Abstract
The precipitation of proteins due to the changes in pH has been a major limiting factor in their utility especially when the precipitation is concurrent with irreversible aggregation. In the present study, an attempt is made to see the effect of glycerol on the pH-induced aggregation of α- globulin which is the major protein fraction (11S) from Sesame (Sesamum indicum L.) seeds. A second order polynomial relation existed between the cosolvent concentration and precipitation which was prevented in presence of the cosolvent. Similarly, there was a second order polynomial relation between 8-anilino 1-naphthalene sulfonic acid (ANS) binding of the protein (as indicated by fluorescence emission at 466 nm) and the cosolvent concentration. The relative precipitation in presence of glycerol is however linearly proportional to the changes in surface hydrophobicity as seen by behavior of ANS with the protein in presence of the cosolvent. A possible role of the cosolvents in prevention of aggregation due to hydrophobicity of the protein is envisaged and the relation between the different parameters is discussed.
INTRODUCTION
The precipitation of proteins during the processing has been a major hindrance in their utility. This is especially the limiting factor when the protein in food stuff has to undergo changes in the pH during the processing steps. Thus, the aggregation of food proteins as a process is of interest to both the food processing industry and the scientists who are looking for the prevention or initiation of such process. It is imperative that effect of the food system on this process (especially in the presence of other major components and inclusions like sugars, etc.) would be an area of major interest since this signifies the role of available components within the system and has an implication on food properties.
α-globulin is the major oilseed storage protein in Sesame (Sesamum indicum L.) seeds which has been well characterized and its behavior in various systems well documented.[Citation1,Citation2,Citation3,Citation4] It is also used extensively to represent the protein in the food systems. It is reported to consist of six acidic and six basic subunits arranged in a hexagonal configuration.[Citation5] These subunits are predominantly held by hydrophobic interactions, [Citation2] which render it susceptible to aggregation.
The effect of cosolvents (sugars and polyols) on the stability of the proteins is well known. [Citation6,Citation7] Similarly, the interaction of α-globulin with sugars and polyols has been studied and these have been shown to decrease the heat-induced coagulation of α- globulin.[Citation8] These have been shown to induce association-dissociation of the protein.[Citation8,Citation9] The increased stability of the protein in presence of cosolvents has been also reported.[Citation9] However, there is no information on the impact of these cosolvents on the acid precipitation of α-globulin. Pintado and Malcata[Citation10] have shown that acid precipitation can be optimized to obtain whey proteins from milk. Recently, aggregation induced by various salts and their effect on soy protein, which is also an oilseed protein are reported in literature.[Citation11] The present study investigates the possible interference or enhancement effect of the cosolvents on an acid precipitating system and attempt to elucidate the factors responsible for the effect. These have a profound effect on the food properties and its manifestation in the product formulations.
The possibility of the interference of the cosolvents during the precipitation of the protein from acidic as well as alkaline pHs to isoelectric pH is also looked into to see if the effect is independent of pH and similar factors. This is especially important since the seed proteins are more amenable to enzymatic hydrolysis and are comparably more soluble at extreme pHs compared to neutral pH. Thus, the study focuses on the prevention of the aggregation process in glycerol and the mechanism involved therein.
MATERIALS AND METHODS
Materials
Glycerol and 8-anilino 1-naphthalene sulphonic acid were obtained from Sigma Chemical Co., (St. Louis, Missouri, USA). Hydrochloric acid, acetone, sodium chloride, sodium acetate and sodium hydroxide were all AR grade and procured from Qualigens Ltd., (Mumbai,India). Glycine was from E-Merck India (P) Ltd., (Mumbai, India). Triple glass distilled water was used in all of the experiments.
Isolation of Protein
α-globulin was isolated from the seeds of Sesamum indicum L. of the white variety according to the procedure of Prakash and Nandi.[Citation1] The purity of the protein was found to be >99.5%, as determined by biophysical techniques. The isolated protein was lyophilized and stored at 4°C. This protein was used in all experiments.
Precipitation of Protein
The stock protein was prepared by dissolving lyophilized protein powder in 0.02 M sodium acetate buffer (pH 4.0). The solution was centrifuged at 10000 × g in Biofuge Model 15 R for 30 min. and the clear supernatant was dialyzed extensively (at least for 24 h) against the same buffer. This was again centrifuged and the supernatant was used as the stock protein. The stock of the glycerol were prepared in distilled water. Six ml of the cocktail was prepared containing the same amount of protein but various amount of glycerol [(w/v), %] and brought to a final concentration using 0.02 M sodium acetate buffer. To 6 ml of the protein, without any cosolvent, 0.1 N NaOH was added in ten installments till the pH reached 4.9 (isoelectric pH of α-globulin). The exact amount of NaOH needed to reach pH 4.9 was determined. Stirring was continued for another 1 min and then the samples were incubated for 12 hrs at 30 ± 1°C. The pH was also checked again. The samples were centrifuged at 10,000 × g for 30 min. and the protein concentration in the supernatant was determined by measuring the absorbance at 280 nm against the corresponding blank in a Shimadzu UV-Visible double-beam Spectrophotometer. The amount of protein precipitated was calculated as the following equation:
where P is precipitation in presence of cosolvent; P0 is precipitation in control; Ci is the initial protein concentration; Cc is the concentration of the protein in the supernatant of control; and Cs is the concentration of the protein in the supernatant of the sample with cosolvent. Similarly, the protein was prepared in glycine – NaOH buffer and was precipitated with 0.1 N HCl.
Fluorescence Spectra of Protein in the Presence of Glycerol
The protein was dialyzed extensively and centrifuged for 30 minutes in a Hitachi centrifuge at 6000 × g. The concentration of protein was adjusted to 1.2 × 10−6 M by measuring the A 280 nm. and this was used as a stock. Stock solutions of 50% glycerol was prepared. A cocktail of the buffer, stock protein or model compound and stock cosolvent was prepared to give the identical protein concentration in various concentrations of the glycerol. The cocktails were incubated at 30 ± 1°C for 3 h in a Innova shaker. After the incubation the samples excited at 280 nm and the emission spectra recorded between 300–400 nm at 30 ± 1°C. A slit width of 3 nm for excitation and 5 nm for emission was used in a Shimadzu Spectrofluorimeter RF-500.
Fluorescence of ANS Protein Complex in Presence of Glycerol
Samples of the protein in 0.02 M glycine-NaOH buffer with various concentration of glycerol and a final protein concentration of 7.5 × 10−7 M were prepared and incubated for 3 hrs at 30°C. Corresponding blanks without the protein were also prepared. A 1 mM stock solution of 8-anilino 1-naphthlene sulphonic acid (ANS) was also prepared in the same buffer. To 2.5 ml of the protein, the ANS was added in installments of 5 microlitre with constant stirring. The fluorescence spectra of the samples were monitored under time scan in a Shimadzu RF 5000 spectrofluorimeter. The emission intensity at 466 nm was recorded 1 min after excitation at 365 nm. Slit width for both emission and excitation was fixed at 5 nm. The fluorescence intensities were corrected for the dilution factor and also subtracted from the corresponding blanks which were titrated with ANS in a similar manner. The amount of ANS required to saturate the required protein was determined. This volume of ANS was added to the different combinations of glycerol and protein. The relative fluorescence intensity was calculated by taking the intensity of the control sample as 100.
RESULTS AND DISCUSSION
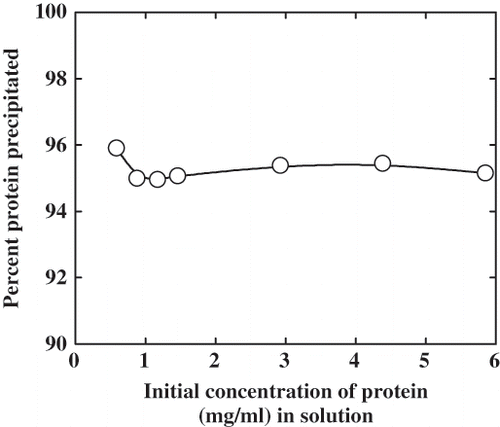
Effect of Protein Concentration on the Precipitation
The effect of protein concentration on the precipitation is shown in . It is seen that there is a linear relationship in the protein concentration and precipitation. This shows that the process would be finite till all the hydrophobic surfaces which are amenable to aggregation are buried in the aggregates. This also indicates that such a process would be dependent on the microstructure of the protein, i.e., the number of hydrophobic patches that are susceptible to aggregation. It is assumed that the protein surface is not entirely hydrophilic but a mosaic of hydrophobic and hydrophilic patches as proposed by Jesior.[Citation12] Once such patches are buried in the interior of the aggregate, they would have lesser accessibility. The constant amount of precipitation as a percentage also reiterates this view. The unprecipitated protein would account for those molecules, which do not reach the critical density/size for precipitation due to small variations in their surface structure. This could also be accounted by the aggregates of α-globulin which are already formed and have no reactive patches prior to the precipitation, due to micro heterogeneity of the system.
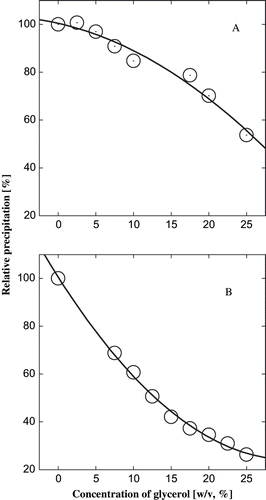
Effect of Glycerol on the Protein Precipitation
The inclusion of glycerol interfered with the precipitation of the protein (). The addition of glycerol had a negative impact on the process of aggregation. There was a second order polynomial relation between the glycerol concentration and precipitation under both conditions. The correlation coefficient R2 was 0.96 for the glycerol concentration and precipitation from alkaline to pI and was 0.98 for the precipitation from acidic pH to pI (). The increasing amount of cosolvent interfered with the aggregation as seen in the decreasing amount of precipitation with the addition of the acid or alkali to the system. The difference in the type of curves of precipitation would be due to the difference in the architecture of the protein in the two pHs, while the similarity is due to the common mechanism underlying both. The initial linear relation between protein concentration and precipitation () does not hold true once the cosolvent is introduced into the system. It is due to the possibility that the effect of the cosolvent on the hydrophobic surface of the protein is not linear. This is because as the cosolvent concentration is increased, there is less and less of hydrophobic residues on the surface to be pushed into the interior. Finally, the saturation is reached where the hydrophobic residues are no more amenable to come under the effect of cosolvent. This may be due to the architecture of the protein or due to the compactness of the protein that leaves little space in the vicinity for any rearrangement, as proposed by Priev et al. [Citation13] The hydrophobicity of actomyosin from fish skeletal muscle decreased with increase in concentrations of polyhydric alcohols, such as sorbitol and mannitol [Citation14] and also the exposure of hydrophobic residues might have been hindered by an interaction of OH groups from the co-solvent molecules.
Figure 2 Effect of glycerol on the isoelectric precipitation of α-globulin. The value P/P0 is the ratio of the precipitation in presence of the cosolvent to the precipitation in the control. A protein concentration of 5.6 × 10−6 M was used. (A) From pH 4.0 to 4.9, and (B) from pH 7.0 to 4.9.
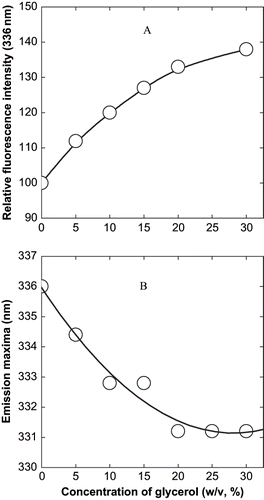
The alteration in the environment of the aromatic chromophores is investigated by fluorescence spectroscopy. Recently, exhaustive compilation of fluorescence spectroscopic technique and its potential to measure and characterize the nutritional components in foods is well reviewed.[Citation15] shows the changes in the emission maxima of the protein as a function of the glycerol concentrations. It is seen that there is a hypsochromic shift in the protein emission maxima. This indicates that the aromatic chromophores are experiencing a more hydrophobic environment in presence of the cosolvents as compared to the control. The movement of the hydrophobic residues to a more hydrophobic region of the protein due to the effect of the cosolvent glycerol is shown by the fluorescence emission of the protein in presence of glycerol (). At pH 4.0, the protein has an emission maxima at 336 nm, thereby indicating presence of some tryptophanyl residues at the aqueous surface. With increasing concentration of glycerol, the wavelength shifts to 331 nm, thus indicating a more hydrophobic environment around these residues. Since the cosolvents are known to be preferentially excluded from the domain of the proteins they would be exerting a force in the opposite direction, thereby pushing more water molecules towards the protein. [Citation16] Among cosolvents, cited in literature trehalose is shown to stabilize proteins more efficiently compared to sucrose and glycerol due to its high glass transition temperature.[Citation17]
Figure 3 Effect of glycerol on the fluorescence spectra of α-globulin at pH 4.0 (0.02 M sodium acetate buffer). (A) Effect on relative fluorescence intensity; and (B) effect on wavelength of maximum emission. The excitation was at 280 nm and a protein concentration used was 3.8 × 10−7 M.
The thermodynamic incompatibility of the hydrophobic (aromatic residues) with the water would tend to push them into the interior of the protein resulting in the less exposure of these residues responsible for aggregation. This is again reflected in the fact that the precipitation decreases in presence of the glycerol. The preferentially hydrated molecule would also be more soluble in the system,[Citation18] thus decreasing the precipitation. It is also possible that these effects would sum up as more number of water molecules around the proteins, which would form the shells, thereby decreasing the net collisions among the molecules resulting in less amount of the precipitate. The increase in fluorescence intensity correlates in a linear manner with precipitation, though in the negative order. This indicates that the increasing intensity due to change in environment of tryptophan was also linked to the decreasing precipitation of the protein (). While, the increase in fluorescence intensity is correlated with the cosolvent concentration in a second order polynomial function, it is directly related to the precipitation. Since there is also an increase in the fluorescence intensity as a function of glycerol concentration, it further indicates that the residues are experiencing a more non-polar environment. Thus, the movement of these residues within a protein molecule would lead to increased hydrophobic interactions within the molecule.
Figure 4 Relation between the precipitation of α-globulin and the change in fluorescence intensity at emission maxima of α-globulin in presence of glycerol at pH 4.0 (0.02 M sodium acetate buffer). The values are plotted from the data of and .
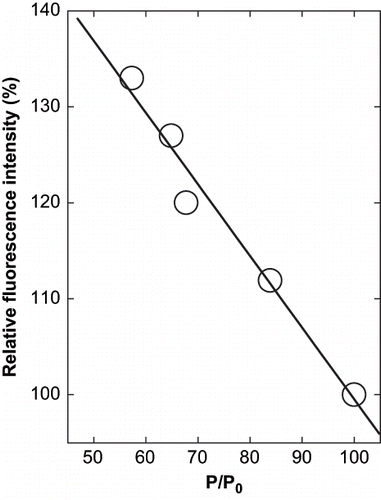
Fischer and Glatz[Citation19] have studied the effect of mixing speeds on the rate of precipitation, particle size distribution and settling behavior in isoelectric fractionation of soy proteins. They considered the speed as a possible parameter to promote aggregation. The inclusion of glycerol is seen to prevent the aggregation in acid precipitation and probably hydrophobic interaction is also decreased at this pH. The effect of hydrophobic residues especially the aromatic side chains of tyrosine, tryptophan, and phenylalanine is crucial in aggregation and their behavior in presence of cosolvents is demonstrated by the fluorescence spectra of the protein in presence of glycerol.
Effect of Glycerol on the Fluorescence of ANS in the Presence of the Protein
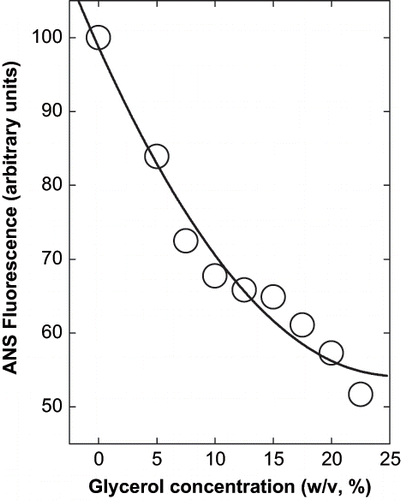
The fluorescence intensity of ANS at 466 nm (which is that of ANS bound to protein) decreased as a function of the glycerol concentration (). This shows that the amount of hydrophobicity in the surface of the protein has decreased as the dye cannot penetrate the interior of the protein. [Citation20,Citation21,Citation22] The correlation between the ANS binding and cosolvent concentration is again a second order polynomial function. This indicates that polyols and sugars lead to increase in hydrophobic interactions within a protein leading to decreased availability of surface residues for protein-protein interaction. This would therefore effectively shield the favorable hydrophobic interactions facilitating for the aggregation. This also explains the decreased aggregation with increasing cosolvent concentration as these could occupy the spaces between the hydrophobic compartments, which are exposed and would have been entropically unfavorable, if there was water instead.
Figure 5 Effect of glycerol on the fluorescence intensity of ANS and protein in presence of glycerol at pH 10.0 (0.02 M glycine-NaOH buffer). The final concentration of protein was 8 × 10−7 M. The excitation wavelength was 365 nm and emission was at 466 nm with a slit width of 5 nm for both.
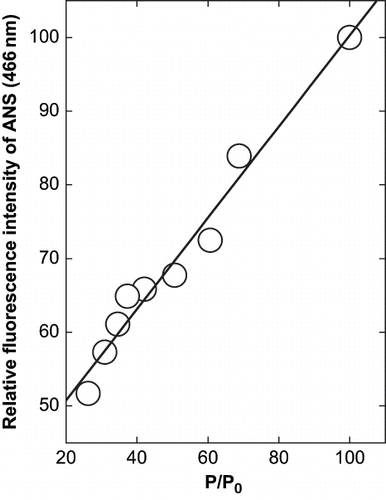
The correlation between the decrease in ANS fluorescence which indicates decreased hydrophobic patches on the surface and precipitation is shown in . The data support the theory that there is a decreased hydrophobic surface leading to less interaction. Though both precipitation and ANS binding are related with the concentration of cosolvent by a second order polynomial function, they are related to each other linearly. It implies that the amount and intensity of aggregation may be dependent on these interactions and their decrease leads to a decrease in both quantity and quality of the precipitate. The subunits of α- globulin have been shown to be primarily held by hydrophobic interactions. While the accessibility of phenylalanine, tryptophan and tyrosine residues on the surface is reflected in fluorescence, the other hydrophobic residues availability upon aggregation is demonstrated by ANS.
CONCLUSION
The tendency to aggregate can be visualized as a process where the hydrophobic surfaces due to unfavorable environment coalesce to decrease the exposure to water. The inclusion of glycerol reduces the degree of this unfavorable environment. It is also possible that the glycerol itself competes for the hydrophobic patches on the protein due to its hydrophobic nature. The results show that aggregation due to pH changes can be effectively encountered by cosolvent like glycerol. It also demonstrates that hydrophobic interactions may be affected by the presence of glycerol in a polynomial manner. The work can open up new vistas wherein the amount of glycerol required to get a defined structure of precipitate perhaps can be predicted. It also shows the relation between the glycerol concentration and the properties like surface hydrophobicity and fluorescence intensity on the protein-protein interaction is facilitated.
ACKNOWLEDGMENTS
RKS gratefully acknowledges the receipt of Senior Research Fellowship from the Council of Scientific and Industrial Research (CSIR), New Delhi, India, during the tenure of the work.
REFERENCES
- Prakash , V. and Nandi , P.K. Isolation and characterization of α-globulin of sesame seed (Sesamum Indicum L.). . Journal of Agricultural and Food Chemistry , 1978 ( 26 ) 320 – 323 .
- Prakash , V. and Nandi , P.K. 1977 . Association-dissociation behavior of sesame α-globulin in electrolyte solution . Journal of Biological Chemistry , 252 : 240 – 243 .
- Prakash , V. 1986 . Effect of sodium chloride on the extractability of proteins from sesame seed (Sesamum indicum L.). . Journal of Agricultural and Food Chemistry , 34 : 256 – 259 .
- Prakash , V. and Nandi , P.K. 1977 . Association-dissociation and denaturation behavior of an oligomeric seed protein α-globulin of Sesamum Indicum L. in acid and alkaline solutions . International Journal of Protein and Peptide Research , 9 : 319 – 328 .
- Plietz , P. , Damaschun , G. , Zirwer , D. , Gast , K. , Schwenke , K.D. and Prakash , V. 1986 . Shape and quarternary structure of α-globulin from sesame (Sesamum indicum L.) seed as revealed by small angle X-ray scattering and quasi-elastic light scattering . Journal of Biological Chemistry , 261 : 12686 – 12691 .
- Arakawa , T. and Timasheff , S.N. 1982 . Stabilization of protein structure by sugars . Biochemistry , 21 : 6536 – 6544 .
- Back , J.F. , Oakenfull , D. and Smith , M.B. 1979 . Increased thermal stability of proteins in the presence of sugars and polyols . Biochemistry , 18 : 5191 – 5196 .
- Lakshmi , T.S. , Nandi , P.K. and Prakash , V. 1985 . Interactions of sugars with α-globulin from Sesamum Indicum L. . Indian Journal of Biochemistry and Biophysics , 22 : 135 – 141 .
- Radha , C. , Muralidhara , B.K. , Ramesh Kumar , P. , Tasneem , R. and Prakash , V. 1998 . Thermal stabilization of multimeric proteins- A case study with α-globulin . Indian Journal of Biochemistry and Biophysics , 33 : 76 – 85 .
- Pintado , M.E. and Malcata , F.X. 1996 . Simplified process for soybean glycinin and beta-glycinin fractionation . Journal of Food Process Engineering , 19 : 457 – 467 .
- Tay , S.L. , Tan , H.Y. and Perera , C. 2006 . The coagulating effects of cations and anions on soy protein . International Journal of Food Properties , 9 : 317 – 323 .
- Jesior , J.C. 2000 . Hydrophilic frame work in proteins? Journal of Protein Chemistry . 19 : 93 – 103 .
- Priev , A. , Almagor , A. , Yedgar , S. and Gavish , B. 1996 . Glycerol decreases the volume and compressibility of the protein interior . Biochemistry , 35 : 2061 – 2066 .
- Mathew , Sijo and Prakash , V. 2005 . Polyhydric alcohols mediated inhibition of calcium activated adenosine triphosphatase activity of fish skeletal muscle actomyosin . International Journal of Food Properties , 8 : 255 – 265 .
- Kulmyrzaev , K.V. , Karoui , R. , De Baerdemaeker , J. and Dufour , E. 2007 . Infrared and fluorescence spectroscopic techniques for the determination of nutritional constituents in foods . International Journal of Food Properties , 10 : 299 – 320 .
- Lee , J.C. and Timasheff , S.N. 1981 . The stabilization of proteins by sucrose . Journal of Biological Chemistry , 256 : 7193 – 7201 .
- Roe , K.D. and Labuza , T.P. 2005 . Glass transition and crystallization of amorphous trehalose-sucrose mixtures . International Journal of Food Properties , 8 : 559 – 574 .
- Arakawa , T. and Timasheff , S.N. 1985 . Theory of protein solubility . Methods in Enzymology , 114 : 49 – 77 .
- Fischer , R.R. and Glatz , C.E. 1986 . Effects of mixing during acid addition on fractionally precipitated protein . Biotechnology and Bioengineering , 28 : 1056 – 1063 .
- Cordone , M. and Puri , N. 1992 . Spetrofluorimetric assessment of the surface hydrophobicity of proteins . Biochemical Journal , 282 : 589 – 593 .
- Terada , H. , Hiramatsu , K. and Aoki , K. 1980 . Heat denaturation of serum albumin monitored by 1-anilino-nopthelene-8-sulphonate . Biochimica et Biophysica Acta , 622 : 161 – 170 .
- Pasdar , N.A. and Li-Chan , E.C.Y. 2000 . Comparison of protein surface hydrophobicity measured at various pH values using three different fluorescent probes . Journal of Agricultural and Food Chemistry , 48 : 328 – 334 .