Abstract
The lipid fraction of Jalisco and Sinaloa chia seeds was analyzed for oil content, fatty acids, squalene and phytosterols. The mean oil content of seeds from these two regions was significantly (p ≤ 0.05) different, but with a similar composition of α-linolenic, linoleic, oleic, palmitic and stearic acids. Total phytosterols in the oil ranged from 7 to 17 g/kg. β-sitosterol accounted for up to 74% of the total unsaponified fraction. The seeds contained less than 0.5 g/kg of squalene. The oil is an attractive source of ω-3 linolenic acid and phytosterols but a poor source of squalene.
INTRODUCTION
Chia seed is native to Mexico where chia plants were cultivated by pre-Columbian communities. For example, chia seed together with corn, amaranth and beans, were important staple crops for the Aztecs. The roasted chia seeds were mixed with water and eaten as a gruel or were ground into flour for baking. They also used chia seed oil in body paints and as an ointment and emollient. A paste made from the mucilaginous moistened seeds was utilized as a poultice for wounds and to remove dirt from the eye. However, as the seed was associated with medicinal and religious practices, as well, its cultivation was banned by the Spanish conquerors.[Citation1, Citation2]
There are several chia plants (Salvia columbaria Benth, Salvia hispanica L., Salvia polystachya), all of them of the Lamiaceae family, but Salvia hispanica L. is the most widely distributed.[Citation3] The outer layer of the seeds contains mucilage that swells and surrounds them in the form of a thick coat when they are soaked in water. In this gelatinous mucilage, xylose, glucose and methyl glucuronic acid have been identified.[Citation4] The seeds are also a significant source of protein (234 g/kg seed) and oil (298 g/kg seed) that is mainly composed of unsaturated fatty acids.[Citation5–7] In Mexico, chia is found mainly in some regions of the western states of Jalisco, Sinaloa and Michoacán. In Argentina the seed has become an increasingly important crop.[Citation7, Citation8] The nutritional benefits of chia seed and one of its main components, i.e., the oil, are being rediscovered and becoming the object of increasing attention by a rising number of those consumers concerned with their health and good nutritional habits.[Citation3]
Our research is concerned with a systematic study of the main macromolecular components of Mexican chia seeds—i.e., fiber, protein, and lipids—of which Salvia hispanica L. is the main species, in order to assess the real value of this crop as a functional food and its potential as a value-added source of nutraceutic components. However, in this paper only the chemical composition of the lipid fraction is reported. The main motivation of this part of our study was based on the potential that chia seed oil can have for preventing cardiovascular disorders because of its important content of polyunsaturated fatty acids.[Citation5–7] Further, we were interested in the possible presence of significant amounts of squalene and phytosterols in the oil. These components are known for hypocholesterolemic and anticarcinogenic effects,[Citation9] but their presence has not been reported previously, although research has been conducted on this oil. The oil content, as well as the composition of the lipid fraction of chia seeds, from Jalisco and Sinaloa are compared. It is expected that the results reported here could provide useful information on the suitability of Mexican chia seed as a regular ingredient of human diet, as well as the potential use of the oil as an ingredient in prepared foods and new food products, with beneficial effects on human health.
MATERIALS AND METHODS
Seeds
The chia seeds (Salvia hispanica L.) were from regions located in the states of Jalisco and Sinaloa. Extraneous matter (dust, vain seeds, and straw from threshed seeds) was separated manually and the clean seeds grounded in a laboratory mill. The resulting flour (mesh 80) was stored in a refrigerator for no more than 24 h in a dark bottle tightly closed under a nitrogen atmosphere to prevent oil oxidation.
Oil Extraction
The oil was extracted in a Soxhlet apparatus according to official procedures.[Citation10] A mass of 1300 g of chia seed flour, placed in an extraction thimble plugged with cotton, was extracted with 7 L of reagent grade hexane (Reasol, Mexico) for about 12 h at 60 ± 2°C. Hexane was separated from oil extracts by vacuum evaporation (Rotavapor, Buchi R-205), and the amount of oil calculated by weight difference. The oil was kept in 25-mL dark screw-cap vials closed under a nitrogen atmosphere and stored at − 4°C until saponification.
Saponification
A mass of 50 g of chia seed oil, with 1 L of sodium hydroxide solution (0.5 M in 95% ethanol) and 30 mg of cholesterol (Sigma-Aldrich) as an internal standard were mixed in a 2 L Erlenmeyer flask. The mixture was magnetically stirred in a nitrogen atmosphere away from light at room temperature (≈ 25 °C) for 20 h.
Extraction of the Unsaponifiable Matter
After saponification, 300 mL of hexane and 100 mL of distilled water were added under continuous stirring to the mixture in the Erlenmeyer. The content of the flask was poured into a separatory funnel and the hexane fraction collected upon phase separation. Another 300 mL portion of fresh hexane was added to the remaining water, and the separation was performed as before. This procedure was carried out two times. All the hexane batches containing the unsaponifiable matter were collected into a single fraction.
Fatty Acids Recovery
The residual aqueous fraction was treated with 100 mL of 6 M hydrochloric acid under magnetic stirring for 5 min. Then, 200 mL of hexane were added to the stirred mixture and the content was poured into a separatory funnel. The fraction of hexane was collected and this procedure repeated once more. The unsaponifiable and saponifiable extracts were dehydrated by filtration through anhydrous sodium sulfate. The remaining solvent was evaporated to dryness in a vacuum evaporator. The residual matter was quantified by transference to 25-mL dark glass vials previously taken to constant weight. Once weighed, the vials were closed under a nitrogen atmosphere, sealed with parafilm and stored at − 4°C.
Derivatization of Fatty Acids
Seven milliliters of 14% boron trifluoride in methanol (Sigma-Aldrich) was added to 350 mg of the saponified fraction in a 25 mL round bottom flask. The flask was placed on a heating mantle and taken to ebullition for 2 min. Then 5 mL of hexane was added and the mixture boiled for another minute. The flask was cooled and 15 mL of a saturated solution of sodium chloride was added. After this procedure, the esters were extracted three times separately with 20 mL of hexane. The hexane batches were collected, filtered through anhydrous sodium sulfate, and evaporated in a vacuum evaporator. The methyl esters of fatty acids were transferred to a dark glass vial previously taken to constant weight. The vial was weighed, closed under a nitrogen atmosphere, and stored in a freezer.
Fatty Acids Profile
The methyl esters of fatty acids were diluted in hexane to about 1 mg/mL and were injected to a gas chromatography unit (Agilent 6890, model G1530A) equipped with an automatic injector tower (model 18593B) with a 100 vials holder and a flame ionization detector. Identification of the fatty acids was carried out in a mass spectrometry unit (Agilent 6850, model G2630A) equipped with a manual controller (model G2629A) and a selective mass detector (Agilent 5973, model G62577A). The column was a high polarity 50% cyanopropyl-methylpolysiloxane (122–2361) 60 m × 0.25 mm, df = 0.15 μm (Hewlett Packard DB-23). The injection temperature was 260°C and the split 130:1. The initial temperature of the column was 170°C and the heating program included a ramp of 2.5°C/min up to 190°C kept for 1 min and a subsequent increase at 1 °C/min to 205°C. Nitrogen was the carrier gas at a flow rate of 30 mL/min. γ linolenic acid was used as internal standard.
Separation of Unsaponifiable Matter Components
The separation of the unsaponifiable matter was carried out in a glass chromatography column (50 mm × 30 cm) packed with silica gel (net: 70–230 Merck, Darmstadt, Germany). A portion of about 0.8 to 1.0 g of unsaponifiable matter mixed with 2.0 g of industrial Celite was placed on the top of the column and eluted with reagent grade petroleum ether. The polarity of the mobile phase was gradually increased by addition of a given amount of ethyl acetate. Fractions of 150 mL were collected in a 250 mL Kitasato flask under vacuum to speed up the elution of each component at a flow rate of about 50 mL/min. Each fraction was applied onto an aluminum thin layer chromatography (TLC) plate to monitor the elution of squalene and phytosterols. Squalene was the first to be eluted after 15 fractions (150 mL each) of petroleum ether. Phytosterols were then eluted using 5250 mL (35 fractions) of a mixture (v/v) of 95:5 petroleum ether:ethyl acetate. Finally, the column was washed with 250 mL of a mixture (v/v) of 75:5 petroleum ether:ethyl acetate and 250 mL of methanol. Every fraction was monitored with two TLC plates. In one of the plates, squalene was eluted with a mixture (v/v) of 99:1 petroleum ether:ethyl ether and developed by spraying the plate with cerium sulfate and “burning” it in a stove. In the second TLC plate, phytosterols were eluted with a mixture (v/v) of 75:25 petroleum ether: ethyl acetate and developed by spraying it with 10% sulfuric acid. The compounds were identified by comparison of the individual Rf to the corresponding reference standards; 0.17 for phytosterols and 0.91 for squalene. The fractions that according to their Rf contained the same compounds were brought together, evaporated to dryness under vacuum, and the percentage of each fraction was determined gravimetrically. The residues were stored in dark vials at −18°C for subsequent quantification by gas chromatography.
Determination of Squalene and Phytosterols by Gas Chromatography
The fractions of the column were diluted to about 1 mg/mL in hexane and subsequently they were injected to the gas chromatography unit described in the previous section. The quantitative identification of the phytosterols was made in the mass spectrometry system described previously. The column was a cross-linked 5% phenyl-methyl-polysiloxane (19091S-433) 30 m × 0.25 mm, df = 0.25 μm (Hewlett Packard HP-5MS). The temperature of the column was 280°C and the heating included an isothermal program. Nitrogen was the carrier gas at a flow rate of 30 mL/min. Squalene and phytosterols were identified and quantified by reference to a standard curve of the following compounds (Sigma-Aldrich): squalene, stigmasterol 95%, stimagstanol 95% and β-sitosterol. Identification was made by reference to a library using the ChemStation software version AA.05.02 673 MX95245561.
Determination of Squalene by HPLC
The fractions containing squalene were diluted with 99:1 (v/v) petroleum ether:ethyl ether and analyzed in an HPLC instrument (Beckman 421) equipped with a photodiode array detector (Beckman 163) at a wavelength of 214 nm. The column was an RP-C18 (Nucleosil 100-C18, 250 m × 4 mm, df = 5 mm). The elution was isocratic with 91.95:8:0.05 (v/v) methanol:2-propanol:acetic acid, an injection volume of 20 μL and a flow rate of 1 mL/min.
Statistical Analysis
All experiments were carried out at least in triplicate. Statistics on a completely randomized design were determined with the SPSS 10.00 for windows procedure. Differences were considered to be statistically significant at p ≤ 0.05.
RESULTS AND DISCUSSION
Oil, Saponifiable, and Unsaponifiable Matter Content of Chia Seeds
shows the oil, saponifiable and unsaponifiable matter content of chia seeds from the two regions examined in this work. The oil content of the Sinaloa and Jalisco seeds was statistically different to a 95% confidence level, with the latter having the greater amount of oil. The difference in oil content could be due to environmental factors.[Citation7] In previous works, where the oil was extracted by the Soxhlet procedure, an oil content of 300 g/kg seed for Salvia hispanica[Citation11] and 298 g/kg seed for Salvia polystachya[Citation5] was reported. These values are in good agreement with those reported here for the Jalisco and Sinaloa seeds but are lower than those reported for Argentinean seeds; 323 to 386 g/kg seed.[Citation7] Unfortunately, the details on the oil extraction procedure, including the solvent used, from which such oil percentages obtained were not given. The oil content of chia seed is lower in comparison with other sources of vegetal oil such as flaxseed, sunflower, and sesame, which have oil contents of about 420, 470, and 530 g/kg, respectively.[Citation12] On the other hand, the saponifiable matter accounts for more than 80% of the oil in the Jalisco and Sinaloa seeds. The saponifiable matter of the Jalisco and Sinaloa seeds is not different to a 95% confidence level. The unsaponifiable fraction represents about 8% of the oil depending on the place of origin.
Table 1 Oil, saponifiable (SM) and unsaponifiable (USM) matter content (mean ± SD; n = 6) of chia seeds
Fatty Acids Profile
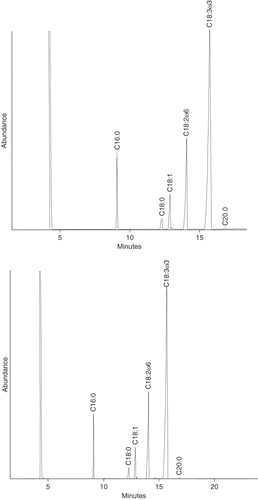
The fatty acids profiles of the saponifiable matter for the Jalisco and Sinaloa seeds are shown in . With reference to a database library, mass spectroscopy revealed that this matter is composed predominantly by unsaturated fatty acids of which α-linolenic is the major component. The same predominance has been reported for Salvia hispanica grown in Argentina.[Citation7] shows that the seeds from Jalisco and Sinaloa have a similar composition. In both of them α-linolenic acid predominates and accounts for about 60% of the total amount of fatty acids present in the oil. The rest is formed by linoleic, oleic, palmitic, stearic, and eicosanoic acids in decreasing order, respectively. The remaining 3% was not identified. As shown in the same table, such percentages are similar to those reported for Argentinean chia seeds[Citation7] and Chia II,[Citation6] but differ greatly from those for Salvia polystachya[Citation5] and Chia I.[Citation6] Taga and coworkers[Citation6] did not specify the variety of their Chia I and Chia II samples, while Ayerza[Citation7] examined presumably seeds of the Salvia hispanica species. The similarity in composition of the samples of these authors with ours suggests that Chia II and Ayerza's seeds are Salvia hispanica. On the other hand the Chia I sample of Taga and coworkes shows noticeable differences with respect to all of the others. Such differences are possibly due to different strains of chia as shown for the Salvia polystachya seeds examined by others.[Citation5]
Table 2 Fatty acids composition of Sinaloa and Jalisco chia oil compared with reported data. Data are expressed as g/100 g of total fatty acid methyl esters
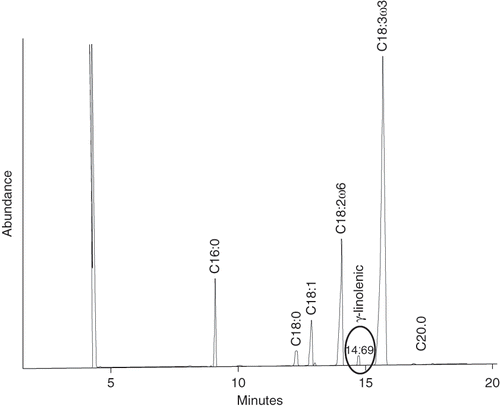
Figure 1 Gas chromatography profile of the saponifiable matter of Jalisco (top) and Sinaloa (bottom) chia seeds. Figures on peaks indicate the number of carbon atoms:number of double bonds of fatty acids.
In the samples analyzed, γ-linolenic acid was not detected. Its percent recovery, determined to asses the effectiveness of the complete analytical scheme, was 80%. This value suggests that during extraction and analysis part of the added acid was lost. shows the profile of the saponifiable matter to which γ-linolenic acid was added as internal standard. The peak having an elution time of 14.69 minutes shows the presence of this acid, confirmed by mass spectrometry. A good resolution of the peaks for α-linolenic and γ-linolenic acids is observed even at low concentrations of the latter. Results suggest that γ-linolenic acid is present in such a small amount in the seeds that it is lost during oil extraction, saponification, recovery and sterification.
Elution Profile of the Unsaponifiable Matter: Presence of Squalene and Phytosterols
The unsaponifiable matter resulting from saponification was analyzed by gas chromatography (). Some components were identified by reference to the retention time of their corresponding standard and others by their mass spectrum using the data library. The amount of squalene in the Sinaloa and Jalisco seeds was less than 0.5 g/kg. Compared with other sources of squalene, such as olive (3.0 to 7.0 g/kg oil),[Citation13] and amaranth oil (60.0 to 80.0 g/kg oil),[Citation14] chia seed oil cannot be considered a good source of this component. Squalene, stigmasterol, β-sitosterol, and stigmastanol were identified by reference to the retention time of their corresponding standards. According to mass spectrometry data, the peak at 9.64 mins can be ascribed to by-products of squalene and sterols biosynthesis. As for squalene, these compounds are derived from farnesol and are named gibberellins.[Citation15] Another possibility is that they are waxes and long chain alcohols as observed in the analysis of the unsaponifiable matter of lipidic substrates.[Citation16] This type of compounds is slightly more polar than squalene owing to the OH groups present in their structure. Sterols represent the major proportion of the unsaponifiable matter. The elution order of phytosterols was in agreement with that reported elsewhere,[Citation17] for the type of columns used in this work: Cholesterol → Cholestane → Brassicasterol → Campesterol → Campestanol → Stigmasterol → Stigmastadienol → Sitosterol → Sitostanol → Stigmastanol. The amount of phytosterols identified is shown in .
Figure 3 Gas chromatography profile of the complete unsaponifiable matter showing the presence of phytosterols and γ-linolenic acid.
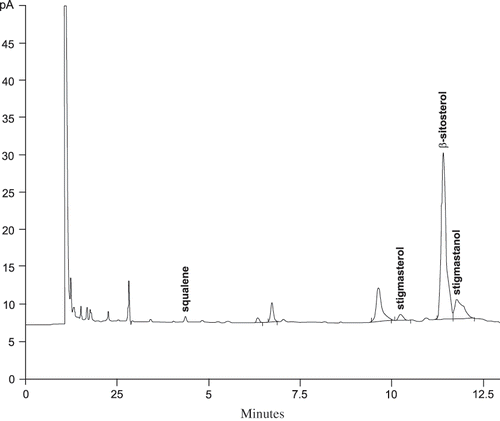
Table 3 Phytsoterols content (mean ± SD; n = 3) of Jalisco and Sinaloa chia seeds
The total sterols content of Jalisco and Sinaloa seeds is 12.6 g/kg and 8.15 g/kg, respectively. These levels are similar to unrefined evening primrose (Oenothera biennis) oil in which the total sterols content is about 10 g/kg. In other unrefined oils of different sources such as borage, olive, peanut, rapeseed, safflower, sesame and sunflower, the total sterols content range from 1.5 to 8 g/kg.[Citation9] The individual and total phytosterols content between the Jalisco and Sinaloa seeds was significantly different to a 95% confidence level. In the seeds from both regions β-sitosterol is the major component over stigmastanol and stigmasterol both of which are the next in decreasing content. In general, β-sitosterol represents around 60% of the total sterols in crude and refined vegetal oils.[Citation9, Citation18] In this work, β-sitosterol accounts for 66% and 74% of the total sterols content of Sinaloa and Jalisco chia seeds, respectively. shows the β-sitosterol and stigmasterol and stigmastanol contents of these seeds together with those for unrefined oils extracted from different seeds. It is well known that sterols are lost during oil refining.[Citation9, Citation19] Refining of chia oil can also lead to lower levels of sterols but the extent to which this could occur remains to be determined. Among the unrefined oils listed in only that from evening primrose (Oenothera biennis) surpasses the amount of β-Sitosterol of chia seed oil. On the other hand, the amount of stigmasterol in chia oil largely surpasses that of the other unrefined oils.
Table 4 Phytosterols content (g/kg) in chia seed oil and other unrefined seed oils
CONCLUSIONS
Chia seed oil is an attractive source of α-linolenic acid and phytosterols in comparison with other vegetal sources with actual important commercial use. The average oil content of the Sinaloa and Jalisco seeds is 25.5 ± 4.55% and 29.7 ± 4.05%, respectively, with α-linolenic acid accounting for about 62% of the total fatty acid methyl esters in the oil, while the total phytosterols content of the Sinaloa and Jalisco seeds is 8.15 ± 2.12 and 12.6 ± 3.80, respectively. The content of individual sterols, β-sitosterol, stigmasterol and stigmastanol, of both seeds is superior to that of peanut, rapeseed, safflower, sesame, and sunflower unrefined oils. The characterization of the lipid fraction of Mexican varieties provided information that could motivate the use of this seeds as an important food source considering the actual trend towards an increasing consumption of functional foods. It also opens the possibility of using the seed as a raw material for industrial purpose.
ACKNOWLEDGMENTS
Thanks are due to Edgar Vázquez Ramos, Lorena López Piña, and Linda Ramírez Muñoz for their technical support along different stages of this work. We are particularly grateful to DGAPA-UNAM (grant IN211602) for financial support and scholarship to L.M. Álvarez-Chávez.
REFERENCES
- Ortiz De Montellano , B.R. 1978 . Aztec cannibalism: An ecological necessity? . Science , 200 ( 4342 ) : 611 – 617 .
- Cahill , J. 2003 . Ethnobotany of chia, Salvia hispanica L (Lamiaceae). . Economic Botany , 574 : 604 – 614 .
- Scheer , J.F. 2001 . Magic of chia: Revival of an Ancient Wonder Food , Berkeley, CA : North Atlantic Books & Frog, Ltd .
- Lin , K.Y. , Daniel , J.R. and Whistler , R.L. 1994 . Structure of chia seed polysaccharide exudate . Carbohydrate Polymers , 23 : 13 – 18 .
- Bushway , A.A. , Belyea , P.R. and Bushway , R.J. 1981 . Chia seed as a source of oil, polysaccharide and protein . Journal of Food Science , 46 : 1349 – 1350 . 1356
- Taga , M.S. , Miller , E.E. and Pratt , D.E. 1984 . Chia seeds as a source of natural lipid antioxidants . Journal of the American Oil Chemists’ Society , 61 ( 5 ) : 928 – 931 .
- Ayerza , R. 1995 . Oil content and fatty acid composition of chia (Salvia hispanica L.) from five Northwestern locations in Argentina . Journal of the American Oil Chemists’ Society , 22 : 1079 – 1081 .
- Ayerza , R. and Coates , W. 2004 . Composition of chia (Salvia hispanica) grown in six tropical and subtropical ecosystems of South America . Tropical Science , 44 ( 3 ) : 131 – 135 .
- Phillips , K.M. , Ruggio , D.M. , Toivo , J.I. , Swank , M.A. and Simpkins , A.H. 2002 . Free and esterified sterol composition of edible oils and fats . Journal of Food Composition and Analysis , 15 : 123 – 142 .
- Association of Official Analytical Chemists . 1995 . Official Methods of Analysis , 16th , Washington, D.C : AOAC .
- Flores , C. 1938 . Estudio analítico de la Salvia hispanica (chía). , FQ-UNAM . Thesis
- United States Department of Agriculture (USDA)-Agricultural Research Service (ARS)-Nutrient Data Laboratory (NDL). USDA National Nutrient Database for Standard Reference. (posted: 2007) http://www.nal.usda.gov/fnic/foodcomp/search/ (Accessed: July 2007 ).
- Newmark , H.L. 1999 . Squalene, olive oil, and cancer: Review and hypothesis . Annals of the New York Academy of Sciences , 889 : 193 – 203 .
- Budin , J.T. , Breene , W.M. and Putman , D.H. 1996 . Some compositional properties of seed oils of eight Amaranth species . Journal of the American Oil Chemists’ Society , 73 ( 4 ) : 475 – 481 .
- Fernandez-Martin , R. , Domenech , C. , Cerdá-Olmedo , E. and Ávalos , J. 2000 . ent-Kaurene and squalene synthesis in Fusarium fujikuroi cell-free extracts . Phytochemistry , 54 : 723 – 728 .
- Gustone , F.D. 1996 . Fatty acid and lipid chemistry , New York : Blackie Academic & Professional .
- Abidi , S.L. 2001 . Chromatographic analysis of plant sterols in food and vegetable oils . Journal of Chromatography A , 935 : 173 – 201 .
- Sidhu , J.S. , Al-Hooti , S.N. , Al-Saqer , J.M. , Al-Amiri , H.A. , Al-Foudari , M. , Al-Othman , A. , Ahmad , A. , Al-Haji , L. , Ahmed , N. , Mansor , I.B. and Minal , J. 2004 . Developing functional foods using red palm olein: Pilot-scale studies . International Journal of Food Properties , 7 : 1 – 13 .
- Patil , R.T. and Ali , N. 2006 . Effect of pre-treatments on mechanical oil expression of soybean using a commercial oil expeller . International Journal of Food Properties , 9 : 227 – 236 .