Abstract
α-Amylase was extracted from honeydew honey and purified by CM-Toyopearl 650M and Toyopearl HW-55F column chromatographies. The molecular weight of the enzyme was estimated to be about 58 kDa by Toyopearl HW-55F gel chromatography and SDS-PAGE, respectively. The optimum pH was 4.0 and optimum temperature 50°C. Inactivation of the enzyme occured when the pH was higher than 8.0 or lower than 3.0. This enzyme activated by Ca2+ and Mn2+, but inhibited by Fe3+, Hg2+, Zn2+, and EDTA. TLC analysis also suggested that the purified enzyme was of the α-type. The relative rate of hydrolysis of the polymeric substance decreased with decreasing percentage of α-1,4-linkages and with increasing percentage of α-1,6-linkages in substrate.
INTRODUCTION
Honey, a viscous and aromatic product appreciated since ancient Grecian times, is prepared by bees mainly from nectar of flowers or honeydew.[Citation1] The characteristics of texture, appearance, flavour, and sweetness of honey, as well as its medicinal properties, have attracted thousands of consumers.[Citation1] Furthermore, a great number of consumers are aware that refined sugar is associated with empty energy and thus they are looking for other more nutritious foods.[Citation2] So, it is anticipated that the world trade of honey will grow consistently in the future. Traditionally, use of honey species in food has been as a sweetening agent. However, several aspects of its use indicate that it also functions as a food preservative. Honeys contain a number of components such as vitamins, phenolics, and enzymes known to act as preservatives.[Citation3,Citation4]
It has been known the presence of enzymes in honeys for many years. In particular, α-amylase (diastase) is one of most important enzyme in honey species, although it contains a small amount of enzymes. The origin of α-amylase in honey is commonly attributed to the bee. The nectar collected is mixed with secretions from the salivary and hypopharyngeal gland of foraging bees. α-Amylase was estimated to account for about 2% of the total protein in the hypopharyngeal gland.[Citation5] This enzyme is largely used in Europe as a measure of honey freshness, because this activity decrease in old or heated honeys.
On the other hand, α-amylase is used in starch liquelaction to produce glucose, fructose, and maltose and in brewing, baking, textile, paper, detergent, and sugar industries.[Citation6] There are few reports about α-amylase in honey species, although this enzyme is beneficial to industrial use. For these reasons, we tried to purify and characterize this enzyme available in honeydew honey.
MATERIALS AND METHODS
Sample
Pure honey from honeydew was obtained from Inoue Yohojo Bee Farm Inc. (Hyogo, Japan) and used in this study.
Chemicals
Neo·amylase test was purchased from Daiichi pure chemicals Co. Ltd. (Tokyo, Japan). CM-Toyopearl 650M and Toyopearl HW-55F were from Tosoh Co. (Tokyo, Japan). Marker proteins for gel chromatography were from Boehringer Mannheim Co. (Tokyo, Japan). Marker proteins for electrophoresis and starch from rice were from Sigma Chemical Co. (St. Louis, MO) and from Amersham Biosciences UK Ltd. (UK). Pure α‐amylase from Bacillus subtilis (20 U/mg, pure β-amylase from barley (32 U/mg), starch (wheat, potato, and sweet potato) were from Wako Chemicals Co., Ltd. (Osaka, Japan). Soluble starch was from Nacalai tesque Inc. (Kyoto, Japan). All reagents were analytical grade.
Measurement of α-Amylase Activity
The α-amylase activity was assayed by the method using blue starch as a substrate. A 0.02 ml of honey solution was mixed with 0.784 ml of substrate solution (a tablet per ml of 0.1 M sodium phosphate buffer; pH 6.0), and then incubated at 37°C for 30 min. The reaction was stopped by the addition of 0.196 ml of 0.5 M NaOH. After centrifugation at 12,000 rpm for 5 min, the supernatants were measured by reading the absorbance at 620 nm. One enzyme unit was defined as the activity (IU/L): one unit (IU/L) = 1.85 × (Somogyi unit/dl). Distilled water was used as negative control.
Protein Determination
The protein content was measured as the method of Lowry et al.[Citation7] using bovine serum albumin as standard. Absorbance at 280 nm was used to uv-monitor protein concentration in column chromatography.
Molecular Weight Determination
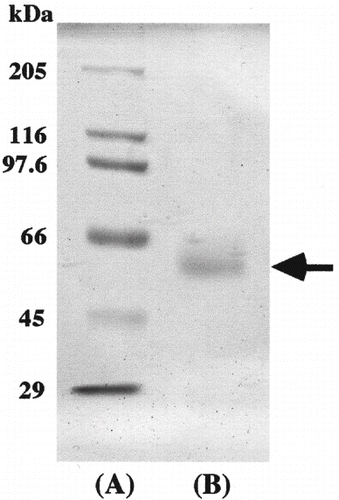
The molecular weight of the purified enzyme was estimated using Toyopearl HW‐55F (1.5 × 75 cm) gel filtration. Ferritin (450 kDa), catalase (240 kDa), aldolase (158 kDa), and albumin (68 kDa) were used as the standard markers for gel filtration. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli[Citation8] using 12.5-% gel. Myosin (205 kDa), β-galactosidase (116 kDa), phosphorylase (97.4 kDa), bovine serum albumin (66 kDa), ovalbumin (45 kDa), and carbonic anhydrase (29 kDa) were used as standards. After electrophoresis, the gel was stained with Coomassie brilliant blue R-250.
Thin-layer Chromatography (TLC) Analysis of Enzymatic Degradation Products
TLC was performed to analyze reaction products of the purified amylase. Twenty microliters of enzyme solution (pure α-amylase from Bacillus subtilis: 0.2 U/ml; pure α‐amylase from barley: 0.04 U/ml; purified amylase from honeydew honey: 0.3 IU/ml) was incubated with 34 µl of 2% unmodified waxy maize starch, 42 µl of 0.2 M sodium acetate buffer (pH 5.0), and 40 µl of 0.5 M NaCl. After at 40°C for 1 h, a 5 µl aliquot of each reaction mixture was loaded on a precoated K6F silica gel 60Å plate (size: 5 × 10 cm; layer thickness: 250 µm: Whatman International Ltd Maidstone England, UK). At the same time, 2 % maltose and 1 % unmodified waxy maize starch were included as controls. The hydrolysates were developed with butanol, ethanol, and water (5:3:2). The plates were sprayed with sulfuric acid-methanol (1:3) solution and then heated it in an oven at 100°C for 30 min to detect the distribution of sugars.
RESULTS AND DISCUSSION
Purification of the Enzyme
Pure honey from honeydew was dialyzed against distilled water at 4°C for 3 days by changing the water once a day. The dialysate was centrifuged at 50,000 × g for 1 h. The supernatants were collected and then lyophilized. The powder sample (400 mg) was dissolved in 40 ml of 10 mM sodium phosphate buffer (pH 6.0). The enzyme solution was applied to a CM-Toyopearl 650M column (1.0 × 5.0 cm) previously equilibrated with the same buffer. Unfortunately, the enzyme was not absorved in this column. The active fractions were pooled and applied to the same column and the enzyme was eluted in the same manner. The active fractions were pooled and concentrated to 0.5 ml using ultrafree-MC (10,000 NMWL Filter Unit: Millipore Co., USA). The concentrated enzyme solution was loaded on a Toyopearl HW-55F column (1.5 × 75 cm) which has been equilibrated with 10 mM sodium phosphate buffer (pH 6.0). The enzyme was eluted with the same buffer. Fractions of 3 ml were corrected at a flow rate of 0.3 ml/min. The enzyme solution was separated into about three protein fractions. The active fractions were pooled and concentrated to 0.5 ml using ultrafree-MC. Finally, the resulting sample was chromatographed on a Toyopearl HW-55F column (1.5 × 75 cm) previously equilibrated with the same buffer, and then the enzyme was eluated with the same buffer. Fractions of 3 ml were corrected at a flow rate of 0.3 ml/ml. The purified enzyme was stored at −85°C until used. The purification procedures are summarized in .
Table 1 Summary of purification of α-amylase from honeydew honey
Molecular Characterization
The molecular weight of the purified enzyme was about 58 kDa, estimated by Toyopearl HW-55F (1.5 × 75 cm) gel filtration (data not shown). According to SDS-PAGE, the purified enzyme appears as a single protein band of molecular weight about 58 kDa (). Babacan and Rand[Citation9] tried to purify amylase from commercially available honey by ultrafiltration with a hollow-fiber cartridge, Sephadex G-100 gel filtration, anion and cation exchange chromatographies. The molecular weight of this enzyme was about 57 kDa by SDS-PAGE. On the other hand, Noman et al.[Citation10] reported that the purification of α-amylase from post-harvest Pachyrhizus erosus L. tuber using DEAE- and CM-cellulose column chromatographies. This enzyme gave a molecular weight of about 40 kDa. Other researchers were also reported that the molecular weight of α-amylase was in the range (38–45 kDa) from shoots and cotyledons of pea (Pisum sativum L.)[Citation11] and thermophilic and alkaliphilic Bacillus sp. TS-23.[Citation12]
Effects of pH and Temperature
The activity of purified enzyme was measured at different pHs at 37°C for 30 min. The optimum pH of the enzyme was about 4.0 (). This value was lower than that (pH 5.5–6.5) of the enzyme from shoots and cotyledons of pea (Pisum sativam L.).[Citation11] On the other hand, Noman et al.Citation10 reported that α-amylase from P. erosus L. tuber showed optimum activity at pH 7.3. Moreover, similar optimum pH (pH 7.0) was observed from vine shoot inter-nodes.[Citation13] The stability of the enzyme was examined at different pHs at 4°C for 24 h. The enzyme retained more than about 80% of the original activity at pH between 3.0 and 6.5, but became extremely unstable when the pH was higher than 8.0 or lower than 3.0 (). It was reported that α-amylase from P. erosus tuber was stable between pH 6.0 and 8.0 for 24 h incubation at 4°C, however, was unstable above pH 9.0.[Citation10]
Figure 2 Optimum pH and temperature on activity and stability of α-amylase from honeydew honey. (A) For each pH, activity was assayed as described under “Materials and Methods” and expressed as relative activity (○). The pH stability curve (•) represents the residual activity after a preincubation period of 1 day at the indicated pH. (B) Enzyme activity was assayed for each temperature (○). Thermal stability of the enzyme was incubated at different temperatures at pH 6.0 for 60 min. After cooling, the residual activity was measured (•)
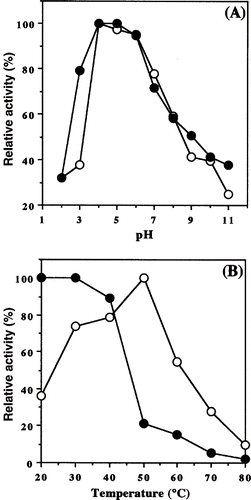
The effect of temperature on the activity of this enzyme was examined at 37°C for 30 min in 0.1 M sodium phophate buffer (pH 6.0). The optimum temperature for the enzyme activity was around 50°C (). On the other hand, the enzyme was incubated at different temperatures at pH 6.0 for 60 min. After cooling, the residual activity was measured. The enzyme was stable when incubated at 40°C for 30 min, but only about 21% of the original activity remained at 50°C for 30 min (). Noman et al.[Citation10] investigated the optimum temperature for amylase activity from P. erosus tuber at temperatures from 10 to 90°C at pH 6.7. As a result, the optimum temperature was about 37°C and this enzyme maintained above 50% activity over a temperature range of 20 to 60°C. Moreover, the enzyme retained about 20% of the original activity at 70°C. Although this enzyme rapidly inactivated above 40°C, it was stable at temperatures up to 40°C for 30 min.
Effects of Metal Ions and SH-blocking Reagents
The effects of metal ions and SH-blocking reagents on the purified enzyme was investigated and the results were indicated as relative activity. That is, the activity was determined by preincubating the enzyme with individual reagent (1 mM) at 4°C for 60 min, followed by incubation with substrate under the standard assay conditions and then assaying the enzyme activity. As a result, the enzyme was strongly activated by Co2+, Mn2+, and DTNB, and was moderately activated by Na+, K+, Ba2+, Ca2+, and PCMB (). In particular, the activity almost doubled in the presence of 1 mM Co2+. On the other hand, Hg2+, Cu2+, Mg2+, Zn2+, EDTA, and CH2ICOOH inhibited the enzyme activity, and Fe3+ almost inhibited the enzyme activity. Noman et al.[Citation10] investigated the effects of various metal ions and EDTA on enzyme activity. Except Na+, Ca2+, Mn2+, and Fe3+, other ions exhibited strong inhibitory effect. Addition of 100 mM EDTA almost completely abolished amylase activity, suggesting this enzyme require metal ions. On the contrary, the presence of 100 mM Ca2+ drastically enhanced the activity; it suggests that calcium is needed for appearance of this activity and stability of the enzyme. The inhibitory effects of other divalent cations might be due to the competition for calcium binding site while monovalent cations and Mg2+ might be poor competitors for calcium binding.[Citation14] Savchenko et al.[Citation15] reported that calcium is required for α‐amylase secretion and synthesis in P. furiosus. Ranwala and MillerCitation16 reported that Hg2+ and Ag+ at 2.0 mM completely inhibited the enzyme. Shaw and Ou-LeeCitation14 reported α-amylase strongly inhibited by Cu2+ and moderately by Li+. From these reports, the purified amylase from honeydew honey was of the α-type, because of strongly inhibition by Zn2+ and EDTA.
Table 2 Effects of metal ions and chemicals on honeydew honey α-amylase activity
Kinetic Constants
Kinetic parameters of the purified amylase for starch as substrate were determined at pH 6.0 and 50°C (optimum temperature). The values of Michaelis constants (Km) and the maximum velocity (Vmax) were calculated by Lineweaver-Burk plots analysis. Apparent Km and Vmax values were 31.9 mg and 44.5 (IU/L), respectively. Noman et al.[Citation10] reported Km and Vmax values were 0.29% and 0.37 µM/min/mg protein, respectively, for starch as substrate. Moreover, Abe et al.[Citation17] also reported the same value in two isozymes from S. oryzae, however, Baker[Citation18] reported about double one in amylase extract from Rhyzopertha dominica.
Substrate Specificity
The substrate specificity of honeydew honey amylase was examined by 7 substances as substrates. As a result, high molecular weight substrate containing the α‐1,4‐linkage such as amylose was good substrate for the enzyme (). As amylopectin, the relative rate of hydrolysis of the polymeric substrate decreased with decreasing percentage of α-1,4-linkages and with increasing percentage of α-1,6-linkages in substrate. Many kinds of starches were used for investigating the substrate specificity. Starches from potato and sweet potato were good substrates: it hydrolyzed at the same rates, although the rates did not amount to that of soluble starch. The relative activities of starches from wheat and rice were about 70–80%. The tendencies of the rate of hydrolysis were similar to those of α-amylase from P. erosus L. tuber.[Citation10]
Table 3 Substrate specificity of α-amylase from honeydew honey
Detection of Enzymatic Degradation Products Using TLC Analysis
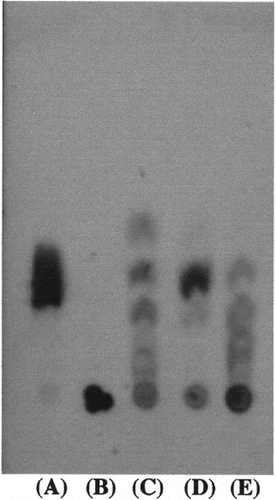
TLC analysis was performed to detect the enzymatic degradation products of purified amylase from honeydew honey and to distinguish between type α- and β-amylase. In comparison, α-amylase from Bacillus subtilis and β-amylase from Barley were used. Although β-amylase produced only maltose from rice starch, α-amylase produced many intermediate products. shows that the purified amylase from honeydew honey not only produced maltose, but also other intermediate products: this enzyme was α-amylase type. Simultaneously, corn starch was degraded using commercially available amylase and the purified amylase, and the products were analyzed using TLC. The results were the same in rice starch ().
Figure 3 Thin-layer chromatogram of the enzymatic hydrolyzing products of rice starch by pure α-amylase, β‐amylase, and purified amylase from honeydew honey after incubation at pH 5.0 and at 40°C for 1 h. The reaction mixture was developted by butanol, ethanol, and water (5:3:2) and the sugars were detected by sulfuric acid:methanol (1:3) solution and heating at 100°C. (A) maltose; (B) rice starch; (C) pure α-amylase; (D) pure β‐amylase; (E) purified amylase from honeydew honey
REFERENCES
- Dustmann , J.H. 1993 . Honey, Quality and Its Control . American Bee Journal , 133 : 648 – 651 .
- Passmore , R. and Eastwood , M.A. 1986 . Human Nutrition and Dietetics. Churchill Livingstone, Edinburgh. Honey Revisited: A Reappraisal of Honey in Pre-industrial Diets . British Journal of Nutrition , 75 : 513 – 520 .
- Ferreres , F. , Garciaviguera , C. , Tomaslorente , T. and Tomasbarberan , F.A. 1993 . Hesperetin C a Marker of the Floral Origin of Citrus Honey . Journal of Science of Food Agriculture , 61 : 121 – 123 .
- Conforti , P.A. , Lupano , C.E. , Malacalza , N.H. , Arias , V. and Castells , C.B. 2006 . Crystallization of Honey at −20°C . International Journal of Food Properties , 9 : 99 – 107 .
- Ohashi , K. , Natori , S. and Kubo , T. 1999 . Expression of Amylase and Glucose Oxidase in the Hypopharyngeal Gland with an Age-dependent Role Change of the Worker Honeybee (Apis mellifera L.) . European Journal of Biochemistry , 265 : 127 – 133 .
- Crueger , W. and Crueger , A. 1990 . Biotechnology: a Textbook of Industrial Microbiology; Sinauer Associates Inc Edited by: Brock , T.D. 191 – 199 . Sunderland, IL
- Lowry , O.H. , Rosebrough , N.J. , Farr , A.L. and Randall , R.J. 1951 . Protein Measurement with the Folin Phenol Reagent . Journal of Biological Chemistry , 193 : 265 – 275 .
- Laemmli , U.K. 1970 . Cleavage of Structural Protein during the Assembly of the Head of Bacteriophage T4 . Nature , 227 : 680 – 685 .
- Babacan , S. and Rand , A.G. 2005 . Purification of Amylase from Honey . Journal of Food Science , 70 : 413 – 418 .
- Noman , A.S.M. , Hoque , M.A. , Sen , P.K. and Karim , M.R. 2006 . Purification and Some Properties of α‐Amylase from Post-harvest Pachyrhizus erosus L. tuber . Food Chemistry , 99 : 444 – 449 .
- Beers , E. and Duke , S. 1990 . Characterization of α-Amylase from Shoots and Cotyledons of Pea (Pisum sativum L.) Seedlings . Plant Physiology , 92 : 1154 – 1163 .
- Lin , L.L. , Chyau , C.C. and Hsu , W.H. 1998 . Production and Properties of a Raw-starch-degrading Amylase from the Thermophilic and Alkaliphilic Bacillus sp. TS-23 . Biotechnology and Applied Biochemistry , 28 : 61 – 68 .
- Berbezy , P. , Legendre , L. and Maujean , A. 1996 . Purification and Characterization of α-Amylase from Vine Inter-nodes . Plant Physiology and Biochemistry , 34 : 353 – 361 .
- Shaw , J.F. and Ou-Lee , T.M. 1984 . Simultaneous Purification of α-Amylase β-Amylase from Germinated Rice Seeds and Some Factors Affecting Activities of the Purified Enzyme . Botanical Bulletin of Academia Sinica , 25 : 197 – 204 .
- Savchenko , A. , Vielle , C. , Kang , S. and Zeikus , G. 2002 . Pyrococcus furiosus α-Amylase is Stabilized by Calcium and Zinc . Biochemistry , 41 : 6193 – 6201 .
- Ranwala , A.P. and Miller , W.B. 2000 . Purification and Characterization of an Endoamylase from Tulip (Tulipa gesneriana) Bulbs . Physiologia Plantarum , 109 : 388
- Abe , R. , Chib , A.Y. and Nakajima , T. 2002 . Characterization of the Functional Module Responsible for the Low Temperature Optimum of Rice α-Amylase (Amy 3E) . Biologia Bratislava , 57 ( Suppl. 11 ) : 197 – 202 .
- Baker , J.E. 1991 . Purification and Partial Characterization of α-Amylase Allozymes from the Lesser Grain Borer Rhyzopertha dominica . Insect Biochemistry , 21 : 303 – 311 .