Abstract
Fermented soybean protein meal hydrolysate (FSPMH) was fractionated by gel filtration on Sephadex G-15. The fractions obtained were subjected to various antioxidant assays, amino acid and molecular weight determinations. Among the seven fractions, the highest antioxidant activity was found in fraction F2, with significant differences (P < 0.01) 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity, hydroxyl radicals (.OH) scavenging and Cu2+ chelating activity. Fraction F2 exhibited scavenging of DPPH (59.43%), .OH (72.80%) and 44.47% Cu2+ chelating activity. All other fractions showed variable activities in different assays. Amino acid analyses of F2 fraction with the strongest antioxidant activity also had the highest percentage of related antioxidative amino acids content (Histidine 3.46, Serine 5.78, Valine 4.08 and Lysine 11.49 g/100 g protein) compared with other six fractions. The molecular weight distribution of F2 was found to vary from 170 to 1500 Da.
INTRODUCTION
In the past few years, there has been increasing interest in research on antioxidants such as phenolic compounds and antioxidant peptides, since they can protect the human body from free radicals and retard the progress of many chronic diseases.[Citation1,Citation2] There is a wide range of oxygen-free radicals and other reactive oxygen species (ROS), which include free radicals such as superoxide anion radicals (O2 .-), hydroxyl radicals (HO.) and non free-radical species such as hydrogen peroxide (H2O2) and singlet oxygen ( | O2) which may form in the human body and in the food system. These radicals induce not only lipid peroxidation that causes deterioration of foods, but also cause oxidative damage by oxidizing biomolecules leading to cell death and tissue damage, such as atherosclerosis, cancer, emphysema, cirrhosis and arthritis[Citation3]; because of increasing demand for natural antioxidant substances, several investigations aimed at assessing the antioxidant potential of biologically active peptides from protein hydrolysates, such as soy protein,[Citation4–6] whey protein,[Citation7,Citation8] zein protein,[Citation9,Citation10] wheat protein,[Citation11,Citation12] porcine myofibrillar protein[Citation13] have been conducted. The antioxidant properties of these hydrolysates have been ascribed to the cooperative effect of a number of properties, including their ability to scavenge free radicals, to act as metal–ion chelator, oxygen quencher or hydrogen donor, and to the possibility of preventing the penetration of lipid oxidation initiators by forming a membrane around oil droplets.[Citation14,Citation15]
Bioactive peptides can be released by the microbial activity of fermented food or through enzymes derived from microorganism.[Citation16] Zhu et al.[Citation17] reported significant findings with 2,2´-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activities, reducing power, and higher peptide of Meitauza kojis obtained through fermentation. Studies have confirmed the degradation of soybean allergens during fermentation by microbial proteolytic enzymes in soy sauce, miso, soybean ingredients, and feed-grade soybean meals.[Citation18–20] Fermentation has the capacity to improve nutritional and functional properties compared to original products. Frias et al.[Citation21] shows that soybean flour fermented with Lactobacillus sp. (L. plantarum) was able to further break down and use available proteins as nutrient sources.
The objective of this study was to investigate the antioxidant potential of different fermented soy protein meal hydrolysate (FSPMH) fractions by determining its reducing power, metal chelating and free radical (DPPH. and OH.) scavenging activities of the different fractions. Furthermore, amino acid composition and molecular weight (MW) distribution were also evaluated to determine their relationship with the antioxidation properties.
MATERIALS AND METHODS
Materials
Soybean protein meal ((SPM) commercial protein isolate) was obtained from Sun-Green Biotech Co. Ltd (Nantong, China). Trinitrobenzenesulfonic acid (TNBS), L-leucine, 1, 1-diphenyl-2-picrylhydrazyl (DPPH) and protease were purchased from Sigma-Aldrich, Inc (St. Louis. MO); the strain Lactobacillus plantarum Lp6 was obtained from the culture collection of Jiangnan University (Wuxi, china). All other chemicals were of analytical grade.
Fermentation
The microorganism Lactobacillus plantarum Lp6 used was stored at 4oC before its use and then cultured for 18 h at 37°C in Man-Rogosa-Shape (MRS) broth [Shensi Biotech Co. Ltd (Shanghai, China)] prior to use for fermentation. A 0.025 mL (1 × 107 colony forming units cfu/g) of strain L. plantarum Lp6 was prepared in sterilized distilled water and then mixed with 25 g of soybean protein meal fortified with soluble starch (0.4 g/g SPM) and protease (0.01 g/g SPM) in polyethylene bag (140 mm x 200 mm) and vacuum sealed. Also 2 mg of disodium phosphate (Na2HPO4.12H2O) was added to improve the activity of L. plantarum Lp6, and then solid-state fermentation was performed at 37°C for 72 h.
Preparation of Fermented Soy Protein Meal Hydrolysate (FSPMH) and Determination of its Degree of Hydrolysis
FSMPH was prepared according to the method described by Ye et al.[Citation22] Five grams of fermented soy protein meal were mixed with 50 mL of distilled water, homogenized for 1 min and incubated at 37°C for 60 min. The incubated mixture was centrifuged at 9600 rpm for 2 min and the residue was washed with 20 mL distilled water, centrifuged again at the same speed and time and the supernatants combined. The supernatants containing peptides was poured out and degree of hydrolysis (DH) was determined using 0.004 mL TNBS in water as described by Adler-Nissen[Citation23] and Spellman et al.[Citation24] Aliquots (0.25 mL) of test or standard solutions were added to test tubes containing 2.0 mL of sodium phosphate buffer (0.2125 M, pH 8.2). TNBS reagent (2.0 mL) was then added to each tube, followed by mixing and incubation at 50°C for 30 min in a covered water bath (in order to prevent light). The reaction was stopped by the addition of 0.1 M Na2SO3 (2 mL) to each tube. Samples were then allowed to cool at room temperature for 30 min and absorbance was measured at 420 nm using a spectrophotometer (model 2.4, 2002 UNICO, WFZ UV−2102 Shanghai, China). L-Leucine (0–2.5 mM) was used to generate a standard curve. DH values were calculated using the following equation:
where htot is the total number of peptide bonds per protein equivalent; and h is the number of hydrolyzed bonds. htot = 7.8 mM/g for soybean protein.[Citation25]
Gel Filtration on Sephadex G-15 of FSPMH
FSPMH (20 mg) was subsequently loaded onto Sephadex G-15 gel filtration column (45 cm × 2 cm, Amersham Pharmacia Biotech AB, Sweden) pre-equilibrated with phosphate buffered saline (pH 7.4). Sample was eluted with the same buffer at a flow-rate of 0.5 mL/min and eluted fractions (5.0 mL) were pooled after spectrophotometric measurement at 220 nm.
Reducing Power
The reducing power of FSPMH was measured according to Wu et al.[Citation26] The sample (0, 1, 5, and 10 mg/mL) was added to 2 mL of 0.2 M phosphate buffer (pH 6.6) and 2 mL of 1% (w/v) potassium ferricyanide. The mixture was incubated at 50°C for 20 min, and then, 2 mL of 10% (w/v) trichloroacetic acid (TCA) was added. The mixture was centrifuged for 10 min at 3000 g, and 2 mL of the supernatant was mixed with 2 mL of distilled water and 0.4 mL of 0.1% (w/v) FeCl3. After reaction for 10 min, the absorbance of the solution was read at 700 nm. Increase in the absorbance of the reaction mixture indicated increased reducing power.
DPPH Radical-Scavenging Activity
The scavenging effect of FSPMH fractions on 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical was measured according to the method of Shimada et al.[Citation27] with little modification. Two milliliters of each sample solution (5.0 mg/mL) were added to 2 mL of 0.1 mM DPPH dissolved in 95% ethanol. The mixture was shaken and left for 30 min at room temperature, and the absorbance of resulting solution was read at 517 nm. A lower absorbance represents a higher DPPH scavenging activity. The scavenging effect was expressed as shown in the following equation:
Hydroxyl Radical-Scavenging Activity
The hydroxyl radical-scavenging assay was carried out using the method described by de Avellar et al.[Citation28] with some modifications. Both 1,10-phenanthroline (0.75 mM) and FeSO4 (0.75 mM) were dissolved in phosphate buffer (pH 7.4) and mixed thoroughly. H2O2 (0.01%) and FSPMH fractions (5.0 mg/mL) were added. The mixture was incubated at 37°C for 60 min, and the absorbance was measured at 536 nm. Results were determined using the following equation:
where AS, absorbance of the sample; A1, absorbance of control solution containing 1,10-phenanthroline, FeSO4 and H2O2; and A0, absorbance of blank solution containing 1,10-phenanthroline and FeSO4.
Metal Chelating Activity
The ability of FSPMH fractions to chelate prooxidative Cu2+ was investigated according to the procedure of Wang and Xiong[Citation4] with slight modifications. In the chelation test, 1 mL of 2 mM CuSO4 was mixed with 1 mL of pyridine (pH 7.0) and 20 L of 0.1% pyrocatechol violet. After the addition of 1 mL of samples (10 mg/mL), the disappearance of the blue color, due to dissociation of Cu2+, was recorded by measuring the absorbance at 632 nm at 5 min of the reaction. The Cu2+ chelating activity of the FSPMH samples was calculated as:
Amino Acid Composition
The lyophilized hydrolysate fractions were digested with HCl (6 M) at 110°C for 24 h under nitrogen atmosphere. Reversed phase high performance liquid chromatography (RP-HPLC) analysis was carried out in an Agilent 1100 (Agilent Technologies, Palo Alto, CA, USA) assembly system after precolumn derivatization with o-phthaldialdehyde (OPA). Each sample (1 μL) was injected on a Zorbax 80 A C18 column (i.d. 4.6 × 180 mm, Agilent Technologies, Palo Alto, CA, USA) at 40°C with detection at 338 nm. Mobile phase A was 7.35 mmol/L sodium acetate/triethylamine/tetrahydrofuran (500:0.12:2.5, v/v/v), adjusted to pH 7.2 with acetic acid, while mobile phase B (pH 7.2) was 7.35 mmol/L sodium acetate/methanol/acetonitrile (1:2:2, v/v/v). The amino acid composition was expressed as g of amino acid per 100 g of protein.
Determination of Molecular Weight Distribution
The FSPMH fraction with the strongest antioxidant and free radical-scavenging activities was analyzed for MW distribution using a Waters TM 600E Advanced Protein Purification System (Waters Corporation, Milford, MA, USA). The hydrolysates were loaded onto TSK gel G2000 SWXL column (i.d. 7.8 × 300 mm, Tosoh, Tokyo, Japan), eluted with 45% (v/v) acetonitrile containing 0.1% (v/v) trifluoroacetic acid at a flow rate of 0.5 ml/min and monitored at 220 nm. A molecular weight calibration curve was obtained from the following standards from Sigma: cytochrome C (12,500 Da), aprotinin (6500 Da), bacitracin (1450 Da), tetrapeptide GGYR (451 Da), and tripeptide GGG (189 Da). Results were processed using Millennium 32 Version 3.05 software (Waters Corporation, Milford, MA 01757, USA). 2.12.
Statistical Analysis
All experiments were conducted at least in triplicate. Analysis of variance (ANOVA) was performed and differences in mean values were evaluated by Tukey's test at P < 0.05 or P < 0.01 using SPSS version 13.0 (SPSS, Chicago, IL, USA).
RESULTS AND DISCUSSION
FSPMH Fractionation
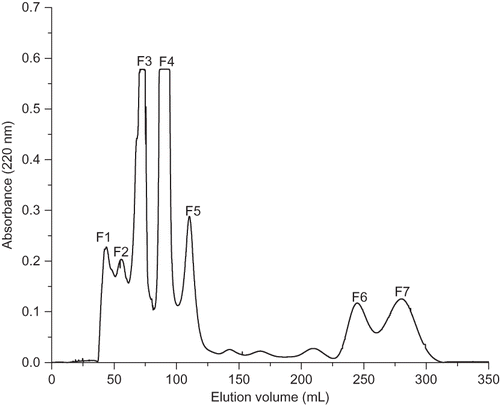
The soybean protein meal contained 77.83% protein and Equationequation (1) exhibited 22.3% degree of hydrolysis. Gel filtration column chromatography (Sephadex G-15) was used to fractionate FSPMH into seven fractions F1, F2, F3, F4, F5, F6, and F7 as shown in the . Each fraction was freeze-dried and stored at −20°C until further use.
Reducing Power
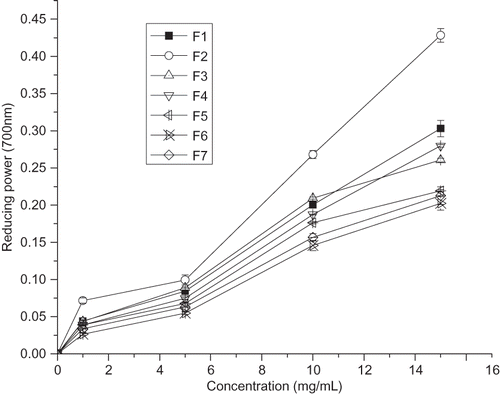
Studies showed earlier that antioxidant activity and reducing power were directly related.[Citation29,Citation30] The results of this research showed that the reducing power of the F2 fraction was the highest, followed by F1, F4, F3, F5, F7, and F6, respectively (). The presence of reducers (i.e., antioxidants) causes the reduction of the Fe3+/ferricyanide complex to the ferrous form.[Citation31] The reducing power of FSPMH fractions increased with increasing concentrations. A similar observation has been reported by Li et al.[Citation32] on chickpea protein hydrolysate (CPH).
DPPH Radical-Scavenging Activity
The estimation of DPPH radical scavenging activity was used for rapid antioxidant evaluation of the different fractions of FSMPH. The scavenging effects of different fractions on the DPPH radical are illustrated in . The different scavenging patterns for DPPH and hydroxyl radicals were likely related to the structure of peptides eluted at different stages of fractionation.[Citation10] DPPH• is an oil-soluble free radical that becomes a stable product after accepting an electron or a hydrogen atom from an antioxidant. These antioxidants donate hydrogen to free radicals, leading to non-toxic species and therefore to inhibition of the propagation phase of lipid oxidation.[Citation33] The results () revealed that F2 at 5.0 mg/mL exhibited a significant (P < 0.01) DPPH radical-scavenging activity (59.43%), which was higher than that of alcalase-treated zein hydrolysate.[Citation10] The other fractions also showed good DPPH radical-scavenging activities in the order of F3 > F4 > F1, whereas F5, F6 and F7 reacted poorly to this assay. The FSPMH fractions possibly contained some substrates, which were electron donors and could react with free radicals to convert them to more stable products and terminate the radical chain reaction.
Table 1 Antioxidant activity of fractionated FSPMH
Hydroxyl Radical-Scavenging Activity
Free radicals such as hydroxyl radical are generated from sequential reduction of oxygen during the normal course of aerobic metabolism. Overabundant radicals cause oxidative stress, which can lead to cell injury and tissue damage.[Citation34] When a hydroxyl radical reacts with aromatic compounds, it can add on across a double bond, resulting in hydroxycyclohexadienyl radical. The values of the hydroxyl radical-scavenging abilities in showed significant differences (P < 0.01) among the fractions at a dosage of 5 mg/mL. However, F2 exhibited the highest value (72.80%) for hydroxyl radical-scavenging activity compare to the others fractions (F1, F3, F4, F5, F6, and F7) with various values at the same concentrations (59.77, 65.13, 58.24, 44.06, 31.80, and 38.70%), respectively. A similar findings has been reported by Moure et al.[Citation35] on soy protein fractions. The fraction (F2) can be a potential source of natural antioxidant. In addition, the incorporation of protein hydrolysate to foods could confer desirable nutritional and functional properties.[Citation36,Citation37]
Metal Chelating Activity
The F2 fraction exhibited significant (P < 0.01) Cu2+ chelating activity of 44.47% () which was in accordance with Zhu et al.[Citation10] findings on zein hydrolysate at a concentration of 10 mg/mL. It was reported that in metallothionein, which excludes surrounding water, would allow segments of the thiol protein to bind more Cu2+ than a structure that was lost.[Citation38] It could be possible that fermentation with Lactobacillus plantarum Lp6 disrupted the spatial structure of FSPMH peptides conducive to binding and trapping of Cu2+, resulting in reduced chelation capability. The finding that metal chelating activity of soy protein meal enhanced after fermentation was in consistence with findings obtained by Moktan et al.[Citation39] where the chelating ability of soybean was affected by bacillus fermentation to kinema. Peptides reported to stabilize prooxidative transitional metal ions include carnosine,[Citation40,Citation41] potato peptides,[Citation4] and peptides rich in Histidine.[Citation42]
Amino Acid Composition
The amino acid composition of these fractions revealed that they are rich in Glutamic acid, Aspartic acid, Tyrosine, Valine, Phenylalanine, Lysine, Leucine, Alanine and Proline.[Citation5] shows that the fraction F2 had the highest amount in Histidine (3.46), Serine (5.78), Valine (4.08), and Lysine (11.49 g/100 g protein), most of which reportedly have relation to antioxidant properties either in their free form or as residues in proteins and peptides;[Citation8,Citation13,Citation43]. This probably accounted for the highest antioxidative activity exhibited by fraction F2 even though F2 did not exhibit the highest contents of total hydrophobic amino acids (THAA) and hydrophobic indices (ΔΦ) calculated according to Adler-Nissen.[Citation25] The variations in the total hydrophobic amino acid (THAA) are shown in , where it can be seen that there was significantly different among the fractions (P < 0.05). As regards hydrophobic index (ΔΦ), the fractions also differed from each other (P < 0.05). Although the differences were significant in the case of hydrophobicity.[Citation44,Citation45] However fraction F2 still showed higher antioxidant properties than the other fractions (). Furthermore, our previous investigations showed significant increase in total free amino acids profile (unpublished data) after fermentation with Lactobacillus plantarum Lp6.
Table 2 Amino acid composition of FSPMH fractions separated by gel filtration on Sephadex G-15
Molecular Weight Distribution
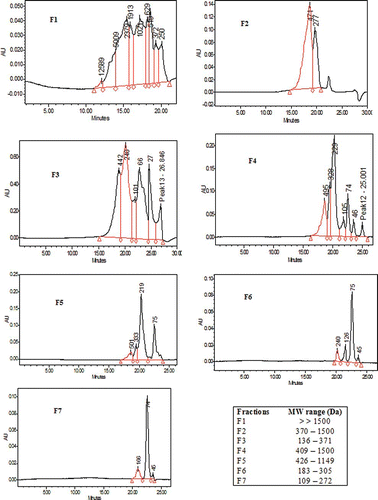
The size of peptides is known to be a significant factor in the overall antioxidant activity of hydrolyzed proteins. Considering that F2 was found to possess the highest antioxidant activity, this fraction was therefore compared its MW distribution to other six fractions (). The chromatographic data indicated that this fraction was composed of MW peptides of which major peaks were located at 370–1500 Da (69.90%) and 162–370 Da (30.10%). Several studies have shown that short peptides with MW ranging from 370 to 1500 Da were responsible for the higher antioxidant activity in commercial whey protein hydrolysate in a liposome oxidizing system.[Citation7] Wu et al.[Citation26] reported that a peptide from mackerel protein hydrolysate with MW of approximately 1400 Da possessed stronger antioxidant activity compared to higher MW peptides. Chen et al.[Citation46] claimed that six peptides of soybean protein hydrolysates with 5 to 16 amino acid residues were highly effective in exhibiting antioxidant activity. These findings are in agreement with observations from other studies and support the fact that functional properties of antioxidative peptides are highly influenced by their molecular mass.[Citation35,Citation37]
CONCLUSION
Based on the findings obtained in this study, the FSPMH fractions obtained by gel filtration exhibited different antioxidative capacities and free radical scavenging activities. The higher antioxidant activities observed with the fraction F2 could be related to its molecular weight distribution of peptide mainly located at 370 to 1500 Da. Moreover the peptides size, the high percentages of antioxidative amino acid residues present, free amino acids and hydrophobicity appeared to collectively contribute to the strong bioactivities of fractionated FSPMH. Therefore, the benefits of F2 fraction could be extended beyond its higher antioxidative ability. Further investigations are undergoing on the purification of peptide isolated from the fractions as well as the mechanisms of their antioxidant activities.
ACKNOWLEDGMENTS
This research was supported by the National Natural Science Foundation of China (No.30671525), the National High Technology Research and Development Program (“863”Program) of China (No. 2007 AA10Z325), 111 project-B07029 and China Scholarship Council (CSC); Ms. Dai Hui is also gratefully acknowledged for her help in doing the experiments.
REFERENCES
- GöktürkBaydar , Ö.G. and Yaşar , S. 2007 . Evaluation of the antiradical and antioxidant potential of grape extracts . Food Control , 18 ( 9 ) : 1131 – 1136 .
- Nagai , T. , Nagashima , T. , Abe , A. and Suzuki , N. 2006 . Antioxidative activities and angiotensin I-Converting enzyme inhibition of extracts prepared from chum salmon (Oncorhynchus Keta) cartilage and skin . International Journal of Food Properties , 9 ( 4 ) : 813 – 822 .
- Kehrer , J.P. 1993 . Free radicals as mediators of tissue injury and disease . Critical Reviews in Toxicology , 23 : 21 – 48 .
- Wang , L. and Xiong , Y.L. 2005 . Inhibition of lipid oxidation in cooked beef patties by hydrolyzed potato protein is related to its reducing and radical scavenging ability . Journal of Agricultural and Food Chemistry , 53 : 9186 – 9192 .
- Chen , H.M. , Muramoto , K. , Yamauchi , F. , Fujimoto , K. and Nokihara , K. 1998 . Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein . Journal of Agricultural and Food Chemistry , 46 : 49 – 53 .
- Wang , L.J. , Dong , L. , Zou , L. , Chen , X.D. , Cheng , Y.Q. , Yamaki , K. and Li , L.T. 2007 . Antioxidative activity of douchi (a Chinese traditional salt-fermented soybean food) extracts during its processing . International Journal of Food Properties , 10 ( 2 ) : 385 – 396 .
- Peňa-Ramos , E.A. and Xiong , Y.L. 2001 . Antioxidative activity of whey protein hydrolysates in a liposomal system . Journal of Dairy Science , 84 : 2577 – 2583 .
- Peňa-Ramos , E.A. , Xiong , Y.L. and Arteaga , G.E. 2004 . Fractionation and characterization for antioxidant activity of hydrolyzed whey protein . Journal of the Science of Food and Agriculture , 84 : 1908 – 1918 .
- Kong , B. and Xiong , Y.L. 2006 . Antioxidant activity of zein hydrolysates in a liposome system and the possible mode of action . Journal of Agricultural and Food Chemistry , 54 ( 16 ) : 6059 – 6068 .
- Zhu , L. , Chen , J. , Tang , X. and Xiong , Y.L. 2008 . Reducing, radical scavenging, and chelation properties of in vitro digests of alcalase-treated zein hydrolysate . Journal of Agricultural and Food Chemistry , 56 : 2714 – 2721 .
- Zhu , K.X. , Zhou , H.M. and Qian , H.F. 2006 . Antioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase . Process Biochemistry , 41 : 1296 – 1302 .
- Wang , J.S. , Zhao , M.M. , Zhao , Q.Z. and Jiang , Y.M. 2007 . Antioxidant properties of papain hydrolysates of wheat gluten in different oxidation systems . Food Chemistry , 101 : 1658 – 1663 .
- Saiga , A. , Tanabe , S. and Nishimura , T. 2003 . Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment . Journal of Agricultural and Food Chemistry , 51 ( 12 ) : 3661 – 3667 .
- Amadou , I. , Yong-Hui , S. , Sun , J. and Guo-Wei , L. 2009 . Fermented soybean products: some methods, antioxidants compound extraction and their scavenging activity . Asian Journal of Biochemistry , 4 ( 3 ) : 68 – 76 .
- Moure , A. , Dominguez , H. and Parajo , J.C. 2006 . Antioxidant properties of ultrafiltration-recovered soy protein fractions from industrial effluents and their hydrolysates . Process Biochemistry , 41 : 447 – 456 .
- Korhonen , H. and Pihlanto , A. 2003 . Food-derived bioactive peptides- opportunities for designing future foods . Current Pharmaceutical Design , 9 ( 16 ) : 1297 – 308 .
- Zhu , Y.P. , Cheng , Y.Q. , Wang , L.J. , Fan , J.F. and Li , L.T. 2008 . Enhanced antioxidative activity of Chinese traditionally fermented Okara (Meitauza) prepared with various microorganism . International Journal of Food Properties , 11 ( 3 ) : 519 – 529 .
- Hong , K.J. , Lee , C.H. and Kim , S.W. 2004 . Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybean and feed soybean meals . Journal of Medicinal Food , 7 : 430 – 435 .
- Kobayashi , M. 2005 . Immunological functions of soy sauce: Hypoallergenicity and antiallergenic activity of soy sauce . Journal of Bioscience and Bioengineering , 100 : 144 – 151 .
- Yamanihi , R. , Huang , T. , Tsuji , H. , Bando , N. and Ogawa , T. 1995 . Reduction of the soybean allergenicity by the fermentation with Bacillus natto . Food Science and Technology International , 1 : 14 – 17 .
- Frias , J. , Song , Y.S. , Cristina , M.V. , De Mejia , E.G. and Conception , V.V. 2008 . Immunoreactivity and amino acid content of fermented soybean products. Journal of Agricultural and Food Chemistry . 56 ( 1 ) : 99 – 105 .
- Ye , Y.T. , Xue , M. , Lin , S.M. , Wang , Y.H. , Luo , L. and Tian , J.S. 2003 . Enzymolysis kinetics of digestive enzyme from intestine and hepatopancreas in grass carp to four kinds of raw feed materials . Journal of Fishery Sciences of China , 10 ( 2 ) : 470 – 473 . (in Chinese, with English abstract)
- Adler-Nissen , J. 1979 . Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid . Journal of Agricultural and Food Chemistry , 27 : 1256 – 1262 .
- Spellman , D. , McEvoy , E. , O'Cuinn , G. and FitzGerald , R.G. 2003 . Protease and exopeptidase hydrolysis of whey protein: Comparison of the TNBS, OPA and pH stat methods for quantification of degree of hydrolysis . International Dairy Journal , 13 : 447 – 453 .
- Adler-Nissen , J. 1986 . Enzymic hydrolysis of food proteins , 57 – 109 . New York : Elsevier Applied Science Publishers .
- Wu , H.C. , Chen , H.M. and Shiau , C.Y. 2003 . Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) . Food Research International , 36 : 949 – 957 .
- Shimada , K. , Fujikawa , K. , Yahara , K. and Nakamura , T. 1992 . Antioxidative properties of xanthan on the antioxidation of soybean oil in cyclodextrin emulsion . Journal of Agricultural and Food Chemistry , 40 : 945 – 948 .
- de Avellar , I.G. , Magalhaes , M.M. , Silva , A.B. , Souza , L.L. , Leitao , A.C. and Hermes-Lima , M. 2004 . Reevaluating the role of 1,10-phenanthroline in oxidative reactions involving ferrous ions and DNA damage . Biochimica et Biophysica Acta , 1675 : 46 – 53 .
- Duh , P.D. 1998 . Antioxidant activity of burdock (Arctium lappa Linne): Its scavenging effect on free radical and active oxygen . Journal of the American Oil Chemists’ Society , 75 : 455 – 465 .
- Duh , P.D. , Tu , Y.Y. and Yen , G.C. 1999 . Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat) . LWT- Food Science and Technology , 32 : 269 – 277 .
- Ferreira , I.C.F.R. , Baptista , P. , Vilas-Boas , M. and Barros , L. 2007 . Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity . Food Chemistry , 100 ( 4 ) : 1511 – 1516 .
- Li , Y. , Jiang , B. , Zhong , T. , Mu , W. and Liu , J. 2008 . Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH) . Process Biochemistry , 41 : 1296 – 1302 .
- Jao , C.L. and Ko , W.C. 2002 . 1, 1-dipheny1-2-picrylhydrazyl (DPPH) radical scavenging by protein hydrolysates from Tuna cooking juice . Fisheries Science , 68 : 430 – 435 .
- Halliwell , B. and Gutteridge , J.M.C. 1999 . Free radical in Biology and Medicine , 3 , 530 – 533 . Oxford, , UK : Oxford University Press .
- Moure , A. , Domínguez , H. and Parajó , J.C. 2006 . Antioxidant properties of ultrafiltration-recovered soy protein fractions from industrial effluents and their hydrolysates . Process Biochemistry , 41 : 447 – 456 .
- Sidhu , J.S. , Kabir , Y. and Huffman , F.G. 2007 . Functional foods from cereal grains . International Journal of Food Properties , 10 ( 2 ) : 231 – 244 .
- Kim , S.Y. , Je , J.Y. and Kim , S.K. 2007 . Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion . Journal of Nutritional Biochemistry , 18 : 31 – 38 .
- Duncan , K.E.R. , Ngu , T.T. , Chan , J. , Salgado , M.T. , Merrifield , M.E. and Stillman , M.J. 2006 . Peptide folding, metal-binding mechanisms, and binding site structures in metallothioneins . Experimental Biology Medicine , 231 : 1488 – 1499 .
- Moktan , B. , Saha , J. and Sarkar , P.K. 2008 . Antioxidant activities of soybean as affected by Bacillus-fermentation to kinema . Food Research International , 41 : 586 – 593 .
- Chan , W.K.M. , Decker , E.A. , Lee , J.B. and Butterfield , D.A. 1994 . EPR spin-trapping studies of the hydroxyl radical scavenging activity of carnosine and related dipeptides . Journal of Agricultural and Food Chemistry , 42 : 1407 – 1410 .
- Decker , E.A. and Faraji , H. 1990 . Inhibition of lipid oxidation by carnosine . Journal of the American Oil Chemists’ Society , 67 : 650 – 652 .
- Wade , A.M. and Tucker , H.N. 1998 . Antioxidant characteristics of L-histidine . Journal of Nutritional Biochemistry , 9 : 308 – 315 .
- Saito , K. , Jin , D.H. , Ogawa , T. , Muramoto , K. , Hatakeyama , E. , Yasuhara , T. and Nokihara , K. 2003 . Antioxidative properties of tripeptide libraries prepared by the combinatorial chemistry . Journal of Agricultural and Food Chemistry , 51 : 3668 – 3674 .
- Strazzullo , G. , De Giulio , A. , Tommonaro , G. , La Pastina , C. , Poli , A. , Nicolaus , B. , De Prisco , R. and Saturnino , C. 2007 . Antioxidative activity and lycopene and β-carotene contents in different cultivars of tomato (Lycopersicon Esculentum) . International Journal of Food Properties , 10 ( 2 ) : 321 – 329 .
- Cano , A. and Arnao , M.B. 2005 . Hydrophilic and lipophilic antioxidant activity in different leaves of three lettuce varieties . International Journal of Food Properties , 8 : 521 – 528 .
- Chen , H.M. , Muramoto , K. and Yamauchi , F. 1995 . Structural analysis of antioxidative peptides from soybean β-conglycinin . Journal of Agricultural and Food Chemistry , 43 : 574 – 578 .