Abstract
Eight different combinations of pretreatments and storage methods (freezing rate, maltose treatment and storage temperature) were applied to frozen kiwifruit slices, and the effect of these treatments on the qualities (color, water content, chlorophyll content, ascorbic acid content and cell structure) were evaluated. Color, water content, chlorophyll and ascorbic acid contents of frozen kiwifruit stored below the glass transition temperature (Tg′) were higher than those of kiwifruit slices stored above the glass transition temperature, and the cell structure of the former were more integrated than that of the later. The effects of freezing rate and sugar treatment on the qualities of frozen kiwifruit slices showed diversity according to the experimental factors and index.
INTRODUCTION
Kiwifruit can be considered as a highly nutritional product due to its high level of vitamin C and a wide number of phytonutrients including carotenoids, lutein, phenolics, flavonoids and chlorophyll.[Citation1] The main goal of the kiwifruit processing is to obtain safe and stable products able to retain the uniqueness of the fresh fruit as much as possible, in addition to retention of its green color, aroma, nutritional value and structural characteristics.[Citation2] However, these quality characteristics of kiwifruit products are liable to deterioratation during the course of necessary processing and storage. Chlorophylls, the major natural pigments in kiwifruit, may convert to pheophytins and pyropheophytins, causing the disappearance of green color.[Citation3] Ascorbic acid loss and cell wall destruction also occurs during the fruit processing and storage.[Citation4]
As one of the special type of frozen storages, storage at semi-crystalline glassy state condition is a new technology developed at the end of last century.[Citation5] A frozen food is composed of crystalline water and unfrozen water concentrated in solutes. The unfrozen water is in glassy state if cooled below its glass transition temperature (Tg′). The stability of the product depends on the status of this highly concentrated unfrozen fraction.[Citation6] The theory of “food polymer science” focuses on food glassy state and glass transition temperature, creating a new way for food science research.[Citation7,Citation8] The glass transition temperature (Tg) is a very important physical parameter in food materials, which refers to the maximally freeze-concentrated solids (T′g) in the case of frozen foods.[Citation9] Under T′g, foods exist in a crystalline-glassy mixture. The rates of all diffusion-limited chemical reactions causing food deterioration are slow down or do not take place below Tg′, thus the qualities (color, structure, nutrition, etc.) of frozen foods can be maximally retained. Most high-moisture content foods have a low glass transition temperature (Tg′), therefore some sugars (e.g., maltose) are often added to raise the Tg.[Citation10,Citation11] This helps to prolong the stability of the foods at a given storage temperature.
Many factors during processing and storage can affect the qualities of frozen kiwifruit. There are some studies being reported in many other frozen fruits and vegetables while none is found in the case of kiwi fruit. The objective of this study was to determine the effects of sugar pretreatment and storage conditions (above or below Tg′) on the quality of frozen kiwifruit slices.
MATERIALS AND METHODS
Raw Material
Kiwifruits (cv. A.deliciosa) were purchased in a local market. The fruit flesh was visually green and the soluble solid content was 11.3%(w/w).
Determination of Glass Transition Temperature
Glass transition temperature of kiwifruit (Tg′) was determined following the method described by Bell et al.[Citation12] using a differential scanning calorimeter (DSC) (TA-50H, Shimadzu, Kyoto, Japan). The program for running DSC was as follows: 1) sample was cooled at the rate of 20°C/ min from 30.00°C to −40.00°C; 2) then heated at the heating rate of 1°C /min from −40°C to −5°C; 3) cooled at 10°C/min from −5°C to −35°C; (4) kept at −35°C for 10 min; 5) cooled at 5°C /min from −35°C to −40.00°C; and 6) heated at the rate of 3°C /min from −40°C to 30°C.
The Storage Methods for Frozen Kiwifruit Slices
In order to evaluate the effects of storage at glassy state condition, 8 different treatment methods were designed by changing the freezing rate (slow freezing and quick freezing using liquid nitrogen), storage temperature (above and below Tg′) and pretreatments (maltose vacuum infusion and no infusion)in this study () as reported by Liu et al.[Citation13,Citation14] for strawberries.
Table 1 Storage methods for frozen kiwifruit
Frozen Storage of Kiwifruit Slices
The process flow implemented was: fresh kiwifruit→peeling, coring, and slicing→blanching→ sugar pretreatment (yes/no) →cooling→ packaging→freezing→frozen storage. Key points applied in the process were 1) peeling and slicing: the fruits were sliced 1cm thick and peeled, the center white core was removed; 2) blanching: samples were blanched in boiling water for 15; 3) maltose vacuum infusion: samples S5, S6, S7, S8 () were infused in 65% (w/w) maltose aqueous solution for 30 min under vacuum condition until soluble solid content of kiwifruit slices achieved approximately to 20%; 4) freezing: samples S1, S2, S5, S6 () were frozen slowly by putting samples into a −20°C refrigerator (Haier Co.,Ltd, Qingdao, China) and letting the temperature decrease naturally. The samples S3, S4, S7, S8 were frozen quickly by immersing in liquid nitrogen, followed by freezing in a −20°C refrigerator; and (5) frozen storage: samples S1, S3, S5, S7 () were stored frozen at −20°C refrigerator and four other samples were stored frozen at another −40°C refrigerator (Haier Co.,Ltd, Qingdao, China).
Determination of Chlorophyll a and b
Chlorophylls a and b were determined simultaneously by using the double-wavelength spectrophotometric method.[Citation15] Briefly, fresh kiwifruit was washed, peeled and chopped. Five grams of chopped kiwifruit were weighed into a mortar, and then a small amount of calcium carbonate and 80% (v/v) acetone were added. The mixture was ground into puree in the mortar and filtered through absorbent cotton. Residues were washed by the 80% (v/v) acetone and filtered again. The filtrate was combined in a 100 mL volumetric flask and made up to the volume by the 80% (v/v) acetone. The absorbance at 663 (A663) and 645 nm (A645) were measured using a 752 model UV-visible spectrophotometer (Precise Scientific Instrument Co., Ltd. Shanghai, China). The concentrations were calculated by the following equations:[Citation15]
where Ca and Cb are the concentration (mg/L) of chlorophyll a and b respectively. Finally, chlorophyll a and b contents of kiwifruit samples were calculated from following equations:
where 0.1 represents 0.1 L extract of chlorophyll, m is the kiwifruit sample weight (g).
Determination of Vitamin C
The vitamin C content was determined according to the 2, 6-dichlorophenol indophenol method.[Citation16]
Determination of Colour
For the most important color of frozen kiwifruit which should been detected during storage was green, so only Hunter a values were determined by using a WSC-S color difference meter (Precise Scientific Instrument Co., Ltd. Shanghai, China)[Citation17] to evaluate the colour quality of the frozen kiwifruit slices.
Observation the Cell Structure by Light Microscopy
Cell structure of frozen kiwifruit slices was observed with the following procedures:[Citation18,Citation19] 1) frozen kiwifruit slices were fixed in FAA (formalin-acetic acid-alcohol) solution for at least 24, and then dehydrated in a graded series of ethanol solutions (30%, 50%, 70%, 90%, and 100%) for 2 hours each and gradationally cleared in ethanol:dimethylbenzene (1:1) solution and 100% dimethylbenzene solutions, and then, the slices were embedded in paraffin; 2) sections were cut about 10 µm thick on a rotary microtome (Yidi medical facilities factory, Jinhua, China); 3) sections were deparaffined in dimethylbenzene solution, and then rehydrated through graded concentration of ethanol; and 4) all sections were stained with hematoxylin solution for about 1∼3 hours, then observed and photographed with a XSP-8C light microscope (Precise Scientific Instrument Co., Ltd. Shanghai, China).
Statistical Analysis
Data analyses were determined using the SPSS, and analyses of variance were conducted by ANOVA procedure. Mean values were considered significantly different when P ≤ 0.05.
RESULTS AND DISCUSSION
Determination of Glass Transition Temperature of Kiwifruit Slices
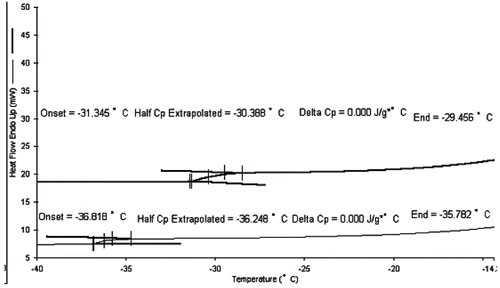
The key to the investigation of retention of quality parameters of kiwifruit in glassy state during storage lies in the determination of its glass transition temperature. shows the DSC curves of maltose-vacuum infused (top curve) and no maltose-infused (bottom curve) kiwifruit slices. The glass transition temperature (Tg′) of vacuum-maltose infused kiwifruit slice was −31.3°C, while that of no maltose-infused kiwifruit slice was −36.8°C. Therefore, maltose vacuum infusion could increase the Tg′ of kiwifruit slices, thus increase their storage stability. It is possible to raise the Tg′ of kiwifruit slices by addition of more maltose. However, when the concentrations of maltose aqueous solution used for pretreatment agent increased from 65% to 80% (w/w), the Tg′ of kiwifruit slices only increased from −31.3°C to −29.6°C (data not shown), which implied that the increase of maltose concentration is not linearly influence the increase of Tg′. According to the Tg′ of kiwifruit determined, −40°C (below Tg′) and −20°C (above Tg′) were chosen as the storage temperatures.
Effect of Storage Methods on the Hunter a Values of Frozen Kiwifruit Slices
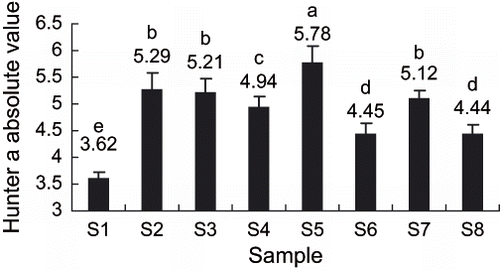
Hunter a value is an important index of kiwifruit sensory quality. The negative a value indicate green colour, and the absolute value is bigger (in negative), the color is greener. showed the effect of different treatment methods on Hunter a absolute values of kiwifruit slices. The Hunter a absolute value of sample S5 (slow freezing, storage temperature > Tg′, maltose vacuum infusion) was the highest, implied that the color of sample S5 was the greenest; while hunter a absolute value of sample S1 (slow freezing, storage temperature > Tg′, no maltose infusion) was the least, implied that the color change of sample S1 was the largest. As shown in , there were significant differences among different treatment, and the results showed that addition of maltose could retain the kiwifruit color quality comparatively better.
Effect of Storage Methods on the Water Contents of Frozen Kiwifruit Slices
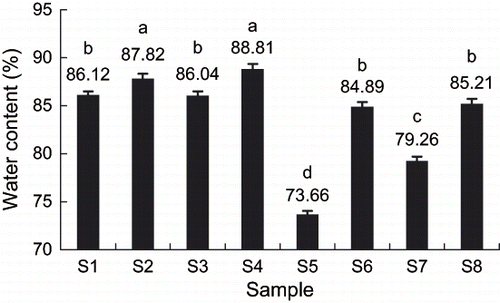
Water content is also an important physicochemical index of frozen kiwifruit slices during storage. showed the effect of storage methods on water content of frozen kiwifruit slices. During storage process of frozen kiwifruit, the main reason for water loss is the sublimation of ice crystals on the surface of kiwifruit. Sample S4 (quick freezing, storage temperature < Tg′, no maltose infusion) had the highest water content, while sample S5 (slow freezing, storage temperature > Tg′, maltose vacuum infusion) had the lowest water content. The results suggested that no maltose infusion, quick freezing and storage temperature below Tg′ were all favorable to retain water contents in kiwifruit slices.
Effect of Storage Methods on Chlorophyll Contents of Frozen Kiwifruit Slices
Chlorophyll is responsible for the green color of kiwifruits, which may be lost by various mechanisms resulting in decrease of color intensity during processing and storage.[Citation20,Citation21] Thus, chlorophyll content is an important physicochemical index to evaluate the color quality of kiwifruits. The less chlorophyll losses, the better color quality maintains. Moreover, showed that, different storage methods affect water contents of kiwifruit slices significantly. Considering the effect of water content on the chlorophyll content, the dry weight (d. w.) was adopted to express the chlorophyll content of kiwifruit.
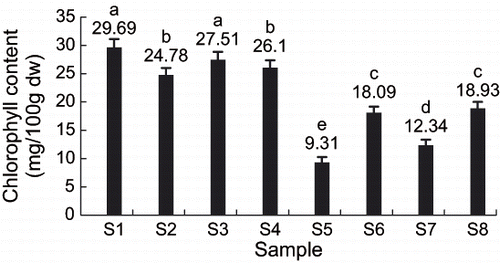
shows the effect of 8 treatments on the chlorophyll content. Chlorophyll content of sample S1 (slow freezing, storage temperature > Tg′, no maltose infusion) was the highest, while chlorophyll content of sample S5 (slow freezing, storage temperature > Tg′, maltose vacuum infusion) was the lowest. Furthermore, the chlorophyll content of all no maltose infused samples of S1, S2, S3, and S4 were higher than those of maltose infused samples of S5, S6, S7, and S8, which implied that the maltose pretreatment decreased the chlorophyll contents of kiwifruit slices. The main reason for this phenomenon was that pretreatment by maltose caused large quantity of juice lost, and then chlorophyll content was decreased.
Effect of Storage Methods on the Ascorbic Acid Contents of Frozen Kiwifruit Slices
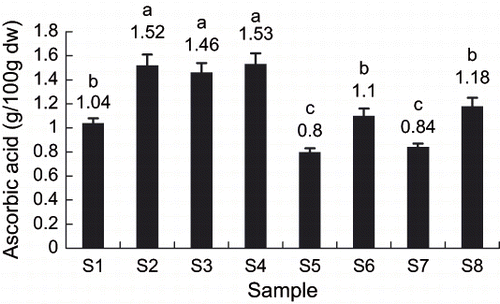
Kiwifruits are rich in ascorbic acid.[Citation1] However, ascorbic acid is easy to be oxidized and lost during processing and storage. Like chlorophyll content, dry weight contents were adopted to express ascorbic acid contents of frozen kiwifruit slices. shows the effect of storage methods on ascorbic acid contents. Ascorbic acid content of sample S4 (quick freezing, storage temperature < Tg′, no maltose infusion) was the highest, while ascorbic acid content of sample S5 (slow freezing, storage temperature > Tg′, maltose vacuum infusion) was the lowest. The results showed that quick freezing, storage temperature below Tg′and no maltose infusions are favorable to retain the ascorbic acid in kiwifruit slices.
Orthogonal Experiment for the Storage of Frozen Kiwifruit Slices
In order to know more clearly and definitely the effects of all factors on the qualities of frozen kiwifruit slices, range analysis of orthogonal experiment with three-factor two‐level design was performed. The factors and levels of orthogonal experiment are shown in . shows the range analysis of the effects of all factors on the Hunter a absolute values, water contents, chlorophyll contents and ascorbic acid contents of kiwifruit slices. Freezing rate (R1) was the main factor affecting Hunter a values, followed by maltose pretreatment and storage temperature. Moreover, as can be seen from the means (K11 and K12), Hunter a value was the highest under the conditions of quick freezing, storage temperature < Tg′ and maltose vacuum infusion, e.g. storage below Tg′ was advantageous to the green color of kiwifruit slices. Kiwifruit slices of quick freezing used liquid nitrogen could pass rapidly through the maximum ice crystal formation zone, decreasing or avoiding formation of big ice crystals, thus caused the least damage to the cell structure and least detrimental to the color quality.
Table 2 Factors and levers of orthogonal experiment
Table 3 Orthogonal experiment design and range analysis
Maltose pretreatment (R2) was the main factor affecting the water content of kiwifruit slices, followed by the factor of storage temperature, but freezing rate had no obviously significant effects on the water content of kiwifruit slices. As can be seen from the means (K21 and K22), water content was the highest under slow freezing, storage temperature < Tg′ and no maltose infusion condition. also showed that water contents of quick freezing kiwifruit slices were lower than those of slow freezing kiwifruit slices possibly due to the cracking phenomenon occuring in the formers. It was also possible that the dehydration effect of maltose caused the decrease of water content to a certain extent, and solids of the kiwifruit also increased meanwhile. When the storage temperature of kiwifruit slices was below Tg′, water content could be maintained constant because samples are transformed to glassy state, at which the water molecule movement is extremely slow preventing the water loss.
R3 of shows that pretreatment was the main factor affecting the chlorophyll content of kiwifruit slices, followed by freezing rate and storage temperature. As can be seen from the means (K31 and K32) chlorophyll content was the highest under the conditions of slow freezing, storage temperature < Tg′ and no maltose infusion. The chlorophyll contents were higher in no maltose impregnated slices than those in the maltose impregnated ones. The main reason may be that soaking in maltose solution caused the loss of chlorophyll from the slices into the solution.
As for the ascorbic acid contents of kiwifruit slices, storage temperature (R4) was the main factor, followed by pretreatment and freezing rate. The means of K41 and K42 showed that ascorbic acid content was the highest under conditions of quick freezing, storage temperature < Tg′ and no maltose infusion. Because quick freezing could pass rapidly through the maximum ice crystal formation zone, the cell damage and consequent loss of juice and ascorbic acid can be very low during thawing. Kiwifruit storage below Tg′ is advantageous to retain the ascorbic acid because of the less molecular movement. The reason why ascorbic acid content decreased after sugar infusion was the same as chlorophyll, which was, soaking in maltose solution caused the loss of ascorbic into the solution. In conclusion, all of these results showed that stored below Tg′ (storage in glassy state) improved the quality of frozen kiwifruit slices.
Effect of Storage Methods on Cell Structure of Frozen Kiwifruit Slices
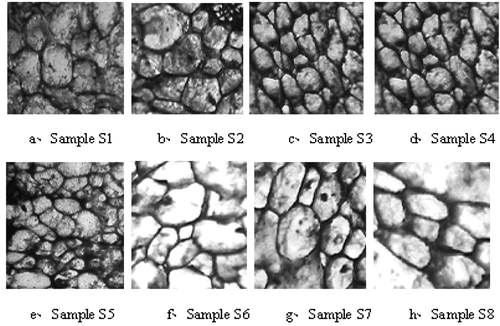
Cell structure is an important index to measure the quality of frozen fruits and vegetables.[Citation22] Cell structures more intact in histological observations, indicates less cell damage during processing and storage. showed the effect of various treatments on the cell structures of frozen kiwifruit slices, where picture a, b, c, d, e, f, g, and h were the light micrographs of samples S1, S2, S3, S4, S5, S6, S7, and S8, respectively.
The cell structures of samples S5, S6, S7 and S8 (maltose pretreated) were more integrated than those of samples S1, S2, S3, and S4 (no maltose pretreated) (). These results suggested that the sugar pretreatment prior to freezing of kiwifruit slices was in favor of the integrity of cell structures. Moreover, comparing samples S2, S4, S6, and S8 (stored below T′g, −40°C) with samples S1, S3, S5, and S7 (stored above T′g, −20°C), the cell structures of the former were also more integrated than those of the latter, also suggesting that kiwifruit slices stored below T′g was favorable to retain the integrity of cell structure. Finally, when comparing samples S3, S4, S7 and S8 (quick freezing) with samples S1, S2, S5, and S6 (slow freezing), the cell structures indicated that quick freezing can also help to maintain the integrities of cell structure.
To sum up, quick freezing, storage below T′g and maltose infusion were favorable to retain the integrity of cell structures of frozen kiwifruit slices. The following factors would have contributed to this effect: 1) when the samples were quickly frozen, they passed rapidly through the maximum ice crystal formation zone, avoiding the cell compression or damage caused by ice crystals and maintaining cell structures to a large extent; 2) samples partly dehydrated by maltose pretreatment before freezing, can cause less ice crystals formation during freezing, helping to retain the cell structures; and 3) When samples were stored below T′g, solutions in cells were converted into glassy state, no new ice crystals will formed or the existing crystal will grow to damage the cell structures because the molecular mobility is limited at this state.
CONCLUSIONS
Chlorophyll content, ascorbic acid content, water content and Hunter a values of frozen kiwifruit slices stored below the glass transition temperature were higher than those stored above the glass transition temperature, and the cell structures of the former were obviously more integrated than those of the latter.
Chlorophyll, ascorbic acid and water contents of maltose pretreated frozen kiwifruit slices were lower than those of no maltose pretreated kiwifruit slices. But, Hunter a values of the sugar pretreated frozen kiwifruit slices were higher than those of the no sugar pretreated kiwifruit slices. Ascorbic acid content and Hunter a values of quick frozen kiwifruit slices were higher than those of slow frozen kiwifruit slices. But, chlorophyll content and water content were lower in quick frozen kiwifruit slices than those in slow frozen kiwifruit slices. The light micrograph of cell structures indicated that quick freezing, stored below T′g and maltose pretreatment were beneficial to maintain the integrities of cell structures of frozen kiwifruit slices.
ACKNOWLEDGMENTS
The authors acknowledge the financial supports from National Natural Science Foundation of China (No. 20776062).
REFERENCES
- Cassano , A. , Figoli , A. , Tagarelli , A. , Sindona , G. and Drioli , E. 2006 . Integrated Membrane Process for the Production of Highly Nutritional Kiwifruit Juice . Desalination , 189 : 21 – 30 .
- Gianotti , A. , Sacchetti , G. , Guerzoni , M.E. and Dalla Rosa , M. 2001 . Microbial Aspects on Short-time Osmotic Treatment of Kiwi Fruit . Journal of Food Engineering , 49 : 265 – 270 .
- Schwartz , S.J. and Lorenzo , T.V. Chlorophyll Stability during Continuous Aseptic Processing and Storage . Journal of Food Science 1991 , 56 ( 4 ) 1059 – 1062 .
- Llano , K.M. , Haedo , A.S. , Gerschenson , L.N. and Rojas , A.M. 2003 . Mechanical and Biochemical Response of Kiwifruit Tissue to Steam Blanching . Food Research International , 36 : 767 – 775 .
- Levine , H. and Slade , L. A . 1989 . Food Polymer Science Approach to the Practice of Cryostabilization Technology . Comments on Agricultural & Food Chemistry , 1 ( 6 ) : 315 – 396 .
- Bhandari , B.R. and Howes , T. 2000 . Implication of Glass Transition for Food Stability . Food Australia , 52 ( 12 ) : 579 – 585 .
- Lu , M.L. and Shi , M. 1998 . Basic Theory of Glass Transition in Food Processing and Preservation . Food and Fermentation Industries , 24 ( 4 ) : 69 – 73 .
- Roe , K.D. and Labuza , T.P. 2006 . Glass Transition and Crystallization of Amorphous Trehalose-Sucrose Mixtures . International Journal of Food Properties , 8 : 559 – 574 .
- Roos , Y.H. 1995 . Glass Transition-related Physicochemical Changes in Foods . Food Technology , 49 ( 10 ) : 97 – 102 .
- Torreggiani , D. , Forni , E. , Guercilena , I. and Maestrelli , A. 1999 . Modification of Glass Transition Temperature through Carbohydrates Additions: Effect upon Colour and Anthocyanin Pigment Stability in Frozen Strawberry Juices . Food Research International , 32 ( 6 ) : 441 – 446 .
- Righetto , A.M. and Netto , F.M. 2005 . Effect of Encapsulating Materials on Water Sorption, Glass Transition and Stability of Juice from Immature Acerola . International Journal of Food Properties , 8 : 337 – 346 .
- Bell , L.N. and Touma , D.E. 1996 . Glass Transition Temperatures Determined Using a Temperature-cycling Differential Scanning Calorimeter . Journal of Food Science , 4 : 807 – 810 .
- Liu , B.L. , Hua , Z.Z. and Xu , J.J. 1999 . Experimental Study on the Glass State Storage of Strawberries . Journal of University of Shanghai for Science and Technology , 21 ( 2 ) : 180 – 183 .
- Liu , B.L. , Xu , J.J. , Hua , Z.Z. , Ren , H.S. , Miu , S. and Feng , Z.Z. 1998 . The Effect of Two Frozen Storage Methods on the Quality of Strawberries . Science and Technology of Food Industry , 19 ( 2 ) : 22 – 23 .
- Wang , Z.G. and Wang , J. 1999 . Preliminary Study on Simultaneous Determination of Chlorophyll a, b by Double-wavelength Spectrophotometric Method . Environmental Monitoring in China , 15 ( 5 ) : 21 – 22 .
- Huang , X.Y. and Liu , L.W. 2001 . Integrative Experiment of Food Chemistry , Beijing, , China : China Agriculture University Press .
- Huang , G.R. 1999 . Relationship between Color and Chlorophyll Content in Green Vegetables . Jiangsu Food and Fermentation , 5 : 11 – 13 .
- Li , Z.L. 1987 . Section Making Technology for Plant , Beijing, , China : Science Press .
- Rui , J.S. and Chen , H.M. 1980 . “ Technology of Tissue Section ” . Beijing, , China : The People's Education Press .
- Lin , X.D. , Zhang , Q. , Ma , L. and Li , D.S. 2002 . Study on Protecting the Color of Kiwifruit in Processing . Journal of Hubei Polytechnic University , 17 ( 1 ) : 70 – 72 .
- Leunda , M.A. , Guerrero , S.N. and Alzamora , S.M. 2000 . Color and Chlorophyll Content Changes of Minimally Processed Kiwifruit . Journal of Food Processing and Preservation , 24 ( 1 ) : 17 – 38 .
- Yan , S.Q. , Liu , B.L. , Hua , Z.Z. , Peng , H.Z. , Miu , S. and Zhou , P.G. Experimental Study on the Effect of Blanching and Frozen on Cellularity and Texture of Potato . Food Science 2000 , 21 ( 8 ) 51 – 54 .