Abstract
In this study, the effect of some ingredients such as skimmed milk powder, whey, sodium caseinate, calcium caseinate, whey protein concentrate (35, 60 kg/100 kg dry solids), whole milk powder, condensed milk and transglutaminase (TGase) on the properties of set-style yogurt was investigated. These protein and dry matter sources (2%) and TGase (1 U/g milk protein) were added into pasteurized milk and incubated prior to fermentation for 2 h at 40°C. After fermentation, enzyme action was stopped by heating for 1 min at 80°C. The control groups were conducted with addition of these materials into milk without TGase. All of the milk samples were inoculated with yogurt cultures at 45°C, until the pH was dropped to 4.4. Syneresis, gel-strength, acetaldehyde amounts, and the degree of TGase reaction were determined. As a result, yogurt products made from enzyme-treated milk showed increased gel strength and less syneresis. SDS-PAGE results showed that the enzyme TGase produced crosslink formation between different protein fractions of milk. In addition, it was also determined that TGase application caused a decrease in acetaldehyde amounts.
INTRODUCTION
TGase (EC 2.3.2.13) is known as an enzyme that is capable of forming inter- and intramolecular crosslinks in many proteins and catalyzes acyl transfer reaction between carboxylamine groups of glutamine and various primary amines.[Citation1] As a result of this cross-linking, it can be obtained large molecular weight polymers which have different functional properties. Many studies have showed that milk proteins are substrates of TGase suggesting that the enzyme has a potential usage in dairy products.[Citation2–7] Especially caseins cross-linked easily because of their flexible, random-coil structures, while whey proteins having compact globular structures and the disulphide bonds cross-linked less effectively.[Citation8–11] There are differences between β-lactoglobulin and α-lactalbumin with respect to crosslinking. Faergemand and Qvist[Citation5] reported that β-lactoglobulin was able to cross-link with the reduction of its disulphide bonds, whereas α-lactalbumin could be cross-linked without the reduction. Although whey powder and whey protein concentrate have been produced on a large scale, they can be used as a cost-effective alternative to other ingredients.[Citation12] Kuraishi et al.[Citation13] reported that TGase affected on the gel strength and viscosity of set type yogurt as a result of improved water-holding properties. Yogurt produced with TGase found to be more palatable by the consumer. The studies have also shown that the gel strength of yogurt samples from transglutaminase-treated milk was higher while the corresponding whey syneresis was lower. Set-style yogurt samples from enzyme-treated milk showed a dry, smooth and whiter shining surface, as well as a milder taste, than products from nontreated milk.[Citation14] This article focuses on the effect of enzymatic cross-linking of milk proteins on the properties of set-type yogurt products.
MATERIALS AND METHODS
Preparation of Yogurt
Raw milk was obtained from the UN-SUT dairy plant of the Suleyman Demirel University. Protein and dry matter contents were increased either by condensation of milk or the addition of skimmed milk powder, whey, sodium caseinate, calcium caseinate, whey protein concentrate (35 kg/100 kg dry solids and 60 kg/100 kg dry solids) and whole milk powder, respectively. These ingredients (ENKA Milk Factory) were added into milk (2%). In addition to that, whole milk was used for yogurt production without increasing protein and dry matter contents. Then the milk samples were pasteurized at 85°C for 5 min. Protein analyses were made with formal titration method.[Citation15] TGase was added into milk according to the milk protein ratios (1 U/g milk protein). TGase activity was 1000 U/g. TGase was obtained from Activa (Ajinomoto Europe Sales GmbH, Hamburg, Germany). These protein sources (2%) and TGase were added into milk and incubated prior to fermentation for 2 h at 40°C. Enzyme action was stopped by heating for 1 min at 80°C. The control groups were conducted with addition of these materials into milk without TGase. All of the milk samples were inoculated with a commercially available traditional thermophilic yogurt cultures (Visby 709) at 45°C and incubation was terminated when pH reached 4.4.[Citation16–18] After 18 h of cold storage pH dropped 4.06–4.26. The experiment was conducted in duplicate.
Gel-Strength
The gel strength of set-style yogurt products was determined at 4–6°C by penetration measurements (Lloyd LF Plus Nexygen 4.1). The instrument was adjusted to the following conditions: conical probe (15 × 25 mm) sample passed at the speed of 24.00 mm/min, with a penetration speed of 1.00 mm/min. The measurement was carried out by compressing the sample until rupture. Breaking strength was evaluated from the force of rupture and expressed in N/cm2 of probe area.[Citation19] Gel strength was determined in triplicate.
Syneresis
The level of spontaneous whey separation in undisturbed set yogurt (syneresis) was determined using drainage method.[Citation14] A cup of set yogurt was taken out from the cold room. Approximately 30 g of the gel was cut in a single action by using a stainless steel ladle and the gel was weighed and drained on a 1 mm2 pore size sieve for 2 h at 6°C. The whey was weighed and the syneresis was expressed as the percent weight of the whey separated from the gel over the initial weight of the gel. Double determinations were carried out.
SDS-PAGE
The yogurt aliquots (600 mg) were taken into an eppendorf tube and boiled for 5 min at 100°C for protein denaturation. The poliacrilamid gel was prepared and 20 μL samples running for 1.5 h at 75 mA. An electrophoresis unit (9 × 12, Biorad) was used in the protein analysis. The gels were stained with Coomassie Brilliant Blue (0.25%). The electrophoretic bands were quantified from the normalized peak areas expressed as intensity mm2 mg−1 protein applied. The protein marker was Sigma M 4038.[Citation20]
Determination of Acetaldehyde
The flavor substances were analyzed by a gas chromatograph (Perkin Elmer Autosystem ×L). The system was equipped with a flame ionization detector (Perkin Elmer) with a 50 m CP WAX 52 capillary column (inner diameter: 0.32 mm; film thickness: 1.2 μm; Carbowax 20M, Varian). The chromatograph was connected to an automatic head-space sampler (Model: Turbo Matrix 16, Perkin Elmer). The operating parameters of the chromatograph were as follows: head pressure at 25 psi, injector temperature at 95°C and detector temperature at 250°C. The oven temperature was held at 70°C for 1 min; the temperature was increased at increments of 4°C/min up to 150°C with a total cycle time of 5.5 min. The parameters of headspace sampler were as follows: sample temperature at 70°C, thermostat time of 30 min, needle temperature at 80°C, transfer line temperature at 90°C, pressurization time of 3 min. and injection time of 0.08 min. Determination of acetaldehyde was carried out in duplicate.
Statistical Analysis
For statistical analysis, SPSS for Windows (SPSS Inc., Chicago IL, USA) version 10 was performed on the nine batches and the corresponding replicates. Duncan test was used for mean comparison.
RESULTS AND DISCUSSION
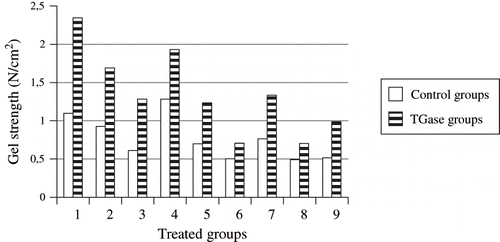
To improve the water holding capacity of the yogurt gel, generally an addition of dry matter sources or evaporated milk is used. TGase is a new alternative to develop the physical properties of yogurt gel. The cross-linking properties of some milk proteins such as casein, α-lactalbumin and β-lactoglobulin with TGase have previously been studied on the model systems.[Citation7,Citation21–23] However, there is a very few studies has been made of the possible industrial use of TGase. In our study, yogurt was manufactured from milk which was enriched with various milk products such as skimmed milk powder, whey, whole milk powder, sodium caseinate, calcium caseinate, whey protein concentrate (35 kg/100 kg dry solids and 60 kg/100 kg dry solids), and condensed milk with and without TGase. To determine the amounts of TGase to be added into the milk, protein analyses of all samples were made (). The results indicate that the condensed milk had the highest protein ratio compared to the others. The changes in gel strength, whey syneresis and amount of acetaldehyde of set style yogurt from untreated and TGase treated milk are shown in . It was observed that the syneresis of the enzyme treated yogurt samples was significantly reduced in comparison with the untreated samples. All products with TGase had higher gel strength and lower whey syneresis. According to statistical results, condensed milk and sodium caseinate yogurts showed the best whey syneresis properties according to the other supplements in yogurt with TGase while the whey syneresis from condensed milk yogurts were lower than the other supplements in untreated yogurt samples (). All supplements significantly affected the gel strength of the untreated yogurt samples (p < 0.05). However, there were no significant differences among sodium caseinate, condensed milk and calcium caseinate (p > 0.05) which had the best gel strength properties. Condensation of milk is one of the most important processes affecting the texture and consistency of yogurt. Heat treatment and condensation process promote their interactions with casein micelles via hydrophobic interactions and the formation of intermolecular disulfide bonds involving whey proteins and some types of caseins. This affects the physicochemical properties of the resulting yogurts.[Citation24] As shown in , although condensed milk had the highest protein ratio, sodium caseinate increased the strength of the TGase treated yogurt samples in comparison with condensed milk because of its' higher casein content. Similar effects of TGase on gel strength of yogurt with Na-caseinate were also reported previously.[Citation14] Lorenzen et al.[Citation14] performed the studies on the effect of enzymatic cross-linking of milk proteins with and without inactivation of the enzyme on the properties of set-type yogurt products. Skimmed milk was enriched with sodium caseinate (0.5–1.5%), with skimmed milk powder (1–3%) made from milk heated to high temperatures or with skim milk powder (1–3%) made from UHT milk. Gel strength of TGase treated samples with increasing dry matter was approximately twice as high as untreated samples, except the sample, which was fortified with skimmed milk powder from UHT milk. It is, therefore, concluded that the thermal impact during preheating might have reduced the number of available glutamine and lysine residues necessary for the action of TGase. Similar results are available from Faergemand et al.[Citation25] who observed higher values of firmness for the TGase treated yogurt with increasing enzyme concentration (up to 0.15%) and protein content (3.7, 4.3 or 4.7%). It was also concluded, in other studies,[Citation19,Citation26] that only a small amount of casein oligomerization was necessary for a significant enhancement of yogurt firmness. The enzymatic cross-linking changed the consistency of the yogurt gels.
Table 1 Protein amounts of all groups
Table 2 Gel strength, whey syneresis and acetaldetyde amounts of yogurt samples without (A) and with (B) TGase
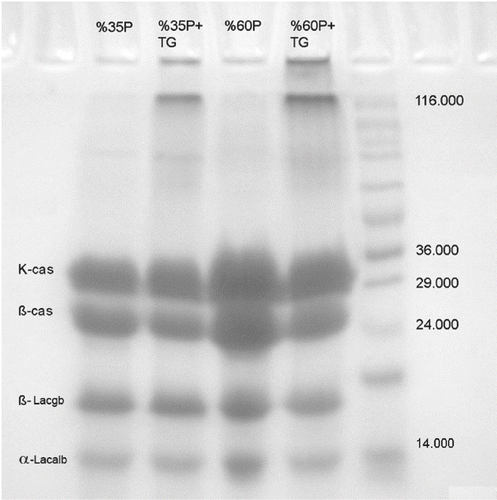
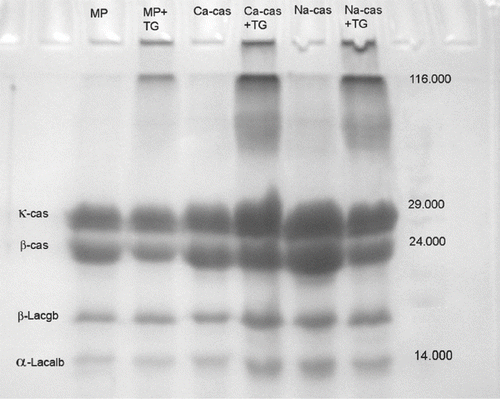
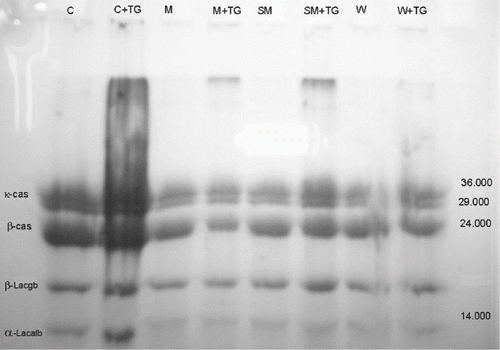
Figure 1 Gel strength of samples with or without transglutaminase treatment. 1 = Na-caseinate, 2 = Ca-caseinate, 3 = skim milk powder, 4 = condensed milk, 5 = whole milk powder, 6 = whey, 7 = 60% protein, 8 = 35% protein, 9 = milk.
There is general agreement in the literature that the aroma and flavor of yogurt are basically due to the production of non-volatile and volatile acids and carbonyl compounds. The typical yogurt flavor is caused by lactic acid, which imparts an acidic and refreshing taste, and a mixture of various carbonyl compounds like acetone, diacetyl, and acetaldehyde of which the latter is considered the major flavor component. In natural yogurt, acetaldehyde is the chemical compound most responsible for providing the characteristic “natural style” or “green apple-like” flavor.[Citation27] In this study, the amounts of acetaldehyde in the yogurt with TGase were lower than yogurt without TGase, except for yogurt made with condensed milk. Lorenzen et al.[Citation14] reported that the sensory properties of skimmed milk yogurt made from TGase-treated milk were less acidic, less intense in odor and taste and firmer than the control yogurt. Owing to the fact that the relationship between the conformational changes of proteins and their interactions with aroma components was previously reported,[Citation28] lower acetaldehyde levels were detected in the yogurt with TGase. However, there were non-significant differences between two experimental groups A and B (p > 0.05).
SDS–PAGE analysis revealed that crosslinking of milk proteins occurred in yogurt samples treated with TGase. Immobile protein polymers accumulated at the top of the lanes of the samples treated with TGase, while these extra bands could not be observed in the other samples (Fig ). Aboumahmoud and Savello[Citation8] reported that reduction of protein contents, appearance of new protein bands and accumulation of large molecular size proteins at the gel origin were the indication of the extent crosslinking. Lorenzen and Neve[Citation29] also discovered that the concentration of reactive lysine and glutamine residues could be increased by thermal modification of milk and, by performing SDS–PAGE of high temperature-heated skim milk proteins incubated with mTGase, observed a decrease in casein and whey protein monomers with increasing incubation time. Twenty minutes of enzyme treatment was sufficient to generate protein bands visible at the top of the gels, which were taken as an indicator for a sufficient amount of cross-linked dipeptides. This may explain the differences in band thickness between yogurt samples with and without TGase in our study. The electrophoretic analysis by SDS-PAGE showed two bands corresponding to the major whey proteins (α-lactalbumin and β-lactoglobulin), and two bands corresponding to caseins (β-casein and κ-casein).
CONCLUSIONS
From the data presented, it was shown that condensed milk and sodium caseinate appeared to be superior applications for yogurt production with and without TGase. In recent years, TGase has been used to catalyzing cross-linking of milk proteins. The results of our studies, it should be mentioned that condensed milk and sodium caseinate are the effective substrates for TGase. However the use of TGase treatment during yogurt preparation or the remaining of activity in food, must comply with the Novel Food Regulations.
ACKNOWLEDGMENT
We thank Prof. Dr. Ender Olcayto for editing of the manuscript.
Notes
15. Kirdar, S. Süt ve ürünlerinde analiz metotlari, SDU Yayin No: 18, Isparta, Turkey, 2001.
REFERENCES
- Meiying , Z. , Guocheng , D. and Jian , C. 2002 . pH Control Strategy of Batch Microbial Transglutaminase Production with Streptoverticillium mobaraense . Enzyme Microb Tech , 31 : 477 – 481 .
- Ikura , K. , Kometani , T. , Yoshikawa , M. , Sasakiand , R. and Chiba , H. 1980 . Crosslinking of Casein Components by Transglutaminase . Agr. Biol. Chem. , 44 : 1567 – 1573 .
- Nio , N. , Motoki , M. and Takinami , K. 1985 . Gelation Mechanism of Protein Solution by Transglutaminase . Agr. Biol. Chem. , 50 : 851 – 855 .
- Sakamoto , H. , Kumazawa , Y. and Motoki , M. 1994 . Strength of Protein Gels Prepared with Microbial Transglutaminase as Related to Reaction Conditions . J. Food Sci. , 59 : 866 – 871 .
- Faergemand , M. and Qvist , K.B. 1997 . Transglutaminase: Effect on Rheological Properties, Microstructure and Permeability of Set Style Acid Skim Milk Gel . Food Hydrocolloid , 11 : 287 – 292 .
- Faergemand , M. , Otte , J. and Qvist , K.B. 1997 . Enzymatic Crosslinking of Whey Proteins by a Ca+2 Independent Microbial Transglutaminase from Streptomyces lydicus . Food Hydrocolloid , 11 : 19 – 25 .
- Sharma , R. , Chr Lorenzen , P. and Qvist , K.B. 2001 . Influence of Transglutaminase Treatment of Skim Milk on the Formation of ε (γ-Glutamyl) Lysine and the Susceptibility of Individual Proteins towards Crosslinking . Int. Dairy J. , 11 : 785 – 793 .
- Aboumahmoud , R.M. and Savello , P.A. 1990 . Cross-linking of Whey Protein by Transglutaminase . J. Dairy Sci. , 73 : 256 – 263 .
- Traore , F. and Meunier , J.C. 1992 . Cross-linking Activity of Placental F Xiiia on Whey Proteins and Caseins . J. Agr. Food Chem. , 40 : 399 – 402 .
- Matsumura , Y. , Chanyongvorakul , Y. , Kumazawa , Y. , Ohtsuka , T. and Mori , T. 1996 . Enhanced Susceptibility to Transglutaminase Reaction of Alpha-Lactalbumin in the Molten Globule State . Biochim. Biophy. Acta , 1292 : 69 – 76 .
- Matsumura , Y. , Lee , D.S. and Mori , T. 2000 . Molecular Weight Distributions of a-Lactalbumin Polymers Formed by Mammalian and Microbial Transglutaminases . Food Hydrocolloid. , 14 : 49 – 59 .
- Ramirez-Suaraz , J.C. and Xiong , Y.L. 2003 . Effect of Transglutaminase-Induced Cross-Linking on Gelation of Myofibrillar/ Soy Protein Mixtures . Meat Sci. , 65 : 899 – 907 .
- Kuraishi , C. , Yamazaki , K. and Susa , J. 2001 . Transglutaminase: its Utilization in the Food Industry . Food Rev. Int. , 17 : 221 – 246 .
- Lorenzen , P.C. , Neve , H. , Mauther , A. and Schlimme , E. 2002 . Effect of Enzymatic Cross-Linking of Milk Proteins on Functional Properties of Set-Style Yogurt . Int. J. Dairy Technol. , 55 : 152 – 157 .
- 15. Kirdar, S. Süt ve ürünlerinde analiz metotlari, SDU Yayin No: 18, Isparta, Turkey, 2001.
- Tamime , A.Y. and Robinson , R.K. 1985 . Yogurt: Science and Technology , Oxford, , UK : Pergamon Press .
- Jumah , R.Y. , Abu-Jdayil , B. and Shaker , R.R. 2001 . Effect of Type and Level of Starter Culture on the Rheological Properties of Set Yogurt during Gelation Process . Int. J. Food Proper. , 4 ( 3 ) : 531 – 544 .
- Krasaekoopt , W. , Bhandari , B. and Deeth , H. 2005 . Comparison of Gelation Profile of Yogurts during Fermentation Measured by Rva and Ultrasonic Spectroscopy . Int. J. Food Proper. , 8 : 193 – 198 .
- Lauber , S. , Henle , T. and Klostermeyer , H. 2000 . Relationship Between the Crosslinking of Caseins by Transglutaminase and the Gel Strength of Yogurt . Eur. Food Res. Technol. , 210 : 305 – 309 .
- Sambrook , J. , Fritsch , E.F. and Maniatis , T. 1989 . Molecular Cloning, A Laboratory Manual , 2nd , New York : Cold Spring Harbor Laboratory Press .
- Imm , J.Y. , Liari , P. and Lee , C.M. 2000 . Gelation and Water Binding Properties of Transglutaminase-Treated Skim Milk Powder . J. Food Sci. , 65 : 200 – 205 .
- Lorenzen , P.C. , Schlimme , E. and Roos , N. 1998 . Crosslinking of Sodium Caseinate by a Microbial Transglutaminase . Nahrung , 42 : 151 – 154 .
- O'Connell , J.E. and de Kruif , C.G. 2003 . β-Casein Micelles: Cross-Linking with Transglutaminase . Colloid Surface A , 216 : 75 – 81 .
- El-Zahar , K. , Chobert , J.M. , Sitohy , M. , Dalgalarrondo , M. and Haertle , T. 2003 . Proteolitic Degradation of Ewe Milk Proteins during Fermentation of Yogurts and Storage . Nahrung , 3 : 199 – 206 .
- Faergemand , M. , Sorensen , M.V. , Jorgensen , U. , Budolfsen , G. and Qvist , K.B. 1999 . Transglutaminase: Effect on Instrumental and Sensory Texture of Set Style Yogurt . Milchwissenschaft , 54 ( 10 ) : 563 – 566 .
- Mautner , A. , Meisel , H. , Lorenzen , P.C. and Schlimme , E. 1999 . Bestimmung des Dipeptids e-(g-glutamyl) lysin aus Transglutaminasevernetzten Proteinen mittels Aminosäureanalyze . Kieler Milchwirtsch. Forschungsber , 51 : 155 – 163 .
- van Aardt , M. 2000 . “ Effect of Shelf-life and Light Exposure on Acetaldehyde Concentration in Milk Packaged in HDPE and PETE Bottles ” . Blacksburg, VA : Virginia Polytechnic Institute and State University, (VA TECH) . Msc Thesis
- Fares , K. , Kandy , P. , Guilard , R. and Vailley , A. 1998 . Physicochemical Interactions between Aroma Compounds and Milk Proteins: Effect of Water and Protein Modification . J. Dairy Sci. , 81 : 82 – 91 .
- Lorenzen , P.C. and Neve , H. . Enzymatic crosslinking of proteins in the manufacture of fermented milk . Fermented Milk, Proceedings of the IDF-seminar on Aroma & Texture of Fermented Milk . Kolding, Denmark. pp. 241 – 249 .