Abstract
The anti-pancreatic lipase and antioxidant activity of Momordica charantia, Morinda citrifolia fruit, and Centella asiatica extract were evaluated for potential use as an anti-obesity agent. Antioxidant activity of the extracts was determined using 2,2-diphenyl-1-picrylhdrazyl and ferric-reducing antioxidant power assays. Inhibition of pancreatic lipase was measured in vitro. Results from this study showed that Momordica charantia, Morinda citrifolia fruit, and Centella asiatica extract exhibited different levels of antioxidant activity, with IC50 ranging from 0.90 ± 0.1 to 3.7 ± 0.8 mg/mL of extracts. All extracts were found to inhibit pancreatic lipase activity, with Momordica charantia, Morinda citrifolia fruit, and Centella asiatica extract demonstrating 21.0 ± 1.3, 25.8 ± 0.1, and 25.3 ± 0.4% inhibition, respectively.
INTRODUCTION
As the world becomes more and more technologically advanced, the issue of obesity seems to be affecting more and more countries of the world, both among developing and developed countries. Across the world, 1.1 billion people are overweight and 12 million are obese.[Citation1] Governments and health agencies are extremely concerned about obesity due to the multitude of problems associated with it, such as hypertension, hyperlipidemia, diabetes mellitus, cardiovascular disease, cancer, and metabolic disorders.[Citation2,Citation3] In the cases where diets and increased physical activity fail to show positive results, pharmaceuticals, such as Orlistat®, an analogue of lipstatin obtained from Streptomyces toxitricini,[Citation4,Citation5] is often used. Orlistat® is a potent inhibitor of gastric, pancreatic, and carboxylester lipase and has been proven to be effective for the treatment of obesity.[Citation6] It is understood that pancreatic lipase (PL) is the most important enzyme responsible for the digestion of triacylglycerols into mono- and diacyglycerols and fatty acids to be absorbed by the body. Researchers and health professionals believe that the inhibition of PL can reduce digestion of fats, hence, their assimilation and absorption. This can mimic a reduced calorie intake in obese patients and help in preventing additional weight gain.[Citation3] It is also believed that an inhibition of pancreatic lipase activity in the body can help in cases of hyperlipidemia.[Citation7]
Antioxidants have been shown to be very important in both the food industry and general health. Antioxidants from fruits and vegetables are thought to have protective effects in degenerative diseases, such as cardiovascular disorders, cancers, and also in the ageing process. There has been a lot of interest in natural antioxidants since the possible side effects of synthetic antioxidants, such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT), have been associated with health risks and toxicity.[Citation8] Rababah and coworkers proposed grape seed extract (GSE) to be used as antioxidants to retard lipid oxidations in food as antioxidant activities of GSE ranged from 66.4 to 81.4% as compared to 94.7% for BHT.[Citation9] Moreover, a low antioxidant status has been linked with obesity and eventual obesity-related disorders, such as cardiovascular diseases, diabetes mellitus, and hypertension.[Citation10,Citation11]
The undesirable effects of some lipase inhibitors and antioxidants have prompted a lot of researches in the potential use of natural compounds as lipase inhibitors in managing obesity and oxidative stress. Presence of lipase inhibitors has also been reported in many other natural sources like marine algae,[Citation12] soybean, and wheat.[Citation13] Recently, Moreno and coworkers[Citation14] reported that the extract of Arachis hypogaea (peanut) nutshell showed inhibitory effects on pancreatic lipase and lipoprotein lipase. Natural antioxidants, such as tocopherols, β-carotene, phenolics, and ascorbic acid, have also been the subject of research.
Tropical herbs, like Momordica charantia, Morinda citrifolia, and Centella asiatica, are widely consumed in Malaysia as part of the diet (salad or vegetable dish) and also in traditional medicine. Hence, the objectives of this study were to assess the antioxidative activity of Momordica charantia fruit extract (MCE), Morinda citrifolia fruit extract (MCFE), and Centella asiatica leaf extracts (CAE) and their effects on pancreatic lipase activity in vitro.
MATERIALS AND METHODS
Preliminary Preparation
Fresh plant materials were obtained from different sources. Momordica charantia (“Peria Katak”) and Centella asiatica (“Pegaga”) were purchased from Pasar Borong in Selangor, Malaysia. Mature Morinda citrifolia fruits (“Mengkudu”) were obtained from the Agricultural Farm, Universiti Putra Malaysia, Selangor, Malaysia. Samples were collected before each experiment. Momordica charantia extract (MCE), Morinda citrifolia fruit extract (MCFE), and Centella asiatica extract (CAE) were obtained using a modified version of the method of Chang and coworkers.[Citation15] In brief, fresh plant samples were cut, deseeded, and washed under running water. They were then diced and oven dried for two days at 40 ± 1°C. The dried plant materials were ground to a fine powder using a domestic grinder (Model BL330; Khind, Kuala Lumpur, Malaysia) and stored at −20°C for future use. Each dried material (10 g) was extracted with absolute ethanol (100 mL) for 24 h at 40°C. Preliminary studies showed that extraction efficiency was better when absolute ethanol was used. The extracts were then filtered and solvent was removed using a rotary evaporator. The resulting viscous substance obtained was diluted to required concentration for all the experiments.
Determination of Antioxidant Activity
2,2-Diphenyl-2-picrylhydrazyl hydrate (DPPH) scavenging activity was determined according to a modified method of Brand Williams.[Citation16] Briefly, 3.5 mL of 6 × 10−5 mol of DPPH solution were reacted with 0.5 mL of different concentrations of MCE, MCFE, and CAE dissolved in methanol. The mixtures were vortexed and allowed to stand for 30 min at room temperature in the dark. Pure methanol was used as the control. The absorbance value was read at 517 nm by a Shimadzu UV-1650 PC UV-Vis spectrophotometer (Kyoto, Japan).
A ferric-reducing antioxidant power (FRAP) test was carried out using the method described by Benzie and Strain[Citation17] with slight modifications. FRAP reagent was prepared by mixing 300 mM sodium (24.09 g) acetate buffer at pH 3.6, 10 mM TPTZ (3.12 g) solution, and 20 mM (5.40 g) FeCl3.6H2O solution (10:1:1/w:v:v). Three milliliters of FRAP reagent were then added to 200 μL of extracts and allowed to react for 30 min at 37°C. Absorbance was measured at 590 nm using a Shimadzu UV-1650 PC UV-Vis spectrophotometer (Kyoto, Japan). Results were expressed as μM Trolox equivalent (TEAC)/g dried weight of sample.
Quantification of Catechin by HPLC
Catechin determination was carried out according to the method adapted from Hertog and coworkers[Citation18] with a slight modification. Hydrolysis of the methanolic extract of plant materials were achieved utilizing 6 M HCl. The extraction mixture was heated to 95°C on a steam bath and refluxed for 2 h. The mixture was allowed to cool and was then filtered through a 0.45 μm (Whatman) nylon membrane filter prior to injection into a HPLC system. The samples were prepared and analyzed in triplicate. The catechin standard was prepared by dissolving a 1–6 mg accurate weight catechin in 10 mL HPLC grade methanol. Each solution was sonicated for 5 min.
HPLC Analysis
The hydrolyzed samples and standards were analyzed using an HPLC system, which consisted of a Millennium (version 3.05.01) chromatography manager, a Waters 2487 dual wavelength absorbance detector with gradient using a Waters 600 pump (Milford, MA, USA). A Waters reverse-phase symmetry C18 column (150 × 3.9 mm, 5 μm; Milford, MA, USA) was used and separations were carried out at room temperature. The mobile phase consisted of deionized water with trifluoroacetic acid (TFA) HPCL grade (Leicestershire, UK) (pH 2.5) as solvent A and 100% methanol as solvent B. The gradient was used as follows: 0–20 min, 100–50% solvent A, 20–30 min, 50–40% solvent A, 30–40 min, 40–100% solvent A. The flow rate was kept at 1.0 mL/min and the detector was set at 280 nm.
Pancreatic Lipase Activity
Porcine PL was dissolved in 0.01 M Tris-HCl buffer (25 units/mL). Plant extracts were dissolved in 0.01 M Tris-HCl buffer at different concentrations (7.81–250 ppm). A modified method of Fox and Stepaniak[Citation19] was used for substrate preparation. Briefly, olive oil (10% v/v) was mixed with Arabic gum (10 g) mixture (10% w/v in 0.1 M), (1.57 g) Tris-HCl buffer, pH 2, 0.5 M (2.92 g) NaCl, and 20 mM (0.02 g) CaCl2 using a homogenizer.
PL activity was determined using a modified method of Fukumoto et al.[Citation20] Briefly, 0.2 mL of lipase solution (25 units) was allowed to react with 0.5 mL of plant extract for 30 min at 4°C. Two mL of reconstituted substrate emulsion was added and the mixture was incubated for 30 min at 37°C. The reaction was stopped using acetone and ethanol mixture (1:1/v:v) and titrated with 0.02 M NaOH to pH 9.4. Titrations were carried out using an automatic titrator (785 DMP Titrino, Metrohm, Herisau, Switzerland). All the readings were taken in triplicate and the experiment was repeated three times.
Analysis of Result
The amount of FFA liberated was assayed using the amount of base required by the incubation mixtures. Thus, PL activity is equivalent to the amount of base added. The control sample is equivalent to 100% enzyme activity. The inhibitory effect was defined as the lowering of relative activity (%) compared to the control's activity:
Statistical Analysis
All comparisons of means were carried out using a One Way ANOVA (Duncan, SPSS version 16). Significance was set at p < 0.05. Each reading was taken in triplicate (n = 3).
RESULTS
Catechin Content and Antioxidant Activity of MCE, MCFE, and CAE as Measured by DPPH and FRAP Assays
The antioxidative potential of all three extracts were determined using the DPPH and FRAP assays. For both assays, MCFE was found to have a more potent antioxidative activity compared to that of MCE and CAE. The antioxidative potential of MCFE, MCE, and CAE are presented in . All three extracts were found to be rich in catechin (). A strong correlation (y = −0.026x + 6.175, R 2 = 0.962) was found between catechin and free radical scavenging activity as measured by the DPPH assay. As catechin content increased, the ability of scavenging free radicals increased. There was also a close correlation (y = 0.255x + 7.012, R 2 = 0.778) between catechin content and antioxidant activity as measured by FRAP assay.
Table 1 Antioxidant activities of extracts as measured by DPPH and FRAP assays
Table 2 Catechin content of Momordica charantia, Morinda citrifolia, and Centella asiatica
Effect of MCE, MCFE, and CAE on Pancreatic Lipase Activity in Vitro
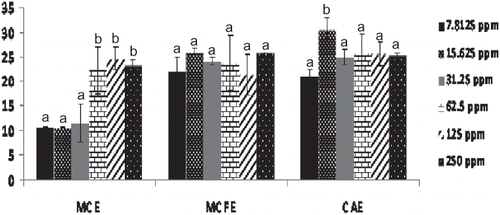
The effect of the extracts on PL activity was assessed in vitro. Results of the study showed that all the extracts exhibited various inhibitory effects on PL activity. Epicatechin and Orlisat were used as natural and synthetic positive controls, respectively. At a concentration of 250 ppm (0.25 mg/mL), there was no significant (p < 0.05) difference in the inhibitory effects of epicatechin, Orlistat, MCE, MCFE, and CAE (). At a concentration of 15.62 ppm, CAE was found to have the highest inhibitory effects (30.45 ± 2.51%) on PL activity. At a concentration of 7.81 ppm (0.007 mg/ML), MCFE (22.1%), and CAE (20.95%) exhibited significantly better inhibition than that of epicatechin (9.7%) and Orlistat (15.28%) (). The effect of increased concentration of extracts on PL activity was determined and different trends were observed. There was no significant (p < 0.05) difference in the inhibition exhibited by MCFE as the concentration was increased (7.81 to 250 ppm). In the case of MCE, there was an increase in inhibitory effect as concentration increased; for CAE, the middle concentration of 15.62 ppm had the highest inhibition ().
Figure 1 The inhibitory effect (%) of Epicatechin, Orlistat, MCE, MCFE, and CAE at a concentration of 0.25 mg/mL on PL activity. Values with different letters indicate a significant difference (p < 0.05), Duncan test, SPSS version16 (n = 3). MCFE: Morinda citrifolia fruit extract; MCE: Momordica charantia extract; CAE: Centella asiatica extract; PL: Pancreatic lipase.
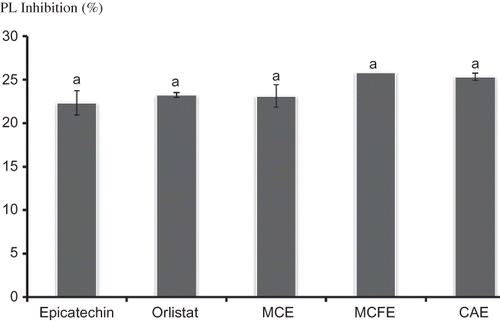
Figure 2 The inhibitory effect (%) of Epicatechin, Orlistat, MCE, MCFE, and CAE at a concentration of 0.007 mg/mL on PL activity. Values with different letters indicate a significant difference (p < 0.05), Duncan test, SPSS version16 (n = 3). MCFE: Morinda citrifolia fruit extract; MCE: Momordica charantia extract; CAE: Centella asiatica extract; PL: Pancreatic lipase.
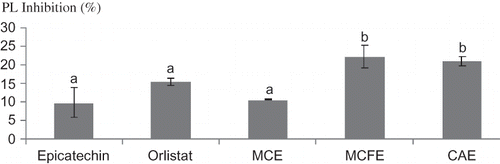
Figure 3 The inhibitory effects of MCE, MCFE, and CAE on PL activity at different concentrations. Values with different letters indicate a significant difference (p < 0.05), Duncan test, SPSS version16 (n = 3). MCFE: Morinda citrifolia fruit extract; MCE: Momordica charantia extract; CAE: Centella asiatica extract; PL: Pancreatic lipase.
DISCUSSION
Antioxidant Activity of MCE, MCFE, and CAE
The body has a complex antioxidant defense system in minimizing free radical damage. Antioxidants prevent oxidation by scavenging or inhibiting free radicals formation. Since synthetically produced antioxidants have been associated with side effects, much effort and money is invested to find antioxidants from natural sources. Although oxidative stress has been linked to many chronic diseases, such as cardiovascular diseases and diabetes mellitus, the relationship with obesity is not well established. Obesity, especially in males, was found to be partly associated with decreased antioxidant status and is thought to have implications in the development of obesity-related problems.[Citation11] It is reported that non-enzymatic glycation in metabolic disorders related to obesity, enhance superoxide formation and inhibit superoxide dismutase. Hyperlipidemia has also been shown to increase endothelial superoxide production and, therefore, is involved in the metabolic consequences of obesity.[Citation21] There is evidence suggesting that obesity leads to oxidative stress. In a study of 76 males (BMI >30 kg/m2) and 24 volunteers, a positive correlation was found between malondialdehyde (MDA) and glucose and an inverse relationship between erythrocyte GSH-PX and Cu/Zn activities and glucose. Disturbances in glucose and insulin metabolism are related to altered oxidative status.[Citation11] Another study done by Reitman and coworkers[Citation10] assessed the plasma content of carotenoids and vitamin E in obese patients with a BMI of 35 kg/m2. Fat soluble antioxidants, vitamin E, and carotenoids were significantly (p < 0.05) lower in obese patients as compared to non-obese ones. There was no difference, however, in the content of water-soluble vitamins in obese and lean subjects. The decline in antioxidant status in obese patients can explain to some extent why people with increased BMI can be more prone to cardiovascular diseases. A decrease in antioxidant activity and increase in oxidative stress can be associated with obesity with insulin resistance, elevated BP, and cardiovascular diseases.[Citation22] Since obesity may lead to oxidative stress, which contributes to obesity-related diseases, such as atherosclerosis, diabetes mellitus, or hypertension, it will be an added benefit to have anti-obesity extracts with antioxidant properties. In the present study, all three extracts studied demonstrated varying antioxidative potentials, with MCFE having the highest antioxidant activity as measured by DPPH and FRAP assays. The DPPH and FRAP assays measure the ability of extracts to scavenge free radicals in the system and the ability to reduce ferric ions, respectively. In the FRAP assay, antioxidant activity was reported as a quantity relative to Trolox.
The different methods used by different researchers to assess the antioxidant activity of herbs do not make it an easy task to compare work from different researchers. The antioxidant activity demonstrated by MCFE was comparable to other herbs, such as Roselle, Pucuk Paku, Rue, and Ketumbar Jawa, while the antioxidant activity of MCE and CAE was comparable to that of celery, West Indian Pea Tree, Spring onion, and Petai.[Citation23] In this study, however, a stronger correlation existed between catechin content and antioxidant activities. MCFE, being the richest in catechin (193 ± 40.1 mg/g), had the highest antioxidant activity as compared with that of CAE (84.5 ± 1.1 mg/g), which had the lowest catechin content and, hence, showed low antioxidant activity. It is, therefore, very tempting to attribute the antioxidative potential of these extracts to the presence of catechin, which was the predominant flavonoid in all three tropical herbs tested. The antioxidant potential of catechin (Trolox equivalents) is compatible with that of other reported values. The value obtained, 2.3 mM Trolox equivalent as compared to 2.4, was reported by Rice Evans et al.[Citation24] Catechin has also been shown to be one of the major flavonoids responsible for the antioxidative properties of teas in a dose-dependent manner.[Citation25] The strong correlation obtained in the study suggests that catechins are powerful antioxidants and are the major antioxidant compounds in these tropical herb extracts. The amount of catechin in MCFE was higher than that reported by Pak Dek et al.[Citation26] This disparity may reflect differences in geographical and environmental conditions, different methods of sampling, and analytical procedures.
The Effects of MCE, CAE, and MCFE on Pancreatic Lipase Activity in Vitro
The effects of MCE, MCFE, and CAE on pancreatic lipase activity were evaluated in this study. Results indicated that MCE, MCFE, and CAE inhibited PL activity in vitro. One of the causes of obesity is believed to be a disruption in lipid metabolism, and enzymes involved can be used as a basis to develop anti-obesity agents. Inhibition of PL to prevent absorption of triacylglycerols, thus suggested that it could help in the management of obesity. A current anti-obesity drug in the market, Orlistat (Roche Laboratories, Kuala Lumpur, Malaysia), works by inhibiting PL activity and mimicking reduced calorie intake. The possibility of natural compounds to inhibit PL has been well documented and includes saponins, polyphenols, plant extracts, terpenes, and microbial sources.[Citation27]
Compared to other plant extracts, MCE, MCFE, and CAE appear to be better lipase inhibitors. Moreno and co workers[Citation28] reported the inhibitory effect of GSE to be 22% at a concentration of 100 ppm. At a lower concentration (62.5 ppm), MCE, MCFE, and CAE demonstrated a similar inhibition as compared to that of GSE with 22.4, 23.8, and 25.4%, respectively. At a concentration of 10 ppm, GSE inhibited PL activity by 3% only, whereas at a concentration of 7.81 ppm, inhibition by MCE, MCFE, and CAE were higher at 10.5, 22.1, and 20.9%, respectively. These results indicate that the herb extracts studied were better PL inhibitors than GSE at these selected concentrations. In 2006, the same authors[Citation14] reported the effect of peanut shell extracts on PL activity that was 29% at 10 ppm, which is comparable to that of MCE, MCFE, and CAE at the same concentration. Many other extracts from plants have been shown to inhibit lipase activity in vitro and some of the studies are also backed by in vivo and clinical studies. An extract from Nomame Herba, CT-II (Saitama, Japan) was found to have potent anti-PL activity (50% at 0.1 mg/mL) and reduced weight gain in rats fed a high fat diet. The anti-obesity effect of CT-II was related to its ability to inhibit lipase activity.[Citation29] Tea saponin, a lipase inhibitor, was also found to suppress an increase in body weight, adipose tissue weight, and diameter of adipocyte of rodents fed a high fat diet. The excretion of triacylglycerols in the feces was also increased. The authors suggested that the anti-obesity effects of tea saponins may be mediated through reduced absorption of dietary fat, as a result of an inhibition of pancreatic lipase activity.[Citation30] Very few clinical studies have been carried out to test the efficacy of lipase inhibitors' rich extracts for the treatment of obesity. The effect of green tea extract AR25 (Exolise, Arkopharma Laboratories, Carros, France) was evaluated in moderately obese patients. In vitro, the extracts were found to be potent PL inhibitors and increased thermogenesis. In clinical trials, after 3 months of consumption of 375 mg catechins, body weight was decreased by 4.6% and waist circumference by 4.48%. The results showed that Exolise, with catechin as its active component, can be a natural anti-obesity agent through its anti-PL activity and increase in thermogenesis.[Citation31]
The inhibitory effect on PL activity has been attributed to the presence of various bioactive compounds present in the plant extracts. Saponins, such as Platycodin saponins from Platycodi Radix, have been shown to have some anti-obesity effects, correlating with the anti-lipase activity. The fraction, Platycodin D, had the most potent inhibitory effect in a dose-dependent manner.[Citation32] Teas have long been used in Chinese traditional medicine to treat various ailments, such as obesity and lipid disorders. It is now known that tea saponins, predominantly present in Oolong tea, have a good inhibitory effect on PL activity, validating the popularity of teas in slimming diets.[Citation30] Water extracts of roots and stems of S. reticulata have been shown to inhibit PL activity with IC50 of 0.26 μg/mL and are also shown to prevent further weight gain in obese rats. The anti-obesity effects of the extracts was attributed to the presence of polyphenols: mangiferin, catechins, and tannins.[Citation33] Polyphenols have also been implicated in the anti-lipase activity of some plant extracts, due to its affinity to bind with proteins by hydrogen or hydrophobic bonds. The inhibitory effects of polyphenols on enzymes can, therefore, be explained by aggregation of enzyme protein.[Citation27] GSE's effect on lipase activity was attributed to the synergistic effect of numerous compounds in the extracts, including flavonoids, procyanidins, and antioxidative metabolites.[Citation28] Stem and leaf extracts of Mangifera indica inhibited lipoprotein lipase (LPL) and PL in vitro and were suggested to affect absorption and uptake of fatty acids. In addition, in vivo studies showed increased fecal fat excretion and reduced serum and insulin levels in obese rats. The polyphenolic-rich extracts were also shown to down-regulate some obesity-related genes, LPL, hormone sensitive lipase, fatty acid synthase, and resistin in liver and epididymal fat.[Citation14]
Since MCE, MCFE, and CAE were all shown to contain phenolic compounds and flavonoids, in particular catechin, their inhibiting effect on PL activity can be attributed to the presence of the bioactive compounds. Orlistat, a well known anti-obesity drug, is associated with numerous side effects, such as abdominal discomfort, flatus, oily stools, and fecal inconsistencies.[Citation34] These side effects seem to justify the quest for PL inhibitors from natural sources in order to promote weight loss without any side effects.
CONCLUSION
This study reported on the antioxidant and anti-PL activity of Momordica charantia extract (MCE), Morinda citrifolia fruit extract (MCFE), and Centella asiatica extract (CAE). MCFE was found to have the most potent antioxidative activity among all three samples tested. The inhibitory effects of the extracts on PL activity indicated the potential use of these herbs in the management of obesity. Although the exact mechanism of the inhibition was not determined, it is suggested that synergistic effects of several bioactive compounds are responsible for this effect.
ACKNOWLEDGMENTS
The authors would like to thank The Ministry of Science, Technology and Innovation of Malaysia for financing the project and Universiti Putra Malaysia for the laboratory facilities provided.
REFERENCES
- Hossain, P.; Kawar, B.E.L.; Nahas, M. Obesity and diabetes in the developing world—A growing challenge. 2007 www.nejm.org (http://www.nejm.org) (Accessed: 9 August 2008 ).
- Ferraro , K.F. , Su , Y. , Gretebeck , R.J. , Black , D.R. and Badylack , S.F. 2002 . Body mass index and disability in adulthood: A 20-year panel study . American Journal of Public Health , 92 : 834 – 840 .
- Mukherjee , M. 2003 . Human digestive and metabolic lipase—A brief review . Journal of Molecular Catalysis B: Enzymatic , 22 : 369 – 376 .
- Toplak , H. and Marhardt , K. 1998 . Reduction of obesity and improvement in metabolic parameters by inhibition of intestinal lipases: Current results with Orlistat . Acta Medica Austriaca , 25 : 142 – 145 .
- Hadavary , P. , Lengsfeld , H. and Wolfer , H. 1988 . Inhibition of pancreatic lipase in vitro by covalent inhibitor tetrahydrolipstatin . Journal of Biochemistry , 256 : 357 – 361 .
- Sjostrom , L. , Rissanee , A. , Anderse , T. , Boldrin , M. , Golay , A. , Koppeschaar , H.P.F. and Krempt , M. 1998 . Randomised placebo controlled trial of orlistat for weight loss and prevention of weight regain in obese patients . Lancet , 352 : 167 – 172 .
- Kahl , R. and Kappus , H. 1993 . Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E . Z Lebensm Unters Forsh , 196 ( 4 ) : 329 – 338 .
- Sharma , N. , Sharma , V.K. and Seo , S.Y. 2005 . Screening of some medicinal plants for anti lipase activity . Journal of Ethnopharmacology , 97 : 453 – 456 .
- Rababah , T.M. , Ereifej , K.L. , Mahasneh , M.A. , Ismaeal , K. , Hidar , A.G. and Yang , W. 2008 . Total phenolics, antioxidant activities and anthocyanins of different grape seed cultivars grown in Jordan . International Journal of Food Properties. , 11 ( 2 ) : 472 – 479 .
- Reitman , A. , Friedrich , I. , Amotz , A.B. and Levy , Y. 2002 . Low plasma antioxidants and normal plasma B vitamins and homocysteine in patients with severe obesity . IMAJ , 4 : 590 – 592 .
- Ozata , M. , Mergen , M. , Oktenli , C. , Aydin , A. , Sanisoglu , S.Y. , Bolu , E. , Yilmaz , M.I. , Sayal , A. , Raskin , I. and Ozdemi , C. 2002 . Increased oxidative stress and hypozincemia in male obesity . Clinical Biochemistry , 35 : 627 – 631 .
- Bitou , N. , Ninomiya , M. , Tsujita , T. and Okuda , H. 1999 . Screening of lipase inhibitors marine algae . Lipids , 34 : 441 – 445 .
- Gargouri , Y. , Julien , R. , Sugihara , A. , Verger , R. and Sarda , L. 1984 . Inhibition of pancreatic lipase and microbial lipases by proteins . Biochimica et Biophysica Acta—Lipids and Lipid Metabolism , 795 : 326 – 331 .
- Moreno , D.A. , Illic , N. , Poulev , A. and Raskin , I. 2006 . Effects of Arachis hypogaea extract on lipid metabolic enzymes and obesity parameters . Life Sciences , 78 : 2797 – 2803 .
- Chang , S.S. , Ostric-Matijaseric , B. , Hsieh , O.A.L. and Huang , C.L. 1977 . Natural antioxidant from rosemary and sage . Journal of Food Science , 42 : 1102 – 1106 .
- Brand Williams , W. , Cuvelier , M.E. and Berger , C. 1995 . Use of free radical method to evaluate anti oxidant activity . LWT—Food Science and Technology , 28 ( 1 ) : 25 – 30 .
- Benzie , I.F.F. and Stain , J.J. 1996 . The ferric reducing ability of plasma (FRAP) as a measure of “anti oxidant power”. The FRAP assay . Analytical Biochemistry , 239 : 70 – 76 .
- Hertog , M.G.L. , Hollman , P.C.H. and Venema , D.P. 1992 . Optimization of a quantitative HPLC determination of potentially anticarcinogenic flavonoids in vegetables and fruits . Journal of Agricultural and Food Chemistry , 40 : 1591 – 1598 .
- Fox , P.F. and Stepaniak , L. 1983 . Isolation and some properties of extracellular heat-stable lipases from Pseudomonas fluorescens strain AFT 36 . Journal of Dairy Research , 50 ( 1 ) : 77 – 89 .
- Fukumoto , J. , Iwai , W. and Tsujiska , Y. 1963 . Studies on lipase I purification and crystallization of a lipase secretion by Aspergillus niger . Journal of General and Applied Microbiology , 9 : 353 – 361 .
- Failla , M.L. , Kennedy , M.L. and Chan , M.L. 1998 . Iron metabolism in genetically obese (ob/ob) mice . Journal of Nutrition , 118 : 46 – 51 .
- Lopes , H.F. , Martin , K.L. , Nashar , K. , Morrow , J.D. , Goodfriend , T.L. and Egan , B.M. 2003 . DASH diet lowers blood pressure and lipid induced oxidative stress in obesity . American Heart Journal , 41 : 422 – 430 .
- Wong , S.P. , Leong , L.P. and Koh , J.H.W. 2006 . Anti oxidant activities of aqueous extracts of selected plants . Food Chemistry , 99 : 775 – 783 .
- Rice Evans , C.A. , Miller , N.J. and Paganga , G. 1996 . Structure–antioxidant activity relationships of flavonoids and phenolic acids . Free Radical Biology and Medicine , 20 : 933 – 956 .
- Meyer , A.S. , Heinonen , M. and Frankel , E.N. 1998 . Antioxidant interactions of catechin, caffeic acid, quercetin and ellagic acid on human LDL oxidation . Food Chemistry , 61 ( 12 ) : 71 – 75 .
- Pak Dek , M.S. , Abdul Hamid , A. , Osman , A. and Soh , C.S. 2008 . Inhibitory effect of Morinda citrifolia L on lipoprotein lipase activity . Journal of Food Science , 78 : C595 – C598 .
- Birari , R.B. and Bhutani , K.K. 2007 . Pancreatic lipase inhibitors from natural sources: Unexplored potential . Drug Discovery Today , 12 : 879 – 889 .
- Moreno , D.A. , Ilic , N. , Poulev , A. , Brasaemle , D.L. , Fried , S.K. and Raskin , I. 2003 . Inhibitory effects of grape seed extract on lipases . Nutrition , 19 : 876 – 879 .
- Yamamoto , M. , Shimura , S. , Ohsaka , T. and Inoue , S. 2000 . Anti obesity effects of lipase inhibitor CT-II, an extract of edible herbs, Nomame Herba, on rat fed on a high fat diet . International Journal of Obesity , 24 : 758 – 764 .
- Han , L.K. , Kimura , Y. , Kawashima , M. , Takaku , T. , Taniyam , T. , Hayashi , T. , Zheng , Y.W. and Okuda , H. 2001 . Anti obesity effect in rodent of dietary tea saponins, a lipase inhibitor . International Journal of Obesity , 25 ( 10 ) : 1459 – 1464 .
- Chantre , P. and Lairon , D. 2002 . Recent findings of green tea extract AR25 (Exolise) and its activity for the treatment of obesity . Phytomedicine , 9 : 3 – 8 .
- Xu , B.J. , Han , L.K. , Zheng , Y.N. , Lee , J.H. and Sung , C.K. 2005 . In vitro inhibitory effect of triterpenoidal saponins from Platycodi Radix on pancreatic lipase . Archives of Pharmacological Research , 28 : 180 – 185 .
- Masayuki , Y. 2002 . Salacia reticulata and its polyphenolic constituents with lipase inhibitory and lyophilic activities have mild anti obesity effects in rats . Journal of Nutrition , 132 : 1819 – 1834 .
- Davidson , M.H. , Hampton , J. and Digirolomo , M. 1999 . Weight control and risk factor reduction in obese subjects treated for 2 years with Orlistat . Journal of the American Medical Association , 281 : 235 – 242 .