Abstract
The individual and interactive effects of temperature (20–50°C), and total soluble solids (TSS) (50–70°Brix), on rheological characteristics of sumac (Rhus coriaria) concentrate were studied using a computer controlled rheometer. Sumac concentrate exhibited Newtonian characteristics for TSS of 50–60°Brix, with a slight deviation corresponding to 70°Brix. The rheological parameters met the criteria of good fit (r2 > 0.9983) and provided information for characterizing the effect of temperature and TSS on the flow behavior of sumac concentrate. It was observed that TSS, and temperature considerably affected the apparent viscosity index individually.
INTRODUCTION
Sumac, or summac, or sumach, is the name given to numerous shrubs and small trees of the botanical genus Rhus (family Anacardiaceae), which comprises about 150 species, natives chiefly in warm regions.[Citation1,Citation2,Citation3] Rhus coriaria, also known as tanner's sumac, is native to the Mediterranean region with red-colored fruits or berries or drupes. Sumac is very popular in the Mediterranean and Arabic countries and is utilized mainly as condiment. The berries can be dried, ground and sprinkled into the cooking, or macerated in hot water and mashed to release their juice, the resulting liquid being used as one might use lemon juice.
The juice extracted from sumac is popular in salad dressings and marinades but it was observed to be very susceptible to moulding at concentration below 50% total soluble solids and it was therefore concentrated to 60–70° Brix to avoid any microbial deterioration. The product prepared this way is named as sumac concentrate in this study.[Citation4]
Processing of juices from fruits is a complex operation with many variables that influence the final product quality. In addition to chemical and physical properties, the rheological behavior of fruit juices is important for the design of processing equipment, quality control during processing, consumer acceptance, and understanding of the structure of food and raw agricultural materials. The viscosity of food products cannot be predicted theoretically, due to complicated physical and chemical structures. Therefore, experimental measurements of viscosity are necessary for the characterization of fluid foods. In practice, rheology stands for viscosity measurements, characterization of flow behavior, and determination of material structure. Basic knowledge of this subject is essential process design and product quality evaluation[Citation5,Citation6].
Rheological measurements have also been considered as an analytical tool to provide fundamental insights on the structural organization of food. Numerous studies have been conducted on the rheological properties of fruit and vegetable products.[Citation7,Citation8,Citation9,Citation10,Citation11,Citation12] concentrates include temperature[Citation13,Citation14,Citation15], total soluble solids (TSS), [Citation7,Citation16,Citation17] particle size[Citation18,Citation19], addition of enzyme[Citation8], and pH[Citation20]. Various rheological models have been used to represent the flow behavior of fluid food foods. The power law model is one most commonly used to describe the flow of food products without yield stress, while Casson and Herschel-Bulkley models have been employed for foods with definite yield stress. Fruit juices are mostly Newtonian in nature and commonly represented by power law model (flow behavior index = 1.0). Fruit juice concentrates and purees with higher total soluble solids have been well described by Herschel-Bulkley model.
The viscosity of food products cannot be predicted theoretically, due to their complicated physical and chemical structures. Experimental measurements are necessary for the rheological characterization of fluid foods. The present study was undertaken to accomplish the task of “characterization of rheological behavior of sumac concentrate”.[Citation21]
MATERIALS AND METHODS
Samples
Sumac concentrate was prepared from sumac berries which were initially screened to remove any small immature fruits and foreign matter such as dust, leaf, and soil. The berries were macerated for 24 h in distilled water at 25°C followed by filtration to separate any suspended particles, and obtain a clear extract with bright red color. This extract was then concentrated to a total soluble solids(TSS) of 60–70° Brix using two types evaporators[a rotary evaporator (Bibby, RE 100, Rotary Evaporator) and a laboratory type rising film evaporator (Armfield FT22-A, Rising Film Evaporator) at a vacuum pressure of 30 mm Hg. Samples with lower TSS were prepared by diluting the concentrated juice with distilled water.
Analytical Measurements
Percent soluble solids and colour values of the samples were determined using Pocket PAL-1, Pocket Refractometer, ATAGO, and a HunterLab Colorflex (A60–1010–615 HunterLab, Reston, VA, USA) model colorimeter respectively. Density measurements were determined using densometer. pH was measured by Janway 3010 pH meter. Moisture content was determined by using vacuum oven.
Total Monomeric Anthocyanin by the pH-Differential Method
Monomeric anthocyanin contents of samples were determined using the pH-differential method.[Citation22]
First of all spectrophotometer was turned on. The instrument was allowed to warm up at least 30 min before taking measurements. The appropriate dilution factor was determined for the sample by diluting with potassium chloride buffer, pH 1.0, until the absorbance of the sample at the λvis-max was within the linear range of the spectrophotometer (Perkin Elmer, USA). Final volume of the sample is divided by the initial volume to obtain the dilution factor.
After obtaining dilution factor, the spectrophotometer was made zero with distilled water at all wavelengths that will be used (λvis-max and 700 nm).
Two dilutions of the sample were prepared, one with potassium chloride buffer, pH 1.0, and the other with sodium acetate buffer, pH 4.5, diluting each by the previously determined dilution factor. These solutions are equilibrated for 15 min. The absorbance of each dilution are measured at the 523 nm and at 700 nm (to correct for haze), against a blank cell filled with distilled water.
Finally results are recorded and absorbance of the diluted sample and monomeric anthocyanin pigment concentration in the original sample were calculated based on cyaniding-3-glucoside with molecular weight of 445.2 and extinction coefficient of 29,600.[Citation22]
Rheological Measurements
The rheological characteristics of sumac concentrate were evaluated using a RheoStress RSI (Haake, Karlsruhe, Germany) model, stress rheometer. The temperature of the bottom plate was controlled with TCP/P peltier unit and a thermostat. A thin film of a vegetable oil was applied to the sample surfaces to prevent any evaporation loss due to heat application. The measuring system was a cone and plate sensor with a 3.5 cm diameter and an angle of 2°. Shear rate was in the range of 0–600 s−1. The rheological behaviors of samples with total soluble solids (TSS) of 50–70 Brix were studied at 20, 30, 40, and 50°C.
Experimental results were analyzed by using a Rheowin Pro Data Manager Version 2.64 (Rheowin, Haake, Karlsruhe, Germany).
Statistical Analysis
The parameters of the models were calculated by the NLIN procedure of the Sigma Plot (Scientific Graph System, version 8.00).
A one-way analysis of variance (ANOVA) was conducted to determine the effect of concentration and temperature on the viscosity of the sumac concentrate using SPSS 8.0. To determine which means are significantly different from each other, the least significance difference (LSD) multiple range test method was used. Trends were considered significant when the means of compared parameters differed at p < 0.05 significance level.
The effect of total soluble solid and temperature, on the constants of Herschel-Bulkley model, which were K and n were determined with the application of multiple regression using STATGRAPHICS Plus 5.1 version.
RESULTS AND DISCUSSION
General Properties of Sumac Concentrate
Knowledge of physical and chemical properties of foods is fundamental in the design and control of food processes, and in quality control assessment. Physical and chemical characteristics of the sumac concentrate are presented in .
Table 1 Analytical data for 70°Brix sumac concentrate
Rheological Characteristics of Sumac Concentrate
Firstly, in order to determine the rheological behaviour of sumac concentrate, Newtonian equation, EquationEq. (1)(1) as applied. EquationEq. (1)
(1) is as follows:
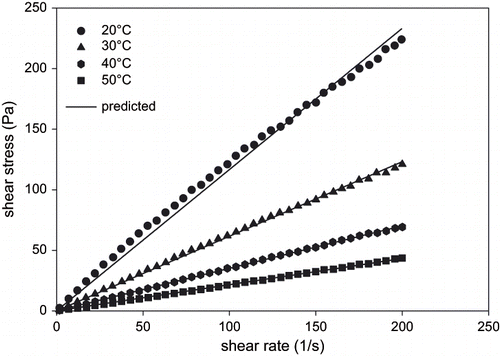
From this law, flow types were determined. A slight deviation from linearity for 70° Brix indicated the existence of a very small yield stress.
In addition to the power law, the generalized Herschel-Bulkley model have proven useful in developing mathematical models to solve food process engineering problems involving wide shear rate ranges.[Citation23] The flow behavior of sumac concentrate was evaluated firstly by fitting the shear stress-shear rate data to this model.
Table 2 Parameters of the Hershley-Bulkey model describing dependency of viscosity TSS at different temperatures for sumac concentrate
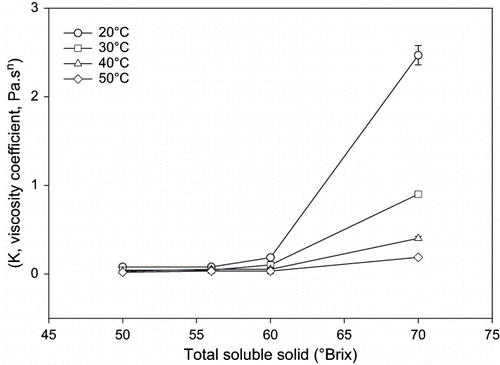
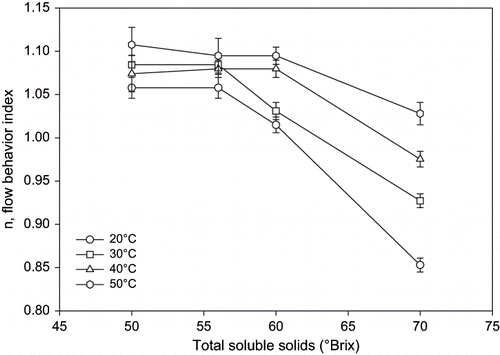
For only 70° Brix sumac concentrate, there was a significant effect of total soluble solid on the flow behavior (p < 0.05). There was a significant increase of viscosity coefficient at this concentrate. Also a slight decrease was observed in the flow behavior index, n; only at 70°Brix concentration. Mostly, flow behavior index is approximately 1.0 for fruit concentrates.
When the results presented in are examined together with , sumac concentrate seems to exhibits a slight yield stress (σ0 = 3.05 pa) with shear-thinning behavior (n = 0.85) corresponding to the most concentrated (70°Brix) sample at the lowest temperature (20°C) studied. As expected, dilution gave rise to conform to the Newtonian behavior.
It should be pointed out that the existence of yield stresses is controversial; they may be artifacts resulting from high Newtonian viscosity at low shear rates as it was the case here. The rest of the more dilute samples showed essentially Newtonian behaviors as it is apparent from the linearity of the curves in .[Citation24] For this reason the same data were also fitted to power law model which is a special case of Herschel-Bulkley model with no shear yield. R2 values were higher than 0.99 for both models.
Effect of TSS on Rheological Behaviour of Sumac Concentrate
Variation of viscosity of sumac concentrate with TSS is presented in for four different temperatures. Concentration of both soluble and insoluble solids is reported to have a strong effect on the viscosity of Newtonian fluids or the consistency coefficient and the apparent viscosity of non-Newtonian fluid foods.[Citation5] As expected, this effect is more noticeable at lower temperatures.
Table 3 Viscosity values of the sumac concentrate obtained from Newtonian model at different TSS and temperature
Two different models were used to evaluate the variation of viscosity of sumac concentrate with TSS: The power law model,
and the exponential model,
Here, η, and C are the viscosity and concentration in Pa.s and °Brix, respectively, while η1, η2, b1 and b2 are the parametric constants to be determined from the fitted data.
The numerical values of the parameters appearing in EquationEqs. (3)(3) and Equation(4)
(4) are given in and respectively for a temperature range including 20, 30, 40, and 50°C. The exponential model was observed to give a slightly better fit than the power law as it was deduced from the higher R2 values (0.997 vs. 0.994) at each temperature in question. It can be observed that; the b2 parameter decreases as the temperature increases, which indicates that the effect of the effect of the concentration on viscosity is more pronounced at lower temperatures.[Citation25] This is in agreement with the fact that, the power law equation tends to give better results for puree-type foods, whereas the exponential model is used in concentrated fruit juices having small amounts of insoluble solids.[Citation5,Citation26]
Table 4 Parameters of the power law model describing dependency of viscosity on TSS at different temperatures for sumac concentrate
Table 5 Parameters of the exponential model describing dependency of viscosity on TSS at different temperatures for sumac concentrate
Effect of Temperature on Rheological Behaviors of Sumac Concentrate
The variation of viscosity with temperature could be described by Arrhenius type relationship:
Table 6 Parameters of Arrhenius equation for sumac concentrate with different total soluble solids
Combined Effect of Temperature and Concentration on Rheological Behaviors of Sumac Concentrate
It is very useful to obtain a simple equation describing the combined effect of temperature and concentration on the material's viscosity.[Citation5]
To evaluate the effect of both the temperature and the soluble solid content on the viscosity of sumac concentrate, two models were used:
Finally the equations which allow the viscosity values to be obtained at different temperatures and soluble solid content for the sumac concentrate were proposed as:
It seems that the EquationEq. (8)(8) gives slightly better fit than the EquationEq. (9)
(9). Since it has higher values of R2 coefficients. Therefore, EquationEq. (9)
(9) was selected to show the combined effect of soluble solids and temperature.
When multiple regression analysis were performed for the viscosity coefficient (K value) and flow behaviour index (n); both total soluble solid and temperature have significant effect at p < 0.05 significance level.
Each temperature at each concentration has significant effect (p < 0.05) on the K values. For 56 and 50 Brix sumac concentrates; flow behavior index (n value) were not significantly different, but for 70 and 60 brix sumac concentrates, were significantly different from each other and also, significantly different from 56 and 50 Brix sumac concentrates (p < 0.05).
CONCLUSIONS
Sumac concentrate, which can be classified as a clarified fruit juice, exhibited Newtonian characteristics in the concentrationtion (50–60°Brix) and temperature (20–50°C) ranges studied with a slight deviation from this behaviour corresponding to 70°Brix at 20°C. The dependence of viscosity on temperature correlated well with an Arrhenius-type relationship. This dependency was observed to increase with TSS as expected. The activation energy values increased with increasing soluble solids concentration. The effect of soluble solids on viscosity was best described by an exponential model.
The data fitted well to the Herschel-Bulkley and power law models with R2 values being greater than 0.99 for all cases. A single equation was formulated to indicate the combined effect of soluble solids and temperature on viscosity.
ACKNOWLEDGMENT
The authors are grateful to Prof. Dr. Ahmet Kaya for his helps in this study for the usage of rheometer and also for the evaluation of data.
Notes
2. Bayram, Ö. Spray Drying of Sumac Flavor using Different Carriers. M.S. Thesis, April 2000. University of Gaziantep, Graduate School of Natural & Applied Sciences, Turkey.
REFERENCES
- 1957 . Encyclopedia Britannica , Chicago : Chicago Press . “Sumac”
- 2. Bayram, Ö. Spray Drying of Sumac Flavor using Different Carriers. M.S. Thesis, April 2000. University of Gaziantep, Graduate School of Natural & Applied Sciences, Turkey.
- Zalacain , A. , Pradanov , M. , Carmona , M. and Alonso , G.L. 2003 . Optimisation of Extraction and Identification of Gallo Tannins from Sumac Leaves . Biosystems Engineering , 84 ( 2 ) : 211 – 216 .
- MacCarthy , D. 1986 . Concentration and Drying of Foods , Elsevier Science Publishing Co., Inc. .
- Juszczak , L. and Fortuna , T. 2003 . Viscosity of Concentrated Strawberry Juice. Effect of Temperature and Soluble Solids Content . Electronic Journal of Polish Agricultural Universities Series Food Science and Technology , 6 ( 2 )
- Kaya , A. and Sözer , N. 2005 . Rheological Behaviour of Sour Pomegranate Juice Concentrates (Punica Granatum L.) . International Journal of Food Science and Technology , 40 : 223 – 227 .
- Rao , M.A. 1977 . Rheology of Liquid Foods—A Review . Journal of Texture Studies , 8 : 135 – 168 .
- Khalil , K.E. , Ramakrishna , P. , Nanjundaswamy , A.M. and Patwardhan , M.V. 1989 . Rheological Behaviour of Clarified Banana Juice: Effect of Temperature and Concentration . Journal of Food Engineering , 10 : 231 – 240 .
- Truong , V.D. and Walter , W.M. 1994 . Physical and Sensory Properties of Sweet Potato Puree Texturized with Cellulose Derivatives . Journal of Food Science , 59 : 1175 – 1180 .
- Godfrey , U.A.M. , Bourne , M.C. and Rao , M.A. 1995 . Blanch Temperature Time Effects on Rheological Properties of Applesauce . Journal of Food Science , 60 : 1289 – 1291 .
- Bhattacharya , S. 1999 . Yield Stress and Time Dependent Rheological Properties of Mango Pulp . Journal of Food Science , 64 : 1029 – 1033 .
- Ahmed , J. , Shivhare , U.S. and Debnath , S. 2002b . Colour Degradation and Rheology of Green Chilli Puree during Thermal Processing . International Journal of Food Science and Technology , 37 : 57 – 64 .
- Saravacos , G.D. 1970 . Effect of Temperature on Viscosity of Fruit Juices and Purees . Journal of Food Science , 35 : 122 – 125 .
- Holdsworth , S.D. 1971 . Applicability of Rheological Models to the Interpretation of Flow and Processing Behaviour of Fluid Food Products . Journal of Texture Studies , 2 : 393 – 418 .
- Vitali , A.A. and Rao , M.A. 1984 . Flow Properties of Low Pulp Concentrated Orange Juice: Effect of Temperature and Concentration . Journal of Food Science , 49 : 882 – 888 .
- Harper , J.C. and El-sahrigi , A.F. 1965 . Viscometric Behaviour of Tomato Concentrates . Food Technology-Chicago , 30 : 470 – 476 .
- Hernandez , E. , Chen , C.S. , Johnson , J. and Carted , R.D. 1995 . Viscosity Changes in Orange Juice after Ultrafiltration and Evaporation . Journal of Food Engineering , 25 : 387 – 396 .
- Tanglertpaibul , T. and Rao , M.A. 1987 . Rheological Properties of Tomato Concentrate as Affected by Particle Size and Methods of Concentration . Journal of Food Science , 52 : 141 – 145 .
- Ahmed , J. , Shivhare , U.S. and Singh , G. 2000 . Chlorophyll and Colour of Green Chilli Puree as Affected by Mesh Size and Temperature . International Journal of Food Properties , 3 : 305 – 315 .
- Dik , T. and Ozilgen , M. 1994 . Rheological Behaviour of Bentonite-Apple Juice Dispersions . Lebensm. Wiss. Technology , 27 : 55 – 58 .
- Juszczak , L. and Fortuna , T.R. 2004 . Effect of Temperature and Soluble Solid Content on the Viscosity of Cherry Juice Concentrate . International Agrophysics , 18 : 17 – 21 .
- Giusti , M.M. and Wrolstad , R.E. 2001 . “ Characterization and Measurement of Anthocyanin by UV-Visible Spectroscopy ” . In Current Protocols in Food Analytical Chemistry , 1 – 13 . New York : Wiley .
- Steffe , J.F. 1992 . Rheological Methods in Food Process Engineering , 2nd , East Lansing : Freeman Press .
- Barnes , H.A. and Walters , K. 1985 . The Yield Stress Myth? . Rheologica Acta. , 24 : 323 – 326 .
- Ibarz , A. 1994 . Rheology of Clarified Fruit Juices. III. Orange Juices . Journal of Food Engineering , 21 : 485 – 494 .
- Ibarz , A. , Macro , F. and Pagon , J. 1993 . Rheology of Permission Juices . Fruit Processing , 5 : 182 – 187 .