Abstract
In this research, conjugated linoleic acid (CLA) as the total of cis-9, trans-11, and trans-10, cis-12 isomers of conjugated linoleic acid and α-tocopherol contents of traditional Turkish dessert “Hosmerim” were determined by Gas-liquid chromatography (GC) with a flame ionization detector (FID) and reversed phase high performance liquid chromatography (RP-HPLC) with UV detection respectively, during 7 days of storage. Processing of lor cheese into hosmerim resulted in 1.5-fold increase in CLA concentration. The contents of CLA and α-tocopherol were found as 7.13 ± 0.06 mg/g lipid and 0.73 ± 0.03 mg/100 g, respectively, after manufacturing of the product. Hosmerim was evaulated as a good source of CLA and α-tocopherol though both nutraceutical compound decreased during 7 days of storage (p < 0.01).
INTRODUCTION
Recently, the tendency to nutraceutical foods has been considerably increased owing to their nutritional and health promoting effects. Traditional Turkish dessert, hosmerim, is considered as a healthful and nutritional food. Hosmerim is a kind of cheese dessert, which is manufactured from whey cheese (lor), butter, egg yolk, semolina and granulated sugar (saccharose). It is one of the most common and delicious dairy dessert rich for milk fat and protein and produced in Ege and Marmara regions of Turkey.
Dietary milk fats, on account of their higher content of saturated fatty acids, have long been associated with a variety of human diseases; however, recent studies have focused on the healthy components of milk fats, including conjugated linoleic acid (CLA) and α-tocopherol.[Citation1] Milk fat represents good dietary sources of α-tocopherol. The main lipophilic antioxidant, α-tocopherol, inhibits peroxidation of polyunsaturated fatty acids in cell membranes. It is a liposoluble vitamin with an antioxidant capacity, reacting with peroxy radicals and other free radicals.[Citation2,Citation3] High concentrations of α-tocopherol are associated with prevention in the risk of disorders connected to free radicals, such as cardiovascular diseases, cancer, cataracts and cell damage.[Citation4,Citation5]
Milk fat is the richest dietary sources of CLA. CLA, the acronym for conjugated linoleic acid, is a collective term used to describe one or more positional and geometric isomers of linoleic acid that have been recognized antioxidant, anticarcinogen, antiatherosclerosis agent, and an immune system modulator. Of these isomers, cis-9, trans-11-octadecadienoic acid (also referred to as rumenic acid, RA) is the most common natural form with biological activity. Some physiological effects have also been more recently attributed to trans-10, cis-12 isomer.[Citation6,Citation7] Dairy products are recognized as major dietary sources of CLA and cheese and butter, ingredients utilized in Hosmerim manufacture, contains higher CLA content in dairy products.[Citation8,Citation9,Citation10,Citation11]
Several factors have been identified that affect the formation of CLA in dairy products. The addition of whey proteins or dry milk powder increased the CLA content of processed cheese and non-fat yoghurt. The added proteins function as hydrogen donors to enhance isomerisation reactions at the initial stage of the biohydrogenation pathway and the conversion of linoleic acid oxidation radicals to CLA.[Citation8,Citation12] Garcia-Lopez et al.[Citation13] found that heating was the only step in processing that increased CLA content in a natural cheese. In brief, it was reported that the processing technology, especially heat treatment and whey protein addition are the factors increases the content of CLA in cheese.
Negligible research has been done on the composition of hosmerim. The objectives of this research were to determine the contents of CLA (as the total of cis-9, trans-11 and trans-10, cis-12 isomers) by GC and α-tocopherol by direct lipid extraction using RP-HPLC with UV detection of traditional cheese dessert, hosmerim, during 7 days of storage and examine the effect of manufacturing process on CLA content of the product.
MATERIALS AND METHODS
Preparation of Dessert Samples
Materials: 500 g of unsalty lor cheese, 2 eggs yolk of 2 middle size of eggs, semolina as 1 water-glass scale (about 160 g), butter as 1 spoonful (15 g) and 750 g of granulated sugar (Preparation time; 10 min and cooking time; 10 min).
Preparation: Lor cheese was pureed and two eggs yolk was added to lor cheese and mixed by a vortex. Then, butter was added to the egg yolk-lor cheese mixture when heating and stirring steadily at about 80°C in a steel saucepan. After 2–3 min, semolina was added gently to the last mixture, and it was continued to mix. When the mixture became the dense consistency, granulated sugar was added to final mixture and continued to mix during 10 min. After the cooling to room temperature (about 24–25°C), it was packaged into 50 g plastic cups and stored at 4ºC for 7 days. Two trials were performed.
Reagents
The CLA standard was purchased from Sigma Chemical Company (St Louis, MO). α-tocopherol (Vitamin E) was obtained from Roche-DSM products. All reagents and organic solvents used were analytical grade (Merck Co., Darmstadt, Germany).
Composition Analyses
Dry matter content: Duplicate samples were dried in an oven at 105 ± 1°C to a constant weight.[Citation14] Protein content: Protein content of dessert was determined by Kjeldahl protein units (“Gerhardt” incineration apparatus and “Gerhardt Vadopest 20” distillation appararus). The protein was calculated as nitrogen (%) × 6.25.[14] Carbohydrate content: Available carbohydrate was determined by the anthrone spectrophotometric method.[Citation15] Energy content: The energy content of the hoşmerim was determined by multiplying the values obtained for total protein, total carbohydrate, and total lipid by 4.00, 3.75, and 9.00, respectively, and summing the results. Lipid extraction and CLA analysis: Total lipids of cheese, butter and hoşmerim samples were determined by a modified method from Akalin et al.[Citation16] 5 g of sample were homogenized with 15 mL of chloroform/methanol (2:1 v/v) mixture at 0°C and then mixed using vortex for 1 min. Homogenized dessert samples were centrifuged at 4000 rpm for 10 min. Chloroform layer containing the extracted lipids was transferred to another tube and the residue was extracted with the same procedure three more times. The combined filtrates were concentrated in a rotary evaporator at 30°C and dried using nitrogen flow to dryness. The total lipid obtained was calculated gravimetrically by the AOAC[Citation14] method. The fatty acid methyl esters were prepared from extracted lipids of “Hosmerim” by esterification reaction with 14% Boron trifluoride (BF3)- methanol complex according to Tokusoglu et al.[Citation17] and Akalin et al.[Citation16]
The extracted lipids of samples were refluxed with 2 mL of 0.05 N NaOH with methanol for up to 5 min and then reacted with 2 mL of BF3 solution for 15 min. Then solutions were equilibrated to room temperature (25°C) and treated with 2 mL of hexane and 2 mL of saturated salt, and separated in a separatory funnel. The separated methyl ester phase was transferred to a GC vial and dried with anhydrous sodium sulfate. The gas-liquid chromatography analysis of methyl esters was carried out using a Perkin Elmer gas chromatograph fitted with a flame ionization detector and an SGE (BP70 X fatty acid column, 60 m, 0.25 μm film thickness, 0.25-mm ID WCOT fused-silica) capillary column. The gas chromatograph was temperature-programmed to start at 150°C for a 0 min isotherm (initial temperature) and increased at 15°C min–1 to 175°C, then held 2 min (Ramp 1), and increased at 2.2°C min–1 to 220°C, and finally held for 15 min (Ramp 2). Both injector and detector temperatures were set at 250°C. Elution time was 20 min. Carrier gas was helium at a flow rate of 1.5 mL min–1, split ratio was 50:1, and the injection amount was 1 μL.
Tocopherol analysis of dessert samples: For tocopherol quantification, lipid analysis was performed above-mentioned procedure. Prior to HPLC injection, the extracts were sonicated for 3 min to remove oxygen and filtered through a 0.50-μm (Acrodisc) filter. The analytical isocratic HPLC method was carried out following the method of Akalin and Tokusoglu.[Citation18] α-Tocopherol in the dessert samples were performed by RP-HPLC using UV detection The chromatographic seperation was performed on a 5-μm Hypersil-ODS column (250 × 4.6 mm) [Phenomenex, CA, USA]. n-hexane/ethyl acetate (90:10, v/v) was used as mobile phase at a flow rate of 1.2 mL min–1. α-Tocopherol was monitored with an absorbance detector set at 296 nm. Detector sensitivity was 0.05 A.U.F.S. and column oven was set at 25ºC. The samples were injected as 10 μL. Three separate extractions were performed and each was injected in triplicate. The quantification of α-tocopherol was determined using external standard calibration and concentrations were calculated by comparison with the peak areas of the standards. The chromatographic methods were repeatable, quick, and sensitive for detection of CLA and α-tocopherol. All analysis were performed in triplicate.
Statistical analysis: Data were subjected to analysis of variance by using Statistica, version 6.0, StatSoft, OK, USA. The significance of the differences between means were evaluated by Duncan's Multiple Range Test taking p < 0.01 as significant.
RESULTS AND DISCUSSION
Prior to CLA and α-tocopherol analysis, proximate composition was evaluated in hosmerim (). Fatty acid (FAs) chromatogram of the dessert is shown in . CLA contents of lor cheese, butter and hosmerim produced from these ingredients were reported in . The contents were obtained by summing the biologically active isomers (cis-9, trans-11 CLA and trans-10, cis-12 CLA) for each sample.
Table 1 Proximate composition of hosmerimFootnote* (1 portion as 100 g)
Figure 1 Fas chromatogram of Turkish dessert “hosmerim” by using of SGE column and FID detector with GC.
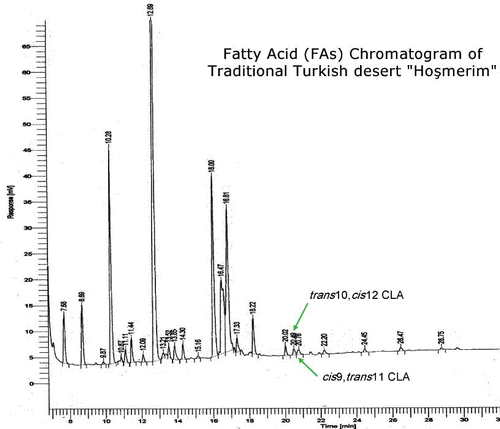
Table 2 CLA levels of lor cheese, butter, and hosmerim.Footnote*
As shown, CLA content of Hosmerim was found significantly higher than those found in butter and lor cheese utilized in that dairy dessert, so an increase in CLA content was obtained following the manufacturing process. The concentration of CLA in dairy products may be increased by processing. Aging, heat treatment and protein quality are among the factors identified as contributing to the formation of CLA in processed dairy products.[Citation12,Citation19] On the other hand, Luna et al.[Citation20] found that processing parameters used in their research had negligible effects on the CLA content of processed cheese. In the present study, CLA can be formed through the oxidation of linoleic acid by free radicals, followed by reprotonation of the radicals by whey proteins during manufacture of hoşmerim. Heat treatment applied for the preparation of the product may also contribute to the formation of CLA by promoting oxidation as reported by Shantha et al.[Citation8] and Collomb et al.[Citation21]
In different varieties of cheese, the CLA contents ranges from 2.7–7.9 mg/g fat[Citation8,Citation9,Citation20,Citation22,Citation23,Citation24,Citation25] and from 4.5–8.1 mg/g fat in butter samples.[Citation8,Citation25,Citation26] The CLA contents in our butter and lor cheese samples are similar to those found by these researchers. CLA and α-tocopherol contents of hoşmerim during storage were shown in . High values for both CLA α-tocopherol were obtained. Both nutraceutical compound significantly decreased after the third day (p < 0.01), and the least values were obtained in the seventh day of storage.
Table 3 CLA and α-tocopherol contents of hosmerim during storage.Footnote*
Although CLA was reported as a stable component during storage of dairy products,[Citation8] Lin et al.[Citation23] found that the CLA content of Cheddar cheese were significantly affected by storage over six months. In this study, a decrease in CLA content was detected in the storage of Hosmerim. As explained above, the free-radical oxidation and biohydrogenation mechanisms are proposed to be the major mechanisms for CLA formation. However, these pathways have been suggested as major pathways for not only the formation of CLA from linoleic and linolenic acids but also the further conversion of CLA to more saturated fatty acids. In another words, the donation of hydrogen to the intermediates of the biohydrogenation pathways enhances formation of CLA, whereas the donation of hydrogen to CLA enhances the conversion of CLA to more saturated fatty acids, causing a decrease in CLA content. On the other hand, tocopherols are sensitive to oxidation, heat and light.[Citation2] α-Tocopherol, the most important component of vitamin E activity, is the major chain breaking antioxidant in dairy products. It was degraded during 7 days of storage at 4°C (p < 0.01) in hosmerim possibly depending on the light exposure and oxidation. There were lower contents of α-tocopherol were determined in some kinds of cheeses by Escriva et al.[Citation27] and Bergamo et al.,[Citation25] while similar α-tocopherol contents were found in processed cheese[Citation28] and in yellow sheep's and cow's cheese.[Citation29]
CONCLUSION
Given these results, hosmerim can be considered as a good source of antioxidant compounds such as CLA and α-tocopherol. At the end of manufacturing, CLA level increased to 7.13 mg/g fat or 1.5 mg/g of sample. In North America, total CLA intake was determined from 3-day food duplicates to be 212 ± 14 and 151 ± 14 mg/day for men and women, respectively.[Citation30] These authors suggest that the daily intake of CLA must be 620 and 441 mg for men and women to exhibit a cancer protective effect. In this study, approximately 150 mg of CLA was obtained in a usual serving size (˜100 g) of hosmerim.
REFERENCES
- Parodi , P.W. 1999 . Conjugated linoleic acid and other anticarcinogenic agents of bovine milk fat . Journal of Dairy Science , 82 : 1339 – 1349 .
- Sies , H. and Stahl , W. 1995 . Vitamin-E and vitamin-C, beta-carotene, and other carotenoids as antioxidants . American Journal of Clinical Nutrition , 62 : 1315 – 1321 .
- Thomas , M.J. 2000 . The role of free radical antioxidants . Nutrition , 16 : 716 – 718 .
- Machlin , L.J. 1995 . Critical assessment of the epidemiologic data concerning the impact of antioxidant nutrients on cancer and cardiovascular disease . Critical Reviews in Food Science and Nutrition , 35 : 41 – 50 .
- Schoene , N.W. 2001 . Vitamin E and fatty acids: effectors of platelet responsiveness . Nutrition , 17 : 793 – 796 .
- Roach , J.A.G. , Mossoba , M.M. , Yurawecz , M.P. and Kramer , J.K.G. 2002 . Chromatographic seperation and identification of conjugated linoleic acid isomers . Analytica Chimica Acta , 465 : 207 – 226 .
- Pariza , M.W. 2004 . Perspective on the safety and effectiveness of conjugated linoleic acid . American Journal of Clinical Nutrition , 79 ( Suppl 1 ) : 1132 – 1136 .
- Shantha , N.C. , Rom , L.N. , O'leary , J. , Hicks , C.L. and Decker , E.A. 1995 . Conjugated linoleic acid concentrations in dairy products as affected by processing and storage . Journal of Food Science , 60 : 695 – 697 . 720
- Jiang , J. , Björck , L. and Fonden , R. 1997 . Conjugated linoleic acid in swedish dairy products with special reference to the manufacture of hard cheeses . International Dairy Journal , 7 : 863 – 867 .
- Fritsche , J. and Steinhart , H. 1998 . Amounts of conjugated linoleic acid (CLA) in German foods and evaulation daily intake . Zeitschrift für Lebensmittel-Untersuchung Und-Forschung , 206 : 77 – 82 .
- Prandini , A. , Geromin , D. , Conti , F. , Masoero , F. , Piva , A. and Piva , G. 2001 . Survey on the level of conjugated linoleic acid in dairy products . Italian Journal of Food Science , 13 : 243 – 253 .
- Shantha , N.C. , Decker , E.A. and Ustunol , Z. 1992 . Conjugated linoleic acid concentration in processed cheese . Analytica Chimica Acta , 69 : 425 – 428 .
- Garcia-Lopez , S. , Echeverria , E. , Tsui , I. and Balch , B. 1994 . Changes in the content of conjugated linoleic acid (CLA) in processed cheese during processing . Food Research International , 27 : 61 – 64 .
- AOAC. Official Methods of Analysis of the Association of Analytical Chemists , Method No: 92307; AOAC: Washington, DC, 1999.
- Osborne , D.R. 1986 . Analisis de los nutrientes de los alimentos , Acribia : Zaragoza .
- Akalın , A.S. , Tokuoglu , O. , Gönc , S. and Ökten , S. 2005 . Detection of biologically active isomers of conjugated linoleic acid in kaymak . Grasas y Aceites. , 56 : 298 – 302 .
- Tokusoglu , Ö. , Akalin , S. and Gonc , S. 2003 . The Investigation on Conjugated Linoleic Acid (CLA, C18:2; cis-9, trans-11, trans-10, cis-12 Octadecadienoic Acid) Amounts in Various Cheeses by Capillary Gas Chromatographic Method . Proceedings of ‘Süt Endüstrisinde Yeni Eğilimler Sempozyumu (SEYES)’ . May 22–23 2003 . pp. 303 – 309 . Bornova , , Izmir : Ege Univ. Fac. Agric., Dep Dairy Techn. . (In Turkish)
- Akalın , A.S. and Tokuşoğlu , O. 2004 . Liquid chromatographic detection of α-, γ-, δ- tocopherols in infant formulas . Milchwissenschaft. , 59 : 244 – 246 .
- Ha , N.L. , Grimm , N.K. and Pariza , M.W. 1989 . Newly recognized anticarcinogenic fatty acids: identification and quantification in natural and processed cheeses . Journal of Agricultural and Food Chemistry , 37 : 75 – 81 .
- Luna , P. , Fuente , M.A. and Juarez , M. 2005 . Conjugated linoleic acid in processed cheeses during the manufacturing stages . Journal of Agricultural and Food Chemistry , 53 : 2690 – 2695 .
- Collomb , M. , Schmid , A. , Sieber , R. , Wechsler , D. and Ryhanen , E-L. 2006 . Conjugated linoleic acid in milk fat: Variation and physiological effects . International Dairy Journal , 16 : 1347 – 1361 .
- Lin , H. , Boylston , T.D. , Chang , M.J. , Luedecke , L.O. and Shultz , T.D. 1995 . Survey of conjugated linoleic acid contents of dairy products . Journal of Dairy Science , 78 : 2358 – 2365 .
- Lin , H. , Boylston , T.D. , Luedecke , L.O. and Shultz , T.D. 1998 . Factors affecting the conjugated linoleic acid content of cheddar cheese . Journal of Agricultural and Food Chemistry , 46 : 801 – 807 .
- Zlatanos , S. , Laskaridis , K. , Feist , C. and Sagredos , A. 2002 . CLA content and fatty acid composition of Greek Feta and hard cheeses . Food Chemistry , 78 : 471 – 477 .
- Bergamo , P. , Fedele , E. , Iannibelli , L. and Marzillo , G. 2003 . Fat-soluble vitamin contents and fatty acid composition in organic and conventional Italian dairy products . Food Chemistry , 82 : 625 – 631 .
- Ledoux , M. , Chardigny , J.M. , Darbois , M. , Soustre , Y. , Sebedio , J.L. and Laloux , L. 2005 . Fatty acid composition of French butters, with special emphasis on conjugated linoleic acid (CLA) isomers . Journal of Food Composition and Analysis , 18 : 409 – 425 .
- Escriva , A. , Esteve , M.J. , Farre , R. and Frigola , A. 2002 . Determination of liposoluble vitamins in cooked meals, milk and milk products by liquid chromatography . Journal of Chromatography A , 947 : 313 – 318 .
- Kristensen , D. , Hansen , E. , Arndal , A. , Trinderup , R. A. and Skibsted , L.H. 2001 . Influence of light and temperature on the colour and oxidative stability of processed cheese . International Dairy Journal , 11 : 837 – 843 .
- Ribarova , F. , Zanev , R. , Shishkov , S. and Rizov , N. 2003 . α-Tocopherol, fatty acids, and their correlations in Bulgarian foodstuffs . Journal of Food Composition and Analysis Food Comp. Anal. , 16 : 659 – 667 .
- Ritzenthaler , K.L. , Mcguire , M.K. , Falen , R. , Shultz , T.D. , Dasgupta , N. and Mcguire , M.A. 2001 . Estimation of conjugated linoleic acid intake by written dietary assessment methodologies underestimates actual intake evaluated by food duplicate methodology . Journal of Nutrition , 131 : 1548 – 1554 .