Abstract
Effect of moisture content on thermo-physical properties of sugarcane, palmyra palm, and date-palm granular jaggery were investigated. Thermal conductivity and diffusivity were determined by line-heat-source transient heat-transfer methodology, while specific heat was calculated from additional data on bulk density of the samples. Thermal conductivity, diffusivity, specific heat, and bulk density was found to vary from 0.08 to 0.39 W m−1 K−1, 0.10 to 0.13 × 10−6 m2 s−1, 1.19 to 2.97 kJ kg−1 K−1, and 510 to 1310 kg m−3, respectively, for a moisture range of 2.0–14.3 (%d.b.); all at an average temperature of 30°C. All these properties except—thermal diffusivity—followed an increasing trend; with the increase in moisture content, each showed a high correlation coefficients. The variation of thermal diffusivity was found to be insignificant.
INTRODUCTION
Jaggery—a sugar-rich food product—is produced all over the world under different names, such as Gur (India), Desi (Pakistan), Panela (Mexico and South America), Jaggery (Burma and African countries), Hakuru (Sri Lanka), and Naam Taan Oi (Thailand).[Citation1] It is basically obtained by evaporation of sugarcane juice, or sap obtained from palmyra palm (Borassus flabellifer L.), or wild date-palm (Phoenix sylvestris L.) trees.[Citation2] The juice contains 10.93% total sugars, mainly comprised of sucrose and small amount of reducing sugars and other minerals and vitamins.[Citation3] After primary filtration for removing suspended particles, the juice or sap is concentrated in an open pan. The concentrated syrup is a thick mass, which is marketed in the form of liquid and solidified mass. The final product so obtained usually contains 65–85% sucrose and 5–15% reducing sugars.[Citation4] It is consumed directly or used for preparation of sweet confectionery items. It is also used in many herbal and traditional medicines.[Citation2] Moreover, the micronutrients present in the jaggery posses antitoxic and anticarcinogenic properties.[Citation5] India produces about 6 million tonnes of jaggery annually, which is 70% of the total production in the world. Sixty-five to seventy percent of the total jaggery is from sugarcane, the remaining 30% is from palms. For better handling, packaging and storage, jaggery in granular form is becoming popular. This could be made either from concentrated juice or solidified jaggery.
Thermal properties of foods or food products, such as specific heat, thermal conductivity, and thermal diffusivity, of food or food products are essential to understand their thermal behaviour and to control heat transfer processes.[Citation6 Citation–7] Thermal properties of particulate foods are mostly affected by bulk density, moisture content, and temperature.[Citation8–11] Singh[Citation12] and Sweat[Citation13] have reviewed thermophysical properties of different vegetables, fruits and its juices. Data on thermal conductivity of various foods have been classified and analyzed.[Citation14] The effective thermal conductivity of various food powders, such as defatted soy flour,[Citation15] skim milk powder,[Citation16] whole milk powder,[Citation17] coffee powder,[Citation18] granular starches,[Citation19] and intermediate moisture granular food[Citation20] were measured at various levels of moisture content, temperature, and bulk density. In most of these studies, the variation of specific heat and thermal conductivity with moisture content has been found to be linear. However, data on thermal properties of granular or powder jaggery is scanty.
The objective of this present work is to study the effect of moisture content on bulk density, thermal conductivity, thermal diffusivity and specific heat of three granular jaggery samples prepared from sugarcane, palmyra, and date-palm juice, and to establish correlations between the moisture content and these properties.
MATERIALS AND METHODS
Preparation of Samples
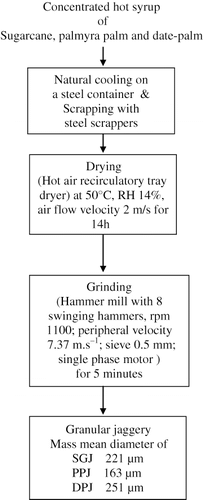
A generalized flow chart for making granular jaggery from concentrated sugarcane, palmyra palm and date-palm juices has been shown in . Particle density and porosity were measured [Citation21] using a multivolume pycnometer (Micromeritics, USA, model 1305). The angle of repose was calculated from the base angle formed by the heap of granules.[Citation22] The particle size distribution of the three granular jaggery samples was determined by sieve analysis technique.[Citation23] Physical characteristics of three granular jaggery samples are given in the . Various constituents in each of the three jaggery samples were determined according to standard methods described elsewhere.[Citation24] The proximate composition of three jaggery samples on moisture free basis is shown in .
Table 1 Physical characteristics of sugarcane, palmyra palm, and date-palm jaggery granules
Table 2 Proximate composition of sugarcane, palmyra palm, and date-palm jaggery samples (moisture-free basis)
Granular jaggery at different moisture contents (about 2–14%) were prepared by equilibrating the samples in different desiccators containing saturated solutions of inorganic salts (MgCl2, MgNO3, NaCl, KCl, and KNO3—all reagent grade chemicals from Loba Chemicals, India). Thus, controlled humidity environment between 30–80% was generated.[Citation25] The moisture contents of each equilibrated jaggery sample (in triplicate) were determined by vacuum oven drying method [Citation24] and average values were noted.
Measurement of Thermal Conductivity and Thermal Diffusivity
Theory of thermal properties measurement by a line source heating
Thermal conductivity and diffusivity of granular jaggery samples have been determined using the line heat source theory of transient heat transfer analysis. The detail theory of the thermal conductivity probe or line heat source technique has been published elsewhere.[Citation26,Citation27] In brief, a line heat-source probe is inserted into the bulk sample (initially at a uniform temperature) and then heated at a constant rate. Temperature adjacent to the line source is monitored. A plot of the logarithm of time versus temperature becomes linear, and its slope could used to calculate the thermal conductivity of the sample. The final equation for the thermal conductivity may be written as
where q is heat produced per unit length per unit time (Wm−1); k is thermal conductivity of the medium (Wm−1K−1); α is thermal diffusivity (m2s−1); t is time (s); γ is Euler's constant; and r is radial distance (m) from the probe.
The diffusivity is obtained from the intersection of the regression line [EquationEq 1] with the t-axis (ΔT = 0). Thus analytically,
Taking the value of to [from the intercept of ΔT versus ln(t)] and finite r, the diffusivity is calculated.
Measurement system
The line heat-source thermal property analyzer (Model KD2, Decagon Devices, Inc., USA) consisted of a 0.9 mm diameter, 60 mm length stainless steel needle, with a line heat-source element, and a temperature sensor. A microcontroller regulated the power to the heating element and measured the probe temperature. The thermal conductivity and diffusivity of the test sample were computed from its own in-built software on the basis of the theory described above. The least count of this instrument was 0.02 Wm−1K−1 for thermal conductivity and that for diffusivity it was 0.1 × 10−6 m2s−1 with the corresponding accuracy of 5% and 10%. The instrument was initially calibrated against distilled water and castor oil, as per the procedure described by the manufacturer. Both these values were compared with the corresponding reported data. Before the sensor needle was introduced into the sample, it was smeared with a silver compound (Arctic SilverTM Visalia, CA) supplied by the manufacturer. This helped better contact between the granular sample and the needle thereby facilitates good heat transfer. The instrument took about 2 min to attain a stabilized value. Each of these measurements was replicated thrice and average values were taken.
Measurement of bulk density
The bulk density of granulated jaggery samples at various moisture contents was determined by the ratio of the weight of material taken in a known volume container.[Citation28] The sample was filled in a known volume cylindrical container and the excess material on the top of it was removed by sliding a knife along the edge. The weight of the sample was noted. This measurement was repeated five times with tapping to ensure equal level of compactness of the granular material, and average of five readings was noted.
Estimation of specific heat
Specific heat (C p ) was estimated from the measured values of thermal conductivity (k), diffusivity (α) and bulk density (ρ) of the granular jaggery[Citation29] following EquationEq. (3)
Statistical analysis
Experimental data of thermal properties have been analyzed with the Origin 6.1 package[Citation30] and Microsoft Excel.[Citation31]
RESULTS AND DISCUSSION
Bulk Density
The bulk density of jaggery granules was found to increase from 570 to 1200 kg m−3; 590 to 1230 kg m−3 and 510 to 1310 kg m−3 with the increase in moisture content from 2.0 to 11.6; 2.1 to 10.2 and 2.9 to 14.3 (%db) for sugarcane, palmyra palm and date-palm jaggery, respectively (); all followed a linear trend (). EquationEqs. (4 Equation–6) show the linear correlation between the bulk density and its moisture contents for sugar cane, palmyra palm and date-palm jaggery, respectively. Bulk density of all these samples was found vary significantly (p < 0.01) each other.
Table 3 Bulk density, thermal conductivity, thermal diffusivity, and specific heat at different moisture contents for three jaggery samples
Figure 2 Variation of (a) bulk density, (b) thermal conductivity, and (c) specific heat of three granular jaggery samples with moisture content at 30°C.
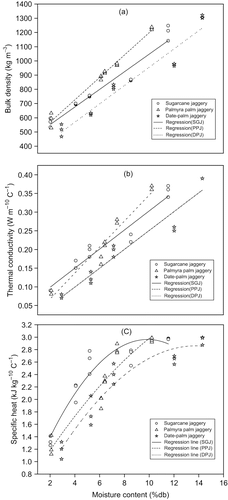
As moisture content in the sample increased, the weight of granular particle increased without any significant increase in volume of the particle, and hence, there was rise in bulk density. Bulk density of palmyra palm jaggery granules was higher compared to that of other two jaggery granules. This might be due to lower mass mean diameter of the particles () resulting close packing of particles in the bed (porosity 65.4%).
Bulk Thermal Conductivity
Bulk thermal conductivity of three jaggery samples increased with the increase in both moisture content and bulk density (). This linear increasing trend ( and ) was in agreement with other food products, like whole and skim milk powder.[Citation32] The thermal conductivity (k) of three jaggery samples was found to posses good linear correlation (p < 0.01) to both moisture content [EquationEqs. (7 Equation–9)] and bulk density [EquationEqs. (10 Equation–12)].
Figure 3 Variation of thermal conductivity of three granular jaggery samples with bulk density at 30°C.
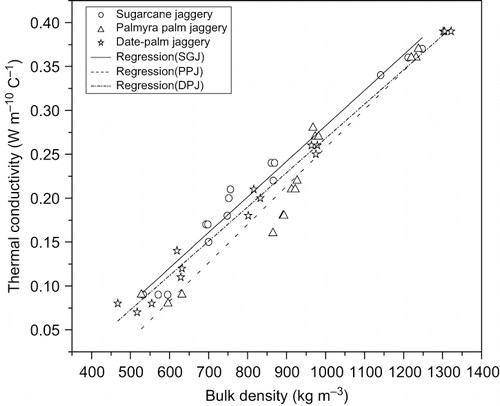
The thermal conductivity of sugarcane jaggery was found to remain higher compared to other two samples upto the moisture content of 5.5%db. After this moisture content, thermal conductivity of palmyra palm sample became higher among all the three samples. It may be noted that, increase in thermal conductivity of palmyra palm, per unit increase in moisture content, was more than that of other two jaggery samples. However, thermal conductivity of date-palm jaggery showed a steady increase but remained lowest and almost parallel to that of sugarcane jaggery for the entire moisture range of 2.0–14.3 (%db). Thermal conductivity of these jaggery samples was found to be in the range of 0.36 to 0.39 W m−1 K−1 at moisture contents ≥10%db, which are close to the reported values for other foods like sugar solutions, syrups, honey, molasses and caramel syrup.[Citation33]
Thermal Diffusivity
Thermal diffusivity values of three jaggery samples varied over a relatively narrow range at 30°C (). It decreased from 0.12 × 10−6 to 0.10 × 10−6, 0.12 × 10−6 to 0.10 × 10−6 and 0.13 × 10−6 to 0.10 × 10−6 m2 s−1 for sugarcane, palmyra palm and date-palm jaggery granules, respectively. The corresponding increase in moisture contents were from 2.0 to 11.6%, 2.1 to 10.2, and 2.9 to 14.3%. All these variations were found to be non-significant (p < 0.05). At lower moisture content (2% db), date-palm jaggery granules showed highest thermal diffusivity compared to that of other two jaggery samples. This might be due to lower bulk density of the former at low moisture content. Higher diffusivity for low bulk density (higher porosity) granular foods has also been observed by some workers.[Citation34]
Specific Heat
Specific heats of three jaggery samples at 30°C, estimated from the experimental values of thermal conductivity, thermal diffusivity, and bulk density, are shown in . It increased from 1.33 to 2.97, 1.24 to 2.95, and 1.19 to 2.95 kJ kg−1 K−1, with the increase in moisture content of the jaggery samples from 2.0 to 11.6 %db (sugarcane), 2.1 to 10.2 %db (palmyra palm), and 2.9 to 14.3 %db (date-palm), respectively. It was found that all these samples showed a rapid change in specific heat up to moisture content between 5.8 and 6.2 (%db). Further increase in moisture content showed a slow change, and approached towards a saturation value at around 10 to13 %db. The higher specific heat at higher moisture content might be attributed to amount of water present in the sample.[Citation33] These values were found to be highly significant at 1% level in the entire moisture range of 2–14%. Specific heats of three granular jaggery samples followed quadratic relationship with moisture content [Equationequations (13 Equation–15)].
CONCLUSIONS
Bulk density, thermal conductivity and specific heat of three granular jaggery samples were found to be dependent on moisture content; increased with the increase in moisture content. Thermal diffusivity of all these samples did not show any significant variation with the change in moisture content. Thermal conductivity of three granular jaggery samples was found to follow linear correlation with the moisture content, while this trend for specific heat was non-linear.
NOMENCLATURE
| Symbols | = | |
| Cp | = |
Specific heat (kJ kg−1 K−1) |
| k | = |
Thermal conductivity (W m−1 K−1) |
| M | = |
Moisture content (%db) |
| q | = |
Heat produced per unit length of the probe per unit time (W m−1) |
| r | = |
Radial distance (m) |
| T | = |
Temperature (°C) |
| t | = |
Time (s) |
| α | = |
Thermal diffusivity ( m2 s−1) |
| ρ | = |
Density (kg m−3) |
| γ | = |
Euler's constant |
| Subscripts | = | |
| SGJ | = |
Sugarcane jaggery |
| PPJ | = |
Palmyra palm jaggery |
| DPJ | = |
Date-palm jaggery |
ACKNOWLEDGMENTS
The first author wishes to thank Dr.S.Raghu Vardhan Reddy, Vice-Chancellor, and Dr. P.Raghava Reddy, Director of Research, ANGRAU for the grant of deputation to carry out the Ph.D programme at IIT, Khragpur, India.
REFERENCES
- Thakur , A.K. 2002 . “ In Potential of jaggery (Gur) manufacturing in Punjab state ” . In Proceedings of the national seminar on status, problems, and prospects of jaggery and khandsari industry in India, IISR, Lucknow, Dec. 2–3, 1999; Singh, J , Lucknow : AICRP on Jaggery and Khandsari .
- Pattnayak , P.K. and Misra , M.K. 2004 . Energetic and economics of traditional gur preparation: A case study in Ganjam District of Orissa, India . Biomass & Bioenergy , 26 : 79 – 88 .
- Dalibard , C. 1999 . Overall view on the tradition of tapping palm trees and prospects for animal production . Livestock Research for Rural Development , 11 ( 1 ) : 1 – 37 .
- Singh , J. 2002 . “ In Research contributions of All India Coordinated Research Project on processing, handling and storage of jaggery and khandsari ” . In Proceedings of the National Seminar on Status, Problems and Prospects of Jaggery and Khandsari Industry in India, IISR, Lucknow, Dec. 2–3, 1999; Singh, J , Lucknow : AICRP on Jaggery & Khandsari .
- Sahu , A.P. and Paul , B.N. 1998 . The role of dietary whole sugar- jaggery in prevention of respiratory toxicity of air toxics and in lung cancer . Toxicology Letters , 95 ( 1 ) : 154
- Rice , P. , Selman , J.D. and Abdul-Rezzak , R.K. 1988 . Effect of temperature on thermal properties of ‘Record’ potatoes . International Journal Food Science and Technology , 23 : 281 – 286 .
- Unklesbay , N. , Unklesbay , K. , Hsieh , F. and Sandik , K. 1992 . Thermophysical properties of extruded beef/corn flour blends . Journal of Food Science , 57 ( 6 ) : 1282 – 1284 .
- Nesvadba , P. and Eunson , C. 1984 . Moisture and temperature dependence of thermal diffusivity of cod minces . Journal of Food Technology , 19 : 585 – 592 .
- Taiwo , K.A. , Akanbi , C.T. and Ajibola , O.O. 1996 . Thermal properties of ground and hydrated cowpea . Journal of Food Engineering , 29 : 249 – 256 .
- Maruolis , Z.B. , Saravacos , G.D. , Krokida , M.K. and Panagiotou , N.M. 2002 . Thermal conductivity prediction for food stuffs: Effect of moisture content and temperature . International Journal of Food Properties , 5 ( 1 ) : 231 – 245 .
- Oliveira , G.S. , Trivelin , M.O. , Lopesfilho , J.F. and Thomeo , J.C. 2005 . Thermo-physical properties of cooked ham . International Journal of Food Properties , 8 ( 2 ) : 387 – 394 .
- Singh , R.P. 1992 . “ Heating and cooling processes for foods ” . In Handbook of Food Engineering , Edited by: Heldman , D.R. and Lund , D.B. 247 – 276 . New York : Marcel Dekker .
- Sweat , V.E. 1974 . Experimental values of thermal conductivity of selected fruits and vegetables . Journal of Food Science , 39 : 1080 – 1083 .
- Krokida , M.K. , Panagiotou , N.M. , Maroulis , Z.B. and Saravacos , G.D. 2001 . Thermal conductivity: Literature data compilation for food stuffs . International Journal of Food Properties , 4 ( 1 ) : 111 – 137 .
- Wallapapan , K. and Sweat , V.E. 1982 . Thermal conductivity of defatted soy flour . Transactions of the American Society of Agricultural Engineers , 25 ( 5 ) : 1440 – 1444 .
- Maccarthy , D.A. 1984 . Effect of temperature and bulk density on thermal conductivity of spray-dried whole milk powder . Journal of Food Engineering , 4 ( 4 ) : 249 – 263 .
- Kent , M. , Christiansen , K. , van Haneghem , I.A. , Holtz , E. , Morley , M.J. , Nesvadba , P. and Poulsen , K.P. 1984 . Cost 90 collaborative measurements of thermal properties of foods . Journal of Food Engineering , 3 ( 2 ) : 117 – 150 .
- Singh , P.C. , Singh , R.K. , Bhamidipati , S. , Singh , S.N. and Barone , P. 1997 . Thermo- physical properties of fresh and roasted coffee powders . Journal of Food Process Engineering , 20 ( 1 ) : 31 – 50 .
- Maroulis , Z.B. , Drouzas , A.E. and Saravacos , G.D. 1990 . Modelling of thermal conductivity of granular starches . Journal of Food Engineering , 11 : 255 – 271 .
- Sabliov , C.M. and Heldman , D.R. 2002 . A predictive model for thermal conductivity of an intermediate moisture granular food . Journal of Food Process Engineering , 25 ( 2 ) : 91 – 107 .
- Tenou , E. , Fitzpatrick , J.J. and Synnott , E.C. 1999 . Characterisation of food powder flowability . Journal of Food Engineering , 39 : 31 – 37 .
- Vega , C. , Esther , K. , Xiao , D.C. and Roos , Y.H. 2005 . Solid-state characterization of spray-dried ice cream mixes . Colloids and Surfaces B: Biointerfaces , 45 : 66 – 75 .
- Linden , G. 1996 . “ Methods of particle size analysis ” . In Analytical Techniques for Foods and Agricultural Products , Edited by: Melcion , J.P. and Monredon , F.De. 229 – 248 . New York : VCH Publishers .
- Association of Official Analytical Chemists . 1998 . Official methods of analysis , Washington, DC : AOAC .
- Jagannadha Rao , P.V.K. , Das , S.K. and Das , M. 2006 . Moisture sorption isotherms of sugarcane, palmyra and date-palm jaggery . Paper presented, American Society of Agricultural and Biological Engineers Annual International Meeting (paper no. 063007) . July 9–12 2006 , Portland, OR.
- Sharma , D.K. and Thompson , T.L. 1973 . Specific heat and thermal conductivity of sorghum . Transactions of the American Society of Agricultural Engineers , 16 ( 1 ) : 114 – 117 .
- Fontana , A.J. , Wacker , B. , Campbell , C.S. and Campbell , G.S. 2001 . Simultaneous Thermal conductivity, Thermal resistivity, and Thermal diffusivity measurements of selected foods and soils . Paper presented, American Society of Agricultural and Biological Engineers Annual International Meeting (paper no. 016101) . July 30–Aug. 1 2001 . Portland, OR
- Perez-Alegria , L.R. , Ciro , H.J. and Abud , L.C. 2001 . Physical and thermal properties of parchment coffee bean . Transactions of the American Society of Agricultural Engineers , 44 ( 6 ) : 1721 – 1726 .
- Sweat , V.E. 1986 . “ Thermal properties of foods ” . In Engineering Properties of Foods , Edited by: Rao , M.A. and Rizvi , S.S.H. 99 – 138 . New York : Marcel Dekker .
- Origin . 2000 . Non-Linear Curve Fit., Ver.6.1 2000 , Northampton, MA : Origin Lab .
- Microsoft Office Professional . 2003 . Microsoft Office Excel 2003 , Redmond, WA : Microsoft, Inc .
- Muramatsu , Y. , Tagawa , A. and Kasai , T. 2005 . Effective thermal conductivity of rice flour and whole and skim milk powder . Journal of Food Science , 70 ( 4 ) : 279 – 287 .
- Singh , R.P. and Heldman , D.R. 2004 . “ Thermal conductivity of selected food products ” . In Introduction to Food Engineering , 3rd , 599 New Delhi : Elsevier .
- Kostaropoulis , A.E. and Saravacos , G.D. 1997 . Thermal diffusivity of granular and porous foods at low moisture content . Journal of Food Engineering , 33 : 101 – 109 .