. 1993 . Floral-type Identification and Quality Evaluation of Some Honey Types . Food Chemistry , 46 : 13 – 17 .
- Yilmaz , H. and Yavuz , O. 1999 . Content of Some Trace Metals in Honey from South Eastern Anatolia . Food Chemistry , 65 : 475 – 476 .
- Crane , E. 1980 . A Book of Honey , Oxford : Oxford University Press .
- White , W. , Plat , L. , Alen-Wardell , G. and Allen-Wardell , C. 1988 . Quality Control for Honey Enterprises in Less-developed Areas: An Indonesian Example . Bee World , 69 : 49 – 62 .
- Anonymous . 1998 . Technical Report of the Beekeeping in Sultanate of Oman, League of Arab States , Khartoum, Sudan : Arab Organization for Agricultural Development .
- Roubik , D. 1995 . Pollination of Cultivated Plants in the Tropics , Rome : Food and Agriculture Organization of the United Nations .
- Low , H. , Vong , K. and Sporns , P. 1986 . A New Enzyme, β-Glucosidase, in Honey . Journal of Apicultural Research. , 25 : 178 – 181 .
- Low , H. , Nelson , L. and Sporns , P. 1988 . Carbohydrate Analysis of Western Canadian Honeys and Their Nectar Sources to Determine the Origin of Honey Oligosaccharides . Journal of Apicultural Research. , 27 : 245 – 251 .
- Suryanarayana , M. , Seethalakshmi , T. and Phadke , P. 1981 . In Pollen Analysis of Indian Honeys—1. Honeys from Litchi (Nephelium litchi) and Jamun (Syzygium Cumini) . Proceedings of 4th International Palynology Conference . 1981 , Lucknow, India. Vol. 3 , pp. 491 – 498 .
- Whitcombe , R. 1984 . In Aspects of the Biology and Management of Apis Florea in Oman . Proceedings of 3rd International Conference on Apiculture of Tropical Climates . 1984 , Nairobi, Kenya. pp. 96 – 103 .
- Ghazanfar , S. 1992 . An Annotated Catalogue of the Vascular Plants of Oman and Their Vernacular Names , Meise, Belgium : National Botanic Garden of Belgium .
- Ghazanfar , S. 1997 . Trees of Oman, an Illustrated Guide to the Native Trees of Oman , Muscat, Oman : Ministry of Regional Municipalities and Environment .
- Hussein , M. 1991 . Beekeeping in Sultanate of Oman , Oman : Ministry of Agriculture and Fisheries Resources .
- Howes , F. 1979 . Plants and Beekeeping , London : Faber and Faber Limited .
- 45. Sajwani. A.M.; Farook, S.A.; Eltayeb, E.A.; Patzelt, A. (Unpublished results, submitted to Palynology) In Press, 31, 2007.
- Vit , P. , Fernandez-Maeso , M. and Ortiz-Valbuena , A. 1998 . Potential Use of the Three Frequently Occurring Sugars in Honey to Predict Stingless Bee Entomological Origin . Journal of Applied Entomology , 122 : 5 – 8 .