Abstract
Rheological properties of Pacific whiting surimi, in the absence and presence of chicken plasma (CP) at different levels (0.3–3.0%, w/w), were studied by dynamic rheological (small strain) and torsion fracture measurements, respectively. The surimi paste exhibited two major distinctive rheological transitions during heating (1°C/min) from 20 to 90°C with peaks observed at 33 and 56°C. The abrupt loss of G′ upon heating from 47 to 57°C, and the occurrence of small peak of phase angle at the same temperature range were prevented by the addition of CP. Nevertheless, the final G′ of the surimi paste added with CP was lower than that of the control. But shear fracture stress of both kamaboko and modori gels increased as the CP levels increased and shear strain increased with the addition of CP up to 2% (P < 0.05). CP inhibited the degradation of myosin heavy chains (MHC) caused by endogenous proteinases as indicated by more retained MHC and lowered TCA-soluble peptide content. Whiteness of gels decreased somewhat with increasing CP levels. CP, thus, could be a helpful additive for improving gelling properties of Pacific whiting surimi
INTRODUCTION
When a myofibrillar protein sol is heated at a constant rate to induce gelation, several transitions in shear modulus development can generally be detected by small-strain rheometry.[Citation1] While such small strain testing has not been shown to highly correlate with sensory texture or fracture strength,[Citation2,Citation3,Citation4] the method has proven useful to study the alteration of gelation properties caused by proteolysis or affected by the addition of protein additives.[Citation5] The storage modulus (G′) and loss modulus (G″) are the measures of material elasticity and viscosity, respectively, at any point during heating or cooling. Tan δ (the ratio of G″/G′) reflects the relative contribution of each to the overall rheological characteristics.[Citation6]
Pacific whiting surimi accounts for about 20% of surimi production in the United States.[Citation7] High proteolytic activity is typical in Pacific whiting muscle and continues after the flesh has been processed into surimi. During heating the proteolysis of myofibrillar protein can prevent development of strong and elastic gels.[Citation8] Rapid heating methods and/or non-muscle protein additives which inhibit these proteases have been used to prevent this. Beef plasma protein (BPP) has been shown to be the most active inhibitor of those tested in whiting surimi, commonly used at about 1–2% concentration in finished surimi-seafood formulations.[Citation9] Seymour et al.[Citation10] reported that the gel-enhancing ability for Pacific whiting surimi by BPP was due to the combined effects of proteolytic inhibition by α2-macroglobulin, covalent cross-linking by transglutaminase (TGase) and gel formation by bovine serum albumin or fibrinogen. Benjakul and Visessanguan [Citation11] reported that pig plasma protein (PPP) showed higher inhibitory activity than BPP or egg white against Pacific whiting protease. More recently the addition of chicken plasma (CP) was found to increase the strength of surimi gels from bigeye snapper due to its protease inhibitory activity.[Citation12] Use of CP to inhibit the greater proteolytic activity of Pacific whiting surimi has not been studied. The purpose of this study was to investigate the effect of CP on the rheological and textural properties of Pacific whiting surimi during heat-induced gelation.
MATERIALS AND METHODS
Chemicals and Surimi
Sodium chloride and trichloroacetic acid were purchased from Fisher Scientific (Pittsburgh, PA, USA). Bovine serum albumin (BSA) and L-tyrosine were procured from Sigma-Aldrich, Inc. (St. Louis, MO, USA). Sodium dodecyl sulfate (SDS) was obtained from Bio-Rad Laboratories (Hercules, CA, USA). Novex precast gels and other electrophoresis reagents were purchased from Invitrogen life technologies (Carlsbad, CA, USA). Frozen surimi grade FA produced from Pacific whiting (Merluccius productus), specifically prepared without added protease inhibitors, was generously provided by Trident Seafoods Corp. (Seattle, WA, USA) and kept at −25°C until used.
Preparation of Chicken Plasma Protein
Chicken blood was obtained from a slaughterhouse in Hat Yai, Thailand. During collection, one-tenth volume of 3.8% (w/v) trisodium citrate was added to prevent coagulation. The blood was centrifuged twice at 1,500 g for 15 min at 4°C using a Sorvall Model RC-B Plus centrifuge (Newtown, CT, USA). The supernatant was then freeze-dried and kept at −18°C until used.
Preparation of Surimi Pastes and Gels
Frozen surimi was partially thawed at 4°C for 2–3 h, cut into small pieces and chopped in a Cuisinart mixer (Cuisinart original food processor, East Windsor, NJ, USA) for 4 min with 2.5% (w/w) NaCl. CP at different levels (0, 0.3, 0.5, 1, 2, and 3%, w/w) was added. The moisture content of surimi paste was adjusted to 80% by the addition of iced water. This paste was used as is to load into the cooking rheometer for small strain testing. For fracture testing, some paste was stuffed into stainless steel tubes with a diameter of 1.9 cm and then incubated at 55°C for 30 min, followed by heating at 90°C for 20 min in a Magni Whirl constant temperature bath (Blue M electrical company, Blue Island, IL, USA). This sample was referred to as modori gel, having been prepared at the optimum temperature for proteolysis to occur.[Citation9] Kamaboko gels were prepared by incubating the paste at 40°C for 30 min prior to heating at 90°C for 20 min; the pretreatment at 40°C being optimum for any endogenous transglutaminase cross linking to occur.[Citation13] A directly cooked gel was heated at 90°C for 20 min. After heating, all gels were immediately cooled in iced water for 30 min. The samples were removed from tubes after cooling, placed in plastic bags and kept in a refrigerator overnight prior to analysis.
Dynamic Test (Small Strain)
Rheological changes of surimi paste during heating were continuously measured using a ATS Rheosystem Stress Tech rheometer (Stresstech, Rheologica instruments AB, Lund, Sweden). The rheometer was equipped with 40 mm, 4 degree slope cone and plate geometry. An oscillation of 0.1 Hz with a resistance stress of 35 Pa was used for testing. This condition was determined to give a linear response in the viscoelastic region. Temperature sweeps were recorded during heating from 20°C (raw sol/paste) to 90°C (cooked gel) at 1°C/min. To avoid evaporation of the sample during heating, a lubricant and cover were used. The elastic modulus (G′), viscous modulus (G″) and phase angle were recorded. The testing was performed in duplicate.
Torsion Test (Large Strain Fracture)
After being equilibrated at 4°C overnight, gel samples were allowed to reach room temperature, and subsequently cut into the length of about 3.0 cm. The prepared samples were attached to mounting discs at each flat end with cyanoacrylate glue. To obtain the dumbbell-shaped samples, the gels were milled to 1.0 cm diameter using a milling machine (Gel Consultants Inc., Raleigh, NC, USA). For testing, 7 milled samples were vertically mounted and twisted to the point of fracture at 2.5 rpm on a Hamann Torsion Gelometer (Gel Consultants Inc., Raleigh, NC, USA). Stress (kPa) and strain (dimensionless) at fracture were calculated with the manufacturer's software for each sample, corresponding to the strength and deformability of the gels, respectively.
Whiteness Measurement
The color attributes of five cooked gel samples from each treatment were measured with a Chroma Meter CR-300 (Minolta Camera Co., Ltd., Osaka, Japan). CIE L* (lightness, 0–100), a* (red/green; “+” being toward the red and “−” being toward the green) and b* (yellow/blue; “+” being toward the yellow and “−” being toward the blue) values were measured. Whiteness index was calculated using the following equation [Citation14]:
Determination of Autolysis in Surimi Gels
In 2 g of finely chopped gel samples, 18 ml of 5% TCA were added and homogenized for 2 min using a Sorvall OMNI-MIXER (Ivan Sorvall Inc, Norwalk, CONN, USA) at the speed of 10,000 rpm. The homogenate was incubated at 4°C for 1 h and centrifuged at 5000 g for 5 min. TCA-soluble peptides in the supernatant were measured using the Lowry method [Citation15] and expressed as μmole tyrosine/g sample.[Citation9]
SDS-PAGE of Cooked Gels
The cooked samples obtained from dynamic and torsion tests were subjected to SDS-PAGE analysis according to the method of Laemmli.[Citation16] In 2 g of sample, 18 ml of 5% (w/v) SDS solution was added. The mixture was then homogenized using a Sorvall OMNI-MIXER (Ivan Sorvall Inc, Norwalk, CONN, USA) at a speed of 10,000 rpm for 1 min. The homogenates were incubated at 85°C in a water bath for 1 h to dissolve total proteins. Solubilized samples were mixed at 1:1 (v/v) ratio with the Novex tris-glycine SDS sample buffer in the presence of reducing agent and boiled for 3 min. The samples (20 μg protein) were loaded into Novex 10% tris-glycine pre-cast gel and subjected to electrophoresis at a current constant at 30 mA/gel using a Xcell SureLock™ Mini-Cell (Novex, San Diego, CA, USA). After separation, the proteins were stained with SimplyBlue™ SafeStain for 3 h. and destained with water. The gel was dried overnight with Gel-Dry™ drying solution.
Statistical Analysis
Data were subjected to analysis of variance (ANOVA). Comparison of means was carried out by Duncan's multiple-range test. Analysis was performed using a SPSS package (SPSS 10.0 for windows, SPSS Inc, Chicago, IL).
RESULTS AND DISCUSSION
Effect of Chicken Plasma on Rheology
Changes in storage modulus (G′) and loss modulus (G″) of Pacific whiting surimi paste during transition from sol to gel as a function of temperature are presented in . The first small peak of G′ appeared at approximately 23°C and G′ slightly declined to reach the minimum value at 29°C. Those changes could derive from the partial denaturation of muscle proteins. Differences in magnitude of G′ were observed when different concentrations of CP were added. Ogawa et al.[Citation17] reported that a small decrease in G′ starting at 25°C during ramp heating indicated the unfolding of α-helix of myosin molecules in the tail portion. G′ substantially increased at 30°C and showed the highest peak at 33°C. Subsequently, G′ decreased until the temperature reached 41°C. However, the rate of decrease was lowered as the addition of CP increased. The sharp increase in G′ at the first transition indicated that myofibrillar protein in surimi started to form a gel network. Ziegler and Acton [Citation18] reported that heating natural actomyosin at temperatures less than 40°C generally resulted in the dissociation of some myofibrillar components, for example tropomyosin, from the F-actin backbone and F-actin from its super helix structure. Egelandsdal et al.[Citation19] reported that initial increase of G′ was attributed to the cross-linking of myosin. Without CP addition, the shoulder was observed at the temperature range of 41–47°C and then markedly decreased to a minimum value at 56°C. Samples containing added CP had no abrupt loss of G′ value over this temperature range. The lowest value of G′ for surimi paste with no added CP occurred at 56°C, and thus, could caused by proteolytic activity in Pacific whiting active at this temperature (the “modori phenomenon”.[Citation9]) However, dissociation of the actin-myosin complex and the denaturation of light meromyosin could also lead to an increased fluidity in this temperature range.[Citation19] The unfolding of myosin may thus cause an increase in fluidity of the semi-gel structure and thereby disrupt protein gel network formed in the early stages of heating, resulting in a decrease in G′.[Citation6] G′ increased sharply in the control sample (no added CP) after exhibiting this modori-like phenomenon, eventually reaching a value of 2970 Pa at the end of testing. For the samples added with CP, a slight increase in G′ was observed and the final values were lower than that of the control. Sample added with 2 and 3% CP showed the lowest value. Egelandsdal et al.[Citation19] suggested that the increase in G′ after 47°C probably attributed to the formation of irreversible gel networks. When muscle proteins at high ionic strength were heated, actomyosin started to unfold and interact with water, consequently exhibiting the viscous nature of the paste. As temperature continuously increased, unfolded actomyosin entangled and formed gel networks as evidenced by an increase of G′ at temperature greater than 55°C. After the gels were cooled down to 25°C, no differences in G′ were obtained between the sample added with 0.3 and 3% CP (data not shown). From the results, a lower G′ was obtained when higher CP contents were used, indicating the dilution effect of muscle proteins by CP addition. Visessanguan et al.[Citation20] also reported that lower gel modulus was obtained with actomyosin added with high level of PPP.
Figure 1 Effect of chicken plasma on changes in (a) storage modulus (G′) and (b) loss modulus (G″) of Pacific whiting surimi paste heated from 20 to 90°C at a rate of 1°C/min. 0.0 (e), 0.3 (▪), 0.5 (Δ), 1.0 (×), 2.0 (*), 3.0% CP (•).
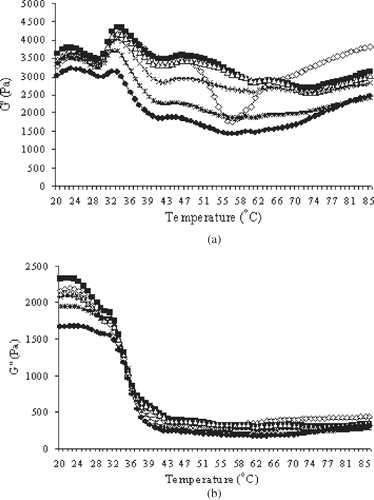
The changes in G″ of Pacific whiting surimi during heat-induced gelation were also monitored (). G″ is a measure of the deformation energy used up in the sample during the shear and lost to the sample afterwards, representing the viscous behavior of a sample.[Citation4] Initially the lowest G″ values were evident in samples containing 3% CP, while the highest values were evidenced by samples containing 0.3% CP. A gradual decrease in G″ was observed for all samples to which CP was added over the temperature range of 20 to 32°C. Thereafter, a marked decrease in G″ value was observed for all samples over the temperature range of 32–43°C. At temperature higher than 45°C, no changes in G″ were observed. At the end of testing, the control sample showed a slightly higher G″ as compared with those to which CP had been added.
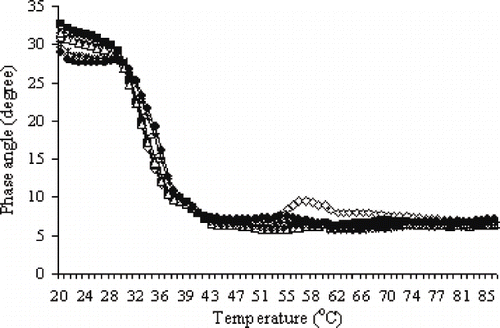
Changes in phase angle of Pacific whiting surimi during ramp heating corresponded to those of G″ (). Changes in phase angle are particularly indicative of the transition from sol to gel. Surimi paste prior to heating behaves as a viscous material, as indicated by a phase angle value of 31°. The phase angle exhibited by these samples gradually decreased over the temperature range of 20–29°C and sharply decreased (from 28° to 7°) from 30 to 43°C, indicating the formation of an elastic material. No marked changes in phase angle of all samples were observed in the temperature range above 43°C. However, a small peak was observed at the temperature range of 53–62°C by the control sample, which coincided with the sharp decrease in G′, apparently caused by extensive proteolytic activity (gel degradation). A gradual increase in phase angle of the control sample as it was heated from 53 to 62°C suggested that this sample exhibited more fluidity as a consequence of proteolytic hydrolysis. No changes in phase angle was observed over this higher temperature range for samples to which CP was added, indicating effective inhibition of proteolysis by the added CP.
Figure 2 Effect of chicken plasma on changes in phase angle of Pacific whiting surimi paste heated from 20 to 90°C at a rate of 1°C/min. 0.0 (e), 0.3 (▪), 0.5 (Δ), 1.0 (×), 2.0 (*), 3.0% CP (•).
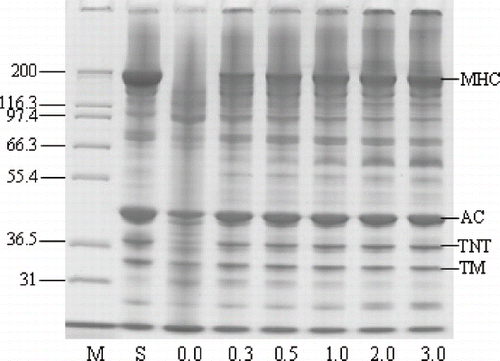
The SDS-PAGE pattern revealed that the degradation of myosin heavy chain (MHC) of surimi paste was inhibited when CP was added, while the MHC band completely disappeared for the control sample (without CP) (). MHC band intensity of Pacific whiting surimi gels increased as the amount of added CP increased. The addition of CP at a level of 3.0% almost completely protected MHC from hydrolysis during gelation of Pacific whiting surimi that occurred upon heating at a rate of 1°C/min from 20 to 90°C. Actin was also degraded to a high extent when the samples were identically ramp heated in the absence of added CP. When MHC was completely hydrolyzed, actin seemingly became the substrate most susceptible to proteolysis. It seems quite apparent that the inhibition of MHC proteolysis by the addition of CP has a direct effect on the rheological properties of surimi paste during heat-induced gelation, which are most likely determined by the chain length of proteins and therefore their ability to interact and form a firm gel.
Effects of Chicken Plasma on the Structural (Fracture)
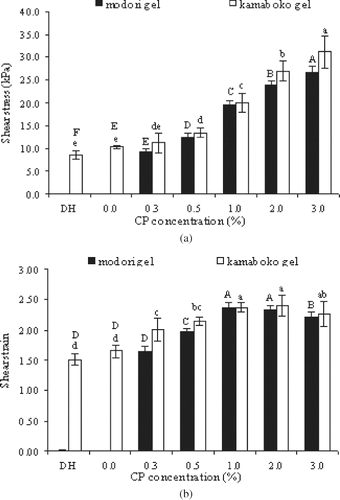
The fracture shear stress of both kamaboko and modori gels made from Pacific whiting surimi increased as the level of added CP was increased up to 3.0% (w/w) (P < 0.05) (). The control modori gel was too weak to exhibit a measurable fracture shear stress and strain. No difference in fracture shear stress was exhibited when comparing the control vs 0.3% CP added kamaboko gels. With the addition of 3% CP, the fracture shear stress of the kamaboko and modori gels increased by 3 and 27 times, respectively, compared with their corresponding controls. Reppond and Babbitt[Citation21] showed that addition of 2% (w/w) BPP increased the strength of gels made from arrowtooth flounder surimi. Morrissey et al.[Citation9] also reported that BPP at a concentration as low as 1% (w/w) strongly inhibited proteolysis and thus resulted in increased strength of gels made from Pacific whiting surimi.
Figure 4 Effect of chicken plasma on the textural properties shear stress (a) and shear strain (b) of Pacific whiting surimi gels. DH: directly heated gel prepared by heating the sol at 90°C for 20 min. Modori and kamaboko gels with various levels of chicken plasma were prepared by incubating the sol at 55°C and 40°C for 30 min, respectively, prior to heating at 90°C for 20 min. Bars represent the standard deviation from five determinations.
Proteolysis can apparently be retarded to different degrees depending upon which protein additives are used. Benjakul and Visessanguan[Citation11] found that PPP exhibited higher inhibitory activity than BPP or egg white against Pacific whiting protease at the same level tested. Blood plasma from any species contains several types of protease inhibitors.[Citation22] When comparing the fracture shear stress of the control, directly heated gels with those of the control kamaboko gel, the latter was 1.2 times higher. This is possibly attributable to the setting phenomenon that often occurs during the low temperature preincubation step used in preparing the kamaboko gels. The setting phenomenon is attributed to TGase activity,[Citation23,Citation24] an endogenous enzyme which at temperatures of 5 to 40°C (dependent on fish species) can induce the formation of ϵ-(γ-glutamyl) lysine linkages between proteins.[Citation13]
A slight increase in fracture shear strain was observed as the amount of CP added was increased up to 1%. No further changes were observed when higher levels of CP were added. The fracture shear strain of the kamaboko and modori gels with 1% and 2% CP increased by 1.44, 1.45, and 2.37, 2.33 times compared with the corresponding controls, respectively. Kassama et al.[Citation25] reported that increasing soy protein concentration up to 5% increased water holding capacity, hardness, and cohesiveness of meat patties. Fracture shear strain of kamaboko gels of Pacific whiting surimi was also higher than that of the directly heated gels. Slight decreases in fracture shear strain of both modori and kamaboko gels were observed when 3.0% CP was added. This was possibly an artifact caused by the lower content of myofibrillar proteins resulting as a consequence of adding CP at such high content. However, Senthil et al.[Citation27] found that incorporated of seaweed powder into fish cutlet upto 10% level have no adversely affecting the textural properties. From these results, the addition of CP to prevent proteolysis, in combination with setting induced by low temperature preincubation (kamaboko gels) was most effective in improving the gel-forming ability of surimi from Pacific whiting.
Though the addition of CP resulted in stronger and more deformable gels, a lower final G′ was observed when CP was added to surimi paste prior to ramp heating, especially evident at the higher level of CP inclusion (). Liu and Xiong [Citation5] mentioned that their gel strength data did not necessarily agree with final values of G′ in dynamic rheological measurements during ramp heating. The final G′ is the measurement of elasticity of myofibrillar gels when the sample is still hot, while fracture gel strength is determined after the formed gel has been cooled. Also, the dynamic rheological measurement is nondestructive, whereas the gel penetration force measurement is destructive. Both measurements represent different aspects of the rheological properties of gelled proteins and hence are not obviously predictive of one another.
Effects of Chicken Plasma on Color
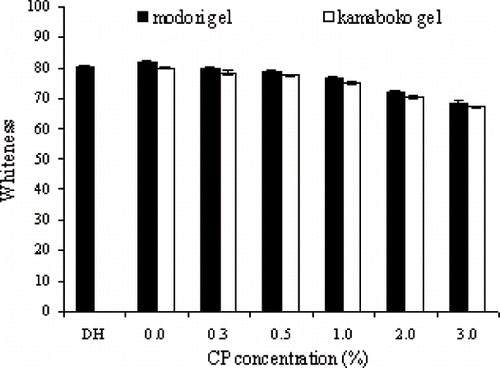
The whiteness of all cooked gels decreased when CP had been added, especially at higher CP concentrations (P < 0.05) (). With the addition of 3.0% (w/w) CP, whiteness of kamaboko and modori gels decreased by 16.37 and 16.28%, respectively, compared with that of gels prepared by the same heating schedules without added CP. This result was in accordance with Benjakul et al.[Citation27] who reported that increased levels of PPP addition resulted in decreased whiteness of surimi gels. The plasma proteins contained some hemoglobin, as well as other pigments with a pale straw color.[Citation11] These pigments possibly reduced the whiteness of resulting gels. The whiteness of directly heated gels was slightly lower than that of kamaboko or modori gels (P < 0.05).
Effect of Chicken Plasma on Protein Degradation
The highest TCA-soluble peptide content, indicative of proteolytic degradation, was observed for modori gels to which no CP had been added (). TCA-soluble peptide content of kamaboko gels decreased as the level of CP added was increased to 1.0% (P < 0.05), and 2% CP addition prevented the formation of TCA-soluble peptides in modori gels. These results indicate greater proteolysis in the modori gels. In general, modori gels exhibited greater protein degradation than kamaboko gels, as indicated by higher TCA-soluble peptide contents. From these results, it is apparent that CP exhibited inhibitory activity towards degradation of myofibrillar proteins at 55°C in Pacific whiting surimi.
Table 1 TCA-soluble peptide content of surimi gels from Pacific whiting added with different levels of chicken plasma
The addition of dried beef plasma hydrolysate similarly prevented this proteolytic “modori phenomenon” in Pacific whiting modori gels (precooked at 60°C plus final cooking at 90°C).[Citation28] Directly heated (to 90°C) gels contained higher TCA-soluble peptide content as compared with that of the control kamaboko gel, but lower than that of the control modori gel. Among all samples, the control modori gel showed the highest TCA-soluble peptide content. It has been reported that 50–60°C is the optimum temperature for heat-activated fish proteinases in Pacific whiting, especially cathepsin L.[Citation29,Citation30]
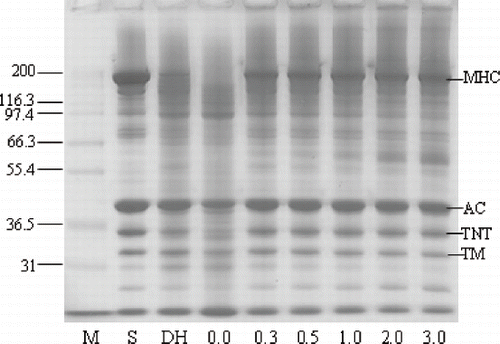
The protein patterns by SDS-PAGE of modori gels made with and without added CP are shown in . The complete disappearance of the MHC band was observed in the control (no CP addition) ‘modori’ gel, while the MHC band was retained to a small extent in directly heated gels. Actin was also hydrolyzed to some extent in the gels to which no CP had been added. MHC was the primary target and most extensively hydrolyzed, followed by troponin-T and tropomyosin.[Citation31] From these results it is apparent that MHC was retained more as the level of added CP was increased to 1.0% while no further changes were observed when higher levels of CP were added. This result was in agreement with the observation that lower TCA-soluble peptide content was measured in modori gels containing high levels of added CP. Changes in actin, troponin and tropomyosin were not observed for samples to which CP had been added; however, the intensity of those bands decreased to some extent when compared with those of the starting surimi.
Figure 6 SDS-PAGE pattern of modori gels from Pacific whiting surimi added with various levels of chicken plasma. M: protein marker; S: surimi; DH: directly heated gel without CP; Numbers designate the level of chicken plasma added. MHC: Myosin heavy chain; AC: Actin; TNT: troponin-T; TM: tropomyosin.
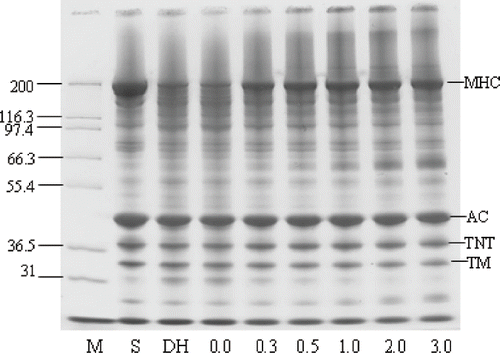
In kamaboko gels an increase in MHC band intensity was also observed when the level of added CP was increased (). Moreover, formation of cross-linked proteins was observed as a band at the the top of SDS-PAGE gels, possibly caused by polymerization of MHC during the setting preincubation.[Citation13,Citation32] It was noted that some MHC was retained in the control (no CP addition) sample and in the directly heated gel. This suggested that polymerized MHC was more resistant to proteolysis. As a result, some MHC was still found in the resulting gels. For directly heated (to 90°C) gels, the MHC band intensity decreased to a lesser extent as compared with the modori gels. No changes in actin, troponin, and tropomyosin were found in these samples, with or without the addition of CP except that the actin band intensity slightly decreased. This also was possibly an artifact caused by the dilution effect of CP addition. Thus, the addition of CP could prevent the degradation of proteins in surimi, which in turn allowed a stronger three-dimensional gel network to be formed.
Figure 7 SDS-PAGE pattern of kamaboko gels from Pacific whiting surimi added with various levels of chicken plasma M: protein marker; S: surimi; DH: directly heated gel without CP; Numbers designate the level of chicken plasma added. MHC: Myosin heavy chain; AC: Actin; TNT: troponin-T; TM: tropomyosin.
CONCLUSIONS
Addition of chicken plasma directly affects the rheological, structural, and colorometric properties of Pacific whiting surimi gels. Though CP addition resulted in the lowered G′ of ramp-heated gels while hot, it caused an increase in fracture shear stress and strain of cooked, cooled gels, both kamaboko and modori gels. The gel enhancing effect of CP addition was most likely due to the inhibition of protein degradation caused by proteases endogenous to the Pacific whiting surimi. CP addition did however contribute to reduced whiteness of gels.
ACKNOWLEDGMENTS
The authors would like to thank Sharon Ramsey (Food Rheology Laboratory) at the Food Science Department, NCSU, for helping in rheological properties measurement training. This work was supported by the Thailand Research Fund under The Royal Golden Jubilee PhD Program to Saroat Rawdkuen (PHD/0039/2544).
REFERENCES
- Xiong , Y.L. 1993 . A comparison of the rheological characteristics of different fractions of chicken myofibrillar proteins . Journal of Food Biochemistry , 16 : 217 – 227 .
- Hamann , D.D. and MacDonald , G.A. 1992 . “ Rheology and texture properties of surimi and surimi-based foods ” . In Surimi Technology , Edited by: Lanier , T.C. and Lee , C.M. 429 – 500 . New York : Marcel Dekker .
- Hamann , D.D. 1988 . Rheology as a means of evaluating muscle functionality of processed foods . Food Technology , 42 ( 6 ) : 66 – 71 .
- Tabilo-Munizaga , G. and Barbosa-Canovas , G. 2005 . Rheology for the food industry . Journal of Food Engineering , 67 : 147 – 156 .
- Liu , G. and Xiong , Y.L. 1997 . Gelation of chicken muscle myofibrillar proteins treated with protease inhibitors and phosphates . Journal of Agricultural and Food Chemistry , 45 : 3437 – 3422 .
- Xiong , Y.L. 1997 . Structure-function relationships of muscle proteins: viscoelastic changes related to heating procedures . Journal of Food Science , 59 : 734 – 738 .
- Guenneugues , P. and Morrissey , M.T. 2004 . “ Surimi resources ” . In Surimi and Surimi Seafood , 2nd , Edited by: Park , J.W . 3 – 32 . New York : Taylor & Francis Group .
- Chang-Lee , M.V. , Pacheco-Aguilar , D.L. , Crawford , L. and Lampila , L.E. 1989 . Proteolytic activity of surimi from Pacific whiting (Merluccius productus) and heat-set gel texture . Journal of Food Science , 54 : 1116 – 1119 . 1124
- Morrissey , M.T. , Wu , J.W. , Lin , D.D. and An , H. 1993 . Effect of food grade protease inhibitor on autolysis and gel strength of surimi . Journal of Food Science , 58 : 1050 – 1054 .
- Seymour , T.A. , Peter , M.Y. , Morrissey , M.T. and An , H. 1997 . Surimi gel enhancement by bovine plasma proteins . Journal of Agricultural and Food Chemistry , 45 : 2919 – 2923 .
- Benjakul , S. and Visessanguan , W. 2000 . Pig plasma protein: potential use as proteinase inhibitor for surimi manufacture; inhibitory activity and the active components . Journal of the Science of Food and Agriculture , 80 : 1351 – 1356 .
- Rawdkuen , S. , Benjakul , S. , Visessanguan , W. and Lanier , T.C. 2004 . Chicken plasma protein affects gelation of surimi from bigeye snapper (Priacanthus tayenus) . Food Hydrocolloids , 18 : 259 – 270 .
- Benjakul , S. and Visessanguan , W. 2003 . Food Research International . Transglutaminase-mediated setting in bigeye snapper surimi , 36 : 253 – 266 .
- Park , J.W. 59 . Functional protein additives in surimi gels . Journal of Food Science 1994 , : 525 – 527 .
- Lowry , O.H. , Rosebrough , N.J. , Farr , L.A. and Randall , R.J. 1951 . Protein measurement with the Folin phenol reagent . Journal of Biological Chemistry , 193 : 265 – 275 .
- Laemmli , U.K. 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage . Nature , 227 : 680 – 685 .
- Ogawa , M. , Kanamaru , J. , Miyashita , H. , Tamiya , T. and Tsuchiya , T. 1995 . Alpha-helical structure of fish actomyosin: changes during setting . Journal of Food Science , 60 : 297 – 299 .
- Ziegler , G.R. and Acton , J.C. 1984 . Mechanisms of gel formation by proteins of muscle tissues . Food Technology , 38 ( 5 ) : 77 – 80 . 82
- Egelandsdal , B. , Fretheim , K. and Samejima , K. 1986 . Dynamic rheological measurements on heat-induced myosin gels: effect of ionic strength, protein concentration and addition of adenosine triphosphate or pyrophosphate . Journal of the Science of Food and Agriculture , 37 : 915 – 926 .
- Visessanguan , W. , Benjakul , S. and An , H. 2000 . Porcine plasma proteins as a surimi protease inhibitor: effects on actomyosin gelation . Journal of Food Science , 65 : 607 – 611 .
- Reppond , K.D. and Babbitt , J.K. 1993 . Protease inhibitors affect physical properties of arrowtooth flounder and walley pollock surimi . Journal of Food Science , 58 : 96 – 98 .
- Karlsrud , T.S. , Bu , L. , Aasen , A.O. and Johansen , H.T. 1996 . Quantification of kininogen in plasma. A functional method based on the cysteine proteinase inhibitory activity . Thrombosis Research , 82 : 265 – 273 .
- Tsukamasa , Y. , Miyaka , Y. , Ando , M. and Makinodan , Y. 2002 . Total activity of transglutaminase at various temperatures in several fish meats . Fisheries Science , 68 : 929 – 933 .
- Wan , J. , Kimura , I. , Satake , M. and Seki , N. 1994 . Effect of calcium ion concentration of the gelling properties and transglutaminase activity of walleye pollack surimi paste . Fisheries Science , 60 : 107 – 113 .
- Kassama , L.S. , Ngadi , M.O. and Raghavan , G.S.V. 2003 . Structural and instrumental textural properties of meat patties containing soy protein . International Journal of Food Properties , 6 ( 3 ) : 519 – 529 .
- Senthil , A. , Mamatha , B.S. and Mahadevaswamy , M. 2005 . Effect of using seaweed (eucheuma) powder on the quality of fish cutlet . International Journal of Food Sciences and Nutrition , 6 ( 5 ) : 327 – 335 .
- Benjakul , S. , Visessanguan , W. and Srivilai , C. 2001 . Porcine plasma protein as proteinase inhibitor in bigeye snapper (Priacanthus tayenus) muscle and surimi . Journal of the Science of Food and Agriculture , 81 : 1039 – 1046 .
- Hamann , D.D. , Amato , P.M. , Wu , M.C. and Foegeding , E.A. 1990 . Inhibition of modori (gel weakening) in surimi by plasma hydrolysate and egg white . Journal of Food Science , 55 : 665 – 609 . 795
- An , H. , Peters , M.Y. and Seymour , T.A. 1996 . Role of endogenous enzymes in surimi gelation . Trends in Food Science and Technology , 7 : 321 – 326 .
- Choi , Y.J. , Kang , I.S. and Lanier , T.C. 2004 . “ Proteolytic enzymes and control in surimi ” . In Surimi and Surimi Seafood , 2nd , Edited by: Park , J.W . 227 – 278 . New York : Taylor & Francis .
- An , H. , Weerasinghe , V. , Seymour , T.A. and Morrissey , M.T. 1994 . Cathepsin degradation of Pacific whiting surimi protein . Journal of Food Science , 59 : 1013 – 1017 . 1033
- Rawdkuen , S. , Benjakul , S. , Visessanguan , W. and Lanier , T.C. 2005 . Combination effects of chicken plasma protein and setting phenomenon on gel properties and cross-linking of bigeye snapper muscle proteins . Lebensmittel.-Wissenschaft und-Technologie , 38 : 353 – 362 .