Abstract
Physicochemical properties of sap from Deglet Nour date palm (Phoenix dactylifera L.) were studied. Composition analysis revealed (on a dry-weight basis) a high content of carbohydrates (94.98 g/100 g of dry matter basis) mainly sucrose, 2.72 g/100g (dry matter basis) of proteins and 2.29 g/100 g (dry matter basis) of ash. Date palm sap also contains 7.64 mg gallic acid equivalent/100 ml of total polyphenol. Thus, date palm sap showed antioxidant activity with a percentage inhibition of the DPPH radical value of 47.64%. Surface and foaming properties were also performed by drop volume and bubbling method, respectively. Equilibrium surface tension of fresh sap was 63.51 mN/m. Freeze-drying method preserved surface activity. Native sap showed better foam power (1.03) and foam stability (1150 s) than solutions prepared from lyophilised sap (5–30 g /100g of solution). Results demonstrated that this natural juice could be regarded as functional food due to its high nutritional value, antioxidant activity, surface activity, and foam power.
INTRODUCTION
The date palm trees (Phoenix dactylifera L.) are grown extensively in the arid and semiarid regions of the world, like northern Africa, the Arabian Peninsula, and Iran. In the south of Tunisia, date palm has an important role since it constitutes the principal source of remuneration and the basis of economy for the people living in Tunisian Sahara. It is also in a privileged position in the national economy. Indeed, the quantity of dates exported in 2004 reached 40.72 mille tons which represent approximately 11,04% of the total quantity of dates exported in the world and 85.91 million US $ in value.[Citation1] ‘Deglet Nour’ cultivar is the most appreciated because of its high sensorial and nutritional properties. It represents more than 60% of cultured Tunisian palm.[Citation2]
Date palm sap “Lagmi ” is a popular juice obtained by tapping palm trees by a local method described previously by Barreveld.[Citation3] The collecting periods is within the spring season and spread out approximately for four months (March-June) at a yield of 8–10 litres per day and palm.[Citation3] “lagmi” is appreciated for its sweet taste and its typical flavor. Fresh sap has a nearly neutral pH that can drop rapidly, when the sap is subject to spontaneous fermentation during the collect time. Several studies were undertaken in order to valorize date palm products such as date flesh, date pits, etc .[Citation4–5] Nevertheless, the literature indicates that little work was carried out in date palm sap although it is traditionally collected and consumed since decades as a fresh juice or alcoholic beverage. It is worth noting that sap derived from several palm trees like Arenga pinnata, Cocos nucifera and Phoenix sylvestris are widely used in Asian countries for production of palm sugar which are appreciated for making cakes, desserts, food coating or to be mixed with drinks.[Citation3,Citation6,Citation7] Others palms such as Borassus flabellifer, Metroxylan sago, Phoenix humilis, Raphia hookeri, and Elaeis guineensis are also tapped to collect the sap which is converted, in Sri Lanka, India, Philippine, Indonesia and in West Africa (Benin, Ivory Coast, Nigeria) into alcoholic beverage obtained from natural fermentation.[Citation8–11]
This work aimed to study the date sap “lagmi” from Deglet Nour variety to provide new information concerning chemical composition and functional properties. Date sap is an unsteady product because of the alcoholic fermentation. The knowledge of its physicochemical characteristics is essential in order to develop some valorization ways such as production of sterilized date sap, date sap syrup, or date sap sugars.
MATERIALS AND METHODS
Sap Collection and Preservation
The sap from the cultivar Deglet Nour was collected by a traditional tapping method from palms in Tozeur region (Tunisia).[Citation3] Sap samples were rapidly stored at a freezing temperature (−50°C) to protect them from fermentation. For some analysis, sap was lyophilized and conserved at 4°C.
Physicochemical Analysis
All analytical determinations were performed in triplicate. Values were expressed as the mean ± standard deviation (X ± SD). pH was measured using a pH meter (HI 8418 pH meter, Hanna instruments, Singapore) at 20°C. Color was determined using a LOVIBOND (Tintometer PFX 195, UK) colorimeter. Results were expressed in accordance with the CIELAB system. The coordinates (L∗, a∗, b∗) were measured. L∗ value is a measure of lightness and varies from 0 (black) to +100 (white), a∗ value varies from −120 (green) to +120 (red), and b∗ value varies from −120 (blue) to +120 (yellow). Dry matter was determined according to the method described by the AFNOR association (Association Française de Normalisation).[Citation12]
Fat content was carried out with a S 306 A Soxtherm (Gerhardt, Germany). Two grams of lyophilized sap were used for lipid extraction, with petroleum ether (40–60°) (Prolabo), in each Soxhlet post. Protein content was determined using Kjeldahl method. The value of 6.25 was used as a protein conversion factor. Ash percentage was measured by ashing about 2 g of dry sap in a Muffle furnace at 550°C for 6 h. The ashes were dissolved in HNO3 and the mineral constituents (Ca, Na, K, Mg, Fe, Zn, and Cu) were determined using an atomic absorption spectrophotometer (Hitachi Z 6100, Japan). Phosphorus content (P) was determined by the phosphomolybdovanadate method.[Citation13]
Total carbohydrates were estimated by difference of mean values, i.e. [total solids − (protein + lipids + minerals)].[Citation14] The separation and identification of sugars was done by High Performance Anion Exchange Chromatography coupled with Pulse Amperometric Detection (HPAEC-PAD) on a Dionex DX500 chromatographic system operating at 1 ml.min−1. 25 μl of a diluted sap (1:100 v/v) were injected. Separation was achieved on a Dionex PA100 column. The mobile phase consisted of sodium hydroxide (150 mM) elution in isocratic mode, followed by a linear gradient with a solution containing both sodium hydroxide (150 mM) and sodium acetate (1 M). The gradient ended by washing with sodium hydroxide 500 mM. Each peak of the chromatogram was identified by spiking with commercially available standard.
Total phenols in date palm sap were determined calorimetrically at 750 nm with the Folin-Ciocalteau reagent as previously done by Gutfinger.[Citation15] Antioxidant activity: DPPH radical scavenging activity of palm sap was estimated according to the method of Turkmen et al.[Citation16] Each sample was diluted with distilled water to precisely 4° Brix using an Abbe refractometer. 0.5 mL of sap sample was mixed with an aliquot of 1.5 mL of 0.1 mM DPPH radical in methanol. Distilled water was used as a control instead of samples. The reaction mixture was vortex-mixed and let to stand at room temperature in the dark for 60 min. Absorbance at 517 nm was measured using a spectrophotometer (Shimadzu UV–vis 1240, China) using methanol as blank. Antioxidant activity was expressed as percentage inhibition of the DPPH radical and was determined by the following equation:
Sap solutions were prepared by dissolving lyophilized sap in distilled water to obtain different dry matter content (5, 10, 30 g /100 g solution). To ensure good solubilisation, each sample was stirred for 20 min. Viscosity measurements of native sap and prepared solutions were performed using a CV 120 Bohlin rheometer (Bohlin instruments, UK). A cone/plate geometry used with a plate radius of 40 mm and a cone angle of 4°. The gap between the cone and plate geometry was set at 150 μm. Shear rates from 0 to 200 s−1 were applied to study the rheological properties of samples. All measurements were made at 20°C and carried out in triplicate.
Dynamic surface tension (air-water) was studied using a drop volume tensiometer TVT (Lauda-Königshöfen, Germany). A detailed description of this method is given elsewhere by Razafindralambo.[Citation17] The automated drop volume tensiometer was used in dynamic mode for the measurements. A drop was formed with a definite volume at the tip of a capillary (radius = 1.055 mm) via a Lauda syringe containing 2.5 ml of solution. All measurements were performed at 20°C. To evaluate surface activity at different concentrations, solutions were prepared with lyophilized sap to provide (5, 10, 21.31, 30, 50, and 60 g /100 g solution). Each solution was stirred for 20 min to ensure the solubilization. Measurements were performed in quadruplicate.
Foaming properties of sap samples were characterized with a Foamscan (Foamscan IT Concept, Longessaigne, France). The method is based on conductimetric and optical measurements.[Citation18] The foam was generated, using 25 ml of sample, in a transparent glass column by blowing air (100 ml/min) through a glass filter (pore size 10–16 μm). The bubbling was automatically stopped after 80 s. At the end of bubbling, foam capacity [FC = Vfoam(f)/Vgas(f)] was calculated, where Vfoam (f) is the final foam volume during bubbling, and Vgas (f) is the final gas volume injected. Whereas the half-drainage time t1/2, time taken to drain the half of the foam volume after the end of the bubbling, was adopted as a criterion to describe the foam stability. In order to investigate the concentration dependence on foam properties, different solutions were prepared from lyophilized sap (5, 10, 21.31, 30 g /100 g solution). Measurements were performed at least in triplicate. Foaming properties were assessed at 20°C.
Statistical Analysis
Statistical analyses were carried out using a statistical software program (SPSS for windows version 11.0). Data were subjected to analysis of variance using the general linear model option (Duncan's test) to determine significant differences between samples (P < 0.05).
RESULTS AND DISCUSSION
Physicochemical Characteristics
The physicochemical properties of the date palm sap are given in . Sap date palm from Deglet Nour variety has a pH value near neutrality is also the case for sap from the wild date palm and coconut.[Citation8–10] The pH indicates the freshness of sap, thus, the lactic acid produced by the bacteria coming from the natural microflora of the sap can significantly lower the pH, in a few hours, at ambient temperature.
Table 1 Physicochemical characteristics of date palm sap of Deglet Nour cultivar
The CieLab (L∗, a∗, b∗) values of date palm sap confirm that this juice is clear and not very coloured. They showed approximately the same values of L∗, b∗ of the sugar palm sap (72.46, 14.86 respectively) but a lower a∗ value (2.80). This means that date palm sap is less red, which may suggests that it contained less red pigments than did the sugar palm sap.[Citation19]
Analysis of composition showed that Deglet Nour cultivar gives a sap rich in total solids (21.31 g/100 g sap) since this sap constituted an organic matter reserve for the production of dates (See Table 2). Dry matter values of others palm saps range generally from 10 to 15 (g/100 g of juice) [Citation6,Citation19,Citation20];, however, this varied with the age of palm tree, the period and the method of tapping and cultivation area.[Citation3,Citation20]
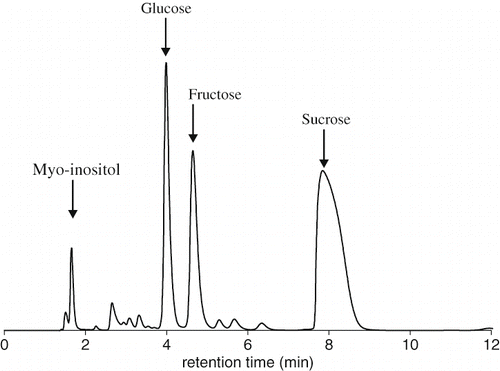
Date palm sap is mainly composed of a mixture of sugars and other minor substances. It contained 94.98 total carbohydrate, 2.72 proteins and 2.29 mineral (g/100 g dry matter basis). Results showed that the sugar composition consist of 95.27% sucrose, 2.51% glucose and 1.61% fructose. A small amount of myo-inositol (0.36%) was also identified (). In maple sap, the relative percentages of sucrose, glucose and fructose were 99.27, 0.38, and 0.38% respectively.[Citation21] Proportion of fructose and glucose in sap date palm was probably due to the inversion of sucrose during the collection time. It is also interesting to note that sucrose is the principal sugar in the fruit of Deglet Nour contrary to most other varieties of dates.[Citation22]
Figure 1 Sugar HPLC elution profile from Deglet Nour palm sap (dilution 1: 100) obtained by HPAEC-PAD chromatography system.
Sap of the Deglet Nour variety holds 0.58 g/100 ml of proteins. This value is higher than protein content values of others palm sap such as Borassus flabellifer (0.35 g/100 ml) and Arenga pinnata (0.31 g/100 ml).[Citation20] Palm sap contained also significant amounts of important mineral elements. Potassium is the most abundant element followed by magnesium and phosphorus. This order was similar to what reported for dates pulps and seeds from the same cultivar.[Citation22,Citation23] Potassium and phosphorus were also the most abundant mineral present in the root of Borassus aethiopum.[Citation24] The other mineral elements in sap, in decreasing order were Ca, Na, Fe, Cu, and Zn.
Total Polyphenol and Antioxidant Activity
Date Palm sap contains 7.64 mg gallic acid equivalent /100 ml of total polyphenol. This value is higher than that obtained by Regnault et al.[Citation25] It is interesting to note that the fresh date pulp of this variety contained a quantity of phenolic compounds (6.73 mg gallic acid equivalent/100 g) more important than that of other varieties of dates.[Citation26] Antioxidant activity observed from the date palm sap was equal to 47.64% ± 2.65%. This value is higher than the value of approximately 35% given for honey.[Citation16] This radical scavenging activity is probably due to the presence of natural phenolic components in sap.
Rheology of Sap Samples
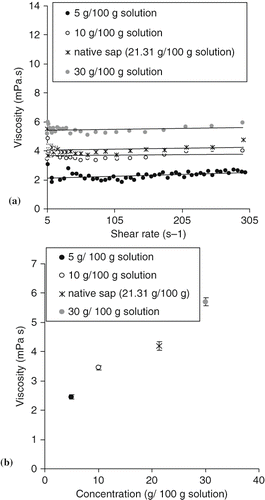
Native sap and lyophilized sap solutions displayed Newtonian behaviour. Thus viscosity increased from 2.45 to 5.7 mPa.s for 5 and 30 g/100g solution, respectively (See ). This could be explained by dominance of sugars (sucrose, glucose, and fructose) in sap. In fact, Quintas et al.[Citation27] showed that supersatured sucrose solutions had a Newtonian behaviour and low viscosity values. The viscosity of the fresh sap from date palm (4.2 mPa s) is higher than that of the sugar palm (1.35 mPa s) because probably of the higher content of dry matter.[Citation19]
Functional Properties
Surface properties
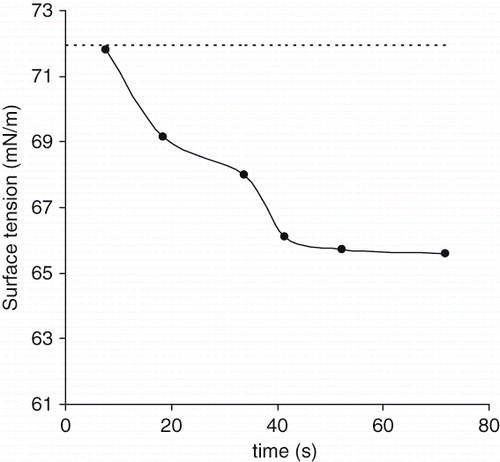
The surface tension evolution over time for the date palm sap of the Deglet Nour variety at the air/water interface is plotted in . The surface tension value decreased, during drop lifetime, approximately from 71.92 to 65 mN/m. It is well known that most of proteins have the ability to diffuse from the aqueous phase to the air/water interface, to adsorb and to unfold at the subsurface forming a monolayer responsible for the reduction of the surface tension.[Citation28,Citation29] Consequently, it was possible that the proteins of date sap are the cause of surface activity observed. The decrease in surface tension observed was lower than that observed for pure surfactants but it was comparable to that of some vegetable proteins solutions (gliadin, glycinin) at short adsorption times.[Citation30,Citation31] According to Damodaran,[Citation29] pure proteins cannot decrease the surface tension of water below 50 mN/m due to their complex structural properties. In our study, there was a mixture of proteins and others components like sugars and minerals that may affect surface properties of sap.
Figure 3 Dynamic surface tension at air-water interface of date palm sap of the Deglet Nour variety (•) at T = 20°C. Surface tension of MilliQ water ∼ 71,92 mN/m (----).
Surface tension (γ) measurements were also carried out using lyophilized sap to study the effect of the dry matter content and freeze-drying on the surface properties of the date palmsap. From dynamic surface tensions, equilibrium surface tensions (γe) have been evaluated by extrapolating the time to infinite (). At the same content of dry matter (21.31 g/100 g), the equilibrium surface tensions of the native sap (63.51 mN/m) and the one of solution prepared from lyophilized sap (62.84 mN/m), were not significantly different (P > 0.05). Possible explanation are related to the presence of low molecular weight sugars like sucrose that are able to protect the proteins from a variety of stresses generated by the lyophilization process (low temperature, freezing, and drying stresses) that remove the protein hydration shell. Presence of a disaccharide like sucrose, which can hydrogen bond to protein in the place of lost water, inhibit dehydration induced denaturation of protein.[Citation32,Citation33]
Figure 4 Equilibrium surface tensions (γe) at the air water interface as a function of dry matter concentration. Values are the mean of 4 determinations.
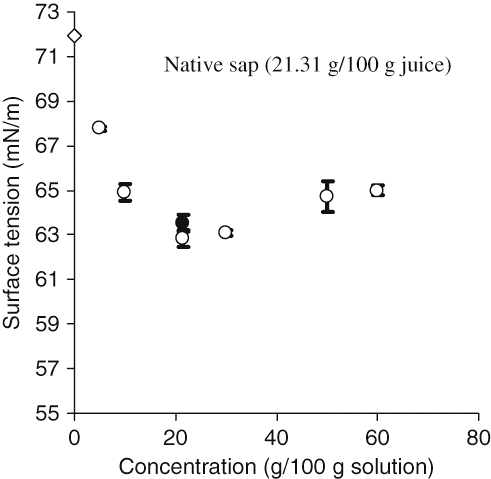
According to our results, it appears that when the concentration increases from 5 to 30 (g /100 g of solution), the equilibrium surface tension values decrease from 67.77 to 63.06 mN/m and consequently surface activity enhances significantly (P < 0.05). These results can be explained by the fact that the dry matter increase induced a rise in the protein content. As already reported in the literature, an increase of protein concentration in the bulk increases the adsorption of the surface active molecules at the air/water interface due to the fact that the protein amount available for the coverage of interface is become more important.[Citation34]
Surprisingly, at higher contents of dry matter (50 and 60 g/100 g of solution), a light increase but statistically significant (P < 0.05) in γe was observed. These solutions contained more proteins and consequently, there should be a more marked surface activity. This could be explained by the fact that the solutions with high percentage of dry matter were also more concentrated in sugars and others components. It has been known that sugars like sucrose and glucose can raise the apparent surface tension of water.[Citation35,Citation36] In the presence of proteins, the effect of sucrose on the surface properties is not yet well elucidated. Indeed, according to Aroulmoji et al.,[Citation35] sucrose may induce a dissociation of some protein aggregates formed in the adsorbed layer and slow down their adsorption at the air/water interface. Nino and Patino[Citation37]demonstrated that the diffusion of globular protein (bovine serum albumin) to the interface increased in aqueous sucrose solutions but the effect decreased with sucrose concentration. Antipova et al.[Citation38] reported that the presence of sucrose decrease the surface activity of other globular protein (ovalbumin). In contrast, the surface activity of sodium caseinate micellar protein increased in the sucrose presence.
The behaviour reported in may be also explained by the fact that increasing in dry matter content changed rheological properties of solution. Thus, the mobility of proteins molecules decreases as viscosity of solution increases. Consequently, the migration and the adsorption of surface-active molecules at the interface are affected.
Foam Properties
Foam properties were carried out using native sap (21.31 g /100 g sap) and solutions prepared with lyophilized sap at a range of 5 to 30 g/100 g solution. At 5 g of lyophilized sap/100 g solution, foam was formed with coarse bubbles. The final foam volume increases and foams resulted with smaller and denser bubbles as concentration of dry matter increases. Native sap represented the highest final foam volume (138 cm3). Foam capacity values (a) increased significantly (P < 0.05) from 0.405 to 0.875, respectively, for samples containing 5 and 10 g/100 g solution. While from 10 to 30 g/100 g solution, the foam power of samples was not significantly different (P 7gt; 0.05).
Figure 5 Effect of dry matter concentration in foam capacity (a) and stability (b) of native sap (21.31 g/100 g sap) and lyophilized sap solutions.
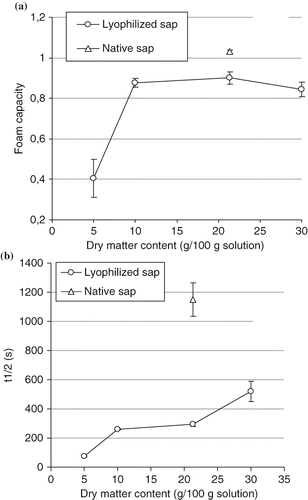
The native sap showed significantly the best foam power (1.03) of all prepared solutions. b shows the half-life time of the different foams at various dry matter contents. Liquid drainage, gas disproportionation and coalescence are the processes of foam destabilization.[Citation29] Results revealed that foam stability was improved significantly (P < 0.05) when content in dry matter increases for the solutions prepared from lyophilized sap. The foam of native sap showed the best stability (1150 s), and was significantly different (P < 0.05) from the foam obtained with lyophilized sap at the same dry matter content (21.31 g/100 g solution). From 5 to 30 g/100 g solution, both surface activity and foam properties were improved. According to Martin et al.,[Citation30] the foam formation is strongly correlated to the rate at which γ can be lowered. However, Rouimi et al.,[Citation39] showed that values of equilibrium surface tension are linked to the foam formation but are not the only criteria. In this way, at the same dry matter (21.31 g/100 g), the native sap and the lyophilized sap solution presented the same surface activity but the foam power and stability were significantly different (P < 0.05). A possible explanation is that the process of freeze-drying could modify the protein's structure, which results in a decline on foam properties, although, the surface properties were preserved.
Increase of dry matter concentration, consequently in level of proteins and sugars, enhances foaming properties. It was assumed that increase in protein content facilitates formation of multilayer cohesive and thicker protein film at the air water interface which resists to disproportionation and coalescence of bubbles decreased the effect of drainage.[Citation30,Citation40,Citation41] The fact that sap contained high level of sucrose perhaps enhanced foaming properties of solutions especially the stability. Indeed, several workers have observed that addition of mono and disaccharides in whey protein solutions, mucuna protein concentrate, rice bran protein concentrate improved the foam stability.[Citation40,Citation42–43] This improvement apparently resulted of the increased in bulk viscosity which decreases the flow in the interior of aqueous lamellae around the bubbles reducing the rate of drainage.[Citation29,Citation40]
CONCLUSION
This preliminary study revealed that date palm sap from Deglet Nour variety is a natural juice wealthy on nutrient components such as carbohydrates, proteins, and mineral elements. It also contains natural antioxidants that are well known to have positive impact for human health. The study and isolation of antioxidative compounds from natural products like palm sap can result in value addition. Results of the present study suggest also sap from date palm present interesting surface activity and foam power that is probably due to the presence of proteins. Freeze drying did not affect surface property but significantly affect the foam stability. It would be interesting to eliminate sugars from the sap to study the functional properties of proteinic concentrates of sap. The plant protein discovery presenting of the interesting functional properties is required in the food field.
REFERENCES
- Food and Agriculture Organization of the United Nations . 2006 . Rome : Agro-statistics Database FAOSTAT .
- Mzali , M.T. , Lasram , M. and Rhouma , A. 2002 . “ Le palmier dattier (Phoenix dactylifera L.) ” . In L'arboriculture fruitière en Tunisie , 201 – 229 . Tunis : ORBIS impression .
- Barreveld , W.H. 1993 . Date palms products.FAO 101 Rome, Italy
- Al- Farsi , M.A. 2003 . Clarification of date juice . International Journal of Food Science and Technology , 38 : 241 – 245 .
- Besbes , S. , Blecker , C. , Deroanne , C. , Lognay , G. , Drida , N.E. and Attia , H. 2005 . Heating effects on some quality characteristics of date seed oil . Food Chemistry , 91 : 469 – 476 .
- Apriyantono , A. , Aristyani , A. , Nurhayati Lidya , Y. , Budiyanto , S. and Soekarto , S.T. 2002 . Rate of browning reaction during preparation of coconut and palm sugar . International Congress Series , 1245 : 275 – 278 .
- Ho , C.W. , Wan Aida , W.M. , Maskat , M.Y. and Osman , H. 2007 . Changes in volatile compounds of palm sap (Arenga pinnata) during the heating process for production of palm sugar . Food Chemistry , 102 : 1156 – 1162 .
- Atputharajah , J.D. , Widanapathirana , S. and Samarajeewa , U. 1986 . Microbiology and biochemistry of natural fermentation of coconut palm sap . Food Microbiology , 3 : 273 – 280 .
- Shamala , T.R. and Sreekantiah , K.R. 1988 . Microbiological and biochemical studies on traditional Indian palm wine fermentation . Food Microbiology , 5 : 157 – 162 .
- Naidu , S.J. and Misra , M.K. 1998 . Production and consumption of wild date palm sap and country liquor in two tribal village ecosystems of eastern ghats of Orissa, India . Bioresource Technology , 63 : 267 – 273 .
- Umerie , S.C. 2000 . Caramel production from saps of African oil palm (Elaeis guineensis) and wine palm (Raphia hookeri) trees . Bioresource Technology , 75 : 167 – 169 .
- AFNO , R. 1999 . Lait et produits laitiers . Détermination de la matière sèche NF V 04-207 , : 137 – 138 .
- AOAC . 1990 . “ Association of the Official Analytical Chemists ” . In In Official methods of analysis of the Association of the Official Analytical Chemists , Edited by: Firestone , D. 1298 Arlington : Association of the Official Analytical Chemists Inc .
- Barminas , J.T. , James , M.K. and Abubakar , U.M. 1999 . Chemical composition of seeds and oil of Xylopia aethiopica grown in Nigeria . Plant Foods for Human Nutrition , 53 : 193 – 198 .
- Gutfinger , T. 1981 . Polyphenols in olive virgin oils . Journal of the American oils chemists Society , 58 : 966 – 968 .
- Turkmen , N. , Sari , F. , Poyrazoglu , E.S. and Velioglu , Y.S. 2006 . Effects of prolonged heating on antioxidant activity and colour of honey . Food Chemistry , 95 : 653 – 657 .
- Razafindralambo , H.L.H. 1996 . Contribution à l’étude des propriétés tensioactives des lipopeptides de Bacillus subtilis , 200 Belgique : Ph.D Thesis Faculté Universitaire des Sciences Agronomiques de Gembloux .
- Fains , A. , Bertrand , D. , Baniel , A. and Popineau , Y. 1997 . Stability and texture of protein foams: a study by video image analysis . Food hydrocolloids , 11 : 63 – 69 .
- Ritthipairote , T. , Youravong , W. and Wanichapichart , P. 2006 . Flux, rejection and fouling during microfiltration and ultrafiltration of sugar palm sap using a pilot plant scale . Songklanakarin Journal of Science and Technology , 28 : 817 – 828 .
- Dalibard , C. 1999 . Overall view on the tradition of tapping palm trees and prospects for animal production, Livestock research for rural development . International Relations Service, Ministry of Agriculture Paris , 11 ( 1 ) : 1 – 42 .
- Akochi , K.E. , Alli , I. and Kermasha , S. 1997 . Characterisation of the pyrazines formed during the processing of maple syrup . Journal of Agriculture and Food Chemistry , 45 : 3368 – 3373 .
- Cheikh-Rouhou , S. , Ben Amara , W. , Besbes , S. , Blecker , C. and Attia , H. 2006 . Essai de valorisation d’écarts de triage de dattes: Elaboration et caractérisation d'un extrait concentré de la pulpe . Microbiologie Hygiène Alimentaire , 18 : 3 – 12 .
- Besbes , S. , Blecker , C. , Deroanne , C. , Drira , N. E. and Attia , H. 2004 . Date seeds: chemical composition and characteristic profiles of the lipid fraction . Food Chemistry , 84 : 577 – 584 .
- Bolade , M. K. and Bello , S.B. 2006 . Selected physicochemical properties of flour from the root of African fan palm (Borassus aethiopum) . International Journal of Food Properties , 9 : 701 – 713 .
- Regnault , R.C. , Hadidane , R. , Biard , J.F. and Boukef , K. 1978 . High performance liquid and thin-layer chromatographic determination of phenolic acids in palm (Phoenix dactylifera) products . Food Chemistry , 25 : 61 – 71 .
- Mansouri , A. , Embarek , G. , Kokkalou , E. and Kefalas , P. 2005 . Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera) . Food Chemistry , 89 : 411 – 420 .
- Quintas , M. , Brandão , T.R.S. , Silva , C.L.M. and Cunha , RL. 2006 . Rheology of supersaturated sucrose solutions . Journal of Food Engineering , 77 : 844 – 852 .
- Van Vliet , T. , Martina , A.H. and Bos , M.A. 2002 . Gelation and interfacial behaviour of vegetable proteins . Current Opinion in Colloid and Interface Science , 7 : 462 – 468 .
- Damodaran , S. 2005 . Protein stabilisation of emulsions and foams . Journal of Food Science , 70 : 54 – 66 .
- Martin , A.H. , Grolle , K. , Bos , M.A. , Stuart , M.A.C. and Van Vliet , T. 2002 . Network forming properties of various proteins adsorbed at the air-water interface in relation to foam stability . Journal of Colloid and Interface Science , 254 : 175 – 183 .
- Bos , M.A. and Dunnewind , B. 2003 . Foams and surface rheological properties of β-casein, gliadin and glycinin . Colloid and Surfaces B: Biointerfaces , 31 : 95 – 105 .
- Allison , S.D. , Chang , B. , Randolph , T.W. and Carpenter , J.F. 1999 . Hydrogen Bonding between Sugar and Protein Is Responsible for Inhibition of Dehydration-Induced Protein Unfolding . Archives of Biochemistry and Biophysics , 365 : 289 – 298 .
- Wang , W. 2000 . Lyophilization and development of solid protein pharmaceuticals . International Journal of Pharmaceutics , 203 : 1 – 60 .
- Makri , E.A. and Doxastakis , G.I. 2007 . Surface tension of Phaselous vulgaris and coccineus proteins and effect of polysaccharides on their foaming properties . Food Chemistry , 101 : 37 – 48 .
- Aroulmoji , V. , Aguié-Béghin , V. , Mathlouthi , M. and Douillard , R. 2004 . Effect of sucrose on the properties of caffeine adsorption layers at the air/solution interface . Journal of Colloid and Interface Science , 276 : 269 – 276 .
- Permprasert , J. and Devahastin , S. 2005 . Evaluation of the effects of some additives and pH on surface tension of aqueous solutions using a drop-weight method . Journal of Food Engineering , 70 : 219 – 226 .
- Nino , R.M.R. and Patino , R.J.M. 2002 . Effect of the aqueous phase composition on the adsorption of bovine serum albumin to the air-water interface . Industrial and Engineering Chemistry Research , 41 : 1489 – 1495 .
- Antipova , A.S. , Semenova , M.G. and Belyakova , L.E. 1999 . Effect of sucrose on the thermodynamic properties of ovalbumin and sodium caseinate in bulk solution and at air-water interface . Colloid and Surfaces B: Biointerfaces , 12 : 261 – 270 .
- Rouimi , S. , Schorsch , C. , Valentini , C. and Vaslin , S. 2005 . Foam stability and interfacial properties of milk protein–surfactant systems . Food Hydrocolloids , 19 : 467 – 478 .
- Adebowale , K.O. and Lawalb , O.S. 2003 . Foaming, gelation and electrophoretic characteristics of mucuna bean (Mucuna pruriens) protein concentrates . Food Chemistry. , 83 : 237 – 246 .
- Chandi , G. K. and Sogi , D.S. 2007 . Functional properties of rice bran protein concentrates . Journal of Food Engineering , 79 : 592 – 597 .
- Firebaugh , J.D. and Daubert , C.R. 2005 . Emulsifying and Foaming Properties of a Derivatized Whey Protein Ingredient . International Journal of Food Properties , 8 ( 2 ) : 243 – 253 .
- Herceg , Z. , Rezek , A. , Lelas , V. , Kresic´ , G. and Franetovic´ , M. 2006 . Effect of carbohydrates on the emulsifying, foaming and freezing properties of whey protein suspensions . Journal of Food Engineering , 79 : 279 – 286 .