Abstract
The antioxidant activities and total phenolic contents of different solvent extracts produced from pickled and dried mustard (Brassica juncea Coss. var. foliosa Bailey) were investigated. For three solvent extracts, the total phenolic content ranged from 18.5 to 23.29 mg of gallic acid equivalent/g of extract with the following order: 70% ethanol extract >70% methanol extract > water extract. All the solvent extracts exhibited good antioxidant activity. Among the various extracts examined, the 70% ethanol extract showed the highest 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity, while the 70% methanol extract showed the highest Fe2+-chelating effect and reducing activity.
INTRODUCTION
Lipid oxidation is one of the most common reasons for deterioration of food products during processing and storage.[Citation1] This reaction could lead to deterioration in taste, flavor, odor, color, texture, and appearance, and a decrease in the nutritional value of the foods.[Citation2] Furthermore, the reaction can induce food poisoning when the fats and oils are severely oxidized.[Citation3] Therefore, from a food quality and food safety perspective, this reaction must be suppressed. In industrial processing, usually synthetic antioxidants, such as butylated hydroxyanisole (BHA) or butylated hydroxytoluene (BHT), are used to decelerate this process and prolong the storage stability of foods. However, these antioxidants are known to have toxic and carcinogenic effects on humans.[Citation4,Citation5] Therefore, consumers generally prefer natural antioxidants over synthetic ones and the interest in natural antioxidants, especially of plant origin, has greatly increased in recent years.[Citation6–8
Plants are good sources of natural antioxidants, which were reported not only to control lipid oxidation in food systems but also to prevent free radical-induced diseases, such as cancer, inflammation, atherosclerosis, etc.[Citation9] It has been demonstrated that the antioxidant properties of plant extracts are mainly attributed to phenolic compounds, such as flavonoids, phenolic acids, tannins, and phenolic diterpenes.[Citation10]
Brassica juncea Coss. var. foliosa Bailey is one of the main crops cultivated in subtropical regions of China. It is not only rich in vitamins, minerals, and dietary fibers, but it also contains a high amount of flavonoids.[Citation11] Jin found that the flavonoids in leaf mustard could prevent or treat diabetes.[Citation12] Wang and Zhu reported that leaf mustard possessed antioxidant activity.[Citation13] Fang et al. demonstrated pickled potherb mustard was a good source of antioxidants.[Citation14] Wang and Zhu also compared the antioxidant activity of fresh and pickled leaf mustards.[Citation15] However, little information is available concerning the antioxidant effect of pickled and dried mustard.
Pickled and dried mustard (PDM, also known as Meigancai in China) is an indigenous Chinese fermented vegetable product and is very popular in south China. It can be stored at room temperature for up to 2 years. Traditionally, PDM is homemade in the beginning of every winter or in the beginning of every spring. After harvesting, the mustards are trimmed, washed, and wilted in the sun, then cut into pieces and mixed with a small amount of pure, granulated, non-iodized pickling salt. After being kneaded with salt to form juice, the vegetable was packed into earthenware pots in layers, and then was pressed tightly by a wooden ladle. The pots are sealed and the mustard is allowed to ferment for a couple of weeks. At the end of pickling, the mustard was taken out and dried in the sun till its stalk had been sundried.
The pickled and dried mustards are usually cooked with meat or other dishes; they are extensively consumed by all social groups in China, especially in the Yangtze River Delta Megalopolis. Steamed pork with PDM is a traditional dish in south China, which has a delicious taste and flavor and can enhance people's appetite. It was reported that steamed pork with PDM could be preserved for over half a year at room temperature,[Citation16] much longer than steamed pork without PDM did. Potential antioxidant activity of the PDM was presumed for partial contribution to these properties.
In the present study, various solvent (70% ethanol, 70% methanol, and water) extracts of pickled and dried mustard were used to evaluate their antioxidant capacities [iron chelation assay, reducing power, and scavenging ability on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals]. The total phenolic content in these extracts was also determined.
MATERIALS AND METHODS
Materials
Pickled and dried mustards were purchased from a rural market in Guangchang County, Jiangxi Province, China. They were crushed in a FW100 Universal high-speed smashing machine (Tianjin Taisite Instrument Co., LTD., Tianjin, China) for 2 min, but at 30-s intervals the process was stopped for 10 s to avoid heating the sample. The ground powder was passed through a 1.0-mm sieve and kept in a desiccator at room temperature until further use (ca.1 d).
Chemicals and Reagents
1,1-Diphenyl-2-picrylhydrazyl (DPPH) was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Folin-Ciocalteu's phenol reagent and 3-(2-pyridyl)-5,6-bis(4-phenyl-sulfonic acid)-1,2,4-triazine (Ferrozine) were purchased from Hefei Bomei Biotechnology Co., Ltd. (Hefei, China). Butylated hydroxytoluene (BHT), ascorbic acid (Vc), potassium ferricyanide, ferric chloride, and sodium carbonate were purchased from Sinopharm Group Chemical Reagent Co., Ltd. (Shanghai, China). Trichloroacetic acid (TCA) was obtained from Xilong Chemical Factory (Shantou, China). Ferrous chloride was purchased from Taishan Chemical Factory Co., Ltd. (Taishan, China). Gallic acid and sodium ethylenediamine tetra acetic acid (Na2EDTA) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). All other reagents used were of analytical grade.
Preparation of Solvent Extracts
The various solvent extracts of pickled and dried mustard were prepared by a method similar to that reported previously by Liu and Yao.[Citation17] Briefly, 10 g of the ground powder of the samples were mixed with selected solvents: 70% ethanol (v/v), 70% methanol (v/v), and distilled water (volume of solvent per g of raw material was 15 ml), respectively. Then the mixture was sealed and left to stand in a water bath for 4 h at 45°C. At the end of extraction, the mixture was filtered by vacuum filtration. The residue was re-extracted as the first extraction. The obtained extraction solutions were combined and evaporated under reduced pressure to give a viscous dark mass. This crude extract was dissolved in water or solvent and used for assessment of antioxidant activity.
Determination of Total Phenolic (TP) Content
TP content in the extracts was determined according to the method of Majhenič, Škerget, and Knez[Citation18] with slight modification. Briefly, the extract was diluted in distilled water to yield 1 mg/ml of test solution. To 1 ml of test solution 2.5 ml of Folin-Ciocalteu reagent (diluted 10 times with distilled water) and 2 ml of Na2CO3 (75 g/l) were added. The mixture was incubated at 50°C for 5 min and then cooled. For a control sample, 1 ml of distilled water was used. The absorbance was measured at 760 nm, by a UV spectrophotometer (Unico UV-4802S UV/VIS double beam spectrophotometer). The results were expressed as the equivalent to mg of gallic acid per g of extract (mg GAE/g extract).
Reducing Power
The reducing power was tested according to the method of Oyaizu.[Citation19] To 1.0 ml extracts with different concentrations (0.2–10 mg/ml), 2.5 ml of sodium phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of potassium ferricyanide (1%, w/v) were added. Afterwards, the mixture was incubated at 50°C for 20 min and then 2.5 ml of trichloroacetic acid (TCA, 10%, w/v) were added to terminate the reaction, followed by centrifugation at 1380× g for 10 min. Aliquots of 2.5 ml of the supernatant were mixed with 2.5 ml of distilled water and 0.5 ml of ferric chloride (0.1%, w/v). The obtained mixture was incubated at room temperature for 30 min and the absorbance was measured at 700 nm against blanks that contained all reagents except the sample extracts. Analyses were run in triplicate. Increased absorbance of the reaction mixture indicated increased reducing power. The EC50 value is the concentration of sample at which the absorbance is 0.5, which was calculated from the graph of absorbance at 700 nm against extract concentration. Ascorbic acid and BHT were used as positive references.
Free Radical Scavenging Activity on 1,1-Diphenyl-2-Picrylhydrazyl
The free radical-scavenging activity of the extract was measured by using the stable radical DPPH according to the method of Liu and Yao[Citation17] with minor modifications. Briefly, 2.0 ml of DPPH radical in ethanol 0.2 mM solution was added to 2.0 ml of extract solution in water at different concentrations (0.2–5 mg/ml). The reaction mixture was shaken and incubated in the dark. Thirty minutes later, the absorbance was measured at 517 nm. Ascorbic acid and BHT were used as the positive references. Lower absorbance of the reaction mixture indicated higher free radical scavenging activity. Radical-scavenging activity was expressed as the inhibition percentage of free radical by the sample and was calculated using the following equation: DPPH radical scavenging activity (%) = [Abscontrol – (Abssample – Abssample background)]/Abscontrol × 100. All the tests were performed in triplicate. The percentage of DPPH radical-scavenging activity was plotted against the extract concentration (mg/ml) to determine the amount of extract necessary to decrease DPPH radical concentration by 50% (called EC50). The EC50 value of each extract was estimated by sigmoid non-linear regression using OriginPro 7.0 software (OriginLab Corp., Northampton, MA, USA).
Iron (II) Chelation Activity
The Fe2+-chelating effect of various extracts was estimated by the method of Dinis, Madeira, and Almeida[Citation20] with slight modifications. Briefly, 0.2 ml of different concentrations of the extracts (0.2–20 mg/ml) and 0.74 ml methanol were added into 0.02 ml of 2 mM FeCl2. The reaction was initiated by the addition of 0.04 ml of 5 mM ferrozine into the mixture, which was then shaken vigorously and left standing at ambient temperature (ca. 25°C) for 10 min. The absorbance of the reaction mixture was measured at 562 nm. Three replicates were made for each test sample. The chelating activity (%) was calculated as the following equation: chelating activity (%) = [Abscontrol – (Abssample – Abssample background)]/Abscontrol × 100. EC50 value is the effective concentration that could chelate 50% of iron(II). Na2EDTA was used as positive control.
Statistical Analysis
Experimental results were expressed as means ± standard deviation (SD) of three parallel measurements. P values < 0.05 were regarded as significant. The statistical analysis was done by the Statistical Package for Social Science (SPSS 11.5, SPSS Inc., Chicago, IL, USA).
RESULTS AND DISCUSSION
Extraction Yield and Total Phenolic Content
Ethanol and water separately or mixed are commonly used solvents for extraction of antioxidants from plants, because they have many advantages, such as nontoxicity and cheapness, over other solvents. Methanol is also a widely used and effective solvent for the extraction of antioxidants, and its residue extracts often exhibited the highest activity and most stable antioxidative activity.[Citation21,Citation22] In this study, distilled water, aqueous methanol, and ethanol separately were employed as the solvent to extract the antioxidants in pickled and dried mustard. The percentage yields of these solvent extracts are presented in . The extraction yield varied from 38.69 to 48.94% (w/w) depending on the extraction solvent with the following order: water > 70% ethanol > 70% methanol. No significant (p < 0.05) difference was found between the yield of 70% methanol extract and 70% ethanol extract. The result agreed with the yield from raisin,[Citation23] lactic-fermented Chinese cabbage,[Citation7] corn tassel, and Rhizoma Smilacis Glabrae,[Citation22] which showed the same order of solvent.
Table 1 Yield and total phenolic content in extracts from pickled and dried mustard
Many studies showed that phenolic compounds contribute to overall antioxidant activities of natural plant extracts. Therefore, it is quite important to evaluate the total phenolic content in tested extracts. In this work, the total phenolic content of various solvent extracts from PDM was evaluated by the Folin-Ciocalteu method. The results were expressed as mg equivalents of gallic acid/g extract and were also given in . The total phenolic content ranged from 18.5 to 23.29 mg of gallic acid equivalent/g extract, depending on the extraction solvent with the following order: 70% ethanol > 70% methanol > water. The amount of total phenolic content of 70% methanol extract in the present study was similar to the amount reported by findings of Fang et al.[Citation14] A similar order of solvent was also found in total phenolic content of extracts from corn tassel[Citation6] and rosemary.[Citation24] No correlation was found between the extraction yield and the content of total phenolics. For example, although water extract had a lower content of total phenolic, it gave a higher yield than the 70% ethanol extract.
Free Radical Scavenging Activity
Due to its simplicity and accuracy, the DPPH assay has become a widely and commonly used method in natural antioxidant studies during recent years.[Citation25] It was reported that almost 90% of recent antioxidant studies applied the DPPH assay combined with other assays. This assay is based on the theory that a hydrogen donor is an antioxidant.[Citation25] The antioxidant effect is proportional to the disappearance of DPPH radical in test samples. Lower absorbance of the reaction mixture indicated higher free radical scavenging activity.
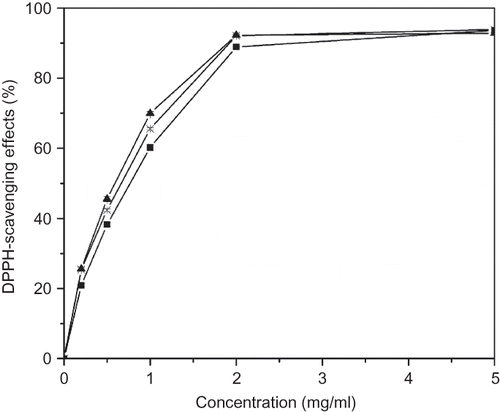
shows the dose-response curve for various solvent extracts of pickled and dried mustard. It can be seen that all the extracts were capable of scavenging DPPH radicals in a concentration-dependent manner; the DPPH radical scavenging effect increased as the concentration of the solvent extract increased to a certain extent, and then leveled off even with further increases in the concentration. The scavenging effect of different solvent extracts from pickled and dried mustard on DPPH radical decreased in this order: 70% ethanol extract > water extract > 70% methanol extract (). At the concentration of 2 mg/ml, the scavenging activities of 70% ethanol extract, water extract, and 70% methanol extract were 92.22, 92.08, and 88.91%, respectively. These results indicated that all the extracts examined had a noticeable effect on scavenging free radical. However, none of the extracts showed a higher scavenging effect than ascorbic acid and BHT did (data not shown). Significant (p < 0.05) difference was observed among the scavenging activity of various solvent extracts at the same amount, indicating that extraction solvent had a significant impact on DPPH radical scavenging activity. shows that the total phenolic content in various solvent extracts decreased in this order: 70% ethanol extract >70% methanol extract >water extract. This order was not coincident with that of the scavenging effect of different solvent extracts on DPPH radical, indicating the antioxidant activity in DPPH assay was not correlated with total phenolic content.
Figure 1 DPPH radical scavenging activity of various solvent extracts from pickled and dried mustard. Symbols: ▪, 70% methanol extract; ▴, 70% ethanol extract; ×, water extract. Values are presented as means ± deviation (n = 3).
EC50 is the efficient concentration of extract required to decrease initial DPPH radical concentration by 50%. A lower EC50 value is associated with a higher DPPH radical scavenging activity. The EC50 values of DPPH radical-quenching activity of various solvent extracts from pickled and dried mustard were presented in . The EC50 value of each extract was estimated by sigmoid non-linear regression using OriginPro 7.0 software (OriginLab Corp., Northampton, MA, USA). It can be seen that 70% ethanol extract (0.55 ± 0.01 mg/ml) had the highest DPPH radical scavenging activity, as shown by the lowest value of EC50, followed by the water extract (0.60 ± 0.01 mg/ml), while 70% methanol extract had the least activity (0.71 ± 0.02 mg/ml). It was evident that the extracts did show the hydrogen donating ability to act as antioxidants. also showed that the EC50 values of scavenging abilities of different solvent extracts on DPPH radicals were far higher than those obtained for ascorbic acid (34.2 ± 1.5 μg/ml) and BHT (20.7 ± 1.1 μg/ml).
Table 2 EC50 values of DPPH radical-quenching activity, reducing power, and Fe2+-chelating activity of various solvent extracts from pickled and dried mustard
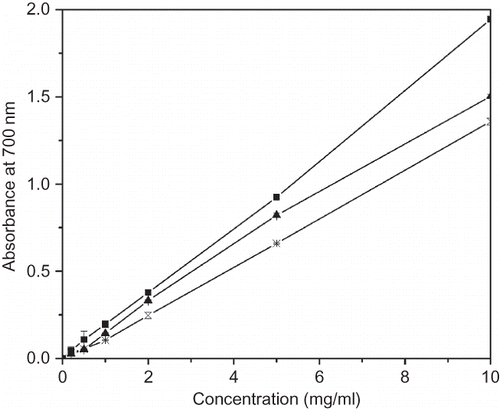
Reducing Power
Numerous studies have shown that the antioxidant activity of certain plant extracts was correlated with their reducing powers.[Citation26] The reducing properties are generally associated with the presence of reductones,[Citation27] which exert antioxidant action by breaking the free radical chains, via hydrogen atom donation.[Citation28] shows the reducing power of different solvent extracts from pickled and dried mustard. An increase in absorbance of the reaction mixture means increased reducing power. As shown in , the reducing power of all the extracts increased with an increasing amount of sample, and all of the amounts showed high activities. Reducing power of various solvent extracts exhibited the following order: 70% methanol extract >70% ethanol extract > water extract. Their reducing power was lower than that of ascorbic acid and BHT (data not shown). shows that the EC50 values of reducing power for 70% methanol, 70% ethanol, and water extracts were 2.60, 3.26, and 3.72 mg/ml, respectively. They were much larger than those obtained for ascorbic acid (91.0 ± 5.2 μg/ml) and BHT (83.0 ± 8.2 μg/ml). Again, the antioxidant activity in reducing power assay was not correlated with total phenolic content. In addition, no correlation was found with the reducing power and DPPH radical scavenging assay.
Iron (II) Chelation
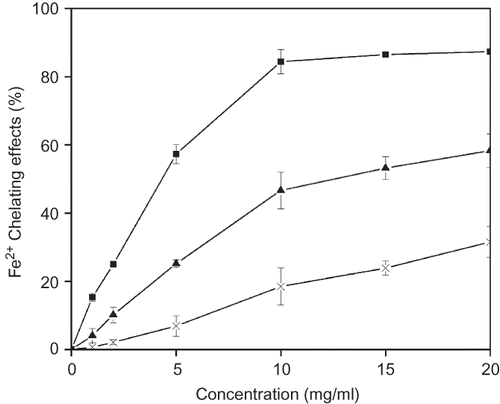
Ferrous ions may be introduced during food processing. These ions were regarded as the most effective pro-oxidants.[Citation29] They can initiate lipid peroxidation, which could induce the deterioration of food quality.[Citation28] These processes can be delayed by iron chelation and deactivation. Therefore, metal chelating activity was claimed as one of the antioxidant activity mechanism.[Citation30] In this work, the ability of the extracts to chelate iron(II) ions was evaluated. The results are presented in It can be observed that all the extracts exhibited an ability to chelate iron(II) ions in a dose-dependent manner. The chelating activity increased with concentration of each sample. Water and 70% ethanol extracts chelated ferrous ions by 18.47 ± 5.45% and 46.63 ± 5.38% at 10 mg/ml, whereas 70% methanol extract showed an excellent chelating ability of 84.45 ± 3.54% at the same concentration. However, none of the extracts appeared to be better chelators of iron(II) ions than the positive control Na2EDTA in this assay system. Na2EDTA showed excellent chelating ability of 93.22 ± 1.42% at a concentration as low as 0.09 mg/ml (data not shown). The sequence for chelating power was 70% methanol extract >70% ethanol extract > water extract. Similar order of solvent was also found in chelating power of extracts from Smilax excelsa L. leaf[Citation31] and mulberry (Morus indica L.) leaves.[Citation21]
Figure 3 Fe2+-chelating ability of various solvent extracts from pickled and dried mustard. Symbols: ▪, 70% methanol extract; ▴, 70% ethanol extract; ×, water extract. Values are presented as means ± deviation (n = 3).
The EC50 values of chelating power for 70% methanol and 70% ethanol extracts were 4.12 and 12.24 mg/ml, respectively (), while the water extract had the highest EC50 value. They all were inferior to Na2EDTA, which had the value of EC50 39.1 μg/ml. The above results have showed that the various solvent extracts from pickled and dried mustard exhibited strong antioxidant activity. It was reported that phenolic acids, such as gallic acid, protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, caffeic acid, p-coumaric acid, ferulic acid, and sinapic acid were identified in fresh potherb mustard.[Citation14] Quercetin and kaempferol were also found as the main flavonols in potherb mustard.[Citation13] These phenolic compounds possess antioxidant activity. During pickling, most of these compounds are still retained. On the other hand, besides phenolics, non-phenolic compounds, such as organic acids, proteins, pigments, sugars,[Citation32] and salts, may be present in the solvent extract. Phenolics in plants are usually found in conjugated forms through hydroxyl groups with sugar as glycosides.[Citation33] Due to the non-negligible activity of the associated phenolic compounds, soluble dietary fibers have also been reported to show potential antioxidant activity.[Citation34] They all may contribute to the antioxidant activity of the extracts from pickled and dried mustard.
Our results also showed that the extracts from pickled and dried mustard had a relatively high total phenolic content. However, no association could be found between total phenolics and antioxidant activities (DPPH radical scavenging effect, reducing power and iron chelation). These results could reasonably induce that various extracts might contain different antioxidants, which exhibited varying reactivity in the three assays used in this work.[Citation35]
CONCLUSIONS
It was demonstrated that various solvent extracts of pickled and dried mustard were capable of directly quenching free radicals, acting as reducing agents, and chelating ferrous ions. The total phenolic content and antioxidant activities of various solvent extracts from pickled and dried mustard varied with the extraction solvents. Among three solvent extracts, 70% ethanol extract demonstrated the highest phenolic content, followed by 70% methanol extract, while water extract had the lowest phenolics content. All the solvent extracts exhibited good antioxidant activity. Amongst the various extracts examined, the 70% ethanol extract showed the highest DPPH radical scavenging activity, whilst the 70% methanol extract showed the highest Fe2+-chelating effects and reducing activity. Variance in the antioxidant activity observed on the different solvent extracts was less correlated to their total phenolics content. Our results indicated that the pickled and dried mustard could be used as a source of natural antioxidant ingredients in the food industry.
ACKNOWLEDGMENTS
This work was supported by The Scientific Research Fund of Hunan Provincial Education Department (Project Number: 08C866), The Scientific Research Fund of Xiangtan University (Project Number: 07QDZ21), and the Aid program for Science and Technology Innovative Researcher Team in Higher Education Institutional of Hunan Province.
REFERENCES
- Huis in't Veld , J.H.J. 1996 . Microbial and biochemical spoilage of foods: An overview . International Journal of Food Microbiology , 33 : 1 – 18 .
- Frankel , E.N. 1988 . Foods. Lipid Oxidation , 187 Dundee : The Oil Press .
- Gotoh , N. , Watanabe , H. , Osato , R. , Inagaki , K. , Iwasawa , A. and Wada , S. 2006 . Novel approach on the risk assessment of oxidized fats and oils for perspectives of food safety and quality. I. Oxidized fats and oils induce neurotoxicity relating pica behavior and hypoactivity . Food and Chemical Toxicology , 44 : 493 – 498 .
- Ito , N. , Hiroze , M. , Fukushima , G. , Tauda , H. , Shira , T. and Tatematsu , M. 1986 . Studies on antioaxidant: Their carcinogenic and modifying effects on chemical carcinogenesis . Food and Chemical Toxicology , 24 : 1071 – 1081 .
- Wichi , H.P. 1988 . Enhanced tumor development by butylated hydroxyanisole (BHA) from the prospective of effect on forestomach and oesophageal squamous epithelium . Food and Chemical Toxicology , 26 : 717 – 723 .
- Mohsen , S.M. and Ammar , A.S.M. 2009 . Total phenolic contents and antioxidant activity of corn tassel extracts . Food Chemistry , 112 : 595 – 598 .
- Sun , Y.P. , Chou , C.C. and Yu , R.C. 2009 . Antioxidant activity of lactic-fermented Chinese cabbage . Food Chemistry , 115 : 912 – 917 .
- Okmen , B. , Sigva , H.O. , Mutlu , S. , Doganlar , S. , Yemenicioglu , A. and Frary , A. 2009 . Total antioxidant activity and total phenolic contents in different Turkish eggplant (Solanum melongena L.) cultivars . International Journal of Food Properties , 12 ( 3 ) : 616 – 624 .
- Shahidi , F. 1997 . “ Natural antioxidants: An overview ” . In Natural Antioxidants: Chemistry, Health Effects and Applications , Edited by: Shahidi , F. 1 – 9 . Champaign , IL : AOCS Press .
- Pietta , P.G. 2000 . Flavonoids as antioxidants . Journal of Natural Products , 63 : 1035 – 1042 .
- Xie , L. and Zhu , Y. 2000 . Study on measurement of total flavonoids in leaf mustard . Instrumentation Analysis and Monitoring (in Chinese) , 3 : 61 – 62 .
- Jin , X. 2003 . Antioxidant activity of leaf mustard . Foreign Medical Sciences (in Chinese) , 25 ( 3 ) : 107
- Wang , P. and Zhu , Z. 2006 . Effect of different harvest seasons on the flavonoids content and antioxidant activities of leaf mustard . Acta Horticulture Sinica (in Chinese) , 33 ( 4 ) : 745 – 750 .
- Fang , Z. , Hu , Y. , Liu , D. , Chen , J. and Ye , X. 2008 . Changes of phenolic acids and antioxidant activities during potherb mustard (Brassica juncea, Coss.) . pickling. Food Chemistry , 108 : 811 – 817 .
- Wang , P. and Zhu , Z. 2006 . Effects of pickling on the contents of antioxidant compounds and antioxidant activities in different cultivars of leaf mustard . Journal of Nuclear Agricultural Sciences (in Chinese) , 20 ( 6 ) : 516 – 520 .
- Xu , Z. 1996 . Steamed pork with pickled and dried vegetables and its storage properties . Science and Technology of Food Industry (in Chinese) , 6 : 64 – 65 .
- Liu , Q. and Yao , H. 2007 . Antioxidant activities of barley seeds extracts . Food Chemistry , 102 : 732 – 737 .
- Majhenič , L. 2007 . Škerget, M . Knez, Ž. Antioxidant and antimicrobial activity of guarana seed extracts. Food Chemistry , 104 : 1258 – 1268 .
- Oyaizu , M. 1986 . Antioxidant activity of browning products of glucosamine fractionated by organic solvent and thin-layer chromatography . Journal of Japanese Society of Food Science and Technology , 35 : 771 – 775 .
- Dinis , T.C.P. , Madeira , V.M.C. and Almeida , L.M. 1994 . Action of phenolic derivatives (acetoaminophen, salicylate and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers . Archives of Biochemistry and Biophysics , 315 : 161 – 169 .
- Arabshahi-Delouee , S. and Urooj , A. 2007 . Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves . Food Chemitry , 102 : 1233 – 1240 .
- Zhang , Q. , Zhang , Z. and Cheung , H. 2009 . Antioxidant activity of Rhizoma Smilacis Glabrae extracts and its key constituent—astilbin . Food Chemistry , 115 : 297 – 303 .
- Zhao , B. and Hall , C. A. III . 2008 . Composition and antioxidant activity of raisin extracts obtained from various solvents . Food Chemistry , 108 : 511 – 518 .
- Pérez , M.B. , Galderón , N.L. and Croci , C.A. 2007 . Radiation-induced enhancement of antioxidant activity in extracts of rosemary (Rosmarinus officinalis L.) . Food Chemistry , 104 : 585 – 592 .
- Moon , J.K. and Shibamoto , T. 2009 . Antioxidant assays for plant and food components . Journal of Agricultural and Food Chemistry , 57 : 1655 – 1666 .
- Meir , S. , Kanner , J. , Akiri , B. and Philosoph-Hadas , S. 1995 . Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves . Journal of Agricultural Food Chemistry , 43 : 1813 – 1819 .
- Duh , P.D. 1998 . Antioxidant activity of Budrock (Arctium lappa Linn): Its scavenging effect on free radical and active oxygen . Journal of the American Oil Chemists’ Society , 75 : 455 – 461 .
- Gordon , M.F. 1990 . “ The mechanism of antioxidant action in vitro ” . In Food Antioxidants , Edited by: Hudson , B.J.F. 1 – 18 . London : Elsevier Applied Science .
- Yamaguchi , T. , Takamura , H. , Matoba , T. and Terao , J. 1998 . HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-dicrylhydrazyl . Bioscience, Biotechnology, and Biochemistry , 62 : 1201 – 1204 .
- Oktay , M. , Gülcin , I. and Küfrevioglu , Ö.I . 2003 . Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts . Lebensmittel-Wissenschaft und-Technologie-Food Science and Technology , 36 : 263 – 271 .
- Ozsoy , N. , Can , A. , Yanardag , R. and Akev , N. 2008 . Antioxidant activity of Smilax excelsa L . leaf extracts. Food Chemistry , 110 : 571 – 583 .
- Sun , T. and Ho , C.T. 2005 . Antioxidant activities of buckwheat extracts . Food Chemistry , 90 : 743 – 749 .
- Robbins , R. 1980 . “ Medical and nutritional aspects of citrus bioflavonoids ” . In Citrus Nutrition and Quality , Edited by: Nagy , S. and Attaway , J. 43 – 59 . Washington : American Chemical Society Press .
- Reyes-Caudillo , E. , Tecante , A. and Valdivia-López , M.A. 2008 . Dietary fibre content and antioxidant activity of phenolic compounds present in Mexican chia (Salvia hispanica L.) seeds . Food Chemistry , 107 : 656 – 663 .
- Nsimba , R.Y. , Kikuzaki , H. and Konishi , Y. 2008 . Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds . Food Chemistry , 106 : 760 – 766 .