Abstract
The microstructure of bayberry juice hazes after storage at 25°C for 6 months was examined by light microscopy and transmission electron microscopy. Haze particles were found spherical and elliptical surrounded by translucent membranes. The transmission electron microscopic study indicated that the particles had three substructures: stain impenetrable central “core,” stain penetrable middle part, and floccule–like outer space structure. The “core” and the middle part of the haze were assumed to be the polyphenols or polyphenol/protein complexes, and the outer space was mainly the protein component. These particles were found to be aggregated to form dentrite or leaf–like structures supposedly due to hydrophobic and covalent links.
INTRODUCTION
Bayberry (Myrica rubra Sieb. et Zucc.) fruits have an attractive red color, special sweet sour taste, and exquisite flavor, which are praised as the “precious Southern Yangtze fruits of early summer”.[Citation1, Citation2] Traditionally, bayberry fruits have been used for the treatment of various gastric intestinal problems such as diarrhea and gastroenteritis in China.[Citation1, Citation3] The fruits of most cultivars ripe in the hot and rainy season of May to July and can only be stored with commercial values for 3 days at 20–22°C and 9–12 days at 0–2°C.[Citation4] In order to extend the shelf-life, bayberry fruits are often processed into juice and juice concentrate. However, during storage and commercial circulation, haze and sediments are readily formed in the clarified juice. These are considered as quality defects and limit the utilization and consumption of the product.
In our previous investigation, when bayberry juice was clarified with xanthan/chitosan or gelatin/bentonite followed by ultrafilration (molecular weight cut-off 100 kDa), haze formation was reduced but not eliminated.[Citation5] The storage temperature was a critical factor affecting haze formation, and the juice was more stable when stored at lower temperature of 4°C. Our investigation also revealed that bayberry juice haze was mainly the type of protein–tannin haze. The freeze dried sediments contained about 20.38% of protein, 70.24% of polyphenols, 7.2% of monosaccharides (considered as the glycoside moieties of the polyphenols), and 6.65% of ash.[Citation6] Typically, the polyphenols in the sediment included gallic acid, protocatechuic acid, cyanidin, quercetin and quercetin glycosides, and ellagic acid.
There are many literatures which report the chemical composition of post–bottling haze and sediment in fruit juices.[Citation7–15] Some microscopic studies including light microscopic and electron microscopic structures of fruit juice hazes were reviewed by Beveridge.[Citation16] Some authors also reported chain–like structures in apple juice haze.[Citation17] These micrographs provided a more direct image of the fruit juice hazes, which were useful to interpret their chemical natures and the mechanisms of formation. Based on the authors' knowledge, no such work was reported on bayberry juice. Therefore, in this work, the microscopic structures of bayberry juice hazes were investigated. Their particle morphologies are illustrated and their growth pattern in the juice is discussed.
MATERIALS AND METHODS
Bayberry Juice
Bayberry juice was processed according to the procedure described in our previous work,[Citation18] with a little modification. Briefly, the fresh bayberries were crushed with a horizontal crusher, and pasteurized at 90°C for 1 min in a tubular heater. The pulp was cooled to 35°C for depectinization, and then centrifuged with a decanter centrifuge. The centrifuged juice was finned with 0.2 g/L of gelatin and bentonite, and filtered through a diatomaceous earth filter. Finally, the clarified juice was heat treated at 120°C for 3 s and aseptically packaged in glass jars. The bayberry juice had a soluble solid concentration of 9.5 oBrix and was stored at 25°C for 6 months until analyzed.
Haze Preparation
Haze preparation was followed the modified method of Beveridge, Veto & Harrison.[Citation19] After 6 months of storage, 1 mL of mixed bayberry juice were transferred to 100 mm lengths of 7.5 mm diameter dialysis tubing (D-4003-10, ISC BioExpress, Kaysville, UT, USA) and dialysed against three changes of 500 mL distilled–deionized water for 24 hours. The dialysis temperature was 5°C and the water was stirred constantly.
Analysis of Ash and Protein Contents
For the analysis of the chemical components of bayberry juice haze, the dialysed haze particles were freeze dried, weighed, and kept in a tight desiccator. The contents of ash and total protein were determined by using the AOAC method of 938.08 and 928.08[20], respectively.
Analysis of Total Polyphenols
Fifteen mg of juice haze were dissolved in 100 mL of 4% acetic acid in acetonitrile, shaken at 200 rpm for 1 h at 30°C in a water bath shaker[21]. The extract was centrifuged at 10000 g for 10 min, and the supernatant was used for total polyphenol analysis. Total polyphenols were estimated colorimetrically using the modified Folin−Ciocalteu method as described previously.[Citation18] The results were the average of triplicate analyses and expressed as gallic acid equivalents (GAE) in g/100 g of dry weight (g/100 g dw).
Light Microscopy
Light microscopic study was performed with a XSP-8C microscope (Shanghai Caikon Optics Instrument Co. Ltd., Shanghai, China) equipped with a Panasonic camera (Panasonic Co. Japan) connected to a computer for photographic recording. One drop of well mixed dialysed bayberry juice containing haze particles was placed on a standard microscope slide and covered with a cover-slip. The samples were viewed without staining them.
Transmission Electron Microscopy
One drop of well mixed dialysed bayberry juice was placed on a copper grid (100 mesh, 3.05 mm diameter) for 5 min then touched off to filter paper.[Citation19] A drop of freshly filtered 1.5% phosphotungstic acid ethanol solution was placed on the sample for 2 min and the excess stain touched off to filter paper. The stained grids were observed in a Hitachi H-700 transmission electron microscope (Hitachi Ltd. Japan).
RESULTS AND DISCUSSION
After stored at 25°C for 6 months, bayberry juice was visually very hazy, and sediment was deposited at the bottom of almost each container. Chemical analysis of the haze showed that it is mainly the type of protein–polyphenol haze, which contained about 20.38% of protein, 70.24% of polyphenols, and 6.65% of ash. The results indicated that polyphenols played a major role in bayberry juice haze formation, which were in accordance with our previous investigation.[Citation6] However, in another study, the phenolic extracts from plant leaves had no significant effects on the stability of pineapple juice.[Citation22]
The bayberry juice samples were dialysed for microscopic observation. showed that haze particles existed in the form of sphere or ellipse in the bayberry juice. They were randomly and disorderly suspended in the juice, and the particle size was about 0.2–0.8 μm, which was in the range of apple juice haze of 0.3–1.0μm[13]. Each of the haze particles was surrounded by a translucent membrane. Examination of these haze particles by transmission electron microscope at the magnification of 60 Kv showed three sub-structures (): the floccule-like outer space, middle part and central core. The centre was the stain impenetrable ‘core’, the middle part was fully penetrated by the stain, and the outer space was the floccule-like structure, which was also stain impenetrable. However, the sub-structures of some haze particles had more than one core. The stain impenetrable ‘cores’ and the stainable middle parts were intercrossed each other, but the outer space was always the stain impenetrable floccule -like structures ().
Figure 1 Light micrographs of bayberry juice haze (bar represent 1μm). (a) Spherical and elliptical haze particles surrounded by translucent membranes; (b) haze particles have a tendency of arraying regularly; (c) haze of bar–like or clubbed; (d) longer bar–like hazes; (e) dentritic structure haze with long “tress” and many “branches”; and (f) leaf –like structure haze.
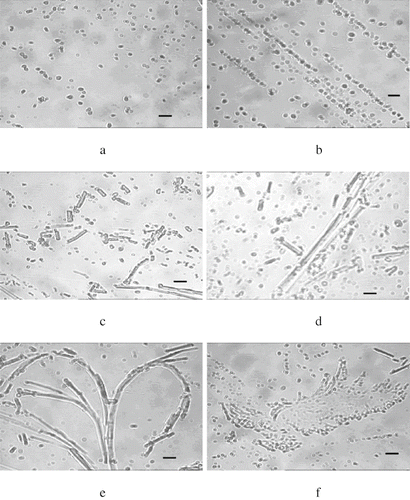
Figure 2 Transmission electron micrographs of bayberry juice haze. (a) Haze particle with three sub-structures: stain impenetrable central “core,” stainable middle part, and floccule–like outer space structure; (b) haze particles with more than one “cores,” the stain impenetrable “cores” dispersed in the stainable middle part; (c) aggregation of haze particles forming a dentrite structure; and (d) aggregation of haze particles forming an erratic structure.
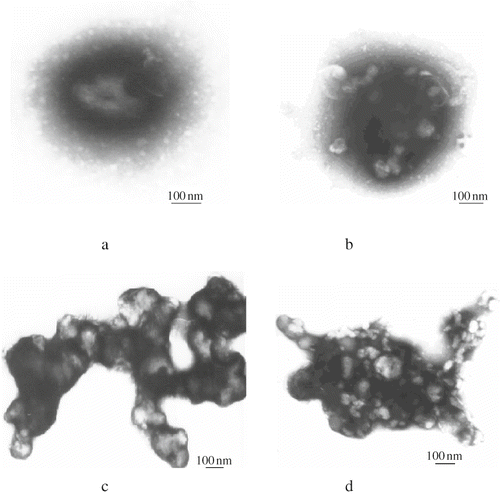
Similarly by transmission electron microscopy, Beveridge, Veto & Harrison observed a space-filling envelope structure in apple juice haze.[Citation17] The authors interpreted that the space-filling structure was surrounded by a series of partially coalesced particles and filled with a thick aqueous suspension of various sizes of insoluble microparticulates. The condensed tannin (procyanidin) making up the envelope boundary and a thin film of protein may provide the foundation of the ‘hyphae’ structures. The protein/tannin interactions built the structure which might have provided the binding sites for particle adhesion. Considering the bayberry juice haze was chemically composed of 20.38% of protein and 70.24% of polyphenols, and based on the micrographs of bayberry juice haze ( and ), we assumed that the “core” and middle part of the haze was the polyphenols or polyphenol/protein complexes, and the outer space was mainly the protein film, which were observed as translucent membranes by light microscopy (). suggested that the polyphenols and proteins may interact each other to form more than one ‘core’ in the haze particles.
Very interestedly, there appeared to be a specific ‘growth pattern’ of the particle microstructure during haze development in bayberry juice. Beginning from , the haze particles have a tendency of arraying regularly. The particulates aggregated linearly, and the disorderly distributed particles became less frequent. As shown in , most of the haze particles were no longer spherical or elliptical, but have the morphologies of bar –like or clubbed, and their lengths were about 1–6 μm. The bar–like hazes became even longer (), and finally aggregated to form a dentrite structure with long ‘tendrils’ and many ‘branches’ (). Moreover, numerous haze particles can aggregate together to form another structure, leaf–like structure (). From electron micrograph, it can also be obviously seen that haze particles were aggregated each other to form a dentrite structure () or erratic structure ().
As stated in the literature, the growth of large particles from small particles is mainly driven by the hydrophobic nature of condensed tannins suspended in the aqueous system.[Citation17] Covalent links may also contribute in oxidizing systems.[Citation23] Bayberry juice haze is the protein/polyphenol type[Citation6] and polyphenoloxidase is involved in haze formation.[Citation24] It is reasonable to deduce that hydrophobicity and covalent links might be the driving forces of haze particle development in bayberry juice. During storage, haze particles growing longer (dentrite structure) and bigger (leaf–like structure), formed large particles visually discernible even without instrument help.
CONCLUSION
Bayberry juice haze was readily formed during storage. The size of the haze particles ranges from 0.2–0.8 μm. The spherical and elliptical particles have three substructures: stain impenetrable central “core,” stain penetrable middle part, and floccule–like outer space structure.These particles are aggregated to form dentrite or leaf–like structures during the juice storage. The aggregation of particles causes an undesirable cloudiness appearance and sedimentation in the fruit juice. This paper presented the microstructure including the sub-structures of such particles. Understanding of the chemical and physical nature of the particles can assist in minimizing the haze in the bayberry juice.
REFERENCES
- Chen , Z. L. 1996 . The history of bayberry . J. Fruit Sci. , 13 ( 1 ) : 59 – 61 .
- Chen , K. , Xu , C. , Zhang , B. and Ferguson , I. B. 2004 . Red bayberry: botany and horticulture . Hortic. Rev. , 30 : 83 – 114 .
- Li , J.R. 2001 . Key technologies of postharvest and processing of red bayberry (Myrica rubra Sieb. et Zucc.) , Ph. D. thesis of Zhejiang University . No. Y425004
- Xi , Y.F. and Zheng , Y.H. 1993 . Effect of temperature on the nutrients and rot of bayberry during post- harvesting . Bull. Sci. Techn. , 9 ( 4 ) : 254 – 256 .
- Fang , Z.X. , Zhang , M. , Du , W.H. and Sun , J.C. 2007 . Effect of Fining and Filtration on the Haze Formation in Bayberry (Myrica rubra Sieb. et Zucc.) Juice . J. Agric. Food Chem. , 55 : 113 – 119 .
- Fang , Z.X. , Zhang , M. , Tao , G.J. , Sun , Y.F. and Sun , J.C. 2006 . Chemical composition of clarified bayberry (Myrica rubra Sieb. et Zucc.) juice sediment . J. Agric. Food Chem. , 54 : 7710 – 7716 .
- Garrido , V.M. , Sims , C.A. and Marshall , M.R. 1993 . Bates, R.P. Factors influencing ellagic acid precipitation in muscadine grape juice during storage . J. Food Sci. , 58 : 193 – 196 .
- Heatherbell , D.A. 1976 . Haze and sediment formation in clarified apple juice and apple Wine. α: The role of polyvalent cations, polyphenolics and protein . Food Technol. NZ. , 11 : 17 – 23 .
- Johnson , G. , Donnelly , B.J. and Johnson , D.K. 1968 . The chemical nature and precursors of clarified apple juice sediment . J. Food Sci. , 33 : 254 – 257 .
- Lee , J.H. and Talcott , S.T. 2002 . Ellagic acid and ellagitannins affect on sedimentation in muscadine juice and wine . J. Agric. Food Chem. , 50 : 3971 – 3976 .
- Singleton , V.L. , Marsh , G.L. and Coven , M. 1966 . Identification of ellagic acid as a precipitate from loganberry wine . J. Agric. Food Chem. , 14 : 5 – 8 .
- Siriwoharn , T. , Wrolstad , R.E. and Durst , R.W. 2005 . Identification of ellagic acid in blackberry juice sediment. J. Food Sci . 70 : C189 – C197 .
- Van Buren , J.P. 1989 . “ Causes and prevention of turbidity in apple juice ” . In Processed apple Products , Edited by: Downing , D.L. 97 – 120 . New York : Van Nostrand Reinhold .
- Wu , L.C. and Seibert , K.J. 2002 . Characterization of haze–active proteins in apple juice . J. Agric. Food Chem. , 50 : 3828 – 3834 .
- Chauhan , S.K. , Tyagi , S.M. and Singh , D. 2001 . Pectinolytic liquefaction of apricot, plum, and mango pulps for juice extraction . Int. J. Food Prop. , 4 : 103 – 109 .
- Beveridge , T. 1997 . Haze and cloud in apple juices . Crit. Rev. Food Sci. Nutr. , 37 : 75 – 91 .
- Beveridge , T. , Veto , L. and Harrison , J.E. 1998 . Formation of chain–like structures in apple juice haze . Lebensm.-Wiss. u.-Technol. , 31 : 74 – 77 .
- Fang , Z.X. , Zhang , M. , Sun , Y.F. and Sun , J.C. 2006 . How to improve bayberry (Myrica rubra Sieb. et Zucc.) juice color quality: the effect of juice processing on bayberry anthocyanins and polyphenolics . J. Agric. Food Chem. , 54 : 99 – 106 .
- Beveridge , T. , Harrison , J.E. and Weintraub , S.E. 1997 . procyanidin contribution to haze formation in anaerobically produced apple juice . Lebensm.-Wiss. u.-Technol. , 30 : 594 – 601 .
- Association of Official Analytical Chemists . 1998 . Official methods of analysis , 16th , Arlington, VA : AOAC .
- Sellappan , S. , Akoh , C.C. and Krewer , G. 2002 . Phenolic compounds and antioxidant capacity of Georgia–grown blueberries and blackberries . J. Agric. Food Chem. , 50 : 2432 – 2438 .
- Arabshahi-Delouee , S and Urooj , A. 2007 . Application of phenolic extracts from selected plants in fruit juice . Int. J. Food Prop. , 10 : 479 – 488 .
- Wall , K.M. , Tait , V.M. , Eastwell , K.C. , Reid , C.A. and Beveridge , T . 1996 . Haze development in aerobically or anaerobically produced clarified apple juices . J. Food Sci. , 61 : 92 – 96 .
- Fang , Z.X. , Zhang , M. , Sun , Y.F. and Sun , J.C. 2007 . Polyphenol oxidase from bayberry (Myrica rubra Sieb. et Zucc.) and its role in anthocyanin degradation . Food Chem. , 103 : 268 – 273 .