Abstract
The viscosities and specific heat capacities of twelve vegetable oils were experimentally determined as a function of temperature (35 to 180° C) by means of a temperature controlled rheometer and differential scanning calorimeter (DSC). Viscosities of the oil samples decreased exponentially with temperature. Out of the three models (modified WLF, power law, and Arrhenius) that were used to describe the effect of temperature on viscosity, the modified WLF model gave the best fit. The specific heat capacity of the oil samples however increased linearly with increase in temperature. The equations developed in the study could be valuable for designing or evaluating handling and processing systems and equipment that are involved in the storage, handling and utilization of vegetable oils.
INTRODUCTION
About 79% of the over 100 million tonnes of edible oils and fats produced worldwide annually are derived from plant sources and are referred to as vegetable oils.[Citation1] Vegetable oils play important functional and sensory roles in food products, and they act as carrier of fat soluble vitamins A, D, E, and K.[Citation2] They also provide energy and essential linoleic and linolenic acids responsible for growth,[Citation3,Citation4] and they are one of the main ingredients used to manufacture soaps, cosmetics, and pharmaceutical products.[Citation5,Citation6]
Vegetable oils are mostly used for cooking and frying of foods and snacks. In both applications, the oils are subjected to elevated temperatures in the range of 35 to 180°C. The optimum design of heating and cooling systems for cooking and frying, and the fundamental understanding of cooking and frying processes require that the thermo-physical properties of the major ingredients involved (such as vegetable oil)[Citation7] in these processes be known. Two of the important thermophysical properties are viscosity and specific heat. The sizing and selection of pumps and pipes for handling the hot oil also require that the viscosity of the oil be known.[Citation8]
It has been well established that temperature has a strong influence on the viscosity of fluid products with viscosity generally decreasing with increase in temperature.[Citation9] Several researchers have reported the viscosity of vegetable oils at room temperature.[Citation9,Citation10,Citation11,Citation12,Citation13] Studies have also been carried out on the effect of temperature on the viscosity of some vegetable oils at temperatures less than 110°C[Citation9,Citation14,Citation15,Citation16] and at temperatures between 150–180°C[Citation17]. The authors did not find any reported study on the viscosity of vegetable oils at temperatures between 110–150°C. Moreover, these studies that have been carried out on temperature effect on viscosity of vegetable oils have been carried out at different temperature ranges.
Additionally, no published data exist on the effect of temperature on the specific heat of vegetable oils throughout the temperature range of 35–180°C. Some of the reported studies on specific heat of vegetable oil include the following: Paul and Mittal[Citation18]—specific heat of canola oil (after being used for frying) at 45–60°C; Maskan and Bagci[Citation7]—sunflower seed oil (after being used for frying) at 0–50°C; and Bhatnagar et al.[Citation19] of vegetable oils of corn, peanut, coconut, soybean and palm at temperatures of 20–100°C. The objectives of this study were to (a) obtain shear stress-shear rate data; and (b) to estimate the viscosity and specific heat of vegetable oils within temperatures range of 35–180°C.
MATERIALS AND METHODS
Rheological Test
The following 12 vegetable oil samples were used in this study: almond, canola, corn, grapeseed, halzenut, olive, peanut, safflower, sesame, soybean, sunflower, and walnut oil. The samples were purchased from a local grocery store in Auburn, AL. Rheological tests were carried out by means of a Bohlin rheometer (Model CVO-100, Bohin Instruments, Gloucestershire, UK)). A programmable water bath (Model F25-HE, Julabo USA Inc. Allentown, PA) was used to ensure correct and stable control of temperature during measurements. The rheometer and the water bath were controlled by means of a software provided by the manufacturer of the rheometer. The concentric cylinder measuring system was used to evaluate the rheological properties of the sample. This measuring system consisted of a 25-mm diameter rotating bob (inner cylinder) located in a 27.5-mm diameter fixed cup (outer cylinder). The bob was used to shear oil samples (∼13 ml) contained in the annular gap between the cup and bob. The samples were sheared at a shear rate of 1 to 100 s−1 and at temperatures of 35–180°C. All measurements were carried out in duplicate.
Specific Heat
A differential scanning calorimeter - DSC (Model Q100, TA Instruments, New Castle, DE) was used to estimate the specific heat of the twelve oil samples. The DSC was calibrated with indium before use. About 8 to 10 mg of oil sample was placed in hermetically sealed aluminum pans. An empty aluminum pan was used as reference. DSC runs were performed from 35–180°C at a scan rate of 20°C/min. Based on the measured amount of energy (heat) absorbed a sample during a run, the DSC manufacturer's software (TA Universal Analysis and TA Advantage Speciality Library) were used to analyze the heat flow data and calculate the specific heat of the oil samples. Results are the average of duplicate samples.
RESULTS AND DISCUSSION
Viscosity
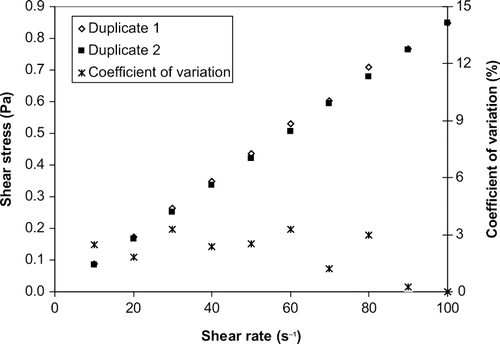
shows that the typical closeness in values of the shear stress—the shear rate data obtained from duplicate measurements. This is confirmed by the plot of coefficient of variation at each shear rate. Similar values of coefficient of variation were obtained at other temperatures and for other oils. Consequently, the average shear stress at each shear rate was used for further data analysis. Within the temperature range of 35–180°C, the linear relationship of shear stress to shear rate indicate that all the vegetable oil samples tested in this study exhibit Newtonian behavior. The viscosities at each temperature were therefore obtained from the slope of the fit of experimental shear stress—shear rate data to the Newton's law of viscosity equation (EquationEq. 1). The values of the estimated viscosities are given in . In each case, the regression coefficient (R2) that was obtained due to the fit of EquationEq. 1 to the experimentally obtained shear stress-shear rate data was greater than 0.995.
Table 1 ViscosityFootnote* (mPa s) of vegetable oil at different temperatures
where σ is shear stress (mPa), is the shear rate (s−1) and μ is viscosity (mPa s). As expected, the viscosities of the oil decreased in an exponential manner with increase in temperature (). The viscosity at 35°C was about 10-to 15-fold of the viscosity at 180°C. This will have significant effects on the energy required to pump the oil and on the rate of heat transfer at elevated temperatures. In addition, the viscosity values obtained in this study were within 12% of the viscosity values reported in literature for soybean, corn and sunflower oil.[Citation9, Citation13, Citation20, Citation21, Citation22]
Table 2 Values of constant ‘A’ and activation energy (Ea) obtained from Arrhenius equation (EquationEqn. 2) for the various samples of vegetable oil
The dependence of the oil viscosities to temperature was modeled using Eqs. Equation2–4. Eq. Equation2 is the Arrhenius model that is commonly used to model temperature dependence of a property.[Citation9, Citation23] Eqs. Equation3 and Equation4 are respectively the modified form of the WLF (Williams-Landel-Ferry) and the power law models. Several researchers have used these equations to describe the viscosity-temperature relationship of food systems.[Citation23,Citation24,Citation26,Citation27,Citation28]
Arrhenius Model
where Ea is the activation energy (kJ/kg), R is universal gas constant (8.314 kJ/kg mol K), T is absolute temperature (K) and A is a constant (mPa s).
Modified WLF Model
where a and b are constants to be determined from EquationEq. 3.
Power Law Model
where k and n are constants. Tref is reference temperature of 273.15 K. Constants A, a, b, k, and n in Eqs. Equation2–4 were estimated by using the non-linear regression procedure NLIN in SAS statistical package.[Citation29] The standard error of estimate (SEE) was computed and used to compare the goodness of fit (an equation with lower SEE value gives a better fit to experimental data compared to an equation with higher SEE value) of the equations to experimental data.[Citation30, Citation31] The lower the estimated SEE for an equation, the better the fit of that equation to experimental data. The values of the estimated constants for EquationEqs. 2 to Equation Equation Equation5 are given in Tables , respectively. For all the models and vegetable oil samples, the correlation coefficient obtained from the non-linear regression procedure was greater than 0.99. However, comparisons of the calculated standard error of estimate (EquationEq. 5) indicate that the temperature-dependence of viscosity for the vegetable oil samples was best described by the modified WLF model. The Arrhenius equation gave the worst fit to the viscosity data. Similar result was obtained by Sopade et al.[Citation25] for the viscosities of Australian honeys when the goodness of fit of the WLF model was compared to three other models (including Arrhenius equation and power law model).
Table 3 Values of constants ‘a’ and ‘b’ obtained from the modified WLF model (EquationEqn. 3) for the various samples of vegetable oil
Table 4 Values of constants ‘k’ and ‘n’ obtained from the power law model (EquationEqn. 5) for the various samples of vegetable oil (EquationEqn. 5)
Table 5 Values of constants ‘m’ and ‘b’ in EquationEq. 6.
Specific Heat
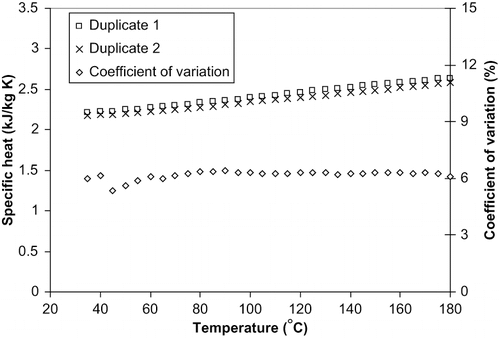
Specific heat of all the vegetable oil samples tested in the study increased linearly with increase in temperature from 35–180°C ( and ). The percent increase in specific heat was about 17% within this temperature range. Therefore, more heat will be required for a unit change in temperature per unit mass of a food that is cooked or fried with oil. It has been postulated that the increase in specific heat of materials with increase in temperature is because of expansion of a substance during heating.[Citation32] Some of the heat being provided is therefore used to furnish the work required for the expansion of that material against the surroundings.[Citation32] Similar increase in specific heat with increase in temperature has been obtained for various food and biological materials.[Citation19,Citation33,Citation34,Citation35]
Table 6 Specific heat (kJ/kg K) of the various oil samples at temperatures of 35 to 180°C
Figure 2 Closeness of duplicate specific heat values for canola oil at temperatures of 35°C to 180°C.
The trendline function in Microsoft Excel was used to fit linear equation (EquationEq. 6) to the specific heat versus temperature.
where Tref is the reference temperature of 273.15 K and T is sample temperature in degrees Kelvin. The values of the slope (m) and intercept (b) of EquationEq. 6 for the various oil samples is given in . A good fit of the experimental data to EquationEq. 6 was obtained as evident by the values of regression coefficients (R2) that were greater than 0.960. The linear equation will enable researchers and food processors to be able to predict the specific heat of these vegetable oils at temperatures between 35–180°C for quality control, and selection and design of equipment and processes that are needed to store, handle, and utilize vegetable oils.
CONCLUSIONS
It can be concluded from this study that temperature affects the viscosity and specific heat of vegetable oils at temperatures between 35–180°C with viscosity decreasing exponentially and specific heat increasing linearly with increase in temperature. The viscosity at 35°C was about 10-to 15-fold of the viscosity at 180°C while the percent increase in specific heat from 35–180°C was about 17%.
REFERENCES
- Hamm , W. and Hamilton , R.J. 2000 . Edible Oil Processing , 281 New York, NY : CRC Press .
- Moreira , R.G. , Castell-Perez , M.E. and Barrufet , M.A. 1999 . Deep-Fat Frying Fundamentals and Applications , 350 Gaithersburg, MD : Aspen Publication .
- Giese , J. 1996 . Fats, Oils and Fat Replacers . Food Tech. , 50 : 78 – 84 .
- Salunkhe , D.K. , Chavan , J.K. , Adsule , R.N. and Kadam , S.S. 1992 . World Oilseeds: Chemistry, Technology and Utilization , 554 New York, NY : Van Nostrand Reinhold .
- Bockisch , M. 1998 . Fats and Oils Handbook , 5 Champaign, IL : AOCS Press .
- Rodenbush , C.M. , Hsieh , F.H. and Viswanath , D.D. 1999 . Density and Viscosity of Vegetable Oils . J. Am. Oil. Chem. Soc. , 76 : 1415 – 1419 .
- Maskan , M. and Bagci , H.I. 2003 . The Recovery of Used Sunflower Seed Oil Utilized in Repeated Deep-fat Frying Process . Eur. Food Res. Tech. , 218 : 26 – 31 .
- Singh , R.P. and Heldman , D.R. 2001 . Introduction to Food Engineering , 659 London : Academic Press .
- Rao , M.A. 1999 . Rheology of Fluid and Semifluid Foods: Principles and Applications , 433 Gaithersburg, MD : Aspen Publication .
- Krishna , A.G.G. 1993 . Influence of Viscosity on Wax Settling and Refining Loss in Rice Bran Oil . J. Am. Oil. Chem. Soc. , 70 : 895 – 898 .
- Kokini , J.L. 1993 . “ Rheological Properties of Foods ” . In Handbook of Food Engineering , Edited by: Heldman , D.R. and Lund , D.B. 1 – 38 . New York, NY : Marcel Dekker .
- Lang , W. , Sokhansanj , S. and Sosulski , F.W. 1999 . Modeling the Temperature Dependence of Kinematic Viscosity for Refined Canola Oil . J. Am. Oil. Chem. Soc. , 69 : 1054 – 1055 .
- Timms , R.E. 1985 . Physical Properties of Oils and Mixtures of Oils . J. Am. Oil. Chem. Soc. , 62 : 241 – 248 .
- Noureddini , H. , Teoh , B.C. and Clements , D.L. 1999 . Viscosities of Vegetable Oils and Fatty Acids . J. Am. Oil. Chem. Soc. , 69 : 1189 – 1191 .
- Coupland , J.N. and McClements , D.J. 1977 . Physical Properties of Liquid Edible Oils . J. Am. Oil. Chem. Soc. , 74 : 1559 – 1564 .
- Toro-Vazquez , J.F. and Infante-Guerrero , R. 1993 . Regression Models that Describe Oil Absolute Viscosity . J. Am. Oil. Chem. Soc. , 70 : 1115 – 1119 .
- Miller , K.S. , Singh , R.P. and Farkas , B.E. 1994 . Viscosity and Heat Transfer Coefficients for Canola, Corn, Palm and Soybean Oil . J. Food Process. Preserv. , 18 : 461 – 472 .
- Paul , S. and Mittal , G.S. 1996 . Dynamics of Fat/oil Degradation during Frying Based on Physical Properties . J. Food Process Engng. , 19 : 201 – 221 .
- Bhatnagar , S. , Gennadios , A. , Hanna , M.A. and Weller , C.L. 1994 . “ Specific Heats of Selected Lipids. Paper 94–6035, American Society of Agricultural Engineers (ASAE) ” . 13 St. Joseph, MI
- Santos , J.C.O. , Santos , I.M.G. and Souza , A.G. 2004 . Effect of Heating and Cooling on Rheological Parameters of Edible Vegetable Oils . J. Food Eng. , 67 : 401 – 405 .
- Telis , V.R.N. , Telis-Romero , J. , Mazzotti , H.B. and Gabas , A.L. 2007 . Viscosity of Aqueous Carbohydrate Solutions at Different Temperatures and Concentrations . Int. J. Food Properties. , 10 : 185 – 195 .
- Sopade , P.A. , Halley , P.J. , D'Arcy , B. , Bhandari , B. and Caffin , N. 2004 . Friction factors and rheological behavior of Australian honey in a straight pipe . Int. J. Food Properties , 7 : 393 – 405 .
- Urbicain , M.J. and Lozano , J.E. 1997 . “ Thermal and Rheological Properties of Foodstuffs ” . In Handbook of Food Engineering Practice , Edited by: Valentas , K.J. , Rotstein , E. and Singh , R.P. 425 – 486 . New York, NY : CRC Press .
- Peleg , M. 1992 . On the Use of the WLF Model in Polymers and Foods. Crit. Rev . Food Sci. Nutr. , 32 : 59 – 66 .
- Sopade , P.A. , Halley , P. , Bhandari , B. , D'Arcy , B. , Doebler , C. and Caffin , N. 2002 . Application of the Williams-Landel-Ferry Model to the Viscosity-Temperature Relationship of Australian Honeys . J. Food Eng. , 56 : 67 – 75 .
- Ollett , A.L. and Parker , R. 1990 . The Viscosity of Supercooled Fructose and its Glass Transition Temperature . J. Texture Studies , 21 : 355 – 362 .
- Soesanto , T. and Williams , M.C. 1981 . Volumetric Interpretation of Viscosity for Concentrated and Dilute Sugar Solution . J. Phys. Chem. , 85 : 3338 – 3341 .
- Williams , M.L. , Landel , R.F. and Ferry , J.D. 1995 . The Temperature Dependence of Relaxation Mechanisms in Amorphous Polymers and other Glass-Forming Liquids . J. Am. Chem. Soc. , 77 : 3701 – 3706 .
- SAS Institute Inc . 2005 . SAS user's guide: Statistics , Cary, NC : Statistical Analysis System Institute Inc .
- Fasina , O.O. and Sokhansanj , S. 1993 . Equilibrium Moisture Relations and Heat of Sorption of Alfalfa Pellets . J. Agric. Engng. Res. , 56 : 51 – 63 .
- Draper , N.R. and Smith , H. 1981 . Applied Regression Analysis , 427 New York, NY : John Wiley and Sons .
- Noggle , J.H. 1989 . Physical Chemistry , 1093 Glenview, IL : Scott, Foresman Co .
- Fasina , O.O. , Farkas , B.E. and Fleming , H.P. 2003 . Thermal and Dielectric Properties of Sweetpotato Puree . Int. J. Food Properties. , 6 : 461 – 472 .
- Tansakul , A. and Chaisawany , P. 2006 . Thermophysical properties of coconut milk . J. Food Engineering. , 73 : 276 – 280 .
- Gratao , A.C.A. , Junior , V.S. , Polizelli , M.A. and Tellis-Romero , J. 2005 . Thermal Properties of Passion Fruit Juice as Affected by Temperature and Water Content . J. Food Process Engng. , 27 : 413 – 431 .