Abstract
Six pomegranate (Punica granatum L.) cultivars belonging to the ‘Mollar’ group were evaluated under homogeneus growing conditions. Chemical parameters, such as sugars, organic acids, and anthocyanin contents, and other quality parameters, including fruit weight, pH, total soluble solids, titratable acidity, and colour, were evaluated. Fruit weight was not significantly affected by cultivar. The highest TSS content was detected in juice from MO6 (15.81 °Brix). All cultivars were sweet, and the ripening index ranged from 59.14 (ME5) to 87.95 (MO6). The composition of pomegranate juice was dependent on the cultivar. Glucose was the major sugar in all evaluated cultivars (60–64% of total sugars), followed by fructose, maltose, and sucrose. Among the detected organic acids, citric acid was the predominant (>54%). The total anthocyanin content ranged from 72 to 200 mg per litre of juice, being the main anthocyanin cyanidin 3-glucoside followed by cyanidin 3,5-diglucoside. The principal component analysis and cluster analysis showed high dissimilarity levels in cultivars ME5, MO6, MA4, and MA5.
INTRODUCTION
Pomegranate has been considered a fruit tree species of minor importance, but the increased consumer demand for high nutritional quality foods opens new perspectives for consumption. Some clinical research studies suggest that pomegranate juice may be helpful against heart disease,[Citation1] Alzheimer's disease,[Citation2] and cancer.[Citation3–5] Furthermore, it is able to change the blood parameters, such as LDL, HDL, and cholesterol.[Citation6–9] The fresh juice contains considerable amounts of total soluble solids (TSS), total sugars, reducing sugars, anthocyanins, phenols, ascorbic acid, and proteins,[Citation10] and has also been reported to be a rich source of antioxidants.[Citation11,Citation12]
Pomegranate fruit maturity status is commonly assessed based on external (rind) colour, juice colour, and acidity of juice.[Citation13] Similarly, the acceptability of pomegranate to the consumer and processor depend on a combination of several quality attributes that are related to the physico-chemical and mechanical properties, including rind colour, lack of physical defects, sugar content, acidity, and flavour.[Citation14] Qualitative and quantitative composition of organic acids and soluble sugars have been often regarded as an indicator of fruit quality traits.[Citation15] In addition, these compounds are important to evaluate fruit maturity, ripeness, and storage conditions, and could be used as chemical markers in distinguishing different cultivars in apricot[Citation16] and adulteration in pomegranate juice.[Citation17]
Fruit rind colour is an attribute that determines consumers' behaviour, and it is accepted as one of the most important external quality parameters.[Citation18] One of the most important quality characteristics of pomegranate is the red pigmentation of seeds and juice. This red colour depends on anthocyanin concentration and on the chemical structure of the individual anthocyanin.[Citation19] To investigate colour quality in a systematic way, it is necessary to objectively determine colour, as well as pigment concentration.[Citation20] The present study was undertaken to develop knowledge on the cultivars from the varietal group ‘Mollar de Elche’ ME. Pomegranate cultivars from the ‘Mollar’ group are mid-season high yielders with large size and sweet fruits, deep red rind colour, and soft seeds.[Citation21] To achieve this objective, organic acids, sugars, anthocyanin contents, chromatic parameters (L*, a*, b*, hue angle, and chroma), pH, titratable acidity, and soluble solids content of pomegranate juices were investigated.
MATERIALS AND METHODS
Plant Material
The plant material belonged to the principal pomegranate gene bank of the EU, which is located at the experimental field station of Universitas Miguel Hernández in the province of Alicante, Spain (02°03′50″ E, 38°03′50″ N, and 25 masl). Six different pomegranate cultivars belonging to the ‘Mollar’ group were chosen for this study: ME5 (Mollar de Elche 5), ME16 (Mollar de Elche 16), ME17 (Mollar de Elche 17), MO6 (Mollar de Orihuela 6), MA4 (Mollar de Albatera 4), and MA5 (Mollar de Albatera 5). The selection of these cultivars was based on the results of previous studies[Citation21] and because they are representative of the ‘Mollar’ group. The fruits of these cultivars were characterized as sweet and presented good organoleptic characteristics, being classified as highly recommendable because of their agronomic and commercial interest and the red colour of their seeds. The experiment was established in a randomised block design with four single-tree replications for each cultivar. Five fruits from each tree were harvested over three consecutive years (2006–2008). Guard rows were used to minimize edge effects. During three consecutive seasons, 20 pomegranate fruits were hand-harvested from October 1–15 when fully mature according to commercial practice to ensure their best flavour and colour.
Analytical Measurements
Chemical composition was determined on the juice obtained by squeezing the seeds (arils). Twenty fruits from each cultivar were weighed and the arils were squeezed with a commercial turmix blender (model JU2000, Moulinex, SEB Iberica, Barcelona, Spain).
Fruit weight
Fruit weights were determined with a Sartorius balance (model BL-600, 0.01 g accuracy, Goettingen, Germany).
pH, total soluble solids, and titratable acidity
The chemical analyses were determined using two juice samples (10 fruits each) for each cultivar. pH was measured with a pH meter (model micropH 2001; Barcelona, Spain). TSS content of the juice was measured with a temperature compensated refractometer (model N-1; Atago Co., Tokyo, Japan), and data were given as °Brix (±0.2 °Brix at 20 °C). Titratable acidity (TA) was determined by titration with NaOH 0.1 N to pH 8.1. Data were expressed as g citric acid/100 mL. The ripening index (RI) was calculated as the ratio of TSS/TA.
Individual sugars, organic acids, and anthocyanins by HPLC
Juice samples were centrifuged at 15,000 rpm for 20 min. One millilitre of the extract was filtered through a 0.45 μm Millipore filter and then injected into a Hewlett-Packard HPLC series 1100 (Wilmington, DE, USA). The HPLC analysis and the individual anthocyanin identification and quantification were performed as reported previously.[Citation22] The anthocyanins were quantified using an external standard of cyanidin 3-rutinoside, with a C-18 column (12.5 × 0.4 cm, 5 μm particle size), using as solvents water + 5% formic acid (solvent A) and HPLC grade methanol (solvent B). Elution was achieved with a gradient starting with 15% B in A, to reach 35% B at 15 min, then isocratic until 20 min with a solvent flow rate of 1 ml/min and detection with a diode array detector. Chromatograms were recorded at 520 nm for quantification. Organic acids were analyzed by the same HPLC equipped with a UV/Vis detector for organic acids analysis. A standard curve of pure organic acids (oxalic, tartaric, malic, ascorbic, acetic, citric, and fumaric acids) purchased from Sigma (Poole, Dorset, UK) was used for quantification.
Sugars were analysed on the same HPLC equipment equipped with a refractive index detector. Sugars analysis was performed on a NH2 column (30 cm × 3.9 mm i.d., Waters, Milford, MA, USA) using acetonitrile/water (85:15, v/v) as the mobile phase. A standard curve of pure sugars (glucose, fructose, sucrose, and maltose) purchased from Sigma (Poole, Dorset, UK) was used for quantification. Results for organic acids and sugars were expressed as g 100−1 juice.
Colour
Juice colour determinations were made yearly by triplicate on two juice samples for the three-year study. Values of the CIELAB L* (brightness or lightness; 0 = black, 100 = white), a* (-a* = greenness, +a* = redness), and b* (-b* = blueness, +b* = yellowness) colour variables were measured using the chromatometer Minolta (model CR-300; Ramsey, NJ). Besides a*/b* ratio, the hue angle [H°* = arctang (b*/a*)] and chroma [C* = (a*2 + b*2)½] were calculated. The hue angle and chroma have been accepted as more intuitively understandable colour variables.[Citation23] The colour index (CI) was calculated as previously reported[Citation24] following the equation: (180 – H°*)/(L* + C*).
Statistical analysis
Statistical analyses were performed using the software package SPSS 18.0 for Windows (SPSS Science, Chicago, IL, USA). A basic descriptive statistical analysis was followed by an analysis of variance for mean comparisons. The method used to discriminate among the means (Multiple Range Test) was the Fisher's Least Significant Difference (LSD) procedure at 95.0% confidence level. Correlation analyses between traits to reveal possible relationships were also carried out over the three years. Principal component analysis (PCA) and cluster analysis (CA) were also performed.
RESULTS AND DISCUSSION
Physical and Physicochemical Parameters
Values of some physicochemical parameters affecting pomegranate quality, including pH, TA, TSS, and RI (ripening index), are shown in . Fruit weight was not significantly affected by cultivar, although the trees of ME16 yielded the highest fruit weight (351.48 g). These results completely agreed with those found by Shwartz et al.[Citation24] and Drogoudi et al.[Citation25] On the other hand, Al-Said et al.[Citation14] on ‘wild’ cultivar grown in Oman, and Al-Maiman and Ahmad[Citation26] on ‘Taifi’ cultivars, declared that the trees produced smaller and lighter fruits. The pH, TSS, TA, and RI were significantly affected by cultivars. The lowest pH value was obtained for MO6 (pH 3.94) and the highest for ME5 (pH 4.07). These results are similar to those for ‘Mollar’ cultivars[Citation27] and for ‘Jabal’ pomegranate.[Citation14] The lowest pH values were detected in fruits from ‘Taifi’ cultivars[Citation26] and ‘Izmir’ sweet cultivars.[Citation15] Concerning TSS, ME17 juice showed the lowest content (14.79 °Brix) while MO6 yielded the highest TSS (15.81 °Brix). Similar TSS values have been reported for ‘Mollar’ cultivars from Spain,[Citation27] ‘Taifi’ cultivars from Saudi Arabia,[Citation26] ‘Tatli’ cultivar from Turkey,[Citation28] ‘Jabal’ cultivars from Oman,[Citation14] and ‘Izmir’ cultivars from Turkey.[Citation15]
Table 1 Fruit weight, pH, total soluble solids (TSS), titratable acidity (TA), and ripening index (RI) of ‘Mollar’ pomegranate juice
Titratable acidity ranged between 0.18 and 0.26%. These results agreed with those found in other ‘Mollar’ cultivars[Citation27] and ‘Izmir’ cultivars,[Citation15] while higher[Citation24,Citation25,Citation28] and lower[Citation14] TA values have been reported. The ripening index (TSS/TA) is used by some authors to classify pomegranate varieties.[Citation27,Citation29] The following classification has been established for Spanish varieties:[Citation21] ripening index (RI) = 5–7 for sour, RI = 17–24 for sour-sweet, and RI = 31–98 for sweet varieties. Taking into consideration this classification, all cultivars were sweet since the RI ranged from 59.14 (ME5) to 87.95 (MO6).
Sugars and Organic Acids
Fructose, glucose, sucrose, and maltose contents were individually analysed, as they play an important role in pomegranate quality. As previously reported for pomegranate juice,[Citation15,Citation24,Citation26,Citation28,Citation30] the two major sugars are glucose and fructose. Glucose was present at the highest concentration as already reported,[Citation15,Citation26] ranging from 60 to 64% of total sugars, followed by fructose, maltose, and sucrose. While values for fructose were in the same range as previously reported in pomegranate juice,[Citation24,Citation26] they were lower than the values obtained for Turkish pomegranates.[Citation15] Sucrose and maltose were detected in all evaluated cultivars. Similar results were previously reported in other Spanish cultivars.[Citation30] Contents of total sugars were not significantly affected by cultivars (). In this study, the mean levels of the glucose/fructose ratio ranged from 1.65 to 1.92; this ratio was lower for ‘Taifi’ pomegranate cultivars (1.14).[Citation26]
Table 2 Individual sugars of ‘Mollar’ pomegranate juice (g 100−1 juice)
Total and individual organic acid contents were significantly affected by cultivar (). The major organic acid in ‘Mollar’ cultivars was citric acid (0.15 to 0.22 g 100−1 juice). These results agreed with other pomegranate juice studies.[Citation15,Citation24,Citation30] Among the six evaluated cultivars, the highest citric acid level was found for ME5 (0.22 g 100−1 juice) and the lowest for ME16, MA4, and MA5 (0.15 g 100−1 juice). Malic acid was found to be the second dominant acid for the cultivars ME5, ME16, and ME17 (ranged from 0.052 to 0.065 g 100−1 juice), while oxalic acid was the second dominant acid for the cultivars MO6, MA4, and MA5 (ranged from 0.047 to 0.051 g 100−1 juice). These results are similar to those obtained on ten cultivars grown in Turkey.[Citation15] Some differences were found regarding citric and malic acids contents compared to those reported by Melgarejo et al.[Citation30] for the same cultivars. Probably, this may be due to different agonomic conditions.
Table 3 Individual organic acids of ‘Mollar’ pomegranate juice (g 100−1 juice)
Tartaric acid was also found in ‘Mollar’ pomegranate juice (ranged from 0.021 to 0.028 g 100−1 juice). Other organic acids, such as ascorbic, quinic, succinic, acetic, lactic, and fumaric acids, were detected in the aril juice at minor or trace amounts.[Citation24,Citation30,Citation31]
Anthocyanins and Colour
Cultivars showed no influence in the colour index of pomegranate juice, but total anthocyanin content was significantly affected (). The individual anthocyanins (delphinidin 3,5-diglucoside; cyanidin 3,5-diglucoside; pelargonidin 3,5-diglucoside; delphinidin 3-glucoside; cyanidin 3-glucoside; pelargonidin 3-glucoside) contents in the juice of selected cultivars were studied as previously reported for pomegranate juice.[Citation22,Citation27] The analyses revealed that all cultivars had a common anthocyanin profile when harvested at commercial maturity, characterized by the six pigments previously identified in other cultivars.[Citation27]
Table 4 Individual anthocyanins of ‘Mollar’ pomegranate juice (mg L−1)
Table 5 Colour of ‘Mollar’ pomegranate juice
The content of total anthocyanins ranged from 72.55 mg L−1 (MA4) to 200.21 mg L−1 (ME5). These values are in the same range as those reported for other pomegranate cultivars: i.e., 50–267 mg kg−1 fresh weight of arils for Spanish ‘Mollar’ cultivars,[Citation22,Citation32] 90–160 mg L−1 in Spanish cultivars,[Citation27] 6–120 mg L−1 in Tunisian pomegranates,[Citation22] 185 mg L−1 in minimally processed pomegranate seeds,[Citation33] and 200 mg L−1 for the ‘Wonderful’ pomegranate.[Citation19] At harvest, the main anthocyanin was cyanidin 3-glucoside for all cultivars except MA5, with cyanidin 3,5-glucoside as the major one. The concentration values of Cy3 ranged from 21.47 mg L−1 (MA4) to 76.42 mg L−1 (ME5). The second dominant anthocyanin was cyanidin 3,5-diglucoside. Its concentration varied between 18.19 mg L−1 (MO6) and 48.83 mg L−1 (MA5). Cyanidin 3-glucoside was reported as the major pigment in four Spanish cultivars as well as in the ‘Wonderful’ variety.[Citation11,Citation22,Citation27] On the other hand, the most abundant pigment was delphinidin 3,5-diglucoside[Citation34] in eight Iranian cultivars. To determine the relationships between anthocyanins levels and juice colour, L*, a*, b* a*/b*, H°*, C*, and CI were calculated. The brightest coloured rind (high L* value) was found in fruits of Elche ‘Mollar’ cultivars (ME5, ME16, and ME17), whereas the darkest coloured rind was found on ME5, MO6, MA4, and MA5 (). According to the colour parameters a*/b*, more attractive fruits were found on ME5, ME16, ME17, and MA5. Similar results were obtained in aril juices of two Israelian commercial accessions: ‘Wonderful’ and ‘Rosh-Hapered’.[Citation24]
Correlation Analysis
The interdependence of the variables was investigated by the analysis of correlation (). As expected, correlation analyses showed a high correlation between pH and TA (r = −0.494, P ≤ 0.05), as well as between TA and malic acid (r = 0.675). Very strong correlations were found between glucose and total sugars (r = 0.948), citric acid and total acids (r = 0.921), and cyanidin 3-glucoside and total anthocyanins (r = 0.945). Delphinidin 3-glucoside was positively correlated with delphinidin 3,5-diglucoside (r = 0.832). The low correlation value obtained between “colour index” and anthocyanins levels suggested that compounds other than anthocyanins as hydroxycinnamic acids influenced aril colour.[Citation24]
Table 6 Pearson product moment correlations between physicochemical characters
Principal Component Analysis (PCA) and Cluster Analysis (CA)
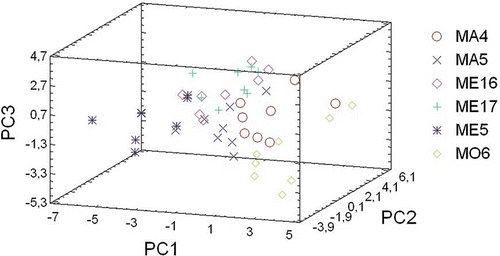
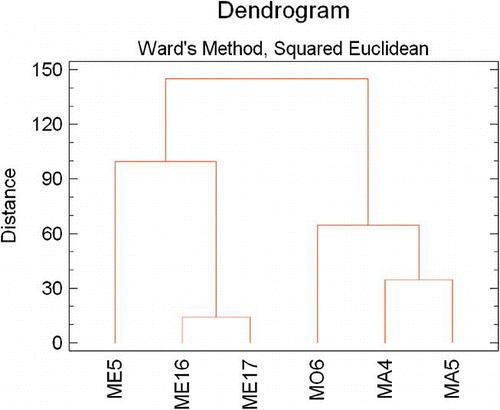
PCA was used to establish genetic relationships among cultivars and to study correlations among fruit traits within sets of pomegranate genotypes.[Citation15] The total variability was explained by the 27 principal components (). By using Kaiser's rule, eight principal components were extracted from the data. Nearly 81% of the variability observed was explained by the first eight components (). showed the correlation between the original variables and the first 3 principal components. PC1 represented mainly anthocyanins (accounting for 22% of the variance). PC2 represented colour parameters and total sugars (account for 15.6% of the variance). PC3 correlated mainly colour parameter a*, Pg3, TSS, and RI (account for 13.5% of the variance). The remaining components explained less variability. represented PC1, PC2, and PC3 plotted on a tridimensional plane. The results obtained from hierarchical CA, using the linkage method between groups, are shown as a dendrogram (), five main groups were clustered. The first main group was made of two cultivars originated from Elche (ME16 and ME17). High dissimilarity levels were found for cultivars ME5, MO6, MA4, and MA5, being highly heterogeneous among the studied cultivars.
Table 7 Eigenvalues and proportion of total variability among pomegranate cultivars as explained by the first 27 principal components
Table 8 Correlation between original variables and the first three principal components (PC)
CONCLUSION
In this study, six pomegranate cultivars were analysed for organic acids, sugars, anthocyanins, chromatic parameters, and other quality attributes including pH, TA, TSS content, and RI. The composition of pomegranate juice was definitively affected by the cultivar. All the evaluated cultivars showed high fruit weight, TSS contents and colour indexes, attractive, quality attributes to enhance pomegranate acceptability by consumers as well as to evaluate fruit maturity of this group of cultivars. Citric and malic acids were found to be the most frequent organic acids in pomegranate juice. Fructose and glucose were the most abundant sugars in Spanish ‘Mollar’ pomegranates. The total anthocyanin content ranged from 72 mg to 200 mg per litre of juice, being cyanidin 3-glucoside the main anthocyanin found followed by cyanidin 3,5-diglucoside. These results may be very useful to food technologists, horticultural researchers, and nutritionists for developing new applications in further pomegranate studies. The results obtained from hierarchical CA led to five main groups. The principal component analysis and cluster analyses showed high dissimilarity levels in cultivars: group of ME16 and ME17, ME5, MO6, MA4, and MA5.
REFERENCES
- Sumner , M.D. , Elliott-Eller , M. , Weidner , G. , Daubenmier , J.J. , Chew , M.H. and Marlin , R. 2005 . Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease . American Journal of Cardiology , 96 : 810 – 814 .
- Singh , M. , Arseneault , M. , Sanderson , T. , Morthy , V. and Ramassamy , C. 2008 . Challenges for research on polyphenols from foods in Alzeheirmer's disease: Bioavailability, metabolism and cellular and molecular mechanism . Journal of Agriculture and Food Chemistry , 56 : 4855 – 4873 .
- Khan , N. , Afaq , F. , Kweon , M.H. , Kim , K. and Mukhtar , H. 2007 . Oral consumption of pomegranate fruit extract inhibits growth and progression of primary lung tumors in mice . Cancer Research , 67 : 3475 – 3482 .
- Malik , A. and Mukhtar , H. 2006 . Prostate cancer prevention through pomegranate fruit . Cell Cycle , 5 : 371 – 373 .
- Seeram , N.P. , Aronson , W.J. , Zhang , Y. , Henning , S.M. , Moro , A. and Lee , R.-P. 2007 . Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localise to the mouse prostate gland . Journal of Agriculture and Food Chemistry , 55 : 7732 – 7737 .
- Aviram , M. and Dornfeld , L. 2001 . Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure . Atherosclerosis , 158 : 195 – 198 .
- Aviram , M. , Dornfeld , L. , Rosenblat , M. , Volkova , N. , Kaplan , M. and Hayek , T. 2000 . Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: Studies in humans and in the atherosclerotic apolipoprotein E-deficient mice . American Journal of Clinical Nutrition , 71 : 1062 – 1076 .
- Aviram , M. , Rosenblat , M. , Gaitini , D. , Nitecki , S. , Hoffman , A. and Dornfeld , L. 2004 . Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation . Clinical Nutrition , 23 : 423 – 433 .
- Kaplan , M. , Hayek , T. , Raz , A. , Coleman , R. , Dornfeld , L. and Vaya , J. 2001 . Pomegranate juice supplementation to atherosclerotic mice reduces macrophage lipid peroxidation, cellular cholesterol accumulation and development of atherosclerosis . The Journal of Nutrition , 131 : 2082 – 2089 .
- El-Nemr , S.E. , Ismail , I.A. and Ragab , M. 1990 . Chemical composition of juice and seeds of pomegranate fruit . Die Nahrung , 34 : 601 – 606 .
- Gil , M.I. , Tomás-Barberán , F.A. , Hess-Pierce , B. , Holcroft , D.M. and Kader , A.A. 2000 . Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing . Journal of Agriculture and Food Chemistry , 48 : 4581 – 4589 .
- Kulkarni , A.P. and Aradhya , S.M. 2005 . Chemical changes and antioxidant activity in pomegranate arils during fruit development . Food Chemistry , 93 : 319 – 324 .
- Cristosto , C.H. , Mitcham , E.J. and Kader , A.A. 2000 . Pomegranate: Recommendations for maintaining postharvest quality. Produce Facts , Davis, CA , , USA : Postharvest Research and Information Centre, University of California .
- Al-Said , F.A. , Opara , L.U. and Al-Yahyai , R.A. 2009 . Physico-chemical and textural quality attributes of pomegranate cultivars (Punica granatum L.) grown in the Sultanate of Oman . Journal of Food Engineering , 90 : 129 – 134 .
- Cam , M. , Hisil , Y. and Durmaz , G. 2009 . Characterisation of pomegranate juices from ten cultivars grown in Turkey . International Journal of Food Properties , 12 : 388 – 395 .
- Bartolozzi , F. , Bertezza , G. , Bassi , D. and Cristoferi , G. 1997 . Simultaneous determination of soluble sugars and organic acids as their trimethylsilyl derivatives in apricot fruits by gas-liquid chromatography . Journal of Chromatography A , 758 : 99 – 107 .
- Tezcan , F. , Gültekin-Özgüven , M. , Diken , T. , Özçelik , B. and Erim , F.B. 2009 . Antioxidant activity and total phenolic, organic acid and sugar content in commercial pomegranate juices . Food Chemistry , 115 : 873 – 877 .
- Ercisli , S. , Orhan , E. , Ozdemir , O. and Sengul , M. 2007 . The genotypic effects on the chemical composition and antioxidant activity of sea buckthorn (Hippophae rhamnoides L.) berries grown in Turkey . Scientia Horticulturae , 115 ( 1 ) : 27 – 33 .
- Holcroft , D.M. , Gil , M.I. and Kader , A.A. 1998 . Effect of carbon dioxide on anthocyanins, phenylalanine ammonia lyase and glucosyltransferase in the arils of stored pomegranates . Journal of the American Society for Horticultural Science , 123 ( 1 ) : 136 – 140 .
- Wrolstad , R.E. , Durst , R.W. and Lee , J. 2005 . Tracking colour and pigment changes in anthocyanin products . Trends in Food Science and Technology , 16 : 423 – 428 .
- Melgarejo , P. 1993 . Selección y tipificación varietal de granado (Punica granatum L.) [Characterisation and tipification of pomegranate cultivars (Punica granatum L.)] , PhD Thesis .
- Gil , M.I. , García-Viguera , C. , Artés , F. and Tomás-Barberán , F.A. 1995 . Changes in pomegranate juice pigmentation during ripening . Journal of the Science of Food Agriculture , 68 : 77 – 81 .
- McGuire , R.G. 1992 . Reporting of objective colour measurements . HortScience , 27 : 1254 – 1255 .
- Shwartz , E. , Glazer , I. , Bar-Ya'akou , I. , Matityalm , I. , Bar-Ilan , I. , Holland , D. and Amir , R. 2009 . Changes in chemical constituents during the maturation and ripening of two commercially important pomegranate accessions . Food Chemistry , 115 : 965 – 973 .
- Drogoudi , P. , Tsipouridis , C. and Michailidis , Z. 2005 . Physical and chemical characteristics of pomegranates . HortScience , 40 ( 5 ) : 1200 – 1203 .
- Al-Maiman , S.A. and Ahmad , D. 2002 . Changes in physical and chemical properties during pomegranate (Punica granatum L.) fruit maturation . Food Chemistry , 76 : 437 – 441 .
- Hernández , F. , Melgarejo , P. , Tomás-Barberán , F.A. and Artés , F. 1999 . Evolution of juice anthocyanins during ripening of new selected pomegranate (Punica granatum L.) clones . European Food Research Technology , 210 : 39 – 42 .
- Ozgen , M. , Durgaç , C. , Serçe , S. and Kaya , C. 2008 . Chemical and antioxidant properties of pomegranate cultivars grown in the Mediterranean region of Turkey . Food Chemistry , 111 : 703 – 706 .
- Martínez , J.J. , Melgarejo , P. , Hernández , F. , Salazar , D.M. and Martínez , R. 2006 . Seed characterisation of five new pomegranate (Punica granatum L.) varieties . Scientia Horticulturae , 110 : 241 – 246 .
- Melgarejo , P. , Salazar , D.M. and Artés , F. 2000 . Organic acids and sugars composition of harvested pomegranate fruits . European Food Research and Technology , 211 : 185 – 190 .
- Poyrazglu , E. , Gökmen , V. and Artik , N. 2002 . Organic acids and phenolic compounds in pomegranates (Punica granatum L.) grown in Turkey . Journal of Food Composition and Analysis , 15 : 567 – 575 .
- Artés , F. , Tudela , J.A. and Gil , M.I. 1998 . Improving the keeping quality of pomegranate fruit by intermittent warming . Zeitschrift fur Lebensmittel -Untersuchung und -Forschung , 207 ( 4 ) : 316 – 321 .
- Gil , M.I. , Martínez , J.A. and Artés , F. 1996 . Minimally processed pomegranate seeds . LWT—Food Science and Technology , 29 ( 8 ) : 708 – 713 .
- Mousavinejad , G. , Emam-Djomeh , Z. , Rezaei , K. and Khodaparast , M.H. 2009 . Identification and quantification of phenolic compounds and their effects on antioxidant activity in pomegranate juices of eight Iranian cultivars . Food Chemistry , 115 : 1274 – 1278 .