Abstract
Sorghum starch was modified using acetic anhydride at various levels (1.25–6.25 g/100 g of starch) and evaluated for its structure and functional properties. The acetylated starches showed degree of substitution and acetyl content between 0.05 to 0.081 and 1.31 to 2.1%, respectively. Acetylation led to surface roughness, formation of cavities and fusion of few starch granules, and a slight change in amylose content. Acetylation increased swelling power and solubility, and decreased gelatinization temperatures, enthalpy of gelatinization, paste viscosity, gel hardness, and syneresis. A change in starch structure was revealed in starches acetylated using acetic anhydride at ≥5.0% (w/w).
INTRODUCTION
Starches have possessed many diverse applications both in food production and industrial applications. But in the food industry, the use of native starches is limited because of their low shear stress resistance, thermal decomposition, high retrogradation, and syneresis.Citation[1] Numerous chemical modification techniques have been used to prevent most of these problems and produce starches with improved functional properties. Acetylation, which is the esterification of starch polymers with acetyl groups to form starch acetates,Citation[2] is one common starch modification technique. Acetic anhydride in the presence of alkaline agent, such as sodium hydroxide, is used to produce acetylated starches.Citation[3] The number of acetyl groups incorporated into the starch molecule during acetylation depends upon number of factors, such as reactant concentration, starch source,[4] reaction time, and presence of catalyst.Citation[5] Previous studies have reported improved freeze-thaw stability, resistance to retrogradation, decrease in gelatinization temperature, and increased clarity upon acetylation.[6] Acetylated starches are extensively used in a large variety of food and non-food applications. These include the use of acetylated starches in baked goods, canned pie fillings, sauces, retorted soups, frozen foods, baby foods, salad dressings, and snack foods,Citation[7] and wrap-sizing for textiles and surface sizing for papers and gummed tape adhesives.Citation[8]
Sorghum (Sorghum bicolor (L) Moench), a member of the grass family, is an important cereal in semi-arid regions of the world because of its draught resistance.Citation[9] India produces 7.40 × 106 metric tons of sorghum per annum and stands as the third largest producer in the world.Citation[10] It is also referred to as “coarse grain” or “poor people crop” as it can sustain the lives of the poorest rural people.Citation[11] Scientific information generated for this crop by carrying out relevant research work can certainly play a key role in agricultural development in the poorest countries of the world.Citation[12] Furthermore, the commercial processing of sorghum into value-added products is an important driver for economic development in these regions.Citation[13] Despite its importance, our knowledge about sorghum processing is limited as compared to other cereals. Moreover, sorghum being cheaper than corn is exploited as an alternative starch sourceCitation[14,Citation15] for diverse industrial applications. Thus, the present study was undertaken to produce acetylated sorghum starches using acetic anhydride at different levels, with an objective to compare their functional properties with the native sorghum starch.
MATERIALS AND METHODS
Materials
Sorghum cultivar (M-35) was procured from the National Research Centre for Sorghum (NRCS), Hyderabad, India.
Starch Isolation
Starch was extracted by using the method of Beta et al.Citation[16] Sorghum grain (100 g) was steeped in 200 ml of NaOH (0.25%, w/v) at 5°C for 24 h. The steeped grains were washed and ground with an equal volume of water using a blender for 3 min. The slurry was filtered through a 200-mesh screen. The material remaining on the sieve was rinsed with water. Grinding and filtering processes were repeated on this material. After rinsing, the material still remaining on the sieve was discarded. The filtrate was allowed to stand for 1 h. The filtrate was centrifuged at 760 × g for 10 min. The grey colored, top protein-rich layer was removed using a spatula. Excess water was added to re-suspend the sample, and centrifugation was done for 5 min. Washing and centrifugation were repeated several times until the top starch layer was white. The starch was dried for 24 h at 40°C.
Acetylation Treatment
The starch was acetylated using the method of Phillips et al.Citation[17] Starch (100 g) was dispersed in distilled water (225 ml) and stirred for 1 h at 25°C. NaOH (3%) solution was used to adjust the suspension pH to 8.0. Acetic anhydride (1.25, 2.5, 3.75, 5.0, 6.25 g) was added dropwise to the stirred slurry, while maintaining the pH within the range of 8.0–8.4 using 3% NaOH solution. The reaction was allowed to proceed for 10 min after the completion of acetic anhydride addition. The slurry was then adjusted to pH 4.5 with 0.5N HCl. After sedimentation, it was washed free of acid twice with distilled water and then oven dried at 40°C.
Acetyl (%) and Degree of Substitution
The percent acetylation (% acetyl) and degree of substitution (DS) were determined titrimetrically following the method of Wurzburg.Citation[3] Acetylated starch (1.0 g) was placed in a 250 ml flask and 50 ml of 75% ethanol in distilled water was added. The loosely stoppered flask was agitated, warmed to 50°C for 30 min, cooled, and 40 ml of 0.5 M KOH was added. The excess alkali was back titrated with 0.5 M HCl using phenolphthalein as an indicator. The solution was stood for 2 h and then any additional alkali, which may have leached from the sample, was titrated. A Blank, using the original unmodified starch, was also used.
Blank and sample were titration volumes in millilitre, sample weight was in gram. DS is defined as the average number of sites per glucose unit that posses a substituent group.Citation[18]
Fourier Transform Infrared (FT-IR) Spectra
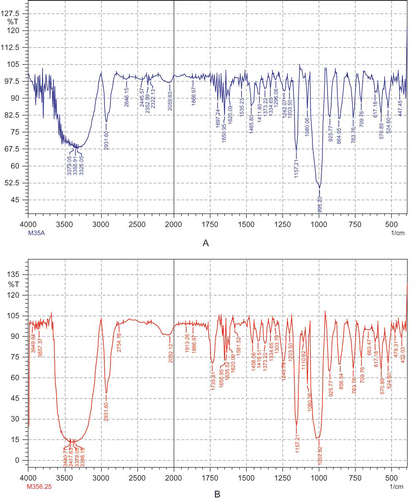
The FT-IR spectra of native and acetylated starches were acquired on a Shimadzu FT-IR instrument (Shimadzu corporation, Kyoto, Japan) using a potassium bromide (KBr) disc containing 1% finely ground sample.
Amylose Content, Swelling Power, and Solubility
Amylose content of native and acetylated starches was determined by using the method of Williams et al.Citation[19] Swelling power and solubility were determined using 2% aqueous suspension of the starch by the method of Leach et al.Citation[20]
Morphological Properties
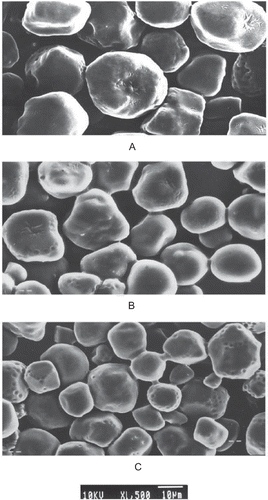
Scanning electron micrographs of native and acetylated starches were obtained with a scanning microscope (Jeol JSM-6100, Jeol Ltd., Tokyo, Japan). Native and acetylated starches were suspended in ethanol to obtain 1% suspension. One drop of the starch-ethanol suspension was applied on an aluminum stub, and the starch was coated with gold-palladium (60:40). An acceleration potential of 10 kV was used during micrography.
X-Ray Diffraction
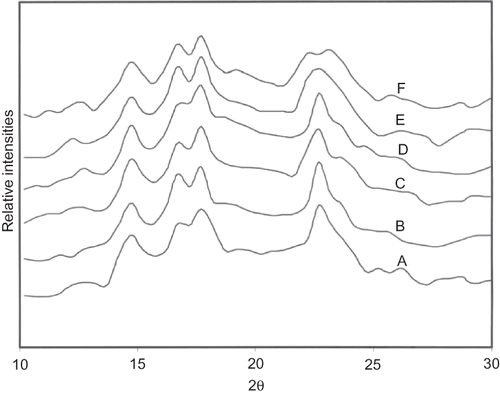
X-ray diffractograms of fully moistured starch granules (exposed to 100% relative humidity for three days) were recorded by a copper anode X-ray tube using an Analytical Diffractometer (Pan Analytical, Phillips, Holland).Citation[21] The diffractometer was operated at 30 mA and 40 kV with a scanning speed of goniometer of 4°/min.
Thermal Properties
Thermal properties of native and acetylated starches were analyzed using a DSC-821e (Mettler Toledo, Switzerland) equipped with a thermal analysis data station. Starch (3.5 mg, dwb) was weighed in a 40 μl capacity aluminum pan (Mettler, ME-27331) and distilled water was added with the help of a Hamilton microsyringe to achieve a starch-water suspension containing 70% water. Samples were hermetically sealed and allowed to stand for 1 h at room temperature before heating in the DSC. The DSC was calibrated using indium and an empty aluminum pan was used as reference. The pans containing starch samples were heated at a rate of 10°C/min from 35 to 100°C. Onset temperature (To ), peak temperature (Tp ), conclusion temperature (Tc ), and enthalpy of gelatinization (ΔHgel ) were calculated automatically. Enthalpies were calculated on starch dry basis.
Pasting Properties
The pasting properties of starches were evaluated with a Rapid Visco Analyser (RVA-4, Newport Scientific, Warriewood, Australia) using RVATM Crosslinked and Substituted Method, No. 9, Version 4.Citation[22] Starch (3 g, 14% mb) was weighed directly in the aluminum RVA sample can, and distilled water was added to a total constant sample weight of 28 g. A programmed heating and cooling cycle was used where the samples were held at 50°C for 1 min, heated to 95°C in 3.7 min, held at 95°C for 2.5 min before cooling to 50°C in 3.8 min, and holding at 50°C for 2 min. Parameters recorded were pasting temperature (PT); peak viscosity (PV); hot paste viscosity (HPV) (minimum viscosity at 95°C); cool paste viscosity (CPV) (final viscosity at 50°C); breakdown (BD) (= PV – HPV); and set back (SB) (= CPV – HPV). All measurements were replicated thrice.
Instrumental Textural Properties
Textural properties of RVA starch gelsCitation[23] were evaluated using the TA/XT2 Texture Analyzer (Stable Micro Systems, Surrey, England). The starch slurry formed in the canister after RVA testing was covered and kept at 4°C overnight and allowed to gel. The gel formed in the can (37 mm diam., 20 mm height) was used directly for texture analysis. The gels were compressed to a distance of 10 mm using a flat cylindrical probe (5 mm dia.) at a cross-head speed of 30 mm/min. The textural parameters of hardness, cohesiveness, springiness, gumminess, and chewiness were computed using a textural profile analysis. The average of 12 repeated measurements was reported.
Syneresis
Starch suspension (5%, w/w) was heated at 90°C for 30 min in a temperature controlled water bath, followed by rapid cooling in an ice water bath to room temperature. The starch sample was stored for 24, 72, and 120 h at 4°C. Syneresis was measured as amount of water (%) released after centrifugation at 3200 × g for 15 min.
Statistical Analysis
The data reported in Tables and are an average of triplicate observations. The data were subjected to statistical analysis using Minitab 14 Statistical Software (Minitab Inc., USA).
Table 1 Acetyl (%) and DS of acetylated starchesa
Table 2 Physicochemical properties of native and acetylated starchesFootnote a
Table 3 Gelatinization properties of native and acetylated starchesa
Table 4 Pasting properties of native and acetylated starchesFootnote a
RESULTS AND DISCUSSION
Acetyl and DS
The DS indicates the amount of substituent introduced in the chemically modified starch during the reaction. DS and percentage acetylation (acetyl, %) increased from 0.050 to 0.081 and 1.31 to 2.10%, respectively, with the increase in the level of addition of acetic anhydride from 1.25 to 6.25 g/100 g (). The increase in DS with acetic anhydride followed a linear increase with regression coefficient value of 0.968. Saartrat et al.Citation[24] also observed a similar trend with increase in acetic anhydride level for canna starches. The acetyl (%) observed was less than the maximum permissible level of 2.5% permitted for use in food formulation.Citation[25] Sodhi and SinghCitation[26] reported acetyl content between 2.26 to 3.68% for acetylated rice starches prepared using 6% of acetic anhydride under reaction conditions similar to the present study. Acetyl content between 4.68 to 5.97% and 3.43 to 4.68%, respectively, for acetylated potato and corn starches, using 2 to 12% w/w acetic anhydride has been reported earlier.Citation[4] Acetyl content of acetylated starches from other sources (potato, waxy corn, corn, wheat, field pea, and lentil) has been reported between 1.01 to 2.80% using 5 and 10% of acetic anhydride.Citation[27] These differences in acetyl content may be due to the use of varied concentrations of reactants and the difference in starch structure from their botanical sources. The introduction of acetyl moiety during acetylation of starches was also substantiated using the FT-IR spectral analysis. An introduction of acetyl moiety through a band at 1735.8 cm–1 was observed for acetylated starches as compared to the native starch ().
Physicochemical Properties
Amylose content of native sorghum starch was 18.7%, which increased to 19.5% upon acetylation with a maximum amount of acetic anhydride (6.25% w/w) (). These observations are consistent with previous reports wherein an increase in amylose content upon acetylation of starches from Canavalis ensiformis,Citation[28] corn, and potatoCitation[4] has been reported. Contrarily, a decrease in amylose content upon acetylation of riceCitation[6] and jackbeanCitation[29] starches has also been reported. These different patterns may be attributed to the interference of acetyl group with the functioning of amylose and amylopectin fractions of starch affecting absorption of iodine during determination of amylose.Citation[18,Citation28] Swelling power and solubility provide evidence of the magnitude of the interaction between starch chains within both the amorphous and crystalline domains. The extent of interaction has been reported to be influenced by the amylose to amylopectin ratio, as well as amylose and amylopectin characteristics in terms of molecular weight distribution, degree of branching, length of branches, and conformation of molecules.Citation[30] Swelling power and solubility of native and acetylated starches ranged between 6.2 to 16.9 g/g and 11.9 to 14.7%, respectively. Acetylation significantly increased swelling power of starch, whereas solubility of starches acetylated with 3.75 g and higher amount of acetic anhydride were significantly higher than native starch. The changes in swelling power and solubility upon acetylation may be attributed to the introduction of hydrophilic substituting groups that retained water molecules to form hydrogen bonds in the starch granules.Citation[28] Moreover, the access of water molecules to the amorphous regions could have also been increased by the introduction of acetyl groups. A similar increase in swelling power and solubility upon acetylation has been reported in earlier investigations carried out on corn,Citation[31] corn and potato,[4,32] rice,[6,26] potato,Citation[33] wheat,Citation[34] and sword bean[35] starches.
Morphological Properties
Scanning electron micrographs of native starch showed presence of irregular, elongated, and spherical shaped granules (). Upon acetylation, a change in external morphology leading to surface roughness, formation of cavities, and fusion of few starch granules was observed. The size of granules was also observed to slightly decrease with the acetylation treatment. These changes may be attributed to the gelatinization of the surface of starch granules with NaOH during the acetylation treatment. Surface roughness caused by acetylation of starches of corn, potato,Citation[32] and rice starchesCitation[6] has been reported earlier. However, in some studies no significant effect of acetylation on external morphology except aggregation of few granules has been observed.Citation[4,Citation26] The aggregation of granules observed in the present study may be attributed to the introduction of hydrophilic groups to the starch molecules.Citation[4]
X-Ray Diffraction
X-ray diffractograms of native starch showed a typical A-type pattern (). Acetylation of starch with acetic anhydride up to 3.75 g/100 g of starch level did not show any significant change in the diffraction patterns. Similar observations have been reported earlier for acetylated corn,Citation[36] new cocoyam,Citation[37] and waxy maize starches.Citation[38] A peak observed around 22.5° was broadened upon acetylation with 5 g/100 g acetic anhydride, which changed to dual peak upon acetylation with 6.25 g/100 g. It seems that the introduction of acetyl group up to 1.59% did not bring any change in structure of crystals. Contrarily, a change from B to C pattern upon acetylation of sword bean starch has been reported earlier.Citation[35]
Thermal Properties
DSC results of native and acetylated starches are shown in . To, Tp, Tc , and ΔHgel , which represent the amount of energy involved in gelatinization, were observed to decrease upon acetylation treatment. Native starch showed To, Tp , and Tc of 68.4, 72.1, and 76.16°C, respectively. To, Tp , and Tc of acetylated starches ranged between 63.3 to 67.9, 67.6 to 71.25, and 72.3 to 75.51°C, respectively. ΔHgel of native starch was 11.47 J/g, which decreased to 11.32, 9.45, and 9.35 J/g, respectively, upon the addition of 1.25, 3.75, and 6.25 g of acetic anhydride per 100 g of starch, respectively. The decrease in To, Tp, Tc, and ΔHgel upon acetylation of starch from rice,Citation[26,Citation39] hybrid maize,Citation[40] corn and potato,Citation[4,Citation32] jack bean,Citation[29] and peaCitation[41] starches has already been reported. LawalCitation[40] attributed these changes to the introduction of bulky groups in the backbone of the biopolymer, which enhances structural flexibility. Moreover, these acetyl groups may have also caused destabilization of granular structure, resulting in a decrease in gelatinization temperature.Citation[34]
Pasting Properties
The pasting properties are dependent on the rigidity of starch granules, which in turn effect the granule swelling potentialsCitation[42] and amount of amylose leaching out in the solution.Citation[43] PV, HPV, and CPV progressively decreased with increase in the concentration of acetic anhydride used (). A decrease in PV upon acetylation has been reported earlier for jack bean,Citation[29] cassava,Citation[44] new cocoyam,Citation[37] hybrid maize,Citation[40] and canna starches.Citation[24] The decrease in PV may be attributed to a change in hydrophilicity of the starch, resulting due to disruption of inter and intra molecular hydrogen bonds by introduction of acetyl groups. However, an increase in PV upon acetylation has also been reported for wheat, potato and maize,Citation[45] rice,Citation[26] pea and waxy maize,Citation[46] normal maize,Citation[31] and waxy corn hybrid starches.Citation[47] The results indicated that the change in PV with acetylation mainly varied with the source of starch.Citation[24] HPV, which is reported to be influenced by the rate of amylose exudation, amylose-lipid complex formation, granule swelling, and competition between exudated amylose and remaining granules for free water,Citation[48] also decreased upon acetylation. Acetylated starches also showed a significant reduction in BD, which is a measure of fragility of starch.Citation[49] The same may be attributed to the degradation caused to the acetylated starch during the modification process.Citation[29] SB was also expected to decrease following acetylation because of prevention of close parallel alignment of amylose chains due to acetyl groups. However, such an effect was not observed during the cooling period. Hoover and SosulskiCitation[50] suggested that the increased amylose exudation and the shearing action of the blades might have nullified any steric effects of acetyl groups in lowering SB. Acetylated starches prepared using ≥3.75 g of acetic anhydride showed significantly lower PT as compared to native starch, indicating their usefulness in reducing the energy demands during processing.Citation[28]
Instrumental Textural Properties
The textural parameters of native and acetylated starch gels differed significantly (). Native starch gels showed hardness, gumminess, and chewiness of 49.3 g, 14.84 g, and 13.95 g, respectively. The gels of acetylated starches showed significantly lower hardness, gumminess, and chewiness as compared to native starch gel. These changes caused by acetylation were observed to depend upon the DS and acetyl content of the starch. Acetylated starch with DS and acetyl content of 0.05 and 1.31%, respectively, showed hardness, gumminess, and chewiness of 37.7 g, 14.36 g, and 13.21 g against 12.8 g, 6.96 g, and 6.62 g for acetylated starch with DS of 0.081 and acetyl content of 2.1%. Gel strength has been reported to mainly depend upon the extent of amylose gel network and deformability of swollen granules.Citation[51] Therefore, a decrease in gel strength is caused by the inhibition of amylose gel network formation because of the introduction of acetyl groups into starch molecules. A similar effect of acetylation on gel strength of corn,Citation[31] rice,Citation[26] and canna starchesCitation[24] has been reported earlier.
Table 5 Textural properties of gels of native and acetylated starchesa
Retrogradation Properties
The retrogradation tendency of native and acetylated starch gels was measured by determining syneresis (%) during storage up to 120 h at 4°C. The syneresis of gels from native and acetylated starches progressively increased with the increase in storage duration (). Perera and HooverCitation[52] attributed the increase in syneresis during storage to interaction between leached out amylose and amylopectin chains. Acetylation considerably reduced the syneresis (%) and negligible release of water was observed even after 120 h of storage at 4°C at 6.25 g level of acetic anhydride. The significant reduction in syneresis among acetylated starches may be attributed to the introduction of acetyl groups. This improvement in water retention capacity has been attributed to impediment of interchain interaction between the macromolecules by acetyl groups.Citation[24] González and PérezCitation[6] reported that the prevention of alignment and association between starch molecules by acetyl groups has led to reduced retrogradation tendency in acetylated rice starch. Moreover, the syneresis results obtained in the present study more clearly supports the effectiveness of acetylation in reducing the retrogradation than set back data obtained using RVA.
Table 6 Syneresis (%) of native and acetylated starchesa
CONCLUSION
The acetylated sorghum starches prepared using varying levels of acetic anhydride showed significant differences in their functional properties. The results revealed that with the increase in levels of acetylation, swelling power and solubility increased, whereas gelatinization temperatures, enthalpy of gelatinization, paste viscosity, gel strength, and retrogradation reduced significantly. Hence, sorghum starch with desirable functional properties suitable for a particular application in a processed food industry could be prepared by critically selecting the level of acetylation.
ACKNOWLEDGMENTS
The authors wish to thank Dr. N. Seetharama, Director, National Research Centre for Sorghum (NRCS), Hyderabad for providing the sorghum samples for this research work. The research facilities provided by Regional Sophisticated Instrumentation Centre (RSIC), Panjab University, Chandigarh for Scanning Electron Microscopy; and Indian Institute of Technology (IIT), Delhi for X-ray diffraction are also acknowledged.
REFERENCES
- Betancur , A.D. and Chel , G.L. 1997 . Acid hydrolysis and characterization of Canavalia ensiformis starch . Journal of Agricultural and Food Chemistry , 45 ( 11 ) : 4237 – 4241 .
- Jarowenko , W. 1986 . “ Acetylated starch and miscellaneous organic esters ” . In Modified Starches: Properties and Uses; Wurzburg, B.O , 55 – 77 . Boca Raton , FL : CRC Press .
- Wurzburg , O.B. 1978 . “ Starch, modified starch and dextrin ” . In Products of the Corn Refining Industry; Seminar proceedings , 22 – 32 . Washington , DC : Corn Refiners Association, Inc .
- Singh , N. , Chawla , D. and Singh , J. 2004 . Influence of acetic anhydride on physicochemical, morphological and thermal properties of corn and potato starch . Food Chemistry , 86 ( 4 ) : 601 – 608 .
- Agboola , S.O. , Akingbala , J.O. and Oguntimein , G.B. 1991 . Production of low substituted cassava starch acetates and citrates . Starch , 43 ( 1 ) : 13 – 15 .
- González , Z. and Pérez , E. 2002 . Effect of acetylation on some properties of rice starch. Starch . 54 ( 3–4 ) : 148 – 154 .
- Wurzburg , O.B. 1995 . “ Modified starches ” . In Food Polysaccharides and Their Applications; Stephen, A.M , 83 – 85 . New York : Marcel Dekker .
- Sanford , P.A. and Baird , J. 1983 . “ Industrial utilization of polysaccharides ” . In The Polysaccharides , Edited by: Aspinall , G.O. Vol. 2 , 411 – 490 . New York : Academic Press,Inc .
- Watson , S.A. 1970 . “ Wet milling process and products ” . In Sorghum Production and Utilization , Edited by: Wall , J.S. and Ross , W.M. 602 – 626 . Westort , CT : Avi .
- FAOSTAT. Production; ProdSTAT; Crops http://faostat.fao.org/site/567/default.asp#ancor (http://faostat.fao.org/site/567/default.asp#ancor)
- AO Sorghum and Millets in Human Nutrition . 1995 . FAO Food and Nutrition Series No. 27, ISBN 92-5-103381-1
- Palmer , G.H. 1992 . Sorghum-food, beverage and brewing potentials . Process Biochemistry , 27 ( 3 ) : 145 – 153 .
- Taylor , J.R.N. 2004 . “ Grain production and consumption: Africa ” . In Encyclopedia of Grain Science , Edited by: Wrigley , C. , Corke , H. and Walker , C.E. 70 – 78 . London : Elsevier .
- Singh , H. , Sodhi , N.S. and Singh , N. 2009 . Structure and functional properties of acid thinned sorghum starch . International Journal of Food Properties , 12 ( 4 ) : 713 – 725 .
- Singh , H. , Sodhi , N.S. and Singh , N. Characterisation of starches separated from sorghum cultivars grown in India. Food Chemistry . 2010 , 119 ( 1 ) 95 – 100 .
- Beta , T. , Corke , H. , Rooney , L.W. and Taylor , J.R.N. 2001 . Starch properties as affected by sorghum grain chemistry . Journal of the Science of Food and Agriculture , 81 ( 2 ) : 245 – 251 .
- Phillips , D.L. , Huijum , L. , Duohai , P. and Corke , H. 1999 . General application of Raman spectroscopy for the determination of level of acetylation in modified starches . Cereal Chemistry , 76 ( 3 ) : 439 – 443 .
- Whistler , R.L. and Daniel , J.R. 1995 . “ Carbohydrates ” . In Food Chemistry , Edited by: Fennema , O.R. 69 – 137 . New York : Marcel Dekker .
- Williams , P.C. , Kuzina , F.D. and Hlynka , I. 1970 . A rapid colorimetric procedure for estimating the amylose content of starches and flours . Cereal Chemistry , 47 ( 4 ) : 411 – 420 .
- Leach , H.W. , McCowen , L.D. and Schoch , T.J. 1959 . Structure of the starch granule. I. Swelling and solubility patterns of various starches . Cereal Chemistry , 36 ( 6 ) : 534 – 544 .
- Ikawa , Y. , Glover , D.V. , Sugimoto , Y. and Fuwa , H. 1981 . Some structural characteristics of starches of maize having a specific genetic background . Starch/Stärke , 33 ( 1 ) : 9 – 13 .
- Scientific , Newport . 1998 . “ Applications Manual for the Rapid ViscoTM Analyser ” . In Newport Scientific , Australia : Warriewood .
- Bhattacharya , M. , Zee , S.Y. and Corke , H. 1999 . Physicochemical properties related to quality of rice noodles . Cereal Chemistry , 76 ( 6 ) : 861 – 867 .
- Saartrat , S. , Puttablek , C. , Rungsarthong , V. and Uttapap , D. 2005 . Paste and gel properties of low-substituted acetylated canna starches . Carbohydrate Polymers , 61 ( 2 ) : 211 – 221 .
- Code of Federal Regulations . 1994 . “ Food additives permitted in food for human consumption ” . In US Government Printing Office Washington, DC , , USA Title 21, Chapter 1. Part 172: Section 172.892, Food Starch
- Sodhi , N.S. and Singh , N. 2005 . Characteristics of acetylated starches prepared using starches separated from different rice cultivars . Journal of Food Engineering , 70 ( 1 ) : 117 – 127 .
- Vasanthan , T. , Sosulski , F.W. and Hoover , R. 1995 . The reactivity of native and autoclaved starches from different origins towards acetylation and cationization. Starch/Stärke . 47 ( 4 ) : 135 – 143 .
- Betancur , A.D. , Chel , G.L. and Harnandez , C.E. 1997 . Acetylation and characterization of Canavalia ensiformis starch . Journal of Agricultural and Food Chemistry , 45 ( 2 ) : 378 – 382 .
- Lawal , O.S. and Adebowale , K.O. 2005 . Physicochemical characteristics and thermal properties of chemically modified jack bean (Canavalia ensiformis) starch . Carbohydrate Polymers , 60 ( 3 ) : 331 – 341 .
- Ratnayake , W.S. , Hoover , R. and Warkentin , T. 2002 . Pea starch: Composition, structure and properties—A review . Starch , 54 ( 6 ) : 217 – 234 .
- Liu , H. , Ramsden , L. and Corke , H. 1997 . Physical properties and enzymatic digestibility of acetylated ae, wx, and normal maize starch . Carbohydrate Polymers , 34 ( 4 ) : 283 – 289 .
- Singh , J. , Kaur , L. and Singh , N. 2004 . Effect of acetylation on some properties of corn and potato starches . Starch/Stärke , 56 ( 12 ) : 586 – 601 .
- Kim , J.T. and Noh , W.S. 1992 . The retrogradation and swelling power of modified potato starches . Journal of Korean Agriculture Chemical Society , 35 ( 5 ) : 404 – 409 .
- Wootton , M. and Bamunuarachchi , A. 1979 . Application of differential scanning calorimetry to starch gelatinization. I . Commercial native starch and modified starches. Starch/Stärke , 31 ( 6 ) : 201 – 204 .
- Adebowale , K.O. , Afolabi , T.A. and Olu-Owolabi , B.I. 2006 . Functional, physicochemical and retrogradation properties of sword bean (Canavalia gladiate) acetylated and oxidized starches . Carbohydrate Polymers , 65 ( 1 ) : 93 – 101 .
- Choi , H.S. , Kim , H.S. , Park , C.S. , Kim , B.Y. and Baik , M.Y. 2009 . Ultra high pressure (UHP)-assisted acetylation of corn starch . Carbohydrate Polymers , 78 ( 4 ) : 862 – 868 .
- Lawal , O.S. 2004 . Composition, physicochemical properties and retrogradation characteristics of native, oxidized, acetylated and acid-thinned new cocoyam (Xanthosoma sagittifolium) starch . Food Chemistry , 87 ( 2 ) : 205 – 218 .
- Wang , Y.J. and Wang , L. 2002 . Characterization of acetylated waxy maize starches prepared under catalysis by different alkali and alkaline-earth hydroxides . Starch/Stärke , 54 ( 1 ) : 25 – 30 .
- Singh , N. , Sodhi , N.S. , Bhambri , V. and Singh , H. 2006 . Effect of acetic anhydride on physicochemical, thermal, retrogradation and pasting properties of rice starches differing in crystallinity . Journal of Food Science and Technology , 43 ( 6 ) : 594 – 598 .
- Lawal , O.S. 2004 . Succinyl and acetyl starch derivatives of a hybrid maize: Physicochemical characteristics and retrogradation properties monitored by differential scanning calorimetry . Carbohydrate Research , 339 ( 16 ) : 2673 – 2682 .
- Huang , J. , Schols , H.A. , Jin , Z. , Sulmann , E. and Voragen , A.G.J. 2007 . Pasting properties and (chemical) fine structure of acetylated yellow pea starch is affected by acetylation reagent type and granule size . Carbohydrate Polymers , 68 ( 3 ) : 397 – 406 .
- Sandhya Rani , M.R. and Bhattacharya , K.R. 1989 . Rheology of rice-flour pastes: Effect of variety, concentration, and temperature and time of working . Journal of Textural Studies , 20 ( 2 ) : 127 – 137 .
- Morris , V.J. 1990 . Starch gelation and retrogradation . Trends in Food Science and Technology , 1 : 2 – 6 .
- Aiyeleye , F.B. , Akingbala , J.O. and Oguntimein , G.B. 1993 . Chemical factors affecting acetylation of cassava starch. Starch/Stärke . 45 ( 12 ) : 443 – 445 .
- Gunaratne , A. 2007 . Corke, H. Influence of prior acid treatment on acetylation of wheat, potato and maize starches . Food Chemistry , 105 ( 3 ) : 917 – 925 .
- Biliaderis , C.G. 1982 . Physical characteristics, enzymatic digestibility and structure of chemically modified smooth pea and waxy maize starches . Journal of Agricultural and Food Chemistry , 30 ( 5 ) : 925 – 930 .
- Wilkins , M.R. , Wang , P. , Xu , L. , Niu , Y. , Tumbleson , M.E. and Rausch , K.D. 2003 . Variability in starch acetylation efficiency from commercial waxy corn hybrids . Cereal Chemistry , 80 ( 1 ) : 68 – 71 .
- Olkku , J. and Rha , C. 1978 . Gelatinization of starch and wheat flour starch—A review . Food Chemistry , 3 ( 4 ) : 293 – 317 .
- Thayumanavan , B. and Kumari , S.K. 1998 . Characterization of starches of roso, foxtail, barnyard, kodo and little millets . Plant Foods for Human Nutrition , 53 ( 1 ) : 47 – 56 .
- Hoover , R. and Sosulski , F. 1985 . A comparative study of the effect of acetylation on starches of Phaseolus vulgaris biotypes . Starch/Stärke , 37 ( 12 ) : 397 – 404 .
- Luyten , H. , Van Vliet , T. and Walstran , P. 1992 . Comparison of various methods to evaluate fracture phenomena in food materials . Journal of Texture Studies , 23 ( 3 ) : 245 – 266 .
- Perera , C. and Hoover , R. 1999 . Influence of hydroxypropylation on retrogradation properties of native, defatted and heat moisture treated potato starches . Food Chemistry , 64 ( 3 ) : 361 – 375 .