Abstract
The purpose of the research was to determine the effect of heat-moisture treatment at different phases and temperatures on resistance starch (RS) level on native starches samples of several botanical sources and to evaluate the thermal stability of different granules. Samples of potato, cassava, wheat, and corn starches were moisturized up to 30% wet basis and then treated in a convection oven at 80, 100, and 120°C during 40 and 60 minutes. RS was determined gravimetrically by a modification of Method 991.43 of the AOAC for the determination of total dietary fibre. All samples were submitted to different thermal analysis in a range from 40 to 180°C at 10°C/min. Overall, in the treated samples an increase of RS was observed, being the corn starch sample treated at 120°C and 60 minutes – the one that presented the highest content of RS (4.2%). Other treated samples showed a decrease in the gelatinization enthalpies with the presence of granular fusion, indicating internal re-arrangement, an increase in the gelatinization temperature and the thermo stability below 95°C.
INTRODUCTION
Resistant starch (RS) has been defined as “the sum of starch and products of starch degradation not absorbed in the small intestine of healthy individuals”.[Citation1] Even though RS escapes digestion in the small intestine, it may be fermented in the large intestine by the colonic microflora. [Citation2,Citation3,Citation4,Citation5]
In the last decade, there has been an increased interest in the nutritional implications of RS, not only for its low caloric content, but also because it may have a physiological effect similar to that dietary fibre.[Citation6,Citation7,Citation8] Moreover, the fact that processing treatments may modify the RS content in foods has gained the attention of food technologists.[Citation7,Citation9,Citation10]
RS has been classified as Type I, which results from the physical inaccessibility of enzymes in intact tissues. Type II comes from the physical structure of native starch granules. Type III results from the retrogradation and gelatinization of starch in resistant structures to the digestive enzymes. Type IV is where the resistance results from the chemical modification, which interferes with the enzymatic digestion.
Although several analytical in vitro methods have been developed to determine RS,[Citation11,Citation12,Citation13,Citation14,Citation15] according to the physiologic definition, it cannot be established as in vitro. Nowadays, there is no accepted analytical procedure to determine RS. This represents an important limitation to evaluate RS production processes. The most commonly used method for RS determination[Citation16,Citation17,Citation18,Citation19,Citation20,Citation21] is based on the AOAC method for the measurement of total dietary fibre (TDF).[Citation16] The TDF method detects the undigested starch remaining after 30 min of boiling with thermally stable α-amylase. This method, which was not initially intended for RS determination, detects only the fraction of RS, which survives a boiling treatment. The portion of RS isolated by the TDF method is important because it is not only appears as dietary fibre, but it also is relatively thermally stable because it remains after a thermal processing at 100°C. Although none of the analytical procedures are intended to evaluate the thermal stability of RS, thermally stable RS can be evaluated either by the Englyst method after a boiling step or by the TDF method.[Citation22]
It is known that the quantity of RS can be increased by maintaining the structure of the granule. The addition of commercial RS to foods has allowed food technologists to produce foods with dietary fibre without the fibre flavour, and to improve the functionality in the alimentary processes. To produce commercial RS, the starches are exposed to a combination of biochemical and/or hydrothermal modifications. The biochemical modifications require the use of enzymes or acids to remove the non-resistant parts of the starch. The hydrothermal modifications are treatments combining heat and moisture. Because the effects of biochemical and hydrothermal modifications in the yield of RS are not well understood, the production of RS is based on empirical methods, and consequently the potential is not known to produce RS. Jacobs and Delcour[Citation23] define a hydrothermal modification as any treatment of heat and moisture carried out at temperatures above the glass transition temperature but below the gelatinization temperature of starch, without destroying the granule structure.
The water content to which the starch is treated by heating is important to determine its physiochemical characteristics. Some authors[Citation23,Citation24,Citation25] use the term “annealing” referring to a treatment of heat with high moisture (> 40% wet basis), and “heat-moisture treatment” referring to a heat treatment of at low moisture (HMT) (< 35% wet basis). Moreover, it was also demonstrated that the hydrothermal treatments on the granules of starch increase or decrease the susceptibility to hydrolysis with α- amylase. These effects are related to the botanical source[Citation26,Citation27,Citation28] and to the conditions of treatment.[Citation29,Citation30]
In all thermal treatments, the thermal stability of the granules of starch depends on the temperature and the content of water where these treatments are applied. Therefore, when the conservation of the granular structure is sought, there are conditions of temperature and critical content of water, over which the thermal treatments destroy the structure of the granule, and consequently they should not be exceeded. On the other hand, the relationship between the temperature and water content to which the thermal treatments destroy the granular structure is complex, depending on the source of starch. However, for certain conditions the temperature and water content can be determined experimentally, for example, by means of differential scanning calorimetry. It is known that more energetic modifications of the structure of the granule are achieved when the hydrothermal modifications are carried out at temperatures and water contents next to the critical conditions, over which the structure of the granule is lost.
In the annealing, the starch granules are treated at temperatures below of their gelatinization temperature and with an excess of water. Although there are many combinations of temperature and water contents through which the thermal treatment would correspond to an annealing treatment, the most energetic and quick modifications happen near gelatinization temperatures. In a similar way to the annealing treatment, the heat-moisture treatment involves a thermal treatment of the starch granules below the fusion temperature, where the structure of the granule is lost in reduced water contents and at low content of water. The purpose of this research was to determine the effect of heat-moisture treatment at different phases and temperatures on RS level on native starches samples of several botanical sources and to evaluate the thermal stability of different granules.
MATERIALS AND METHODS
Starches Samples
Starch samples used in this research were potato starch (Number 4251 catalogue, Sigma, St. Louis, MO), wheat starch (Number 5127 catalogue, Sigma, St. Louis, MO), corn starch (Number 5296 catalogue, Sigma, St. Louis, MO), and commercial cassava starch obtained from VALOIS SACIFIA, Gdor. Roca, Misiones, Argentina.
Physiochemical Characterization
The gelatinization and fusion temperatures were determined by differential scanning calorimetry using starch suspensions of 30% (w/w) and 70% (w/w), respectively. Thermal analysis was performed using a differential scanning calorimeter (DSC 7, PerkinElmer Corp., Norwalk, CT) equipped with a thermal analysis data station (PerkinElmer). Indium (m.p. 156.4 °C, ΔHm 28.45 J/g) was used as a calibration standard. The reference cell contained a sealed, empty, stainless steel pan. Starch samples (∼15.0 mg, dwb) were weighed into preweighed stainless steel pans (Perkin-Elmer). Deionized water was added to obtain ∼ 30% (w/w) or ∼ 70% (w/w) starch suspensions. The samples were stirred with a needle. Pans were sealed, the total weights were determined, and the suspensions were stored overnight at room temperature. All samples were heated at 20°C up to 180°C, at a heating rate of 10°C/min. Samples were then quench cooled from 180oC to 20oC at 10oC/min. Gelatinization enthalpy and peak temperatures were calculated (7 Series Software, Perkin-Elmer). Analyses were performed in triplicate and mean values and standard deviation are reported.
Hydrothermal Treatments
Considering initial fusion temperatures of the starches, the samples indicated in were prepared adding deionized water from their initial moisture content up to 30% w/w wet basis. In order to homogenize the distribution of water inside the granules, they were maintained at 25°C during 72 hours. Using a 22 ml stainless steel pressure vessel (model 4701 model, provided by Parr Instrument Co.), approximately 20 g of the hydrated samples were thermostatized in a gravitational convection oven (± 1°C). Immediately after the treatment, the vessel was submerged in a bath of water-ice during 5 min. Then the samples were extracted and dried off in forced air oven at a temperature of 40°C until constant weighed. Then, they were sifted using a 120-mesh sieve (125 μm opening).
Table 1 Conditions of heat-moisture treatment (HMT) studied
Boiling-Stable Resistant Starch Determination
The assay is based on the 991.43 method of the AOAC for the determination of total dietary fibre.[Citation16] The method was adapted for essentially ash and protein-free starch analysis. Starch samples (0.5 g) were suspended in 20 ml MES/TRIS buffer (pH = 8.2) and incubated with 25 μl of thermo stable α-amylase (Megazyme E-BLAAM. Wicklow, Ireland) at 95°C−100°C during 35 minutes. The samples were then cooled at 60°C and adjusted to pH 4.1–4.8 with 5% HCl or 5% NaOH. The samples were then incubated with 100 μl of amyloglucosidase (Megazyme E-AMGDF. Wicklow, Ireland) at 60°C for 30 minutes. Mixtures were vacuum filtered with a #42 filter paper and washed with 10 ml of deionised water, 95% ethanol, and acetone. The boiling-stable portion of RS was determined as the residue remaining after drying the samples in a convection oven at 103°C overnight.
Statistical Analysis
Statistical analysis was made using Minitab 13.20 (Minitab Inc., State College, Pa., USA). To compare values of temperature and gelatinization energy, an ANOVA test was used. To compare individual means an a posteriori test (LSD) was used (95% of significance level).
RESULTS AND DISCUSSION
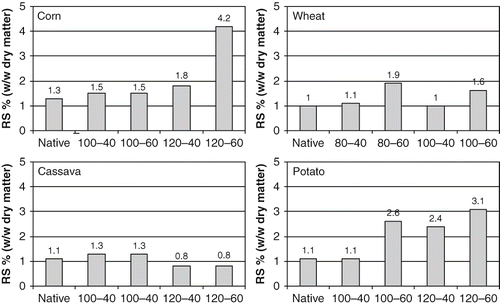
shows the boiling-stable RS content obtained following the pattern of the AOAC method for the determination of total dietary fibre[Citation16] for the different samples of native and hydrothermal modified starches. A significant increase in the corn starch sample 120ºC-60 min in relation to the native was found; but on the other hand, the other samples treated did not present significant difference. Wheat starch samples treated during 60 min had a statistically significant increase in relation to the native, without differences among them. In addition, no significant differences were observed between the treatments of 40 min and the native. The treatments at 100ºC caused no significant increase in the samples of starch cassava, but on the other hand, a significant decrease was observed in the samples treated at 120ºC in relation to the native. In potato starch significant differences were found in three treatments (100ºC-60 min; 120ºC-40 min and 120ºC-60 min) in relation to the native one and also among the samples at 120ºC-40 min and at 120ºC-60 min.
Table 2 Boiling-stable resistant starch contentFootnote a
Relatively high variation coefficients (up to 27%) were obtained due to the low RS content. Overall, the RS determinations showed an increase in the samples treated in relation to their corresponding native starch, except for cassava starch which had a decrease (see ).
Ghua and Ali[Citation31] suggested that the time and severity of thermal processing employed may imparts specific properties to the respective starch-based products due to a partial or complete destruction of crystalline structure starch and considerable macro-molecular degradation. On the other hand, RS overall resistance could be dependent of the structure of the starch granule.[Citation32] Thus, the different RS contents found in the natives and treated samples could be due to the particular gelatinization process of each type of granule to thermal treatments.
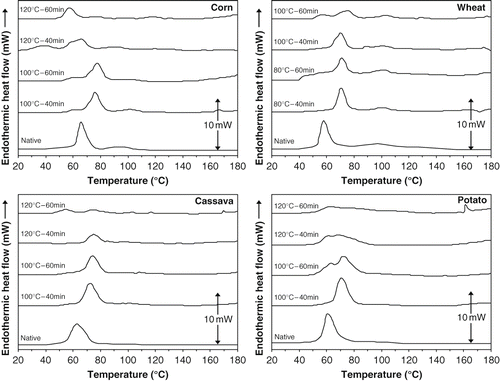
The endotherms of the corn, wheat, cassava and potato samples (native and treated) are shown in . The corn endotherm presents its characteristic of main peak around 65°C[Citation33,Citation34] and also a second component associated with melting of amylose-lipid complexes.[Citation35] The treatments at 100°C tended to increase the peak of the main component of endotherm with light loss of the gelatinization enthalpy (see ). Treatments at 120°C produced a partial fusion of the granule, being the most intense at 60 min, as can be observed in the endotherms of a. The wheat endotherm presents its characteristic of main peak around 57°C[Citation34] and a second component associated with melting of amylose-lipid complexes.[Citation35,Citation36] The treatments at 80°C tended to increase the peak of the main component of the endotherm with partial loss of the gelatinization enthalpy (see ). The thermal treatments at 100°C produced partial fusion of the granule (see b), which was verified with the microscope of polarized light. The cassava endotherm presents its characteristic of an only peak around 63°C.[Citation34,Citation37] The treatments at 100°C tended to increase the peak of the endotherm with light loss of the gelatinization enthalpy (see ). The treatments at 120°C produced an important fusion of the granule, as can be observed in the endotherms of c. The destruction of the granule due to the fusion was verified with the microscope of polarized light. The endotherm of potato starch presents its characteristic of an only peak around 61°C.[Citation33] The treatments at 100°C rose the peak of the endotherm to around 72°C (see b). The thermal treatment at 100°C and 60 min shows fusion principle due to the appearance of two peaks in the endotherm. This suggests the formation of two types of starch crystallites.[Citation33,Citation38] Treatments at 120°C produced a partial fusion of the granule as can be observed in the endotherms of d. The partial destruction of the granule due to the fusion was verified with the microscope of polarized light.
Table 3 Temperature of peak and gelatinization enthalpy
The heat-moisture treatments studied caused an increase in gelatinization endotherms width, with significant reduction of the gelatinization enthalpy (see ), what was also observed by several authors[Citation23,Citation39,Citation40]; and an increase in the gelatinization temperature, except for the corn starch sample treated at 120ºC and 60 minutes, (see ). Perera et al.[Citation41] postulated that an increase in the peak temperature is a reflection of melting of crystallite, which are formed because of amylase-amylose interaction and amylase-amylopectin interactions along the chains, which is stronger in heat moisture treatments. This suppresses the swelling of the granule leading to delayed gelatinization and a high onset, peak and conclusion temperature. The reduction in the gelatinization enthalpy following hydrothermal treatment indicated partial gelatinization of some less heat stable molecules.[Citation38] Hoover and Manuel[Citation28] claimed that amylase content and starch chain length were two significant factors determining the physical properties of the heat moisture treated products. Not only the complex formation between amylose chains and other starch chains (mainly in amorphous regions), may cause physical changes in the treated starches. The structural difference among the amylose chains of the samples and the presence of lipids in corn and wheat starches could result into the different influences by the heat moisture treatment upon the thermal characteristics of the starches.
CONCLUSIONS
The heat-moisture treatments studied in all the starches treated caused an increase of the width of the gelatinization endotherms, with significant reduction of the gelatinization enthalpy, with the presence of granular fusion indicating internal rearrangement. In general, the treatments produced an increase in the gelatinization temperature and they increased the thermo-stability below 95°C. Determinations of boiling-stable RS showed an increase of its content in starches treated samples of corn, potato, and wheat in relation to their corresponding native starch, except for the cassava starch, which had a decrease of the content of RS. The corn starch treated during 60 minutes at 120ºC presented the highest content in RS (4.2% w/w), followed by potato starch (3.1% w/w) with identical treatment. Although the increase of RS in corn and potato starches is important in relation to its corresponding native starch, the increase of RS in the same ones is not as important as we could expect; mainly if it is compared with other kinds of starches, for example with the corn starch of high native amylose and hydrothermally treated.
ACKNOWLEDGMENTS
This work was partially supported by Fundación para el Desarrollo e Investigación Científica y Tecnológica (DINCYT).
REFERENCES
- EURESTA . 1992 . European Flair Action Concerted on Resistant Starch Department of Human Nutrition , The Netherlands : Wageningen Agricultural University .
- Cummings , J.H. , Englyst , H.N. and Wiggins , H.S. 1986 . The role of carbohydrates in lower gut function . Nutr. Rev. , 44 ( 2 ) : 50 – 54 .
- Englyst , H.N. and Macfarlane , G.T. 1986 . Breakdown of resistant and readily digestible starch by human gut flora . J. Sci. Food Agric. , 37 : 699 – 706 .
- Mathers , J. C. 1992 . Energy value of resistant starch . Eur. J. Clin. Nutr. , 46 : S129 – S130 .
- Schulze , J. 1992 . Undigested carbohydrates and intestinal micro-ecology . Eur. J. Clin. Nutr. , 46 : S137 – S138 .
- Biliaderis , C. G. 1991 . The structure and interactions of starch with food constituents . Can. J. Physiol. Pharmacol. , 69 : 60 – 78 .
- Björck , I. and Asp , N.-G. 1994 . Controlling the nutritional properties of starch in foods - a challenge to the food industry . Trends Food Sci. Technol. , 5 : 213 – 218 .
- Brown , I. 1996 . Complex carbohydrates and resistant starch . Nutr. Rev. , 54 ( 11 ) : S115 – S119 .
- Annison , G. and Topping , D.L. 1994 . Nutritional role of resistant starch: chemical structure vs. physiological function . Annu. Rev. Nutr. , 14 : 297 – 320 .
- Englyst , K. and Englyst , H.N. 2005 . Carbohydrate bioavailability . British Journal of Nutrition. , 94 : 1 – 11 .
- Berry , C.S. 1986 . Resistant starch: Formation and measurement of starch that survives exhaustive digestion with amylolytic enzymes during the determination of dietary fibre . J. Cereal Sci. , 4 : 301 – 314 .
- Björck , I. , Nyman , M. , Pedersen , B. , Siljeström , M. , Asp , N.-G. and Eggum , B.O. 1986 . On the digestibility of starch in wheat bread — studies in vitro and in vivo . J. Cereal Sci. , 4 : 1 – 11 .
- Champ , M. 1992 . Determination of resistant starch in food products: interlaboratory study . Eur. J. Clin. Nutr. , 46 ( 2 ) : S51 – S62 .
- Englyst , H.N. , Kingman , S.M. and Cummings , J.H. 1992 . Classification and measurement of nutritionally important starch fractions . Eur. J. Clin. Nutr. , 46 ( 2 ) : S33 – S50 .
- Muir , J.G. and O'Dea , K . 1993 . Validation of an in vitro assay for predicting the amount of starch that escapes digestion in the small intestine of humans . Am. J. Clin. Nutr. , 56 : 540 – 546 .
- AOAC . 1985 . Official Methods of Analysis , 14th , 399 Arlington, VA : AOAC . 1st suppl., Sec. 43 (A14-43, A20)
- Sievert , D. and Pomeranz , Y. 1989 . Enzyme-resistant starch. I. Characterization and evaluation by enzymatic, thermoanalytical, and microscopic methods . Cereal Chem. , 66 ( 4 ) : 342 – 347 .
- Sievert , D. and Pomeranz , Y. 1990 . Enzyme-resistant starch. II. Differential scanning calorimetry studies on heat-treated starches and enzyme-resistant starch residues . Cereal Chem. , 67 ( 3 ) : 217 – 221 .
- Szczodrak , J. and Pomeranz , Y. 1991 . Starch and enzyme-resistant starch from high- amylose barley . Cereal Chem. , 68 ( 6 ) : 589 – 596 .
- Björck , I.M.E. and Siljeström , M.A. 1992 . In-vivo and in-vitro digestibility of starch in autoclaved pea and potato products . J. Sci. Food Agric. , 58 : 541 – 553 .
- Unlu , E. and Faller , J. 1998 . Formation of resistant starch by a twin-screw extruder . Cereal Chem. , 75 ( 3 ) : 346 – 350 .
- Brumovsky , J.O. and Thompson , D.B. 2001 . Production of boiling stable granular resistant starch by partial acid hydrolysis and hydrothermal treatments of high-amylose maize starch . Cereal Chem. , 78 ( 6 ) : 680 – 689 .
- Jacobs , H. and Delcour , J. 1998 . Hydrothermal modifications of granular starch, with retention of granular structure: a review . J. Agric Food Chem. , 46 : 2895 – 2905 .
- Stute , R. 1992 . Hydrothermal modification: The difference between annealing and heat-moisture treatment . starch/stärke. , 44 ( 6 ) : 205 – 214 .
- Knutson , C.A. 1990 . Annealing of maize starches at elevated temperatures. Cereal Chem . 67 ( 4 ) : 376 – 384 .
- Lorenz , L. and Kulp , K. Heat-moisture treatments of starches. Functional properties and baking potential . Cereal Chem. 1981 , 58 49 – 52 .
- Lorenz , L. and L. Kulp , K. 1982 . Cereal and root starch modification by heat-moisture treatment. I Physicochemical properties . Starch/stärke. , 34 : 50 – 54 .
- Hoover , R. and Manuel , H. 1996 . The effect of heat-moisture treatment on the structure and physicochemical properties of normal maize, waxy maize, dull waxy maize and amylomaize . V starches. J. Cereal Sci. , 23 : 153 – 162 .
- Kawabata , A. , Takase , N. , Miyoshi , E. , Sawayama , S , Kimura , T. and Kudo , K. 1994 . Microscopic observation and X-ray diffractometry of heat moisture treated starch granules . Starch/stärke. , 46 ( 12 ) : 463 – 469 .
- Franco , C.M.L. , Ciacco , C.F. and Tavares , D.Q. 1995 . Effect of the heat-moisture treatment on the enzymatic susceptibility of cornstarch granules . Starch/Stärke. , 47 ( 6 ) : 223 – 228 .
- Ghua , M. and Ali , Z. 2002 . Molecular degradation of starch during extrusion cooking of rice. International . Journal of Food Properties , 5 ( 3 ) : 509 – 521 .
- Sajilata , M.G. , Singhal , R.S. and Kulkarni , P.R. 2006 . Resistant starch . A review. Comp. Rev. Food Sci. Food Safety , 5 ( 1 ) : 1 – 17 .
- Lim , S.-T. , Chang , E.-H. and Chung , H.-J. 2001 . Thermal transition characteristics of heat-moisture treated corn and potato starches . Carbohyd. Polym. , 46 : 107 – 115 .
- Rahman , S. 1995 . Food properties handbook , Boca Raton : CRC Press .
- Saif , S.M.H. , Lan , Y. and Sweat , V.E. 2003 . Gelatinization properties of rice flour. International . Journal of Food Properties , 6 ( 3 ) : 531 – 542 .
- Nebesny , E. , Kwaniewska-Karolak , I. and Rosicka-Kaczmarek , J. 2005 . Dependence of thermodynamic characteristics of amylose-lipid complex dissociation on a variety of wheat . Starch/Stärke. , 57 ( 8 ) : 378 – 383 .
- Mishra , S. and Rai , T. 2006 . Morphology and functional properties of corn, potato and tapioca starches . Food Hydrocolloid , 20 : 557 – 566 .
- Hormdok , R. and Noomhorm , A. 2007 . Hydrothermal treatments of rice starch for improvement of rice noodle quality . Lebens. Wiss. Technol. , 40 : 1723 – 1731 .
- Gunaratne , A. and Hoover , R. 2002 . Effect of heat-moisture treatment on the structure and physicochemical properties of the tuber and root starches . Carbohydrates Polymers , 49 : 425 – 437 .
- Sang , Y. and Seib , P.A. Resistant starches from amylose mutants of corn by simultaneous heat-moisture treatment and phosphorylation . Carbohydrates Polymers , 63 167 – 175 . 2006
- Perera , C. , Hoover , R. and Martin , A.M. 1997 . The effect of hydroxypropylation on the structure and physicochemical properties of native, deffated and heat-moisture treated potato starches . Food Res. Int. , 30 ( 3–4 ) : 235 – 247 .