Abstract
Buckwheat, a non-glutinous pseudo-cereal having a long and traditional history as a food source in Asia, Europe, and the United States, has many beneficial health aspects. However it has suffered from declining production during the past few years. In order to prevent further decline of buckwheat production new products need to be developed for the consumer market and more research need to be carried to study the effect of different processing parameters on buckwheat characteristics. This study was carried out to investigate the effect of microwave heat-moisture and annealing processes on buckwheat starch that had been dried to three moisture levels: 30.3, 40.0, and 50.4 kg/100kg. Starch samples were analyzed using a differential scanning calorimeter, a colorimetric amylose leaching tests, and an x-ray diffractometer. Additional moisture levels in the starch treatment groups (15.2% and 25.8%) were produced for the x-ray diffraction tests. DSC data indicated that moisture levels had a significant effect on onset melting temperature, peak melting temperature and enthalpy of fusion. In addition, heat treatment and interaction of moisture with heat treatment both had a significant effect on amylose leaching results. Significant effect on starch was found mainly at the 50.4 kg/100 kg moisture level as compare to the 30.3 and 40.0 kg/100 kg moisture level. X-ray diffraction readings showed a stable D-space placement for all treatment groups. Intensity visibly increased with decreased moisture level and with heat treatment in case of 40.0% and 50.4% moisture level starches. Resistance to amylose leaching and melting at higher temperatures for higher moisture level buckwheat starch samples was attributed to increased networking among the amylose and amylopectin components in the buckwheat starch.
INTRODUCTION
Buckwheat (Fagopyrum esculentum) is a non-glutinous pseudo cereal that is consumed mainly in China, Japan, and Eastern Europe, but could be profitable in the other countries if new uses were found for its products.[Citation1] Buckwheat can grow to be anywhere from two to five feet in height and produces white or pink blossoms with five petals.[Citation2] Buckwheat can be divided into groups of species: annual and multiennal.[Citation3] Although it contains the same tissue components as cereals, but Buckwheat is a dicotyledon as are peas and beans, while grains like wheat and corn are monocots.[Citation4] It has a starch composition similar to cereals, but has higher amounts of amino acids lysine, methionine, and cystine, which are more typical of legumes.[Citation5] The researchers[Citation6] found that buckwheat starch was round and polygonal with some holes and pits on the surface, and was 1.6 to 2.4 times smaller than that from wheat or corn. They also found that buckwheat starch contained a higher amount of apparent amylose (46.6%) compared to that in corn (28.5%) and wheat (27.5%). In order to learn more about processing of buckwheat into consumer products, it is important to find out how its major components such as starch react to different processing techniques. Most processing techniques involve the use of heat and moisture. The effects of several heat and/or moisture processing techniques, such as boiling, baking, and dry-heat, on buckwheat starch composition and characteristics have been studied.[Citation7–Citation8]
One area that is yet to be studied is the effect of microwave annealing and heat-moisture treatments on buckwheat starch properties. Annealing is a heat moisture process that uses treatment of starch at intermediate or excess moisture (40% moisture content and above) at a temperature below the gelatinization one.[Citation9] The theory behind annealing is that it could cause changes in the molecular structure within the starch, creating those that are more resistant to gelatinization.[Citation10] In a study by, Hoover and Vasanthan [Citation11] it was found that annealing led to greater resistance to gelatinization in that amylose leaching decreased and gelatinization temperature increased, especially for starches high in amylose. Since buckwheat is high in amylose content[Citation6] annealing could prove useful in making the starch more resistant to gelatinization. Heat moisture treatment is a process that uses treatment of starch with low moisture (30% or below) content at a temperature below the gelatinization one.[Citation9] The theory behind heat-moisture treatment is that it changes the crystalline structure of the starch, creating crystalline forms more resistant to gelatinization.[Citation10]
Since today's processing techniques require faster modes of treatment, a microwave with a probe was used to process the starch. The effect of the annealing and heat-moisture treatments was studied using a differential scanning calorimeter, an x-ray diffractometer, and an amylose leaching colorimetric method.
This study explored the effects of microwave annealing and heat-moisture treatment on buckwheat starch properties. The factors that effects are moisture content of the starch, temperature at which the starch was heated, and time of starch heated. The buckwheat starch was isolated from a buckwheat flour milling fraction that was produced from the starchy endosperm of the buckwheat plant. Moisture level was established at 30.3, 40.0, and 50.4 kg/100 kg and microwave heating parameters were set at 6 min at 65.6°C and 10% power to allow for changes within the granule but not dry out the starch granules (dextrinize) or cause them to gelatinize. The objectives of our study were to construct and conduct heat-moisture and annealing heating regimens in the microwave. To study the heat-moisture treated and annealed starch using the differential scanning calorimeter, the x-ray diffractometer, and an amylose leaching colorimetric method in order to determine whether starches resistant to further heat and moisture were formed with annealing and heat-moisture treatment.
MATERIAL AND METHODS
Commercially available variety of buckwheat was procured from the local market. The Petroleum ether, sodium hydroxide, hydrochloric acid, paraffin wax, tricholoracetic acid, iodine and potassium iodide (AR grade) were procured from S.D. fine chemicals India limited.
Buckwheat Starch Isolation
In order to obtain an accurate evaluation of the effect that microwave heat moisture and annealing treatments had on buckwheat starch, the starch first had to be isolated from the buckwheat flour. The flour milling fraction used in this experiment contained 72.0% carbohydrate, 9.3% protein, 1.9% fat, 2.2% fiber, and 1.2% ash. The non-starch components were removed to avoid interactions between the starch and other components (lipid, protein).
The first step in starch isolation involved the defatting of the flour. This was performed using petroleum ether. A total of 800 g of buckwheat flour was mixed with 4 ltrs (1:5 w/v) of petroleum ether. Petroleum ether used for fat extraction. It is especially effective with extracting hydrophobic lipids and is safer and less expensive than other fat.[Citation12] In order to continually disperse the flour in the petroleum ether, beaker with stir bar was placed on an magnetic stirrer for two-hour duration. This was done to prevent the buckwheat flour from settling and thereby allowing the petroleum ether to come in contact with flour and extract the lipid. After 2 h of stirring, flour petroleum ether was passed through fitted with Whattman 24.0 cm filter paper and transfers the material in evaporation dishes overnight under room temperature. Evaporation dishes were weighed previous to use in order to help quantify the amount of defatted flour obtained after the defatting process. The protein was removed using a centrifugation technique.[Citation6] Defatted buckwheat flour (∼30 g) was steeped in 0.2% NaOH (1:6 w/v) in 250 mL Erlenmeyer flasks and placed in a 45°C water bath for 90 min. Each flask was stirred with a glass stirring rod in order to suspend the starch in the NaOH prior to placing it in the water bath. The flour/NaOH mixture was then blended in a hand blender for 2 min and sieved through 0.208 mm, 65 inch mesh to remove larger particles. The flour mixture was poured into counter balanced centrifuge tubes and run at 3000 rpm (∼1464 × g) for 15 min in a centrifuge (EltekR RC 41000 India) at 25–35°C. The supernatant was discarded and the top brown-yellow protein layer removed with a metal spatula and water from a distilled water bottle. The white starch layer was resuspended in distilled water, centrifuged, decanted, and cleaned of the top brown-yellow protein layer. This was repeated until there was no longer any visible brown-yellow protein present (usually two to three times). The starch was then resuspended in distilled water and adjusted to within a pH range of 6.5–7.0 using 1 M HCl and calibrated by pH meter. The starch was then washed two to three times with distilled water and dried at ambient temperature after that moisture content of stored samples was determined by an air drying method. Percent moisture content of the various samples was determined by a 4-h drying method. Five gram samples from were weighed into aluminum dishes and placed in a hot air oven at 105°C for 4 h. The aluminum dishes were cooled and then weighed again. Percent moisture was determined by subtracting the weight of original sample (g) from weight of sample post drying (g) divided by weight of original sample multiplied by 100. Starch was immediately placed in a container capped, and sealed with parafilm wax and refrigerated at 4°C.
Microwave Heat-Moisture and Annealing of Buckwheat Starch
Preliminary tests were run to determine the gelatinization temperature (using the differential scanning calorimeter) and amount of time required to appropriately microwave the isolated buckwheat starch. Since the purpose of heat-moisture treatment and annealing treatment was to heat the starch below the gelatinization temperature with less than 35% and at least 40% moisture content respectively, the microwave temperature had to be such to allow the changes to occur within the starch granule without allowing the starch granule to break and amylose to leach out.
Microwave tests were performed in triplicate in a 900 Watt (LG MG-607) convection microwave oven. Approximately 10 g of each sample was placed in 50 mL centrifuge tubes placed in 50 mL containers (for stability) and microwaved at 10% power at 65.6°C (150°F) for 6 min. The temperature for heat treating the starch was determined as per literature search[Citation6] and preliminary testing. A temperature probe was placed in the center of the sample during constant intervals of time to ensure that it did not increase over the desired temperature while heating. Once the sample reached the desired internal temperature it was held for the specified length of time. After the microwave treating completed, samples were immediately capped, wrapped with parafilm wax to prevent moisture loss or gain, and placed in a 25°C water bath to prevent further heating. Once cooled, granules were separated by applying a mortar and pestle to the contents of the centrifuge tubes.
X-ray Diffraction Evaluation of Starch Crystalline Structure
X-ray diffraction is a method used to characterize the crystalline structure of a material Pomeranz.[Citation13] X-rays consist of high energy waves created when a high concentration of electrons hits a heavy target, causing the electrons to penetrate the atoms of the target and give off high energy waves. These waves then penetrate a sample such as a starch granule where they are diffracted by crystalline layers. The spacing of the crystalline layers may be examined by the distance (d) between the wavelengths that are diffracted. The intensity of the D-spacing peaks relates to the concentration of the crystalline phase within the starch granule.[Citation14]
X-ray diffraction was performed on a Scintag PAD-X Advanced Diffraction System X1 (Thermo ARL, Waltham, MA). A small amount of buckwheat starch powder was placed in a plastic x-ray sample holder and flattened with a piece of glass in order to entirely fill the holder and to arrange the sample level with the edges of the holder to reduce scanning errors. The buckwheat was scanned through the 2θ range of 0–40° using Data Scan software. The angles used were similar to those described found by researchers Hoover[Citation15] and are typical of x-ray diffraction starch analyses. D-spacing and intensities were examined in the samples using software. In which contained a manual cursor function that gave d-spacing and intensity data at selected points. For this procedure a starch sample with 15.2 ± 0.04% moisture and a sample with 25.8 ± 0.33% moisture were created in order to have a more complete view of the effect of moisture level and heat treatment on x-ray diffraction analyses of buckwheat starch. Preparations were similar to previous air temperature drying with 30 g of buckwheat starch from the lowest and highest moisture level starches placed in evaporation dishes at ambient temperature for approximately 24 h. New moisture levels were adjusted as previously described.
Differential Scanning Calorimeter Evaluation of Buckwheat Starch
During each test session, three samples of the heated buckwheat starch at the three different moisture levels and one sample of the unheated starch at the three moisture levels were run through a differential scanning calorimeter method using DSC-821° (Mettler Toledo) equipped with DSC cell and thermal analysis data station. It was used to measure the presence of exothermal or endothermal changes as the temperature was increased. Thermal curves of starch gelatnization were obtained by weighing (3–4 mg, dwb) of starch into 40 ml capacity aluminium pans and distilled water was added with the help of Hamilton micro syringe to get starch-water ratio 70:30. Then samples were hermetically sealed and allowed to stand for 1 h at room temperature. The thermal analyzer was calibrated with indium and an empty pan was used as a reference. Sample pans were heated at a rate of 10°C per minute from 20–100°C and then onset temperature (TO), peak temperature (Tp), end set temperature (Tc) and enthalpy of gelatinization (Hgel) were calculated.
DSC was used to observe and measure the temperature ranges at which the starch underwent melting as well as the amount of energy (enthalpy, J/g) required for the melting process. Onset temperature (To) was determined by extrapolation.[Citation16] The peak temperature (Tp) was determined as the temperature at which the DSC reading had reached maximum endothermic transition. Enthalpy of fusion was determined by the software as the area of the transition peak from selected onset temperature to conclusion temperature of transition. The mean of the onset of the melting transition, the peak, and the enthalpy of fusion was calculated for each treatment group. The DSC was used to determine the effect that moisture level and heat treatment had on starch melting characteristics.
Amylose Leaching Colorimetric Measurement
Amylose leaching was carried out in a similar manner to the procedure described by researchers.[Citation11] The method was a modified version of the procedure described.[Citation17] Approximately 20 mg of heat and moisture treated starch was placed in centrifuge test tubes. Add 6 mL of water. Then tubes were placed with caps slightly tightened, in jar at 95°C water bath for 30 min. After the 30 min the tubes were placed in a 25°C water bath to cool. After cooling the tubes were placed in a centrifuge at 25–35°C and run at 2000 rpm (412 × g) for 10 min. After this, 1 mL of supernatant was withdrawn and placed in a small 25-mL Erlenmeyer flask. From this flask, 0.10 mL was withdrawn and added to 5 mL of 0.5% tricholoracetic acid and 0.05 mL of 0.01 N I2-KI solution and mixed. After allowing the samples to stand at room temperature for 30 min, they were run on a spectrophotometer (Shimadzu India, Ltd.) at 620 nm. A calibration curve was prepared using absorption readings from standards containing 0–100% amylose with amylopectin. The calibration curve from the standards was used to determine the percent of amylose that had leached out of the starch granules. Ultraviolet-visible spectrophotometry is a quantitative analytical method that can be used to determine unknown concentrations of a known molecule in a solution.[Citation18]
Statistical Analysis Procedure
In order to determine the effect of heat and moisture content on amylose leaching and the melting temperature parameters of the starch the results were analyzed Minitab for Windows (MINITAB Release 7 version) software. Independent sample t-tests and least significant difference (LSD) was also used to differentiate the samples
RESULTS AND DISCUSSION
Buckwheat Percent Moisture Level Results
Prior to and after heat treatment of the buckwheat starch the moisture content was determined using the hot air oven method. From the original starch three levels of starch hydration were produced using ambient temperature drying at 0, 12, and 24 h after starch isolation. The moisture content in the samples were: 30.3 ± 0.4, 40.02 ± 0.2, and 50.4 ± 0.08 kg/100kg. These values matched the criteria described in Jacobs and Delcour (1998) for heat-moisture treatment (< 35%) and annealing (> 40%). After microwave heat treatment, nine samples were taken from each sample set and tested for changes in hydration level in a hot air oven. The resulting moisture levels were 28.8 ± 0.5%, 38.9 ± 0.2%, and 48.4 ± 0.3%.
X-Ray Diffraction Results
Several x-ray diffraction measurements were taken for unheated and heated buckwheat starch at moisture levels of 15.2%, 25.8%, 30.3%, 40.0%, and 50.4%. Heat treatment involved microwaving the starch at the same parameters as the other tests. All graphs were smoothed using MDI Jade 6.5 in order to better read and compare graphs. and illustrate the difference in x-ray diffraction between moisture levels for unheated and heated samples. The difference in x-ray diffraction between the unheated and heated samples for each moisture level depicted in and . For the unheated 50.4% moisture level two different graphs were presented by the x-ray diffraction machine. reports the d-spacing angles at which the crystalline layer in the starch refracted the x-ray and intensities for the two major peaks found on each graph.
Figure 3 X-ray diffraction of unheated buckwheat starch at moisture levels of: a = 15.2%; b = 25.8%; c = 30.3%; d = 40.0%; and e. 50.4%.
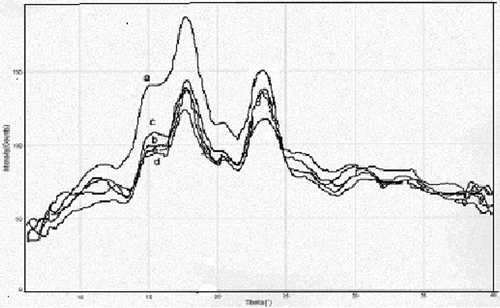
Figure 4 X-ray diffraction of heated buckwheat starch at moisture levels of: a = 15.2%; b = 25.8%; c = 30.3%; d = 40.0%; e = 50.4%.
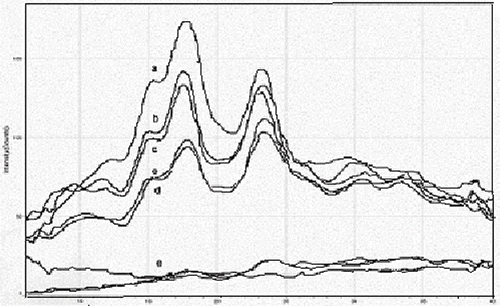
Table 1 X-ray Diffraction data for buckwheat starch heated and unheated in microwave at various moisture levels
Most graphs peaked at 3.8 Å and 5.0 Å with intensities increasing at lower moisture levels for unheated samples. Within each moisture level changes in intensity were not seen with heating except for starch samples with moisture levels 40.0% and 44.4% .The starch did have a cereal A-type crystallinity with two major d-spacing peaks at 5.0 Å (∼17.7°) and 3.8 Å (∼23.4°) and one smaller peak that was not recorded but was visible as a shoulder at about 5.7 Å (∼15.4°). This did not change with percent moisture or heat treatment (see and ). In general the intensity of the x-ray diffraction readings increased as moisture level decreased. X-ray intensity also increased with microwave annealing treatment of buckwheat starch with moisture levels of 40.0% and 50.4%.
Contrary to some of these experiments heat-moisture treatment did not result in significant changes to intensity, while annealing did.[Citation17] A possible explanation for the increased intensity with annealing is that the excess moisture coupled with heat may have been able to more evenly spread the amylose throughout the starch granule, allowing interaction of the amylose and amylopectin branches in the crystalline regions which would account for higher intensity readings between heated and unheated starch at higher percent moisture levels and comparable readings among several heated starches As suggested,[Citation14,Citation11] in interaction between amylose and amylopectin chains may also have occurred at the two moisture levels, which would also account for increased concentration of the crystalline regions. Loss of moisture due to heating was not considered a major factor for increased x-ray diffraction readings.
Differential Scanning Calorimeter Results
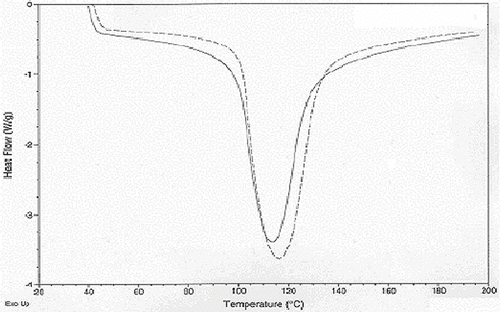
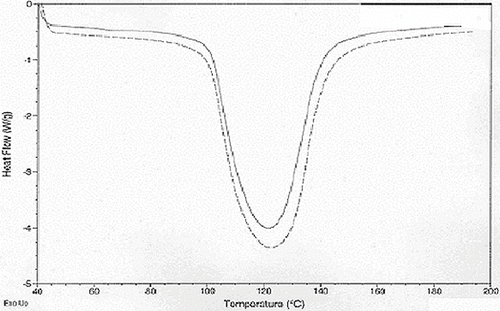
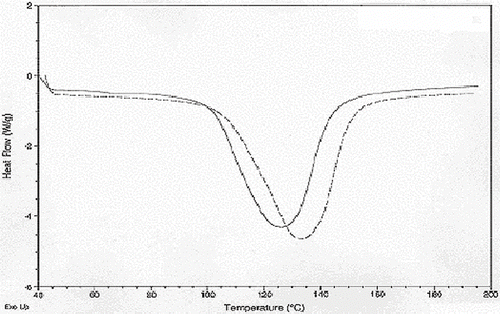
Data analyses for differential scanning calorimeter (DSC) have been given in . The moisture level did have a significant effect on mean onset melting temperature but the 50.4% moisture level starch had a significantly higher mean onset temperature than that for the 30.3% moisture (p < 0.01) level starch while there was no significant difference between the 50.4% and 40.0% (p = 0.252) moisture level starches as well as the 30% and 40% (p = 0.116) moisture level starch. Application of microwave heating did not have a significant effect on mean onset melting temperature. The combined effect of moisture and microwave heat also did not have a significant effect on mean onset temperature. Overall, moisture level of 50.4% gave the starch a significantly higher onset melting temperature readings than the 30.3% starch but not the 40.0% one. The application of heat did not have an influence on or interact with moisture level to influence onset melting temperatures. The 50.4% moisture level had a significantly higher mean peak temperature than the 40.0% and the 30.3% ones. There was no significant difference between the 30.3% and 40.0% moisture level starches. Application of microwave heating did not have a significant effect on the mean peak melting temperature. The combined effect of moisture and microwave heat also did not have a significant effect on mean peak melting temperature. This indicated significant difference among the variances across the different treatment groups and as such this could have an effect on mean peak melting temperature readings. As with onset melting temperatures, peak melting temperatures were also influenced by moisture but not heating.
Table 2 Differential Scanning Calorimeter data for buckwheat starch heated in microwave for 6 min at various moisture levels (n = 6)
DSC enthalpy of the entire set of starches at different moisture levels was found to have a significant effect on mean DSC enthalpy whereas heating was not found to have a significant effect. Interaction between moisture level and microwave heating had an insignificant effect on DSC enthalpy, the moisture levels, 50.4% moisture level starch was found to have a significantly higher mean DSC enthalpy than 30.3% moisture level starch. However, 50.4% moisture level buckwheat starch did have a significantly higher mean DSC enthalpy than 40.0% one however it did not have a significantly higher level mean DSC enthalpy than 30.3% moisture level starch.
, , and represent DSC of buckwheat starches at the different moisture levels. As percent moisture increased, the DSC endotherm peaks widened (increasing enthalpy) and shifted toward the higher temperatures (increasing onset and peak melting temperature). According to previous[Citation18] research DSC readings at lower moisture levels were due to the melting of the majority of the crystalline structure versus the small amount of crystalline structure stripping that takes place at the lower (66°C) endotherm when excess moisture is available. In preliminary tests with buckwheat starch that had higher moisture levels and with some of the 50.4% starch samples some endotherms in the 66°C area were visible. The lowest peak temperature for any of these readings was 67.6°C. Heat treatment temperature was set at 65.6°C (150°F) in order to supply enough heat to cause changes in the crystals without causing gelatinization which did partially occur in some samples as was noted in the results section. DSC endothermic changes did occur, but, as stated in the results, were attributed to moisture level changes, particularly between the 30.3% and 50.4% moisture levels. The shift in higher endothermic parameters is contrary,[Citation19] and other researchers who have studied the effect of moisture content on DSC parameters[Citation21] and found DSC parameters such as onset and peak temperature to decrease with increasing moisture content. Change in enthalpy was more consistent with the findings,[Citation19,Citation21,Citation22] where peaks became smaller with decreased moisture content. Although hard to conclude due to the great amount of variance in especially peak temperatures, buckwheat starch with its higher water binding capacity and higher amylose content may actually form stronger internal bonds between amylose and itself or amylose and amylopectin at higher moisture levels which would contribute to increased resistance to melting. Amylose leaching results focused on the interaction of amylose with itself and other starch granule components.[Citation23] The results of this experiment found that amylose leaching was not significantly affected solely by moisture level as were DSC endotherm readings; rather the amylose leaching was affected more by the use of microwave heat treatment, and the combination of moisture and microwave treatment. This was most significant especially with the 40.0% and 50.4% moisture level annealed starch. Although the unheated 50.4% moisture level.
Amylose Leaching Results
In order to determine the amylose leaching percentage, 0–100% amylose standards were prepared and tested with the same procedure as the treated samples. Since there was a large deviation from 40–60%, these data points were eliminated. The resulting graph gave an equation of y = 0.573×, i.e., the standard equation (y = mx + c) obtained from the standard amylose concentration results points gave the amylose concentration curve and it has a zero intercept value and 0.573 slope value, which was used to determine the percent of amylose that leached out of the starch granules during the test using the absorbance readings from the starch-iodine test.
The microwave heating had a significant effect on mean amylose leaching readings results are shown in and that the interaction between moisture level and microwave heating also had a significant effect on mean amylose readings. However, moisture level alone did not have a significant effect on mean amylose readings. In other words, moisture level alone did not affect mean amylose leaching; however it did have an effect in combination with microwave heating.
Table 3 Amylose Leaching of buckwheat starch microwave heated for 6 min at various moisture level (n = 9)
The results of the t-tests indicated that the mean amylose leaching reading for the unheated 50.4% moisture level starch was significantly higher than that for the heated 50.4% moisture level starch and that the unheated 40.0% moisture level starch and all of the 30.3% moisture level starches were significantly higher than the heated 50.4% moisture level starch. The unheated 50.4% moisture level starch had significantly higher mean amylose leaching than the heated 40.0% moisture level starch. Differences among the other treatments were not significant at the selected alpha level. This means that mean amylose leaching was the lowest for the heated 50.4% moisture level starch, followed by the heated 40.0% moisture level starch, the unheated 40.0% moisture level starch and both treatments of 30.3% moisture level starch, and finally the unheated 50.4% moisture level starch. In examining the results for this experiment it is important to note that, for some buckwheat starch characteristics, heat treatment or the interaction of heat treatment and moisture level had a significant effect, while for other characteristics moisture level alone had a significant effect. Three main moisture levels — 30.3%, 40.0%, and 50.4% — and two heating options — microwave heated or unheated at below the gelatinization temperature — were used to create microwave heat-moisture (30.3%, heated) and annealed (40.0%, 50.4%, heated) samples.
These factors (moisture and heat) created 6 treatment groups which were applied to the buckwheat starch and then used to examine buckwheat starch characteristics. The three tests used in this experiment examined a characteristic, which has to do with amylose interactions in the starch granule and characteristics which have to do with the crystalline region of the starch granule (concentration and stability). Results from these tests showed that buckwheat granule structures can be stabilized in some ways using microwave and moisture heat treatment to make it more resistant to breaking apart from further addition of heat and water.
Starch had the highest mean amount of amylose leaching; it was not significantly different from the other unheated starches. The most significant finding from this test was that annealed starches had significantly lower amylose leaching. This finding is consistent with annealing treatments of different starches[Citation11] but not heat-moisture treatment of different starches.[Citation14] The findings are also consistent with the restrictive swelling properties of buckwheat starch found.[Citation6] Lower swelling relates to lower amylose leaching in that granules that are more resistant to swelling are more resistant to leaching of their components. Higher amounts of amylose, coupled with the effects of annealing conditions, could help to form strong internal bonds between amylose and itself and amylose and other starch granules components which would make the granules more resistant to changes caused by the further addition of heat and moisture.
CONCLUSIONS
The purpose of this experiment was to explore the effect of microwave heat-moisture treatment and annealing on buckwheat starch properties. The hypothesis was that both treatments would make the buckwheat starch granules more resistant to destruction by further heat and moisture application. This hypothesis was tested by isolating buckwheat starch from flour, preparing five moisture levels, and setting up three different tests which looked at the resistance of the buckwheat starch granule to melting from additional heat, the leaching of amylose, a component of starch, with application of heat and water; and the crystalline structure of the starch before and after heat treatment at the different moisture levels. High moisture levels were found to have a significant effect on melting parameters whereas annealing treatment was found to have a significant effect on amylose leaching. There were no changes in d-space angles in x-ray diffraction; however, intensities did increase with lower moisture level and annealing. These findings were attributed to interactions between amylose and other starch components throughout the starch granule.
REFERENCES
- Edwardson , S. 1996 . “ Buckwheat: Pseudo Cereal and Nutraceutical. ” . In Progress in new crops , Edited by: Janick , J. 195 – 207 . Alexandria, VA : ASHS Press . Janick, J., Ed.
- Saeger, E.; Dyck, E. Buckwheat. Minnesota Grown Opportunities. 2001 http://www.mda.state.mn.us/mgo/publication/buckwheat.pdf (Accessed: 16 January 2002 ).
- Li , S. and Zhang , Q.H. 2001 . Advances in the Development of Functional Foods from Buckwheat . Critical Reviews in Food Science and Nutrition , 41 : 451 – 464 .
- Starr , C. 2000 . Biology: Concepts and Applications , 4th , Pacific Grove, CA : Brooks/Cole Thomson Learning .
- Zheng , G.H. , Sosulski , F.W. and Tyler , R.T. 1998 . Wet-milling, Composition and Functional Properties of Starch and Protein Isolated from Buckwheat Groats . Food Research International , 30 : 493 – 502 .
- Qian , J. , Rayas-Duarte , P. and Grant , L. 1998 . Partial Characterization of Buckwheat Starch . Cereal Chemistry , 75 : 365 – 373 .
- Skrabanja , V. , Elmståhl , H.G.M.L. , Kreft , I. and Björck , I.M.E. 2001 . Nutritional Properties of Starch in Buckwheat Products: Studies in vitro and in vivo . Journal of Agricultural Food Chemisty , 49 : 490 – 496 .
- Skrabanja , V. and Kreft , I. 1998 . Resistant Starch Formation Following Autoclaving of Buckwheat (Fagopyrum esculentum Moench) Groats. An in vitro study . Journal of Agricultural & Food Chemistry , 46 : 2020 – 2023 .
- Jacobs , H. and Delcour , J.A. 1998 . Hydrothermal Modifications of Granular Starch, with Retention of the Granular Structure: A Review . Journal of Agricultural and Food Chemistry , 46 : 2895 – 2905 .
- Stute , R. 1992 . Hydrothermal Modification of Starches: the Difference between Annealing and Heat/moisture-treatment . Starch/Stärke , 44 : S205 – 214 .
- Hoover , R. and Vasanthan , T. 1994b . The Effect of Annealing on the Physicochemical Properties of Wheat, Oat, Potato and Lentil Starches . Journal of Food Biochemistry , 17 : 303 – 325 .
- Min , D.B. and Steenson , D.F. 1998 . “ Crude Fat Analysis ” . In Food Analysis , 2nd , Edited by: Nielsen , S.S. 201 – 216 . Gaithersburg, MD : Aspen Publishers, Inc. .
- Pomeranz , Y. and Meloan , C.E. 2000 . “ X-ray Methods ” . In Food Analysis:Theory and Practice , 3rd , 158 – 171 . Gaithersburg, MD : Aspen Publishers, Inc. .
- Cullity , B.D. 1978 . Elements of X-ray Diffraction , 2nd , Reading, MA : Addison-Wesley Publishing Company, Inc. .
- Hoover , R. and Vasanthan , T. 1994 . Effect of Heat-moisture Treatment on the Structure and Physicochemical Properties of Cereal, Legume, and Tuber Starches . Carbohydrate Research , 252 : 3 – 53 .
- Schenz , T.W. and Davis , E.A. 1998 . “ Thermal Analysis ” . In Food Analysis , 2nd , Edited by: Nielsen , S.S. 587 – 598 . Gaithersburg, MD : Aspen Publishers, Inc. .
- Chrastil , J. 1987 . Improved Colorimetric Determination of Amylose in Starches or Flours . Carbohydrate Research , 159 : 154 – 158 .
- Penner , M.H. 1998 . “ Ultraviolet, Visible, and Fluorescence Spectroscopy ” . In Food Analysis , 2nd , Edited by: Nielsen , S.S. 397 – 412 . Gaithersburg, MD : Aspen Publishers, Inc .
- Donovan , J.W. 1979 . Phase Transitions of the Starch-Water System . Biopolymers , 18 : 263 – 275 .
- Li , W. , Lin , R. and Corke , H. 1997 . Physicochemical Properties of Common and Tartary Buckwheat Starch . Cereal Chemistry , 74 : 79 – 82 .
- Rolee , A. and LeMeste , M. 1999 . Effect of Moisture Content on Thermomechanical Behavior of Concentrate Wheat Starch-water Preparations . Cereal Chemistry , 76 : 452 – 458 .
- Singh , V. , Ali , S.Z. , Somashekar , R. and Mukherjee , P.S. 2006 . Nature of Crystallinity in Native and Acid Modified Starch . International Journal of Food Properties , 9 ( 4 ) : 845 – 854 .
- Saif , S.M.H. , Lan , Y. and Sweat , V.E. 2003 . Gelatinization properties of rice starch . International Journal of Food Properties , 6 ( 3 ) : 531 – 542 .