Abstract
During ohmic heating, the heating rate of a product depends on its electrical conductivity, a temperature dependent property. The electrical conductivity of the individual components in a multicomponent food system was determined over the entire sterilization temperature range (25−140°C). The product selected was chicken chowmein, composed of chicken, celery, mushrooms, water chestnuts, bean sprouts, and chowmein-style sauce. A device was developed to measure electrical conductivities of the components over the required temperature range. Results showed that the sauce (2.1 S/m at 27°C to 10.8 S/m at 140°C) was much more conductive than the solid components, i.e., celery (0.1 S/m to 3.4 S/m), water chestnut (0.1 S/m to 2.8 S/m), mushrooms (0.2 S/m to 1.4 S/m), bean sprouts (0.2 S/m to 1.5 S/m) and chicken (0.6 S/m to 3.4 S/m). Variation in electrical conductivity was also observed between different samples of the same component.
INTRODUCTION
In recent years, there has been considerable interest in combining ohmic heating with aseptic packaging to process shelf-stable meals containing particulates.[Citation1] Ohmic heating occurs due to energy dissipation when a current is passed through an electrically conducting food product. Most foods requiring thermal processing contain ionic constituents such as acids and salts; hence, conduct electricity.[Citation2] The rate of heating is directly proportional to the square of the electric field strength and the electrical conductivity.[Citation3] Advantages of ohmic heating lie in the uniform heating of both solid particles and the liquid phase, high energy efficiencies, and the realization of a high-temperature, short time process, which is often not deliverable by conventional means, due to heat transfer limitations.[Citation4]
The critical property influencing ohmic heating is the electrical conductivity of the food. Palaniappan and Sastry[Citation2] reported that electrical conductivity is a linear function of temperature, and the relationship can be expressed as:![]()
Some research has been done on the electrical conductivity of selected solid and liquid foods,[Citation2,Citation5–8] fruit based products,[Citation9–11] and meat products.[Citation12–15] However, relatively little attention has been focused on electrical conductivities of low acid particulate foods.
The present study aims to measure electrical conductivity of a low acid multiphase product as part of a larger effort to develop process filing protocols. The product selected for this study was chicken chowmein, which is used as a ration in the US Army (formulation[Citation16] shown in ). Since the local electrical conductivity of each constituent plays a critical role in how the total product heats, a total effective product electrical conductivity measurement is insufficient to describe the heating of individual components. Components with significantly different electrical conductivity may heat at different rates giving rise to non-uniform heating of the product. Although data exists on the electrical conductivity and its change during ohmic heating of some solid and liquid food components, there is no study comparing the electrical conductivity data of individual components in a multicomponent food product. Thus measurement of the electrical conductivity of each major constituent is important. Multiple samples should also be tested to account for lot to lot differences in the components.[Citation17]
Table 1 Formulation of the chicken chowmein
The objectives of this study were 1) to develop a device for electrical conductivity measurement extending up to a temperature of 140°C during ohmic heating; and 2) to measure and compare the electrical conductivity of the individual components of the chicken chowmein over the sterilization temperature range.
MATERIALS AND METHODS
Experimental Device
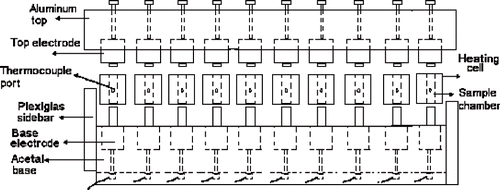
The experimental device is shown in . A base made from acetal was constructed in which ten electrodes were housed. The electrodes were all made from titanium and coated with platinum. An aluminum top was also constructed which could house ten electrodes. Ten ohmic heating cells were constructed from ultem. These cells had a cylindrical sample chamber through their centre which could be fitted over these base electrodes using O-rings. The food samples could then be sandwiched between the base and the top electrodes. A thermocouple opening was provided at the center of the cell to enable temperature measurements. Acrylic Plexiglas® side bars were screwed to the acetal base to support the aluminum top.
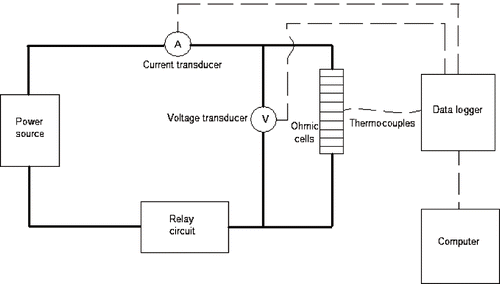
The schematic wiring diagram is shown in . A thermocouple (Cleveland Electric Laboratories, Twinsburg, OH, USA) was used to measure the temperature of each sample at its geometric center. The ohmic cells were connected to a relay switch which directed the order in which the cells were heated. Voltage (Ohio Semitronics, Hilliard, OH, USA) and current (Keithley Instruments Inc., Cleveland, OH, USA) transducers were used to measure the voltage across the samples and the current flowing through them. A data logger (Campbell Scientific Inc., Logan, UT, USA) linked to a computer was used to record voltage, current and temperature data at fixed time intervals. In this manner, ten food samples could be tested at a time in the ohmic heating device. The system was capable of operation at above atmospheric pressure, so that electrical conductivity could be measured at sterilization temperatures.
Methodology
Cylindrical samples of celery, chestnut, chicken and blanched mushroom (twenty samples each) were prepared using a slicer and a set of cork borers. The samples were 0.0079 m in length and 0.0078 m in diameter, which are also the dimensions of the sample chamber. The mushrooms were blanched in water at 100°C for 7 min to preshrink them to prevent their shrinkage during ohmic heating and a loss of contact at the electrodes. These samples were placed in the sample chamber in the heating cells and sandwiched between the electrodes. A thermocouple was then inserted into the cell through the thermocouple port and each sample was heated to 140°C using alternating current of 60Hz and voltage between 15 to 20 V. The temperature, voltage and current were measured continuously and recorded using the data logger linked to the computer. Similarly, twenty samples were also studied for the bean sprouts. However, the bean sprouts, due to their small size, were measured by packing as many of them as possible in the sample chamber and heating them to 140°C.
The chowmein style sauce was prepared using the formulation in , which has been derived from the US Army formulation.[Citation16] First starch slurry was prepared. All the other ingredients were then added to starch slurry and heated while continuously stirring till a brown thick sauce was obtained. This sauce was then poured into the sample chamber to test its conductivity up to 140°C. Twenty samples were studied for the sauce as well.
Table 2 Formulation of the chowmein style sauce
Analysis
The electrical conductivity of the samples was calculated using the dimensions of the cell, voltage and the current, using the formula:
Thereafter, the electrical conductivity was plotted against temperature to yield its electrical conductivity-temperature curve.
Error Estimation
The accuracy of each electrode set was tested, before and after the experiments, by determining the electrical conductivity of three different calibration salt solutions (conductivity standard solution 8974 μS/cm, 12880 μS/cm & 15000 μS/cm, OAKTON Instruments, Vernon Hills, IL, USA). The maximum difference between the measured and the reference value for any heating cell was 8.45%. The temperature at the center of the sample was used as the representative value, and was assumed to be spatially uniform because of the small size of the sample.
RESULTS AND DISCUSSION
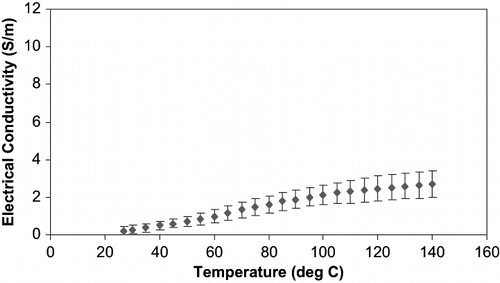
For all samples, electrical conductivity increased with temperature (), as is expected and consistent with literature data. One representative electrical conductivity-temperature curve (for celery) is shown in . Y-error bars shown are two standard deviations. Palaniappan and Sastry[Citation2] measured the electrical conductivity of selected vegetables, and observed that electrical conductivity increased with temperature. Similar observations have also been reported in other work in the literature.[Citation4,Citation7,Citation10,Citation18] The increase in electrical conductivity during heating of the biological tissue occurs due to increase in ionic mobility because of structural changes in the tissue like cell wall protopectin breakdown, expulsion of non conductive gas bubbles, softening, and lowering in aqueous phase viscosity.[Citation19,Citation20]
Table 3 The Electrical conductivity (S/m)Footnote∗ of samples measured at increasing temperatures
There was variation in electrical conductivity between different samples of the same component. In celery, the electrical conductivity varied between 2.25 to 3.41 S/m at 140°C. For water chestnuts, the values were between 1.35 to 2.71 S/m at 140°C. In bean sprouts, this value was between 0.74 and 1.47 S/m at 140°C. This difference may be attributed to the natural variation present in biological tissues. The air trapped in the pores of the vegetables could also lead to differences in the electrical conductivity.
For mushrooms the electrical conductivity at 140°C varied between 0.84 to 1.39 S/m. This difference was lower in magnitude than the other samples and might be attributed to the blanching step. The air in the pores was removed during the blanching process which leads to lesser variation in the samples. Electrical conductivity of chicken varied between 1.77 to 3.34 S/m at 140°C. This difference can be due to the natural biological variation as well as variations in fat content of the samples. The electrical conductivity of pork cuts has been studied and it was observed that lean is highly conductive compared to fat and addition of fat to lean reduced the overall conductivity.[Citation14] Therefore, chicken samples having higher fat content would have a lower electrical conductivity value and vice versa. Variation was also seen in the electrical conductivity of the chowmein style sauce, and may likewise be attributable to the variation in fat content of individual samples.
The electrical conductivity values for chicken obtained in our study are slightly higher than those reported in previous literature.[Citation2,Citation5] The difference might be attributed to the type of meat cut or the amount of fat present on the sample. The samples used in our study were from boneless and skinless chicken breast portion. The source of the chicken pieces has not been identified in the literature mentioned above. The difference between the electrodes used might also account for some of the variation as Palaniappan and Sastry[Citation2] used rhodium plated electrodes compared to our platinized titanium.
Mitchell and de Alwis[Citation5] reported the electrical conductivity of mushroom at 25°C which was lower than our measured values. This difference might be attributed to the type of mushroom used. Our study used white button mushrooms whereas the type of mushroom used in their study was not specified. Also, our study used blanched mushrooms which might further increase their electrical conductivity as the air was removed from the pores.
The conductivity temperature plots show that the chowmein style sauce was much more conductive than the vegetables or the chicken particles. This could lead to uneven processing during ohmic heating. The reason for the high electrical conductivity of the chowmein style sauce may be attributed to salt and highly conductive soy sauce present in it. The electrical conductivity values for soy sauce measured at increasing temperature (average of three samples) are as shown in . Variation in the electrical conductivity of chowmein style sauce was observed which again may be attributed to the variation in fat content of individual samples. The electrical conductivity of the chowmein style sauce varied between 5.8 to 8.8 S/m at 140°C.
CONCLUSIONS
A wide variation in the electrical conductivity of individual components of multicomponent food product like chicken chowmein was observed. Also variation was observed in the electrical conductivity between samples of the same constituent. If a sterilization process based on ohmic heating is to be successful, the formulation needs to be modified so that the components approach a nearly isoconductive state. This may be done by treatment of the solid phase via salt infusion.
NOMENCLATURE
| A | = |
Cross sectional area of the sample (m2) |
| I | = |
Current flowing through the sample (A) |
| L | = |
Length of the sample (m) |
| m | = |
Temperature compensation constant |
| T | = |
Temperature (°C) |
| Tref | = |
Reference temperature (°C) |
| V | = |
Voltage across the sample (V) |
| σ | = |
Electrical conductivity (S/m) |
| σ ref | = |
Electrical conductivity at reference temperature (S/m) |
| σ T | = |
Electrical conductivity at any temperature (S/m) |
ACKNOWLEDGMENTS
Support provided by the USDA National Integrated Food Safety Initiative Grant No. 2003‐51110‐02093, titled: Safety of foods processed using four alternative processing technologies, is gratefully acknowledged. Salaries and research support provided in part by the Ohio Agricultural Research and Development Center, through the Regional Research Project NC-1023. References to products and trade names are made with the understanding that no endorsement or discrimination by The Ohio State University is implied.
REFERENCES
- Zoltai , P. and Swearingen , P. 1996 . Product Development Considerations for Ohmic Processing . Food Technol. , 50 : 263 – 266 .
- Palaniappan , S. and Sastry , S.K. 1991 . Electrical Conductivities of Selected Solid Foods during Ohmic Heating . J. Food Proc. Engr. , 14 : 221 – 236 .
- Sastry , S.K. and Palaniappan , S. 1992 . Mathematical Modeling and Experimental Studies on Ohmic Heating of Liquid-particle Mixtures in a Static Heater . J. Food Proc. Engr. , 15 : 241 – 261 .
- Zareifard , M.R. , Ramaswamy , H.S. , Trigui , M. and Marcotte , M. 2003 . Ohmic Heating Behavior and Electrical Conductivity of Two-phase Food Systems . Innovative Food Science and Emerging Technologies , 4 : 45 – 55 .
- Mitchell , F.R.G. and de Alwis , A.A.P. 1989 . Electrical Conductivity Meter for Food Samples . J. Phys. E. , 22 : 554 – 556 .
- Halden , K. , de Alwis , A.A.P. and Fryer , P.J. 1990 . Changes in the Electrical Conductivity of Foods during Ohmic Heating . Int. J. Food Sci. Tech. , 25 ( 1 ) : 9 – 25 .
- Palaniappan , S. and Sastry , S.K. 1991 . Electrical Conductivity of Selected Juices: Influences of Temperature, Solids Content, Applied Voltage, and Particle Size . J. Food Proc. Eng. , 14 : 247 – 260 .
- Henningsson , M. , Ostergren , K. and Dejmek , P. 2005 . The Electrical Conductivity of Milk—the Effect of Dilution and Temperature . Int. J. Food Prop. , 8 : 15 – 22 .
- Castro , I. , Teixeira , J.A. , Salengke , S. , Sastry , S.K. and Vicente , A.A. 2003 . The Influence of Field Strength, Sugar and Solid Content on Electrical Conductivity of Strawberry Products . J. Food Proc. Engr. , 26 ( 1 ) : 17 – 29 .
- Castro , I. , Teixeira , J.A. , Salengke , S. , Sastry , S.K. and Vicente , A.A. 2004 . Ohmic Heating of Strawberry Products: Electrical Conductivity Measurements and Ascorbic Acid Degradation Kinetics . Innovative Food Science and Emerging Technologies , 5 ( 1 ) : 27 – 36 .
- Icier , F. and Ilicali , C. 2004 . Electrical Conductivity of Apple and Sour Cherry Juice Concentrates during Ohmic Heating . J. Food Proc. Engr. , 27 ( 3 ) : 159 – 180 .
- Wu , H. , Kolbe , E. , Flugstad , B. , Park , J.W. and Yongsawatdigul , J. 1998 . Electrical Properties of Fish Mince during Multi-frequency Ohmic Heating . J. Food Sci. , 63 ( 6 ) : 1028 – 1032 .
- Piette , G. , Buteau , M.L. , De Halleux , Chiu , L. , Raymond , Y. , Ramaswamy , H.S. and Dostie , M. 2004 . Ohmic Cooking of Processed Meats and its Effects on Product Quality . J. Food Sci. , 69 : E71 – 78 .
- Shirsat , N. , Lyng , N.G. and Brunton , N.P. 2004 . Ohmic Processing: Electrical Conductivities of Pork Cuts . Meat Sci. , 67 : 507 – 514 .
- Saif , S.M.H. , Lan , Y. , Wang , S. and Garcia , S. 2004 . Electrical Resistivity of Goat Meat . Int. J. Food Prop. , 7 ( 3 ) : 463 – 471 .
- Yang , T. and Dunne , P. 2004 . Personal Communication by phone and e-mail. ,
- Larkin , J.W. and Spinak , S.H. 1996 . Safety Considerations for Ohmically Heated, Aseptically Processed, Multiphase Low-acid Food Products . Food Technol. , 50 : 242 – 245 .
- Reznik , D. 1996 . Ohmic Heating of Fluid Foods . Food Technol. , 50 : 250 – 251 .
- Bean , E.C. , Rasor , J.P. and Porter , G.C. 1960 . Changes in Electrical Conductivities of Avocados during Ripening . Year Book of Californian Avocado Society , 44 : 75 – 78 .
- Sasson , A. and Monselise , A.P. 1977 . Electrical Conductivity of “Shamouti” Orange Peel during Fruit Growth and Postharvest Senescence . J. Am. Soc. Horti. Sci. , 102 ( 2 ) : 142 – 144 .