Abstract
The purpose of this investigation was to determine, using a capillary viscosimeter, kinematic viscosity data of aqueous solutions of ethanol and glucose, at several temperatures (from 20 up to 45ºC) and concentrations. Glucose solubility strongly depended on ethanol concentration allowing the kinematic viscosity study (from 5% up to 45% w/w ethanol concentration with variable glucose concentrations as a function of its solubility). Kinematic viscosity modelling with solution concentration and temperature simultaneously, based on previous models for respective binary systems, was successfully carried out. The estimated values by this model give low average deviations (2.62%) from the experimental data. Glucose solubility (at 25ºC) in alcohol solutions and index of refraction (at 20ºC) of ternary solutions were also determined and empirical models are proposed in order to evaluate both physical properties with high (R2 > 0.993 and > 0.991, respectively) accuracy.
INTRODUCTION
Determination of the flow behaviour of a fluid is necessary to different engineering applications such as selection, design and operation of processes. Kinematic viscosity is the property of transport of momentum. In this way, common operations as stirring, mixing, pumping, etc are influenced by the kinematic viscosity value. Furthermore, the property transfer rates of other unit operations controlled by mass transfer (absorption, liquid-liquid and solid-liquid extraction, etc) and heat transfer (evaporation, heat exchanger, etc) are also strongly depended on the kinematic viscosity values (of only one or several phases involved).[Citation1]
In many chemical and biological processes, sugar/ethanol/water solutions (with or without other organic or inorganic solutes at lesser concentrations) are presented. Specifically, glucose/ethanol/water solutions are used in different ranges of concentration in many processes such as bio-ethanol production from sugarcane, fermentations in sugar industry,[Citation2,Citation3] fruit preservations in alcoholic solutions with sugar, common systems of solid-liquid extraction and, also, as hypertonic solution usable in osmotic dehydration processes,[Citation4] among others.
The rheological behaviour of the corresponding ethanol/water and glucose/water binary solutions (glucose/ethanol system shows very low solubility) are well studied. In the first case, the kinematic viscosity data of solutions from 0 up to 95.11% w/w ethanol from 20 up to 45ºC was determined and modelled exhibiting great deviations from ideal behaviour.[Citation5] In the second case, experimental kinematic viscosity data at 20ºC can be found in handbooks[Citation6] and at other temperatures were also reported and modelled.[Citation7]
In spite of ternary systems of glucose/ethanol/water systems are Newtonian systems very scarce data on physical properties at different temperatures can be found in the literature. Thermodynamics measurements (solubility, water activity, osmotic coefficient, vapour pressure, etc) and predictions of ternary system from 25 up to 60ºC[Citation8], and refractive index and viscosity values at different concentrations at 30ºC[Citation9] are reported.
The main aim of this work is to determine the kinematic viscosity of ternary solutions of water/ethanol/glucose at different concentrations of each component and temperatures ranging from 20 up to 45ºC. As solubility of sugar in the liquid ethanol/water solutions depends on the concentration of water, experimental data of solubility at 25ºC were also determined. Finally, refractive index data of the solutions at 25ºC were also measured. Kinematic viscosity data modelling was achieved by the use of model equations correlating kinematic viscosity of solutions with concentration and temperature, simultaneously. Previously, kinematic viscosity of aqueous solutions of glucose (from 0 up to 5 mol/kg) were successfully modelled with the model initially proposed by Torok-Rard-Miller[Citation10] and modified in order to take into account the temperature effect (from 20 up to 50ºC) as follows[Citation7]:
where ν G/water (m2/s) is the kinematic viscosity of the aqueous solutions of glucose; m (mol kg−1) is the glucose molality; TR = T (K)/273.1 and νwater (m2/s) is the kinematic viscosity of water given by[Citation11]:
where T is the absolute temperature (K). The average deviation of 1.20% was found between estimated values and experimental data for this binary system. By other hand, kinematic viscosity of solutions ethanol/water were also previously modelled employing Redlich-Kister equation[Citation12] for the concentration effect (ethanol concentration from 0 to 95.11 w/w) and, for the dependence on temperature (from 20 to 45ºC), empirical Vogel-Fulcher-Tamman[Citation13] equation was selected. The final model for this binary solution is given by[Citation5]:
where ν eth/water and ν eth (m2/s) are the kinematic viscosity of aqueous solution and pure ethanol, respectively; and x is the molar fraction of ethanol. The average deviation of 1.61% was found between estimated values and experimental data for this binary system.
MATERIALS AND METHODS
The solutions were prepared by weight, with a Mettler AJ150 balance with a precision of ± 0.0001 g using degassed distilled water, commercial ethanol (Panreac, 96% v/v) and, in the case of ternary solutions, glucose (Merck with purity > 99.5%) previously dried to constant mass in a oven.
Glucose Solubility in Ethanol/Water Solutions
Several aqueous solutions (50 g) at different concentrations (from 0 up to 95.11% w/w) of ethanol were prepared. Small weighted quantities of glucose previously dried, at 70ºC until constant weight, were added and each solution was shaken at least during 24 h at 25ºC. This operation was repeated until the detection of solid sugar in the system after the cited period of time. Glucose solubility was expressed as molar fraction.
Kinematic Viscosity of Glucose/Ethanol/Water Solutions
Kinematic viscosities were determined by using a Schott-Gerate AVS 350 automatic Ubbelohde viscosimeter with the procedure previously described.[Citation11] All measurements were made at least in five replicates. Temperature control was assured by inserting the capillary viscosimeter into a water thermostatic bath with a Selecta Digiterm heating system (precision of ± 0.1ºC). The temperature was varied from 20ºC up to 45ºC by steps of 5ºC. Upper temperature was limited by the presence of volatile compounds (ethanol). During the same experiment, the experimental kinematic viscosities remained constant indicating that solution concentration did not vary. The uncertainty in the measurements was within ± 0.001 m2/s.
The capillaries of the viscosimeter were periodically calibrated by the kinematic viscosity determination of tri-distilled water at several temperatures. Each capillary's specific constant (K) was determined using kinematic viscosity values attained by applying EquationEq. (2) obtained in the same range of temperature. In terms of concentration, the ternary system was studied at several concentrations of ethanol (< 45% w/w due to above this value glucose solubility is very low) and glucose (with variable concentrations depending on the different solubility).
Refractive Index of Glucose/Ethanol/Water Solutions
The refractive index, nD , was measured using the Atago's digital Abbe refractometer DR-A1 thermostatted at 20ºC (± 0.1ºC) at atmospheric pressure and previously calibrated employing water. The uncertainty in the measurements was within ± 0.0001.
RESULTS
Glucose Solubility in Ethanol/Water Solutions
shows the solubility curve of glucose (molar fraction) as function of molar fraction of ethanol at 25ºC. A strong decrease of glucose solubility is found at high concentrations of ethanol. This behaviour was also observed by other authors at other temperatures for different sugars as glucose, fructose and sucrose.[Citation8,Citation14,Citation15] Solubility values of glucose can be acceptability fitted with molar fraction of ethanol by an empirical equation (R2 = 0.993) given by:
Figure 1 Solubility of glucose in ethanol/water solutions vs. molar fraction of ethanol at 25ºC. Line corresponds to EquationEq. (4).
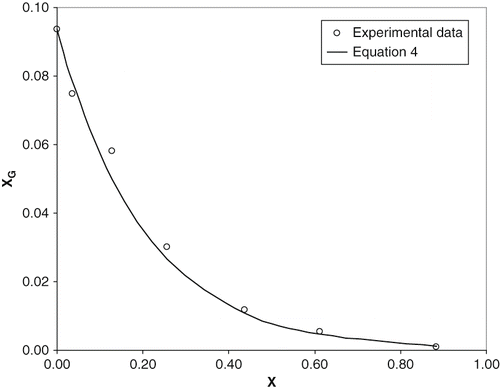
where s (molar fraction) is the glucose solubility in the ternary solution; and s0 (molar fraction) is the glucose solubility in water at 25ºC. The found data for s0 = 0.0936, is according to other reported values at the same range of temperature.[Citation14,Citation15] It is observed that the quantity of glucose added above x = 0.24 (45% ethanol mass fraction) is small and the study of viscosity with glucose concentration is strongly limited. In this way, the study of the kinematic viscosity of ternary systems is restricted from 0 up to 0.24 of molar fraction of ethanol.
Kinematic Viscosity of Glucose/Ethanol/Water Solutions
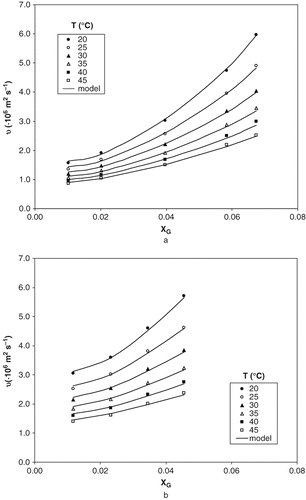
collects the experimental data of the kinematic viscosities of aqueous solutions of ethanol and glucose, in the range of concentrations and temperatures studied. In all cases, kinematic viscosity decreased with temperature at fixed concentration, and increased with glucose concentration at constant temperature. As glucose solubility is higher in water than ethanol, higher changes are observed in systems with higher water concentrations. The effect of ethanol concentration on kinematic viscosity is not as clear as previous behaviours due to aqueous solutions of ethanol show maximum values (as function of temperature) in the range from 45 to 55% (w/w) of ethanol concentration.[Citation5] The modelling of kinematic viscosities corresponding to these ternary solutions was carried out applied:
Table 1 Experimental data of kinematic viscosity of ternary ethanol/water/glucose solutions
where ν solvent is the corresponding kinematic viscosity of binary ethanol/water solution with the same ethanol concentration of ternary solution given by EquationEq. (3), and a, b, and c are parameters. Specifically, at x = 0, a parameter values correspond to the relative kinematic viscosity of the aqueous solutions of glucose given by the EquationEq. (1). So, modelling of the ternary systems is based on the modelling of the respective aqueous binary systems and the b and c parameters fitting. Regarding to b parameter was found that depends only on glucose concentration following a potential function:
where xG is the molar fraction of glucose, and the coefficients were obtained by non-linear fit using TablecurveTM program (AISN software). Finally, c parameter values were fitted to a function of temperature and glucose concentration simultaneously, obtaining the following expression:
shows, as example, the experimental data (dots) and calculated values (lines) of kinematic viscosity of ternary solutions at two different levels of ethanol concentration of the corresponding binary system without glucose, 5 (a) and 35% (w/w) (b). A good agreement between experimental data and calculated values can be observed. The estimated values of kinematic viscosity with the proposed model give an average deviation of 2.62% and maximum deviation of 4.23% in relation to the corresponding experimental data.
Refractive Index of Glucose/Ethanol/Water Solutions
collects the values of the index of refraction of aqueous solutions of glucose (bottom of the table) and ternary systems. Values of aqueous glucose solutions are according to bibliographic sources.[Citation6] In all cases, the values of index of refraction increase with glucose and ethanol concentration. In order to modelling the found results, linear relationships were determined among index of refraction and glucose concentration (wG , expressed in mass fraction), at a fixed ethanol concentration, . In this way, each linear function tends to, when glucose concentration tends to zero, the index of refraction of aqueous ethanol solutions, nDeth/water . These values were obtained from other authors[Citation16] (also collected in ) and considered as the values in the origin of the straight equations. So,
Figure 3 Experimental data and calculated values of index of refraction of ternary solutions and aqueous glucose solutions at 20ºC. Lines correspond to EquationEq. (8).
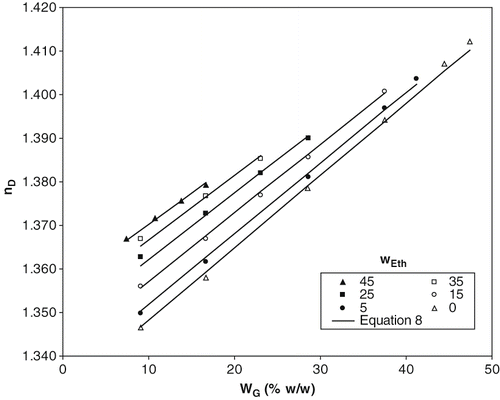
Table 2 Experimental data of refractive index viscosity of ternary ethanol/water/glucose solutions at 20ºC
where m is the slope of each linear equation that it was successfully correlated (R2 = 0.991) with ethanol mass fraction, obtaining as final equation:
This equation allows to evaluate the index of refraction of aqueous solutions with ethanol and glucose up to 45% w/w of ethanol and corresponding solubility values of sucrose at 20ºC with maximum deviations of 0.12%.
CONCLUSIONS
Solubility of glucose in ethanol and water solutions at 25ºC strongly decreases with ethanol concentration. Empirical exponential function was found adequate to evaluate the glucose solubility with low deviations between experimental data and predicted values. Above 45% (w/w) of ethanol the added glucose is low, and viscosity study is restricted to below that value. Kinematic viscosity of ternary solutions was experimentally determined at different concentrations and temperatures. Kinematic viscosity decreased with temperature, increased with glucose concentration, and showed maximum values at determined concentration of ethanol (similar behaviour in mixtures ethanol/water). A semi-empirical model to evaluate kinematic viscosity of ternary systems based on the existing models for the respective aqueous binary systems was developed and satisfactorily applied to experimental data with low deviations in spite of the non-ideal behaviour of the studied systems. Finally, index of refraction data were obtained experimentally at 20ºC. Index of refraction increases with glucose and ethanol concentration. Simple model (linear equations) is proposed to evaluate this optical property with concentration of components.
REFERENCES
- Chenlo , F. , Moreira , R. , Pereira , G. and Bello , P. 2006 . An equation for modeling the kinematic viscosities of binary and ternary solutions with sugars and sodium chloride as a function of concentration and temperature: Experimental data of solutions with lactose . Int. J. Food Proper. , 9 : 149 – 156 .
- Decloux , M. , Labatut , F. , Saska , M. and Godshall , M.A. 2001 . Effect of ethanol on sucrose solubility and molasses exhaustion . Int. Sugar J. , 103 : 547 – 552 .
- Togrul , H. and Arslan , N. 2004 . Mathematical model for prediction of apparent viscosity of molasses . J. Food Eng. , 62 : 281 – 289 .
- Barbosa-Cánovas , V. and Vega-Mercado , H. 1998 . Dehydration of Foods , 330 Gaithersburg, MD : Aspen Publishers Inc .
- Moreira , R. , Chenlo , F. and Correia , G.R. 2007 . Viscosity of water based solutions of ethanol and sucrose . Int. J. Food Proper. , 10 : 435 – 444 .
- Weast , R.C. 1983 . CRC handbook of chemistry and physics , 67th , 962 Boca Raton, FL : Chemical Rubber Publishing .
- Moreira , R. , Chenlo , F. and Pereira , G. 2003 . Viscosities of ternary aqueous solutions with glucose and sodium chloride employed in osmotic dehydration operation . J. Food Eng. , 57 : 173 – 177 .
- Peres , A.M. and Macedo , E.A. 1996 . Thermodynamic properties of sugars in aqueous solutions: Correlation and prediction using a modified UNIQUAC model . Fluid Phase Equil. , 123 : 71 – 95 .
- Flood , A.E. and Puagsa , S. 2000 . Refractive index, viscosity, and solubility at 30ºC, and density at 25ºC for the system fructose + glucose + ethanol + water . J. Chem. Eng. Data , : 902 – 907 .
- Torok , T.I. , Rard , J.A. and Miller , D.G. 1993 . Viscosities, electrolytic conductivities, and volumetric properties of HCl-MgClx-H2O as a function of temperature up to high molar ionic strengths . Fluid Phase Equil. , 88 : 263 – 275 .
- Vázquez , G. , Chenlo , F. , Álvarez , E. , Moreira , R. and Pardo , P. 1994 . Viscosities of solutions of interest for studies of absorption processes . J. Chem. Eng. Data , 39 : 87 – 89 .
- Redlich , O. and Kister , A.T. 1948 . Thermodynamics of nonelectrolyte solutions –X-Y-T relations in a binary system . Ind. Eng. Chem. , 40 ( 2 ) : 341 – 345 .
- Cocchi , M. , Marchetti , A. , Sanna , G. , Tassi , L. , Ulrichi , A. and Vaccari , G. 1999 . Kinematic viscosities of ternary mixtures containing ethane-1,2-diol, 2-methoxyethanol and water from −10ºC to 80ºC . Fluid Phase Equil. , 157 : 317 – 342 .
- Peres , A.M. and Macedo , E.A. 1997 . Solid-liquid equilibrium of sugars in mixed solvents . Entropie , 202/203 : 1 – 5 .
- Bockstanz , G.L. , Buffa , M. and Lira , C.T. 1999 . Solubilities of α-anhydrous glucose in ethanol/water mixtures . J. Chem. Eng. Data , 34 : 426 – 429 .
- Herráez , J.V. and Belda , R. 2006 . Refractive index, densities and excess molar volumes of monoalcohols + water . J. Solution Chem. , 35 : 1315 – 1328 .