Abstract
Tamarind (Tamarindus indica L.) is a member of the dicotyledonous family Leguminosae. The fruit pulp has a pleasant acid taste and rich aroma, and thus, it is used as the chief souring agent for curries, sauces, and certain beverages. The unripe fruit pulp is green in color, while the ripe pulp is light brownish red. In storage, the brown pulp turns slowly to deep brown and finally black in color. In the present study, the cause of color changes during developmental stage, as well as during storage of tamarind pulp was investigated. Tamarind pulp in unripe condition (green pod) shows polyphenol oxidase activity up to 105 days of maturity of the fruit or until it ripens. Thereafter due to ripening there is a marked increase of reducing sugars and available lysine leading to Maillard reaction. This inhibits enzymatic browning in the ripe pulp during subsequent storage.
INTRODUCTION
The pulp of tamarind (Tamarindus indica L.) fruit has tartaric acid, which renders it acidic in taste, and hence, is widely used for domestic and industrial purposes. It also contains reducing sugars, pectin, proteins, fiber, and cellulosic materials. The acid and sugar contents differ from sample to sample; for example, tartaric acid: 8–18%, reducing sugars 25–45%, pectin 2–3.5% and proteins 2–3%.[Citation1] Half of the tartaric acid is in combined form, chiefly as potassium bitartrate and to a small extent as calcium tartrate. The glucose and fructose contents are about 70 and 30%, respectively that comprises the total sugar content while a trace amount of sucrose is also present. The crude protein in pulp is around 3%, and proline and pipecolinic acid are the chief amino acids present in it.[Citation1]
Reaction of sugars with amino acids through Maillard reaction is responsible for the non-enzymatic browning in food and agricultural products. Lysine is the major amino acid that contributes to this type of reaction as it has a free ϵ-amino group that can readily react with reducing sugars.[Citation2] Because of the reversible nature of the Maillard reaction in the early stages, color development of the pulp is negligible; however, some results indicate that the nutritional availability of the amino acid is reduced.[Citation3] The formation of dark colored products in glucose-lysine solutions at pH values ranging between 3 and 9 have been reported to be due to the alkaline degradation of sugar mainly and to a lesser extent to the interaction between lysine and glucose.[Citation2] Moreover, the effect of lysine cannot be simply due only to its characteristics, since the two other basic amino acids, histidine and arginine, did not significantly affect the color development in heated glucose solutions. In a study on the extent of Maillard product formation in parenteral alimentary solutions containing 25% glucose and 4.25% 14C-labeled amino acids, it was observed that proline and other hydrophobic amino acids were less reactive than the other amino acids except tryptophan and hydroxyl amino acids, which reacted rapidly.[Citation2] The complexity of non-enzymatic browning reactions is known to be at least partly due to the sugar caramelization processes.[Citation4]
Maillard reaction causes major food quality losses.[Citation4] It may lead to undesirable changes due to the formation of chemically stable and nutritionally unavailable derivatives known as melanoidins.[Citation5] In addition to amino acid destruction, the Maillard reaction leads to a decrease in the availability of amino acids. At least three mechanisms are responsible for this decreased availability the involvement of an amino acid side-chain in the Maillard reaction, the formation of cross-links between peptide chains through aldol or internal condensations, and the decreased overall digestibility of the protein.[Citation5]
The factors affecting Maillard reaction are amino acid to sugar ratio, water content, pH, and organic acids.[Citation6] The non-enzymatic browning reaction was accelerated at neutral or alkaline pH while the Maillard reaction was favoured by higher temperatures.[Citation7] Some of the chemical components and radical absorbance capacity of ripened tamarind fruit was analysed by Parvez et al.[Citation8] Also Ubbaonu[Citation9] studied some physicochemical changes in velvet tamarind (Dialium guineense wild) fruits during development and ripening.
It is, necessary, therefore, to monitor the color changes in tamarind pulp due to storage, and consequently, to find the actual cause for such changes. The two probable causes appear to be enzymatic and non-enzymatic (Maillard) reactions that are common in many agricultural and food commodities. On the other hand, determination of color changes during storage and understanding the manner of such changes in tamarind pulp are scarce though it is known that tamarind pulp darkens during storage.
The brownish-red color of the tamarind pulp turns darker and within one year, it becomes almost black.[Citation1] The presence of polyphenol oxidase in several agricultural produce products like, potato[Citation10] and apple,[Citation11] has been reported. The reason for the phenomenon of darkening of tamarind pulp is yet to be clearly understood, though its importance is known to be academically interesting and commercially important. Hence, the objectives of the present research are (a) to determine the color changes during developmental stage of tamarind fruit as well as during storage of tamarind pulp; and (b) to find the cause of color changes (non-enzymatic browning Maillard reaction, or enzymatic browning).
MATERIALS AND METHODS
Plant Materials
Tamarind (Tamarindus indica L.) pods were collected from the tree itself at different intervals right from the developmental stage of the fruit (green pod) to the ripened state (brown pod), and during storage of brown pulp for the analyses. After harvesting the tamarind pods (green or brown), the outer hard shell was removed and deseeded. This deseeded pulp (green in unripe stage and light brown color after ripens) was used for all studies. The proximate composition of pulp (unripe green and ripe), as determined following by the AOAC method[Citation12] is shown in . Trypsin, (+)-catechin and tertiary butyl catechol were obtained from Sigma Chemicals, Steinheim, Germany. Trichloro acetic acid (TCA) was obtained from Rankem Chemicals, Ranbaxy Fine Chemicals Limited, S.A.S. Nagar, Punjab, India. An UV-visible spectrophotometer (Model #UV-1601, Shimadzu, Singapore) was used for measuring the absorbance values of the sample solutions at different wavelengths as mentioned in the following sections.
Table 1 Properties and proximate composition of ripe tamarind pulp
Pulp Color
The pulp was taken on a glass slide and its color was determined by employing a Hunter color meter (Model # LABSCAN XE, Hunter Associate Laboratory, Virginia, USA) with a view angle of 100, illuminant D65 and a slit diameter of 15 mm, and the CIELab color parameters (L*, a*, and b* values) were obtained. The values of L* denote brightness, a* indicate redness or greenness with positive and negative values, and b* mean yellowness or blueness with positive and negative values. The overall color change (ΔE) was measured with L*, a*, and b* values using EquationEq. (1).
Storage Studies
The matured pulp samples (brown pods) were stored up to one year (until they turned completely black) in polyethylene pouches at room temperature (25–30°C), and were withdrawn at regular intervals for color measurement and for enzymatic and non-enzymatic browning studies.
Enzymatic Browning
Enzyme activity was determined following the method suggested by Coseteng and Lee.[Citation13] One gram of tamarind pulp sample was dissolved in 20 mL of 0.01 M sodium acetate buffer (pH 6.0). Later, the dispersion was transferred to a dialysis bag and dialyzed against 0.01 M sodium acetate buffer for 24 h under refrigerated conditions. It was maintained at 8°C to remove low molecular weight components like tartaric acid, which inhibits enzymes.
The dialyzed sample was taken in a conical flask and 10 mL of same buffer was added, then it was stirred by employing a magnetic stirrer for 1 hour under refrigerated conditions and followed by centrifugation at 6000 rpm for 15 min. The enzyme extracts in the supernatant were collected for further analysis. Sodium acetate buffer (2.6 mL) was taken in a test tube and 0.3 mL of 0.5 M tertiary butyl catechol (substrate) solution was added to the same and enzyme assay was conducted by adding 0.1 mL of centrifuged enzyme. The optical density was noted from the spectrophotometer at 405 nm immediately after adding enzyme at different intervals such as 0, 1, 2, 3, 4, and 5 min. Enzyme concentrations were varied while keeping the substrate volume, concentration and final volume constant. The other four sample solutions were prepared in a similar manner that had the following compositions like (a) 200 μL enzyme extract +0.3 mL substrate and 2.5 mL buffer (b) 300 μL enzyme extract+0.3 mL substrate and 2.4 mL buffer (c) 400 μL enzyme extract+0.3 mL substrate and 2.3 mL of buffer, and (d) 500 μL enzyme extract+0.3 mL substrate and 2.2 mL of buffer.
Index for Non-Enzymatic Browning
Non-enzymatic browning was determined following the method of Duh et al.[Citation14] One gram of tamarind pulp sample was taken in duplicate, and was dispersed in 10 mL of glass distilled water to which 1 mL of trypsin enzyme containing 2 mg enzyme was added. It was incubated for 1 h at 45°C. Then 1 mL of 10% TCA was added followed by filtration. The filtrate was taken for absorbance measurement at 420 and 550 nm. The browning index was calculated by subtracting the absorbance values obtained at 550 nm from that of 420 nm.
Reducing Sugars
Reducing sugars were estimated by following method of Karel and Labuza.[Citation15] Three replications were performed. One g of tamarind pulp was dispersed in 10 ml of distilled water and extracted for 2 h. Then it was centrifuged at 5000 rpm for 20 min. One ml of supernatant was taken in a test tube and 1 ml of DNS was added. Tubes were boiled for 10 min and cooled. Two ml of distilled water was added and absorbance was read at a wavelength of 550 nm.
Available Lysine
Available lysine was determined by FDNB method of Booth.[Citation16] Three replications were performed. Half gram of tamarind pulp was taken in duplicate and 8 ml of 8% sodium bicarbonate was added. This mixture was shaken for 10 min and 0.4 ml of FDNB (1 fluoro 2, 4 dinitrobenzene) dissolved in 15 ml alcohol was added. Shaking was continued for 2 h. Alcohol was evaporated to dryness and 30 ml of 8.1N HCl was added. This mixture was refluxed on a sand bath for 16 h and then filtered when still hot. Filtrate was made up to 250 ml after cooling. Two ml of clear filtrate was pipetted into stopperd test tubes labeled A and B. Filtrate in tube A was washed with diethyl ether thrice. Ether was removed and the volume was made up to 10 ml with 1N HCl. Filtrate in tube B, after washing with diethyl ether and removing it completely, was mixed with 2 ml of carbonate buffer of pH 8.5 and 5 drops of methoxy carbonyl chloride. Then 0.75 ml of concentrated HCl was added to prevent excess frothing. The contents were washed with diethyl ether thrice and then ether was completely removed. Volume was made up to 10 ml with distilled water. Absorbance was measured at 435 nm. The difference between A and B gives the available lysine content.
Preparation of Tamarind Fruit Phenolic Extract
Approximately 1 g of pulp was placed in centrifuge tubes and soaked for 8 hours in 5 mL of 70% methanol at room temperature (26 ± 2°C). The resultant extract was filtered through filter papers followed by evaporation of alcohol. The residue was extracted with methanol three times for 3 h each as described by Owen et al.[Citation17] This extract was used to determine total phenolic, total flavonoid, and condensed tannin content.
Total Phenolic Content
The total phenolic content (TPC) of the pulp extract was determined by the Folin-Ciocalteu assay according to the method of Singleton and Rossi[Citation18] using gallic acid (GA) as the standard. The total phenolic content was expressed as gallic acid equivalents (mg of GAE/100 g sample).
Total Flavonoid Content
Total flavonoid content (TFC) was determined using the colorimetric method as described in Xu and Chang 2007.[Citation19] The results were expressed as micrograms (+)-catechin equivalents per 100-gram sample (mg of CAE/100 g sample) using the calibration curve of (+)-catechin. Calibration curve was prepared by using 10 to 50 mg (+)-catechin solution in water.
Condensed Tannin Content
Analysis of condensed tannin content (CTC) was carried out according to the method of Broadhurst and Jones[Citation20] with modifications as described by Xu and Chang.[Citation19] The amount of condensed tannin was expressed as mg catechin equivalents (mg of CAE/100 g sample) using the calibration curve of (+)-catechin.
DPPH Radical Scavenging Activity
Hydrogen donating or radical scavenging ability of T. indica fruit extract was determined using the stable radical 2, 2-diphenyl-1-picrylhydrazyl (DPPH) according to Parejo et al.[Citation21] The reaction mixture containing 1.5 mLof a DPPH methanolic solution (0.2 mg/mL) and 0.75 mL of the crude extract (methanol for the control) was incubated at 37 °C for 20 min, and the absorbance was measured spectrophotometrically at 517 nm. The percent of DPPH discoloration of the sample was then calculated. Ascorbic acid (10 μg/mL) was used as a positive control.
Statistical Analysis
The data shown in the tables are the means and standard deviations (SD) of three replicates. Statistical analyses were performed employing Duncan's Multiple Range Test (DMRT) at 5% level of significance.
RESULTS AND DISCUSSION
Properties of Tamarind Fruit
Fresh tamarind fruit is green at the developmental stage but it turns brown after maturation. It becomes dull brownish orange or yellow during storage and finally becomes black if stored for a long time. shows some of the properties of the pulp, and its proximate composition when matured. The high acidity (about 10%) is also reflected by low pH (about 2.5) of the pulp.
Polyphenol Oxidase Acitivity
shows the activity of polyphenol oxidase (PPO) at a pH of 7 at various concentrations (100–500 μL) of green tamarind pulp. An increase in time of treatment increases the absorbance values at all concentrations indicating the presence of polyphenol oxidase in green pulp. shows the activity of polyphenol oxidase in ripe pulp of tamarind fruit at different pH values, and comparison of these values with that of potato pulp. The activity remains unaltered with changes in time of enzyme treatment for tamarind pulp at different pH values demonstrating the absence of PPO in ripe tamarind pulp. On the other hand, potato pulp turns brown due to enzymatic browning showing increased absorbance. Thus, a comparison of tamarind and potato pulps indicates the presence of PPO activity in potato but not in ripe tamarind.
Table 2 Activity of polyphenol oxidase in unripe green pulp of tamarind at different concentrations of pulp extract at pH 7
Table 3 Activity of polyphenol oxidase in ripe pulp of tamarind fruit at different pHs compared to potato pulp
Non-Enzymatic Browning
In a different approach, the existence of Maillard reaction was examined during development of the fruit (). Maillard reaction has not been observed in unripe green tamarind sample. No reducing sugars and free amino acids during the early stages of fruit development were observed (), and their quantity increases as fruit approaches maturity. The melanoidin compounds are responsible for the color formation during storage.[Citation22] Reductone moiety present in the melanoidin structure exhibits both reducing and chelating properties in addition to oxygen scavenging properties.[Citation22] This reductone moiety also prevents browning by reducing copper in polyphenol oxidase. Alternatively, there is a possibility that reductone in the Maillard reaction product may be capable of converting the quinone back to diphenol, and thus, preventing the polymerization of quinone.
Table 4 Browning index of unripe green tamarind pulp
Table 5 Enzymatic and non-enzymatic browning of tamarind during development from unripe green to ripe brown pulp
Proline, serine, β-alanine, phenylalanine, and leucine are the free amino acids present in tamarind pulp. These free amino acids are present in higher quantities in the ripe fruits than in immature fruits and there is an accumulation of free amino acids observed during the stage of maturation and ripening of tamarind.[Citation23] Absence of reducing sugars and free amino acids in the unripe green tamarind pod is the reason for the absence of Maillard reaction. Kotecha and Kadam[Citation24] reported that the activity of polyphenol oxidase and non-enzymatic browning increases during storage of ripe tamarind. However, this trend was not observed in the present investigation.
Browning index, a measure of Maillard reaction, is shown in for tamarind pulp. A marked increase of browning index during storage shows the existence of Maillard reaction in ripe tamarind pulp. Maillard reaction begins in the initial stages of ripening and it progresses during storage of fruit. It may be inferred that tamarind pulp possesses enzymatic browning activity during the development stage without undergoing Maillard reaction. Later, after attaining the stage of maturity and during storage of pulp, Maillard reaction increases while enzymatic activity stops.
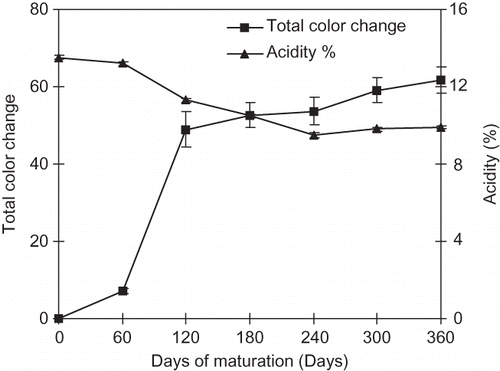
The activity of polyphenol oxidase is responsible for browning in unripe green tamarind pulp. Its activity increases with time of maturity up to 60 days and then decreases markedly to approach a zero value at about 105 days of maturity. No polyphenol oxidase activity has been observed in the matured/ripened tamarind pulp (brown pulp) measured at pH values ranging between 4 and 7. Pulp acidity was initially high for the unripe pulp (), and then it decreased slowly in the stored pulp because free sugar develops after the fruit ripens. Color L* values of the pulp drastically reduced (), and the pulp turned to a deep brown or black color after it ripened and while in storage because of Maillard reaction as it exhibits high browning index (). The negative a* values indicate the green color of the unripe pulp till 90 days of maturation just before it ripens (). Pulp once ripens turns to reddish brown and slowly to deep brown reflected by positive a* values () which then slowly increases due to pigment formation during Maillard reaction (). The increase in positive values of a* and decrease in b* with days of maturation, caused the samples to become more and more dark brown. The overall color change in the tamarind pulp increased sharply after maturation () due to the non-enzymatic browning (Maillard reaction).
Table 6 Tamarind color (L*, a*, b*) during development from unripe green to ripe brown pulp
Total Phenolic Content
Total phenolic content was highest for tamarind pulp stored for the longest period (). Total phenolics compounds are composed of phenol and polyphenols. During the initial stage of development of pulp, the low values of total phenolics are illustrated by low pigmentation and lack of brown color. If the green pod is cut and exposed to air, polyphenol oxidase present in the pod converts phenols to quinones exhibiting a brown coloration. In later stages, i.e., during ripening and storage, there is a marked increase in phenolic compounds because of further quinone formation in tamarind fruit. Priya et al.[Citation25] mentioned that phenolic compounds possess different biomedical properties such as antiallergenic, anti-inflammatory and antiviral. Moreover, it reduces risks against cancer, cataract, and coronary health problems including hypertension. The preventive effect is usually attributed to the antioxidant property of individual effect of phenolics or its interaction or synergistic effect with other antioxidants such as vitamin C, carotenoids, folic acid, and dietary fiber. Observation of high correlation between the level of phenolics and antioxidant property[Citation26] signifies the potential benefit of tamarind phenolic compounds in the prevention and treatment of different human health problems.
Table 7 Concentration of phenolic compounds and their radical scavenging activities at different stages of tamarind fruit development
Total Flavonoids and Condensed Tannin
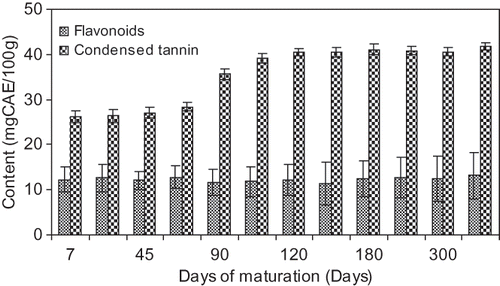
Tamarind pulp contains a good amount of total flavonoids () and there is no significant change (p ≤ 0.05) during ripening or storage. Condensed tannin content in the matured or ripened pulp is significantly higher compared to the unripe sample () to impart more color in the pulp which is evident from the browning index in the ripening stage (). This is due to a cumulative effect of polyphenolic content, total flavonoids, and condensed tannins.
DPPH Radical Scavenging Activity
Ripe tamarind pulp shows higher DPPH radical scavenging activity compared to unripe sample (). Polyphenolic contents of the extracts appear to function as good donor of electron and hydrogen atom, and therefore, are capable of terminating radical chain reaction by converting free radicals and reactive oxygen species to more stable products. The degree of discoloration indicates the scavenging potentials of the antioxidant extract. Similar observation has been reported for several plant extracts including tea.[Citation27,Citation28] DPPH is used as a free radical to evaluate antioxidative activity of some natural sources, and the degree of its discoloration is attributed to hydrogen donating ability of test compounds which is indicative of their scavenging potential.[Citation29] Decrease in the absorbance of the tamarind fruit extract shows an increase in the scavenging activity with an increase in days of maturation. This scavenging activity of tamarind pulp may be related to their presence of flavonoids, condensed tannin, and polyphenols () thus contributing to their electron transfer/hydrogen donating ability. Ripe tamarind is harvested and then the outer hard shell is removed followed by removal of seeds. Otherwise, soon it gets infested. This deseeded pulp (light brown in color) is light and heat sensitive, and becomes brown and finally black during storage. It is possible that this reaction also occur in vivo in the pod (while attached to the tree).
CONCLUSIONS
Unripe tamarind (green pod) shows polyphenol oxidase activity from the initial stage of development until the stage of the onset of ripening up to 105 days. Thereafter, there is an abrupt increase in the content of reducing sugars due to ripening in addition to a steady increase in available lysine content in the ripe pulp. The onset of Maillard reaction takes place during the ripening stage and thereafter during storage. The presence of reducing sugars and available lysine inhibits enzymatic browning. Long time stored tamarind pulp (360 days) is a rich source of antioxidants.
ACKNOWLEDGMENT
The authors thank Dr. Y.N. Sreerama, Scientist, Grain Science and Technology Department, CFTRI, Mysore, for his constructive suggestions in improving the manuscript.
REFERENCES
- Shankaracharya , N.B. 1998 . Tamarind—chemistry, technology and uses: A critical appraisal . Journal of Food Science and Technology , 35 : 193 – 208 .
- Ajandouz , E.H. and Puigserver , A. 1999 . Nonenzymatic browning reaction of essential amino acids; Effect of pH on caramelization and Maillard reaction kinetics . Journal of Agricultural and Food Chemistry , 47 : 1786 – 1793 .
- Adrian , J. , Billaud , C. and Potus , J. 2000 . Nutritional and pathological consequences of the Maillard reaction . Medicine-et-Nutrition , 36 : 69 – 89 .
- Myers , D.V. and Howell , J.C. 1992 . Characterization and specification of caramel colors: an overview . Food and Chemistry Toxicology , 30 : 356 – 363 .
- Mauron , J. 1981 . The Maillard reaction in food: A critical review from the nutritional standpoint . Progress in Food & Nutrition Science , 5 : 5 – 35 .
- Eskin , N.A.M. 1990 . Biochemistry of Foods , 2nd , 240 – 295 . New York : Academic Press .
- Meyer , L.H. 1969 . Food Chemistry , 2nd , 108 – 112 . New York : Van Nostrand Reinhold Publishing Corporation .
- Parvez , S.S. , Parvez , M.M. , Fujii , Y. and Gemma , H. 2003 . Analysis of chemical components and oxygen radical absorbance capacity of Tamarindus indica L . Japanese Journal of Tropical Agriculture , 47 ( 4 ) : 243 – 249 .
- Ubbaonu , C.N. 2005 . Physico-chemical changes in velvet tamarind (Dialium guineense Wild) during fruit development and ripening . Nigerian Food Journal , 23 : 133 – 138 .
- Severini , C. , Baiano , A. , De Pilli , T. , Romaniello , R. and Derossi , A. 2003 . Prevention of enzymatic browning in sliced potatoes by blanching in boiling saline solutions . Lebensmittel-Wisenschaft und- Technologie , 36 : 657 – 665 .
- Roux , E. , Billaud , C. , Maraschin , C. , Brun-Merimee , S. and Nicolas , J. 2003 . Inhibitory effect of unheated and heated D-glucose, D-fructose and L-cysteine solutions and Maillard reaction product model systems on polyphenol oxidase from apple. II. Kinetic study and mechanism of inhibition . Food Chemistry , 81 : 51 – 60 .
- Association of Official Analytical Chemists . 1980 . Official methods of Analysis , 13th , 15 – 211 . Washington, DC : AOAC .
- Coseteng , M.Y. and Lee , C.Y. 1987 . Changes in apple polyphenoloxidase and polyphenol concentrations in relation to degree of browning . Journal of Food Science , 52 : 985 – 989 .
- Duh , P.D. , Yen , G.C. , Yen , W.J. and Chang , L.W. 2001 . Antioxidant effects of water extracts from Barley (Hordeum vulgare L.) prepared under different roasting temperatures . Journal of Agricultural and Food Chemistry , 49 : 1455 – 1463 .
- Karel , M. and Labuza , T.P. 1968 . Nonenzymatic browning in model systems containing sucrose . Journal of Agricultural and Food Chemistry , 16 : 717 – 719 .
- Booth , V.H. 1971 . Problems in the determination of FDNB available lysine . Journal of the Science of Food and Agriculture , 2 : 658 – 666 .
- Owen , R.W. , Haubner , R. , Mier , W. , Giacosa , A. , Hull , W.E. , Spiegelhalder , B. and Bartsch , H. 2003 . Isolation, structure elucidation and antioxidant potential of the major phenolic and flavonoid compounds in brined olive drupes . Food and Chemical Toxicology , 1 : 703 – 717 .
- Singleton , V.L. and Rossi , J.A. 1965 . Colorimetry of total phenolic with phosphomolybdicphosphotungstic acid reagents . American Journal of Enology and Viticulture , 16 : 144 – 158 .
- Xu , B.J. and Chang , K.C. 2007 . Comparative analyses of phenolic composition, antioxidant capacity, and color of cool season legumes as affected by extraction solvents . Journal of Food Science , 72 ( 2 ) : 159 – 166 .
- Broadhurst , R.B. and Jones , W.T. 1978 . Analysis of condensed tannins using acidified vanillin . Journal of the Science of Food and Agriculture , 29 : 788 – 794 .
- Parejo , I. , Viladomat , F. , Bastida , J. , Rosas-Romero , A. , Flerlage , N. , Burillo , J. and Codina , C. 2002 . Comparison between the radical scavenging activity and antioxidant activity of six distilled and nondistilled Mediterranean herbs and aromatic plants . Journal of Agricultural and Food Chemistry , 50 : 6882 – 6890 .
- Tan , B.K. and Harris , N.D. 1995 . Maillard reaction products inhibit apple polyphenoloxidase . Food Chemistry , 53 : 267 – 273 .
- Gunasena , H.P.M. and Hughes , A. 2000 . Tamarind (Tamarindus indica L.) , 32 – 41 . Southampton, , UK : International Centre for Underutilized Crops .
- Kotecha , P.M. and Kadam , S.S. 2003 . Studies on browning in tamarind pulp during storage . Journal of Food Science and Technology , 40 : 398 – 399 .
- Priya , T.I. , Sabu , M.C. and Jolly , C.I. 2008 . Free radical scavenging and anti-inflamatory properties of Lagerstroemia speciosa . Inflammopharmacology , 16 ( 4 ) : 182 – 187 .
- Guo , D.J. , Chen , H.L. , Chan , S.W. and Yu , P.H. 2008 . Antioxidative activities and the total phenolic activities contents of tonic Chinese medicinal herbs . Inflammopharmacology , 16 ( 5 ) : 201 – 207 .
- Amarowicz , R. , Pegg , R.B. , Raim-Mohaddam , P. , Bral , B. and Weil , J.A. 2004 . Free radical scavenging capacity and antioxidant activity of selected plant species from the Canadian Prairies . Food Chemistry , 84 : 551 – 562 .
- Zhu , Q.Y. , Hackman , R.M. , Ensunsa , J.L. , Holt , R.R. and Keen , C.L. 2002 . Antioxidative activities of Oolong tea . Journal of Agricultural and Food Chemistry , 50 : 6929 – 6934 .
- Shimada , K.K. , Fujikawa , K.Y. and Nakamura , T. 1992 . Antioxidative properties of xanthan and autoxidation of soybean oil in cyclodextrin . Journal of Agricultural and Food Chemistry , 40 : 945 – 948 .