Abstract
A mixture design approach was employed to determine the effect of κ - and λ-carrageenans of Oaxaca cheese fat reduction on melting (Schreiber and Melt Area), color, protein, moisture, and yielding. Melting was affected mainly by the fat content, but κ -carrageenan seems to improve this property. Color was affected by fat content, with a relatively profiting effect of λ-carrageenan, related to higher moisture content and higher cheese yielding. Incorporation of low concentrations of carrageenans allowed a considerable fat reduction with no detrimental effect on Oaxaca cheese overall quality.
INTRODUCTION
Oaxaca cheese is a typical pasta filata style of cheese similar to mozzarella, with a sweet milk flavor and excellent melting properties, employed in hot dishes.[Citation1] Oaxaca cheese had a great acceptance and is elaborated in many zones of Mexico, most of the times at artisan level in small cheese dairies in rural regions, with the concomitant natural acidity of the milk. This acid milk is mixed with fresh milk; but since it is a rennet-set cheese manufactured from whole milk, most of the times the acidity of the milk is achieved adding diluted organic acids.[Citation2]
Fat in cheeses contributes to stretch and flow by providing a lubricating effect when melted. When fat content is reduced in cheese manufacture (≤10%) without modification of the regular procedure, that cheese is usually hard, rubbery, translucent and has poor flavor development, with further problem of poor meltability and performance when cooked. These properties can be ameliorated if the water content of the cheese is increased by adding ingredients that bind moisture or impart fat-like characteristics when incorporated into the cheese matrix. The incorporation of fat mimetic ingredients can be used to soften the body of the low-fat cheese.[Citation3,Citation4] These fat mimetic ingredients improve sensory and functional characteristics of low fat cheeses improving texture and yield.[Citation5] However, they cannot fully replace the non polar functional properties of fat such as its flavor-carrying capacity.[Citation6] Among these ingredients, carrageenans are widely employed in milk industry due to their interaction with milk proteins.[Citation7] The functionality of κ-carrageenan[Citation8,Citation9,Citation10,Citation11] or other type of carrageenans (ι and/or λ)[Citation12,Citation13,Citation14] in milk systems are affected by environmental conditions, like temperature, ionic strength, and concentration of ingredient. Discussion between the interaction carrageenans and casein micelles turns around the effect of carrageenans and milk proteins concentrations on the overall system behavior, where the apparently high specific interaction between κ-carrageenan and κ-casein enhancing the gelling properties.[Citation15,Citation16,Citation17,Citation18] Ingredients interaction in processed foods can be studied by the mixture design methodology, where the systematic experimental design validates the importance of ingredients interactions.[Citation19] The objective of this work was to study by a mixture design approach the effect of κ- and λ-carrageenan incorporation in Oaxaca cheese fat reduction on melting and physicochemical properties.
MATERIALS AND METHODS
Cheese Manufacture
Raw milk was proportioned by the Productora Universitaria de Lácteos Farm PROUNILAC (Rancho Universitario, Tulancingo, México). All treatments were elaborated from same milk batch (10.29 kg per treatment). Milk was pasteurized and warmed to 35–38°C, adjusting pH to 5.4–5.6 (28–30°Dornic) using lactic acid (Reasol, Mexico City, Mexico). Fat content was determined using the Gerber test,[Citation20] and adjusted to the respective proportion () in a centrifugal separator Edelcrem 125 (VIGUSA, S.A., México City, México). The corresponding carrageenans proportions (κ and/or λ) were added and mixed according to formulations described in . After 15 min of ripening, 1 ml of rennet (diluted to 10 ml with distilled water) was added. After setting, curd was cut with 1 cm knives and then allowed to heal for 15 min. The whey was drained, and curds were cheddared at 35–38°C during 30 min. The cheddared curd was washed twice in hot water at 70–80°C, hand-stretched until elastic and smooth consistency. Cheese was stretched and cooled in cold water, dry salted, and rolled into a ball of twine, weighted, and stored at 4–6°C until and analysis the next day.
Table 1 Formulation in the three-component constrained simplex lattice mixture design in fractions and concentration
Experimental Design
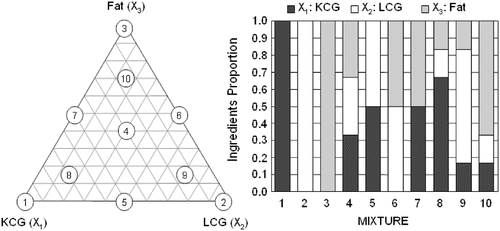
A three component constrained simplex lattice mixture design was used. The mixtures components consisted of Gelcarin-ME913 κ-carrageenan (X1, KCG), Viscarin-GP209 λ-carrageenan (X2, LCG), kindly provided by FMC Biopolymers (Philadelphia, Pennsylvania, USA), and milk fat (X3). Components were expressed as fractions of the mixture and the sum (X1 + X2 + X3) of the proportions was one. The ten points consisted of three single ingredients systems, three two-ingredients mixtures and four thee-ingredient mixtures ().
Melting Tests
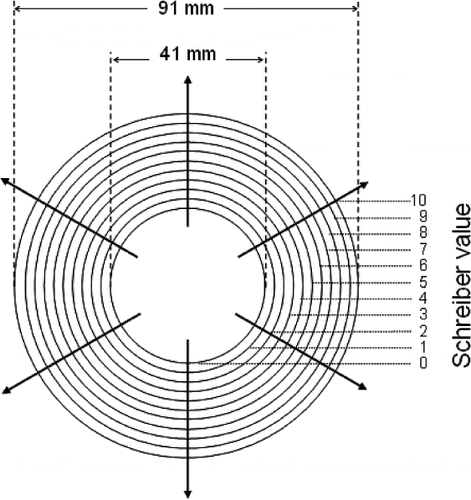
Meltability of cheese samples was determined by the Schreiber test and by the change in cheese melting area. For Schreiber tests,[Citation21] cheese samples were cut in cylinders (41 mm diameter and 5–6 mm height), placed in the center of petri dishes bottoms and heating at 95°C during 15 min in a convection oven FED 53 (WTC Binder, Tuttingler, Germany). The diameter of cheese discs was measured in 6 radial perpendicular directions (in mm) and the average reported as Schreiber values ().
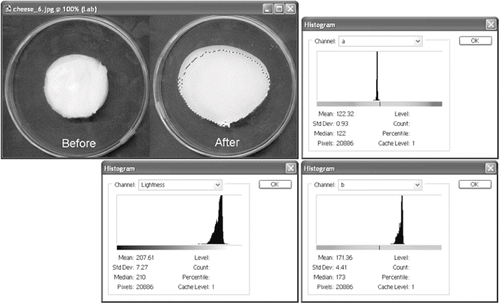
Change in the melt area was determined adapting the technique reported by Wang and Sun.[Citation22] Same cheese samples employed in Schreiber test were scanned, before and after heat treatment, in a Microtek® 3600 flatbed scanner (Microtek International Inc., Redondo Beach, California, USA). Images were analyzed with Adobe Photoshop® software version 7.0 (Adobe System Incorporated, San Jose, California, USA), selecting the cheese area with the Magic Wang Tool, calculating the pixels number form the Image Histogram Window (). The ratio areas (number of pixels) before and after heat treatment was reported as percent of melt area change, where 100% indicated no melting (no change of area), according to:
Color
Color in CIE-LAB coordinates was determined adapting the technique reported by Yam & Papadakis.[Citation23] Images scanned for area determination were employed to determine the color of the samples in CIE-Lab coordinates using Adobe Photoshop® software, obtaining the Lab coordinates from the Image Histogram Window (). Results are the mean of three readings, standardizing the values according to the following EquationEqs. (2 Equation Equation–4):
Protein Content, Humidity, and Cheese Yield
Protein content was determined by Kjeldahl procedure (AOAC Official Method 920.120, conversion factor of 6.38), and moisture according to the AOAC Official Method 948.12.[Citation24] Cheese yields were calculated as the percent weight of finished cheese divided by the weight of the milk used.[Citation25]
Statistical and Data Analysis
Scheffe's canonical special cubic equation for 3 components was fitted to data collected at each experimental point using backward stepwise multiple regression analysis as described by Cornell.[Citation26] This canonical model differs from full polynomial models in that it does not contain a constant term, i.e., it has a zero intercept. Variables in the regression models, which represent two-ingredient or three-ingredient interaction terms, were referred to as “non-linear” terms. Canonical special cubic equation postulated was:
RESULTS AND DISCUSSION
Melting Properties
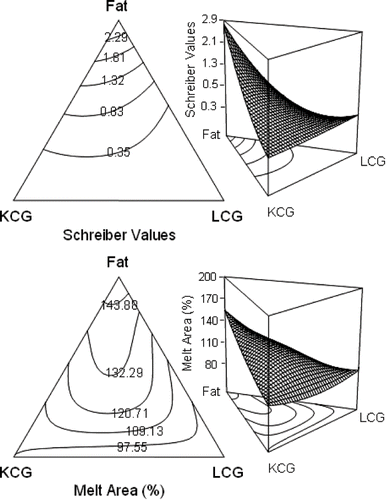
As expected, full-fat samples (mixture 3) obtained higher Schreiber melting scores, and samples with half fat and equal proportions of KCG-LCG (mixture 10) presented same melting values than full-fat sample. In the melt area change, KCG and LCG incorporation in half-fat samples (mixture 10) provoked the higher increase in cheese melting, followed by the full-fat samples (mixture 3). At same fat content, KCG (mixture 8) resulted in better melting properties than LCG (mixture 9), but samples with no fat (mixture 6) and the central point, equal proportion of the ingredients (mixture 4) resulted in a area reduction after heating (). In the Schreiber test regression analysis, only both fat and triple interaction parameters had a positive coefficient, whereas carrageenans interaction resulted in an antagonistic effect on this property. Similar trend was observed for the regression in Melt Area change, although non linear parameters or components interactions presented a negative coefficient (). Fat affected reduced-fat cheese properties more than the moisture content.[Citation27] This can be appreciated in contour plots, where Schreiber scores diminished when fat was decreased, with the poorest values in the KCG-LBG side. Nonetheless, in Melt Area change contour plot the melting behavior reflected a positive interaction between components, since although the same tendency was observed, central point resulted in greater cheese melting. Employing the Melt Area change even at lower fat contents a positive change in cheese melting can be appreciated, in contrast with Schreiber values (). Differences in melting behavior can not be appreciated with the Schreiber test, since in the Schreiber test the meltability index only reflects the state of melted cheese without considering the influence of the initial size of cheese discs, which unavoidably results in errors, since different melting characteristics gave rise to shape distortions that were partly a reflection of the flowability differences between the fully melted and unmelted regions within the specimen.[Citation28] This is why the Schreiber test is less sensitive than computer based methods.[Citation22] Schreiber and other empiric methodologies like Olson and Price test to determine cheese melting are poorly related to computer based methodologies.[Citation29] Nonetheless, results indicate an antagonistic effect of both KCG and LCG on the melting capacity of the cheese samples at lower fat content (ca. < 4.00 %), even above the central point a positive value can be observed in Melt Area but not in Schreiber test, slightly enhanced by KCG probably due to the impeded slippage of proteins provoked by a strong electrostatic interaction caused by the LCG more charged molecule, whereas the not so strong interaction between KCG and milk proteins allowed to protein strands to flow easily.[Citation3] Carrageenans reactivity with proteins depends on number and position of sulfate groups. Anions like sulfate group forms stable colloidal protein-carrageenan complex interacting with the protonated amino group in amino acid side chains. This interaction is more direct than the one between both negatively charged carrageenan sulfate group and the dissociated carboxylic acid group, since they need calcium ions to form a intermolecular chelate bond to interact. In any case, the more presence of sulfate groups in LCG provoked a strong interaction with milk proteins resulting in a poor meltability.
Table 2 Experimental results for the Oaxaca cheeses
Table 3 Regression coefficients and correlation for the adjusted models to experimental data in mixtures design
Color
Luminosity of the Oaxaca cheese was higher when LCG was present in formulation (mixtures 6 and 9). Darker samples were obtained with low fat content with KCG (mixture 10). Cheeses with only KCG (mixture 1) or LCG (mixture 2) were less luminosity as well (). In regression analysis, it seems that non-linear parameters, KCG-fat and KCG-LCG-fat, had an antagonistic effect on cheeses lightness, since negative or lower coefficients values were obtained (). In contour plot, KCG-fat interaction resulted in darker cheese (). Low fat cheeses are more translucent that the full fat cheeses, since translucent can be attributed to lack of fat which provides opacity in cheese.[Citation5]
Figure 5 Isoresponse contour plots and three-dimensional response surface of special cubic model for Oaxaca cheese color.
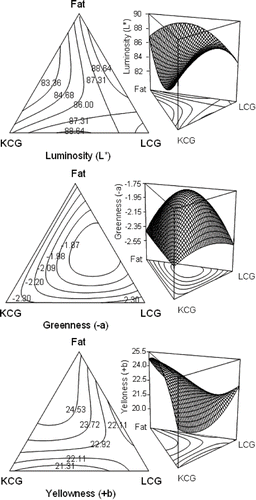
The green component (−a∗) was higher for samples with fat plus KCG (mixture 7) than the samples with fat plus LCG (mixture 6) (). In regression analysis, ingredients interaction seems to make a less green cheese (). In contour plot more green color was obtained when KCG concentration increased (). Yellowness of samples (+b∗) was higher with higher fat content and KCG (mixture 3) and KCG-fat (mixture 7) (). Regression analysis indicated a synergistic effect of the components, since triple interaction parameter presented a higher coefficient. In same manner, KCG-fat interaction enhanced yellow color, but both KCG-LCG and LCG-fat interactions resulted in a detrimental effect on cheese yellowness (). In contour plot, a more yellow color was obtained at high fat content and lower KCG proportion (). As fat content increased, the milk samples were whiter (higher L∗), more blue or less green (higher a∗), and more yellow (higher b∗), but an appropriate fat substitute should compensate this effect,[Citation30,Citation31] since greenness and yellowness color characteristic of cheese are given the amount of milk fat in formulation. Color of the low-fat cheese containing fat mimetics was different form that of the low fat cheeses without them, since they appeared to contribute more to lightness values than to green or yellow values.[Citation5] The color contribution by the carrageenans seems to be more dependent on the fat and KCG concentrations, where at low fat contents KCG produced a slightly reduction in cheese lightness, but make cheeses more green and yellow.
Protein Content, Moisture, and Cheese Yielding
Protein content was increased in cheese when higher proportions of KCG (mixture 8) or LCG (mixture 9) was present in formulation, but carrageenans interaction resulted in the lower protein content (mixture 5) (). This behavior was reflected in regression analysis, where the KCG-LCG interaction presented a negative coefficient, but triple interaction resulted in the higher coefficient (). Contour plot shows a higher protein content in central point that decreased with fat content reduction and carrageenans interaction (). Carrageenans reactivity with milk proteins, both caseins and whey proteins, appeared to be highly dependent on gum concentration and on heat treatment.[Citation32,Citation33] During heating, micelle size increased sharply around 35–40°C, which corresponds to the temperature at which κ-carrageenan helix formation begins (approx. 37°C).[Citation13] The λ-carrageenan in coil form adsorbs onto the casein micelles at temperatures from 25 to 65°C causing an increase in the micelle size even at lowest concentration, since its conformation is not temperature dependent, whilst κ-carrageenans only adsorb at temperatures where they are, at least partially, in the helical form since at 25°C the κ-carrageenan chains form double helix becoming more rigid and undergoing self-association.[Citation11,Citation14] Therefore, the conformational state of the carrageenan macromolecules determines the behavior of the casein/carrageenan system, suggesting a difference especially in relation to interaction with the casein micelles,[Citation18] explained by the formation of ionic linkages between a specific zone of the caseins and carrageenan molecules.[Citation16] Nonetheless, it has been reported that conformation does not appears to have a dominant effect on the binding of carrageenan to casein micelles, suggesting therefore that the micelle are totally covered with the carrageenan molecules intimately wrapped around them.[Citation9,Citation15,Citation34,Citation35] Effect of rennet on κ-casein excision during cheese manufacture allows the interaction of carrageenans with other casein fractions (α and β) in milk, the main contribution is likely to be with κ-casein located on the surface of the micelles.[Citation17] In this view, at relatively high carrageenans concentrations probably their interaction with milk proteins provoked a the lost of protein during whey drained, but at low concentrations (ca. 0.5%) their temperature dependence conformation enhanced protein retention during curding.
Figure 6 Isoresponse contour plots and three-dimensional response surface of special cubic model for Oaxaca cheese protein, moisture, and yielding.
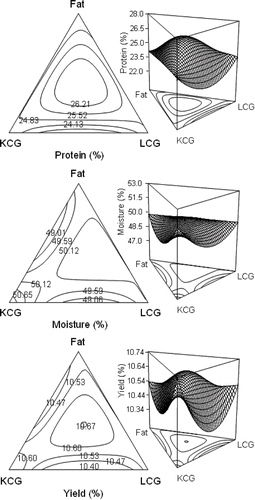
Moisture was higher in cheeses with no KCG in formulation (mixtures 2 and 3). KCG increased moisture when was in combination with other ingredient, as in central point (mixture 4) or with LCG (mixture 5) (). Regression analysis resulted in an antagonistic effect of non-linear terms, marked by KCG presence, but linear terms and triple interaction obtained a positive coefficient (). In contour plot, central region and KCG vertex gave higher moisture content, affect by KCG-LCG and KCG-fat interactions (). Casein precipitation by action of rennet, degradation of surface layer covered with “hairs” of glycomacropeptide of κ-casein as a steric stabilizer, result in water syneresis when gel was cut.[Citation30] Therefore, the increased moisture content of low fat cheese made using fat replacers suggested that curd syneresis was retarded during cheese making, which can occur as a result of water being bound directly to the fat replacer, or the fat replacer may interfere with shrinkage of the casein matrix, thus lowering the driving force involved in expelling water from the curd particles,[Citation3] increasing cheese moisture.
Cheese yielding was higher when equal proportions of KCG-LCG with no fat (mixture 5) or equal proportions of fat and LCG (mixture 6) were employed (). In regression analysis a non-linear terms had an antagonistic effect on yield (). In contour plot, yield followed a comparable behavior to moisture content, with higher yields in pure components vertexes and central point, decreasing markedly in KCG interaction with fat or LCG (). At higher κ-carrageenan concentration, and although interaction between κ-carrageenan and κ-casein cannot be ruled out, κ-carrageenan gelation are influenced by the concentration of milk proteins, milk solid concentration, carrageenans content, ionic environment, temperature and pH,[Citation8, Citation36] where κ-carrageenan gel formation involves mainly carrageenan-carrageenan cross linkages and not carrageenan-casein or casein-casein linkages.[Citation8] The higher electrostatic charge density and the no temperature dependence on its conformation could be key factors determining the strength of the attractive interaction between the λ-carrageenan and the caseins proteins,[Citation14] forces that remains even during and after the rennet action at the acid Oaxaca cheese manufacture conditions, increasing cheese yielding.
CONCLUSION
The electrostatic interaction and conformational temperature of each carrageenan determined their effect on cheese properties. At lower fat levels carrageenans addition affected the properties of Oaxaca cheese, where κ-carrageenan enhanced cheese melting allowing protein strands to slippage and flow, whereas λ-carrageenan interaction affected negatively this property. Nonetheless, components interaction allowed a good melt index determined by Melt Area change at fat content as lower as 4%. Color was more dependent on fat content, with a relatively profiting effect of λ-carrageenan, related to higher moisture content and higher cheese yielding. Incorporation of low concentrations of both carrageenans allowed a considerable fat reduction with no detrimental effect on Oaxaca cheese overall quality.
REFERENCES
- Van Hekken , D.L. and Farke , N.Y. 2003 . Hispanic cheeses: The quest for queso . Food Technology , 57 ( 1 ) : 32 – 34 . 36 38
- Esquivel , A. and Santos-Moreno , A. 1996 . Los quesos típicos mexicanos: queso Oaxaca . Lácteos Cárnicos Mexicanos , 11 ( 4 ) : 4 – 8 .
- McMahon , D.J. , Alleyne , M.C. , Fife , R.L. and Oberg , C.J. 1996 . Use of fat replacers in low fat mozzarella cheese . Journal of Dairy Science , 79 : 1911 – 1921 .
- Johnson , M.E. and Chen , C.M. 1995 . “ Technology of manufacturing reduced-fat cheddar cheese ” . In Chemistry of the Structure-Function Relationship on Cheese , Edited by: Malin , E.L. and Tunick , M.H. 331 – 337 . New York : Plenum Press .
- Drake , M.A. , Boylston , T.D. and Swanson , B.G. 1996 . Fat mimetics in low-fat cheddar cheese . Journal of Food Science , 61 : 1267 – 1270 . 1288
- Banks , J.M. 2004 . The technology of low-fat cheese manufacture . International Journal of Dairy Technology , 57 : 199 – 207 .
- Piculell , L. 1995 . “ Gelling carrageenans ” . In Food Polysaccharides and their applications , Edited by: Stephen , A.M. 205 – 244 . New York : Marcel Dekker .
- Drohan , D.D. , Tziboula , A. , McNulty , D. and Horne , D.S. 1997 . Milk protein-carrageenan interactions . Food Hydrocolloids , 11 : 101 – 107 .
- Oakenfull , D. , Miyoshi , E. , Nishinari , K. and Scott , A. 1999 . Rheological and thermal properties of milk gels formed with κ-carrageenan. I. Sodium caseinate . Food Hydrocolloids , 13 : 525 – 533 .
- Puvanenthiran , A. , Goddard , S.J. , Mckinnon , R. and Augustin , M.A. 2003 . Milk-based gels made with κ-carrageenan . Journal of Food Science , 68 : 137 – 141 .
- Tziboula , A. and Horne , D.S. 1999 . Influence of milk proteins on κ-carrageenan gelation . International Dairy Journal , 9 : 359 – 364 .
- Langendorff , V. , Cuvelier , G. , Launay , B. and Parker , A. 1997 . Gelation and flocculation of casein micelle/carrageenan mixtures . Food Hydrocolloids , 11 : 35 – 40 .
- Langendorff , V. , Cuvelier , G. , Launay , B. , Michon , C. , Parker , A. and De Kruif , C.G. 1999 . Casein micelle/iota carrageenan interactions in milk: influence of temperature . Food Hydrocolloids , 13 : 211 – 218 .
- Langendorff , V. , Cuvilier , G. , Michon , C. , Launay , B. , Parker , A. and De Kruif , C.G. 2000 . Effects of carrageenan type on the behaviour of carrageenan/milk mixtures . Food Hydrocolloids , 14 : 273 – 280 .
- Augustin , M.A. , Puvanenthiran , A. and Mckinnon , I.R. 1999 . The effect of κ-carrageenan conformation on its interaction with casein micelle . International Dairy Journal , 9 : 413 – 414 .
- Bourriot , S. , Garnier , C. and Doublier , J.L. 2000 . “ A description of micellar casein/kappa-carrageenan mixed systems by means of calorimetry and rheology ” . In Gums and Stabilizers for the Food Industry–10 , Edited by: Williams , P.A. and Phillips , G.O. 203 – 210 . Cambridge : Royal Society of Chemistry .
- Schorsch , C. , Jones , M.G. and Norton , I.T. 2000 . Phase behaviour of pure micellar casein/κ-carrageenan systems in milk salt ultrafiltrate . Food Hydrocolloids , 14 : 347 – 358 .
- Snoeren , T.H.M , Paynes , T.A.J. , Jeunink , J. and Both , P. 1975 . Electrostatic interaction between κ-carrageenan and κ-casein . Milchwissenschaft , 30 : 391 – 396 .
- Arteaga , G.E. , Li-Chan , E. , Nakai , S. , Cofrades , S. and Jiménez-Colmenero , F. 1993 . Ingredient interaction effects on protein functionality . Journal of Food Science , 58 : 656 – 662 .
- Secretaria de Salubridad y Asistencia . 1982 . Norma Mexicana NMX-F-387-1982: Fluid milk determination of butterfat by the Gerber Method Mexico City, , Mexico
- Kosikowski , F. 1982 . Cheese and Fermented Milk Foods , 405 – 406 . Brooktondale : F.V. Kosikowski and Associated .
- Wang , H.-H. and Sun , D.-W. 2002 . Correlation between cheese meltability determined with a computer vision method and with Arnott and Schreiber tests . Journal of Food Science , 67 : 745 – 749 .
- Yam , K.L. and Papadakis , S.E. 2004 . A simple digital imaging method for measuring and analyzing color of food surfaces . Journal of Food Engineering , 61 : 137 – 142 .
- Association of Official Analytical Chemist (AOAC) . 1996 . Official Methods of Analysis of AOAC International CD-Rom , 16th , Washington, D.C. : AOAC International .
- Drake , M.A. , Herrett , W. , Boylston , T.D. and Swanson , B.G. 1996 . Lecithin improves texture of reduced fat cheeses . Journal of Food Science , 61 : 639 – 642 .
- Cornell , J.A. 1980 . Experiments with Mixtures: Design Models, and the Analysis of Mixture Data , 2 – 63 . New York : John Wiley & Sons .
- Subramanian , R. , Muthukumarappan , K. and Gunasekaran , S. 2006 . Linear vsicoelastic properties of regular- and reduced-fat pasteurizad process cheese during heating and cooling . International Journal of Food Properties , 9 : 377 – 393 .
- Park , J. , Rosenau , J.R. and Peleg , M. 1984 . Comparison of four procedures of cheese meltability evaluation . Journal of Food Science , 49 : 1158 – 1162 . 1170
- Everard , C.D. , O'Donnell , C.P , Fagan , C.C. , Sheehan , E.M. , Delahunty , C.M. and O´Callaghan , D.J. 2005 . Correlation between process cheese meltability determined by sensory analysis, computer vision method and Olson and Price test . International Journal of Food Properties , 8 : 267 – 275 .
- Dalgeish , D.G. 1997 . “ Structure-function relationship of caseins ” . In Food Proteins and their Application , Edited by: Damodaran , S. and Paraf , A. 199 – 223 . New York : Marcel Dekker .
- Schmidt , K.A. and Smith , D.E. 1992 . Rheological properties of gum and milk protein interactions . Journal of Dairy Science , 75 : 36 – 42 .
- Schmidt , K.A. and Smith , D.E. 1992 . Milk reactivity of gum and milk protein solutions . Journal of Dairy Science , 75 : 3290 – 3295 .
- Dalgeish , D.G. and Morris , E.M. 1988 . Interactions between carrageenans and casein micelles: electrophoretic and hydrodynamic properties of the particles . Food Hydrocolloids , 24 : 311 – 320 .
- Xu , S.Y. , Stanley , D.W. , Goff , H.D. , Davidson , V.S. and Le Maguer , M. 1992 . Hydrocolloid/milk gel formation and properties . Journal of Food Science , 57 : 96 – 102 .
- Puvanenthiran , A. , Goddard , S.J. and Augustin , M.A. 2001 . Gelation of mixed gels containing κ-carrageenan and skim milk components . Journal of Food Science , 67 : 573 – 577 .
- Phillips , L.G. , McGiff , M.L. , Barbano , D.M. and Lawless , H.T. 1995 . The influence of fat on the sensory properties, viscosity, and color of lowfat milk . Journal of Dairy Science , 78 : 1258 – 1266 .