Abstract
A capillary isotachophoresis method was developed and applied for determination of glutamic acid and aspartic acid in tomato juice samples. The leading electrolyte was 10 mmol L−1 L-histidine monohydrochloride including 1% poly (vinylpyrrolidone) adjusted with histidine to pH 5.5. The terminating electrolyte was 5 mmol L−1 2-morpholineethanesulfonic acid adjusted to pH 6.0 with tris(hydroxymethyl)aminomethane. It was proved that the developed method is suitable for a routine analysis of glutamic and aspartic acid in tomato juice. The simplicity and low cost of this analytical procedure makes the technique a good alternative to high performance liquid chromatography. Usefulness of the method was tested on different varieties of tomatoes as well as different ripeness stages of the fruits. Moreover, commercial tomato juices were also analysed.
INTRODUCTION
Glutamic acid is one of the most common amino acids. There are two forms of glutamic acid. It exists in “bound” and “free” form. In the “bound” form glutamic acid is a protein building block together with other amino acids.[Citation1] The proteins are important components of muscles and other tissues. The “free” form of glutamic acid acts as an excitatory neurotransmitter.[Citation2,Citation3] Glutamic acid in its “free” form can be found in plant and animal tissues. There are several foods containing a high level of glutamic acid. The most abundant among them are parmesan cheese, meat (beef, pork, chicken, duck), fish (e.g., salmon or mackerel), fish roes (caviar), peas, tomatoes, mushrooms, and even human milk.[Citation1,Citation4] Protein hydrolysates like those that can be found in fish or soy sauces also contain high amounts of “free” glutamic acid.[Citation1,Citation5] Additionally, fermented soybean contained more glutamic acid than raw soybean.[Citation6] Other types of food processing like cooking, roasting, or autoclaving showed no effect on glutamic acid level as it was reported by Umoren et al. for horse-eye beans.[Citation7]
Glutamic acid salt—monosodium glutamate (MSG)—is used as a palatability enhancer and can be found almost in every seasoning and sauce as well as in many other products.[Citation8–10] In Japan, both glutamic acid and MSG are considered as producing a fifth taste. Here, the name Umami was proposed to describe this taste.[Citation11,Citation12] Independently of what name is used (the fifth taste or the taste enhancer), the taste of sauces and seasonings containing MSG is considered to be better than those without MSG. High amounts of MSG added to commercially available food products leads to increased consumption of glutamate. A lot of studies on glutamate that were undertaken revealed several adverse effects of glutamate. The most important seems to be glutamate excitoxicity.[Citation2,Citation3,Citation13] These concerns resulted in withdrawal of MSG from some instant soup and sauce formulations. Therefore, formulations without MSG but containing dried vegetables like tomato are proposed.[Citation10]
Similar adverse effects can be observed for aspartic acid.[Citation13] This compound is also considered as a source of umami taste.[Citation14,Citation15] However, aspartic acid is not used commercially as the taste enhancer. Thus, only naturally occurring aspartic acid is supplied with food so far. The concentration of free glutamic acid in tomato is relatively high in comparison to other vegetables.[Citation1] Moreover, Okumura et al.[Citation16] reported that both the coexistence and the ratio of glutamic acid and aspartic acid are the most important factors in reproducing tomato taste.[Citation16] Additionally, lack of glutamic acid resulted in obtaining the taste of green tomatoes. Therefore, determination of glutamic acid and aspartic acid is of great importance in analysis of both natural tomato juice and commercial tomato juice samples.
Analysis of aspartic and glutamic acids can be performed with the use of chromatographic techniques. Several applications of HPLC required derivatisation of amino acids, which made possible their UV[Citation17,Citation18] or fluorimetric detection.[Citation19,Citation20] Underivatised aspartic acid and glutamic acid were analysed with the use of mass spectrometric detection.[Citation21,Citation22] The use of GC-MS in analysis of glutamic acid and aspartic acid was also reported. However, derivatisation of samples was required to increase volatility of the analytes and thus enable GC analysis.[Citation18,Citation23] Moreover, enzymatic techniques were also used for analysis of glutamic acid.[Citation24]
This article presents a simple isotachophoretic method useful in analysis of aspartic acid and glutamic acid. Isotachophoresis belongs to the group of electrophoretic techniques and is based on the migration of charged particles in an electric field. In isotachophoresis the sample is introduced between a fast leading electrolyte and a slow terminating electrolyte. During the first stage of the separation process, various components of the sample separate. After having reached complete separation, each particular zone contains only a single substance, which migrates with the same electrophoretic velocity. The analytes can be detected with the use of a conductivity detector. Thus, no derivatisation is needed, which makes the analysis low-cost and simple.
MATERIALS AND METHODS
Apparatus
Isotachophoretic separations were performed by using the electrophoretic analyser EA 100 (Villa Labeco, Spišská Nová Ves, Slovak Republic) equipped with a column coupling system consisting of two capillaries made of fluorinated ethylene-propylene copolymer. First, a preseparation capillary (160 mm × 0.8 mm I.D.) was connected to the analytical capillary (160 mm × 0.3 mm I.D.) via the bifurcation block. The analyser was equipped with a sample valve of 30 μL fixed volume and conductivity detectors placed on both columns 40 mm from the outlet ends. Separations were performed at ambient temperature. The isotachopherograms were evaluated by a personal computer software package supplied with the analyser. Qualitative information in the isotachopherogram is obtained from the relative step heights (RSH—counted as the ratio of the step height of the analyte to the step height of the terminator), while the zone lengths give quantitative information. The concentrations can be calculated by comparing the step length of a compound with the calibration curve of standard solutions.
Isotachophoresis Conditions
The leading electrolyte was 10 mmol L−1 L-histidine monohydrochloride containing 1% poly(vinylpyrrolidone) and adjusted with histidine to pH 5.5. The terminating electrolyte was 5 mmol L−1 2-morpholineethanesulfonic acid adjusted to pH 6.0 with tris(hydroxymethyl)aminomethane. The driving current in the preseparation capillary was 250 μA. The initial driving current in the analytical capillary was 50 μA. During detection, the current was reduced to 20 μA.
Chemicals
Tris(hydroxymethyl)aminomethane, 2-morpholineethanesulfonic acid, L-histidine monohydrochloride monohydrate, L-histidine, and iron (III) chloride were all from Sigma-Aldrich (St. Louis, MD, USA). Water used in preparation of electrolyte systems, solutions of model mixtures, and samples were prepared by reverse osmosis in a Demiwa System from Watek (Ledec nad Sázavou, Czech Republic), followed by double distillation in a quartz apparatus. L-glutamic acid, L-aspartic acid, L-ascorbic acid, and succinic acid were from Sigma-Aldrich, acetic acid was from Merck (Darmstadt, Germany), and sodium phosphate from POCh (Gliwice, Poland).
Sample Preparation
Tomato samples were homogenized and then juice was separated from a pulp by filtration. The samples were filtered and 1 mL of filtrate was added to 10 mg of iron (III) chloride in a 50-mL volumetric flask. After several seconds, the flask was filled to the mark with water and mixed. The diluted sample was injected in triplicate into the isotachopherograph.
RESULTS AND DISCUSSION
Isotachophoresis is an analytical technique, which enables separation and quantitative determination of compounds capable of migrating in electrolyte solutions after voltage is applied. Proper separation of the analytes requires conditions in which all these compounds have different mobilities. Basically, mobility depends on the type of a molecule (its molecular mass, number of acidic and basic groups, etc.). For each analyte, effective mobility depends on several factors. Here, the main role in changing mobility of the analytes can be ascribed to pH of the leading electrolyte. For an acidic analyte, maximum mobility can be achieved by selection of the leading electrolyte pH two units higher than its pKa. On the other hand, selection of the leading electrolyte pH two units below pKa diminishes ionisation of the acid and considerably reduces its mobility. For an amino acid molecule, the situation is more complicated. This class of compounds can be protonised or deprotonised depending on pH. Here, for a certain selected pH amino acid is a neutral molecule. At this point, the isoelectric point amino acid has no mobility in the electric field and isotachophoretic separation cannot be performed. Thus, proper pH of the leading electrolyte must be selected to achieve analytical conditions that enable movement of an ionised molecule in the electric field. For dicarboxylic amino acids, like aspartic acid and glutamic acid, isotachophoretic movement is possible in a wide pH range from 3 to 10.[Citation25] A theoretical possibility of separation can easily be deduced from tabulated RE values (defined as the ratio of the conductivity of the leading electrolyte to the conductivity of the sample zone) collected by Hirokawa et al.[Citation25] Nevertheless, the tabulated RE values are not collected for all analytes. Therefore, practical experiments are required in most analytical tasks.
Sádecká and Polonský proposed analytical conditions for isotachophoretic analysis of several acids, including ascorbic acid, glutamic acid, and acetic acid.[Citation26] The proposed conditions required usage of electrolytes at pH = 3.0. According to tabulated RE values of acetic acid and glutamic acid, however, the two acids should co-migrate in a mixed zone.[Citation24] There was no resolution of ascorbic acid, glutamic acid, and acetic acid achieved during our studies in analytical conditions proposed by Sádecká and Polonský.[Citation26] All three acids eluted in one mixed zone. This problem can be connected with different lots of analytical column used in analysis. The mobility of these three acids at pH = 3.0 is too similar to obtain their separation using all lots of the analytical columns.
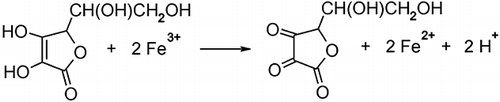
Better separation can be achieved by increasing pH of the leading electrolyte. Acetic acid is well separated from glutamic acid and ascorbic acid at pH = 5.5. However, glutamic acid still co-migrates with ascorbic acid. Analysis of glutamic acid at pH = 5.5 requires removing of ascorbic acid from the sample. This can easily by obtained by oxidising ascorbic acid to dehydroascorbic acid with Fe+3 ions. The reaction proceeds according to . Example isotachopherograms of a mixture of glutamic acid and ascorbic acid before and after reaction with Fe+3 ions is presented in . The above proposed analytical conditions for analysis of glutamic acid allowed to separate several organic acids usually present in fruit and vegetable juices (). Among them aspartic acid can easily be separated and quantified. Thus, glutamic acid and aspartic acid, both responsible for umami taste, can be analysed in one run.
Figure 2 Example isotachopherograms of a mixture of glutamic and ascorbic acids obtained before (A) and after (B) reaction with Fe+3 ions. LE: leading electrolyte; 1: glutamic acid; 2: ascorbic acid; TE: terminating electrolyte.
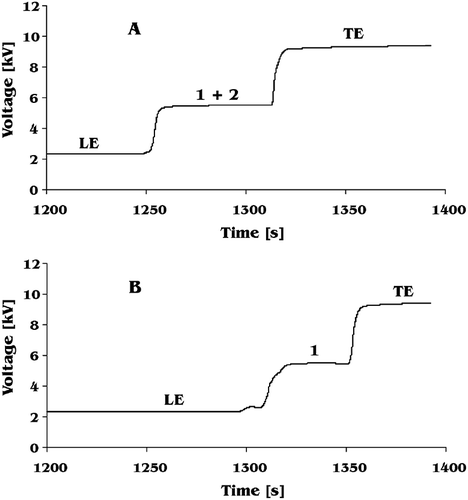
Figure 3 Isotachophoretic separation obtained for a series of organic acids usually present in tomato juice. LE: leading electrolyte; 1: citric acid; 2: succinic acid; 3: acetic acid; 4: phosphates; 5: aspartic acid; 6: glutamic acid; TE: terminating electrolyte.
The analytical method was subjected to validation tests. The calibration curves were measured within the range from 1.5 to 73.6 mg L−1 for glutamic acid and from 1.3 to 66.6 mg L−1 for aspartic acid. The coefficient of determination for both glutamic acid and aspartic acid was above 0.999 (). Limit of detection (LOD) and limit of quantitation (LOQ) were calculated according to the International Conference on Harmonisation recommendations.[Citation27] Briefly, for the limit of detection the value 3.3·σ·S−1 was used, where σ is the standard deviation of the response (the standard deviation of the y-intercept of regression line) and S is the slope of the calibration curve. For the limit of quantitation the value 10·σ·S−1 was used. Both instrumental and method LODs and LOQs are presented in . Lower detection and quantitation limits can be achieved by decreasing the driving current in the analytical capillary, which leads to obtaining longer steps of the analytes. However, due to limited current stabilisation possibilities of the isotachophoretic analyser, driving current should not be lower than 8 μA.[Citation28] In this study, driving current was set at 20 μA to improve precision of analysis. Precision obtained for juice samples analysis was reported in . Also, recovery of the analytes was tested for spiked real juice sample with satisfactory results ().
Table 1 Results obtained during validation tests of the analytical method
Comparison of the results obtained here with those presented in the literature shows good performance of the present method. Instrumental LODs for both aspartic acid and glutamic acid are lower than those achieved in HPLC analysis of underivatised amino acids with the use of NMR, RID, UV, and ELSD detectors.[Citation29] The use of a conductivity detector for HPLC analysis of underivatised aspartic acid and glutamic acid led to LODs equal to 1 and 25 mg L−1, respectively.[Citation29] Only the use of mass spectrometry and chemiluminescent nitrogen detection led to LODs lower than those presented in this article.[Citation29] Lower limits of detection in HPLC can be obtained after derivatisation of amino acids, which is performed for samples containing low amounts of these analytes.[Citation17] Also, concentration of amino acids can be made for samples containing low amounts of these compounds.[Citation17] However, both concentration and derivatisation of amino acids should be performed only if they are needed. Otherwise, additional costs are created.
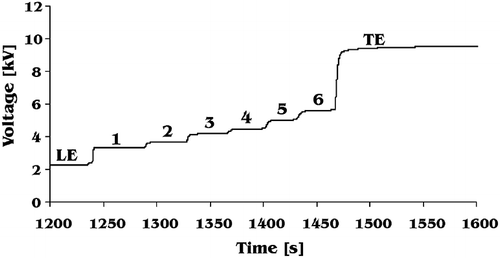
The method was applied to simultaneous determination of glutamic acid and aspartic acid in samples of tomato juice. The results achieved during analysis are presented in and typical isotachopherograms of tomato samples are shown in . Three different varieties of tomato were studied. The ripe fruits of these tomatoes were yellow, orange, and red, respectively. Concentration of glutamic acid in these three varieties was different. The lowest concentration was noted for yellow tomato juice and the highest for red tomato juice. This change of glutamic acid concentration with tomato colour is surprising. Although changes of glutamic acid concentration with changes of tomato colour were already reported,[Citation30] it was believed that an increase of glutamic acid concentration in tomato was connected with its ripening. Here, glutamic acid was determined in samples taken from well ripe tomatoes and still its concentration was dependent on colour of the fruits. The more red was a tomato, the more glutamic acid was analysed in its juice. The above dependence was not obtained for aspartic acid. Its concentration in yellow and orange tomato juice was similar. Only red tomato juice contained considerably more aspartic acid ().
Table 2 Content of glutamic acid and aspartic acid in analysed tomato juice samples
Figure 4 Typical isotachopherograms of juice samples from green (A) and ripe (B) tomato. LE: leading electrolyte; 1: aspartic acid; 2: glutamic acid; TE: terminating electrolyte.
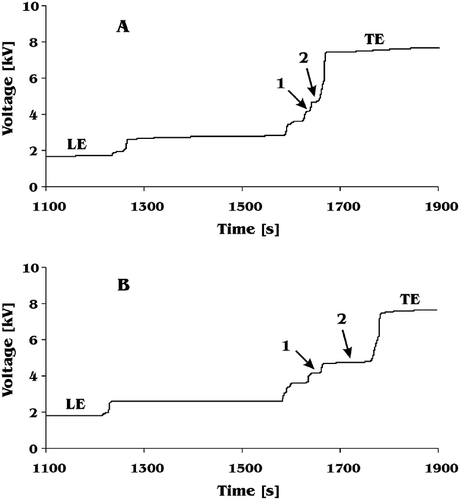
Also, three samples of different commercially available tomato juice were analysed. Both glutamic acid and aspartic acid were determined in all these samples. There was a similar amount of glutamic acid found in each sample. There was also similar concentration of aspartic acid in each of the three samples. Nevertheless, proportion of the two acids in samples of commercial juice was different than in fresh red tomato juice. Concentration of glutamic acid in all commercial juice samples was considerably lower than in fresh red tomato juice. It was analysed at a level between values found for tomato juice samples from ripe and partially ripe tomatoes (). However, concentration of aspartic acid in samples of commercial juice was at a level characteristic for ripe tomato. The reason for this inconsistency remains unknown.
The results obtained here are like those presented in the literature. Similar amounts of glutamic acid (from 638 to 2823 mg L−1) were reported be Vermeir et al. for tomato juice taken from several different tomato cultivars.[Citation24] Mahdi et al. reported glutamic acid in a tomato juice sample at 2100 mg kg−1.[Citation31] Yoshimura et al. found glutamic acid in two tomato cultivars at concentrations from 1364 to 1501 mg kg−1 fresh weight and aspartic acid at concentrations from 179 to 263 mg kg−1 fresh weight.[Citation32] Oruna-Concha et al. reported amino acids separately in tomato flesh and pulp. The mean concentration of glutamic acid was 1260 and 4560 mg kg−1 in flesh and pulp, respectively. Aspartic acid was reported at 410 mg kg−1 in flesh and 520 mg kg−1 in pulp.[Citation23] The results achieved by Yoshimura et al.[Citation32] and Oruna-Concha et al.,[Citation23] as well as the results presented in this article, support the thesis given by Okumura et al.[Citation16] that tomato taste depends on existence and proper proportions of glutamic acid and aspartic acid.
CONCLUSIONS
Glutamic acid and aspartic acid can be successfully determined in tomato juice samples using the present isotachophoretic method with conductivity detection. This method required neither derivatisation nor mass spectrometric detection. There was also no organic solvent needed for elution of the analytes. Only water buffers are used during the analysis, which lowers its cost in comparison to other analytical techniques, such as high performance liquid chromatography. The results obtained in this work proved that concentration of glutamic acid in tomato juice depended on its ripeness stage and variety of tomatoes.
ACKNOWLEDGMENTS
This research was financially supported by grant DS-31-201/2010 from the Ministry of Science and Higher Education (Warsaw, Poland).
REFERENCES
- Löliger , J. 2000 . Function and importance of glutamate for savory foods . The Journal of Nutrition , 130 : 915S – 920S .
- Danbolt , N.C. 2001 . Glutamate uptake . Progress in Neurobiology , 65 : 1 – 105 .
- Platt , S.R. 2007 . The role of glutamate in central nervous system health and disease—A review . The Veterinary Journal , 173 : 278 – 286 .
- Mol , S. and Turan , S. 2008 . Comparison of proximate, fatty acid and amino acid compositions of various types of fish roes . International Journal of Food Properties , 11 : 669 – 677 .
- Bellisle , F. 1999 . Glutamate and the UMAMI taste: Sensory, metabolic, nutritional and behavioural considerations. A review of the literature published in the last 10 years . Neuroscience and Biobehavioral Reviews , 23 : 423 – 438 .
- Handoyo , T. and Morita , N. 2006 . Structural and functional properties of fermented soybean (Tempeh) by using Rhizopus Oligosporus . International Journal of Food Properties , 9 : 347 – 355 .
- Umoren , U.E. , Effiong , O.O. , Onyilagha , J.C. , Ekpe , E.D. and Okiror , S.O. 2008 . Changes in nutritional characteristics of the horse-eye bean [Mucuna Urens (L.) Medic] subjected to different processing methods . International Journal of Food Properties , 11 : 901 – 909 .
- Populin , T. , Moret , S. , Truant , S. and Conte , L.S. 2007 . A survey on the presence of free glutamic acid in foodstuffs, with and without added monosodium glutamate . Food Chemistry , 104 : 1712 – 1717 .
- Kasapis , S. 2009 . Developing minced fish products of improved eating quality: An interplay of instrumental and sensory texture . International Journal of Food Properties , 12 : 11 – 26 .
- Abeysinghe , C.P. and Illeperuma , C.K. 2006 . Formulation of an MSG (monosodium glutamate) free instant vegetable soup mix . Journal of the National Science Foundation of Sri Lanka , 34 : 91 – 95 .
- Ikeda , K. 1909 . New seasonings . Journal of the Tokyo Chemical Society , 30 : 820 – 836 . in Japanese
- Ikeda , K. 2002 . New seasonings . Chemical Senses , 27 : 847 – 849 . English translation
- Blaylock , R.L. 1994 . Excitotoxins—The Taste That Kills , Santa Fe , NM : Health Press .
- Stapleton , J.R. , Roper , S.D. and Delay , E.R. 1999 . The taste of monosodium glutamate (MSG), L-aspartic acid and N-methyl-D-aspartate (NMDA) in rats: Are NMDA receptors involved in MSG taste? . Chemical Senses , 24 : 449 – 457 .
- Delay , E.R. , Sewczak , G.M. , Stapleton , J.R. and Roper , S.D. 2004 . Glutamate taste: Discrimination between the taste of glutamate agonists and monosodium glutamate in rats . Chemical Senses , 29 : 291 – 299 .
- Okumura , S. , Eguchi , S. , Ogawa , W. and Suzuki , K. Methods for preparation of foods, beverages and seasoning having tomato flavour . Japanese Patent, Publication (kokoku) No. 43–11731 . 1968 .
- Timperio , A.M. , Fagioni , M. , Grandinetti , F. and Zolla , L. 2007 . Chemically enhanced liquid chromatography/tandem mass spectrometry determination of glutamic acid in the diffusion medium of retinal cells . Biomedical Chromatography , 31 : 1069 – 1076 .
- Hodisan , T. , Culea , M. , Cimpoiu , C. and Cot , A. 1998 . Separation, identification and quantitative determination of free amino acids from plant extracts . Journal of Pharmaceutical and Biomedical Analysis , 18 : 319 – 323 .
- Tcherkas , Y.V. and Denisenko , A.D. 2001 . Simultaneous determination of several amino acids, including homocysteine, cysteine and glutamic acid, in human plasma by isocratic reversed-phase high-performance liquid chromatography with fluorimetric detection . Journal of Chromatography A , 913 : 309 – 313 .
- Populin , T. , Moret , S. , Truant , S. and Conte , L.S. 2007 . A survey on the presence of free glutamic acid in foodstuffs, with and without added monosodium glutamate . Food Chemistry , 104 : 1712 – 1717 .
- Ma , D. , Zhang , J. , Sugahara , K. , Ageta , T. , Nakayama , K. and Kodama , H. 1999 . Simultaneous determination of γ-aminobutyric acid and glutamic acid in the brain of 3-mercaptopriopionic acid-treated rats using liquid chromatography-atmospheric pressure chemical ionization mass spectrometry . Journal of Chromatography B , 726 : 285 – 290 .
- Schmidt , J. 2003 . Feasibility study: Fast liquid chromatography-mass spectrometry for the quantification of aspartic acid in an aspartate drug . Analytical and Bioanalytical Chemistry , 377 : 1120 – 1123 .
- Oruna-Concha , M.-J. , Methven , L. , Blumenthal , H. , Ch , Young and Mottram , D.S. 2007 . Difference in glutamic acid and 5′-ribonucleotide contents between flesh and pulp of tomatoes and the relationship with umami taste . Journal of Agricultural and Food Chemistry , 55 : 5776 – 5780 .
- Vermeir , S. , Beullens , K. , Mészáros , P. , Polshin , E. , Nicolaï , B.M. and Lammertyn , J. 2009 . Sequential injection ATR-FTIR spectroscopy for taste analysis in tomato . Sensors and Actuators B: Chemical , 137 : 715 – 721 .
- Hirokawa , T. , Nishino , M. , Aoki , N. , Kiso , Y. , Sawamoto , Y. , Yagi , T. and Akiyama , J.I. 1983 . Table of isotachophoretic indices. I. Simulated qualitative and quantitative indices of 287 anionic substances in the range pH 3–10 . Journal of Chromatography , 271 : D1 – D106 .
- Sádecká , J. and Polonský , J. 2001 . Determination of ascorbic and isoascorbic acid in beverages and additives to meat products by capillary isotachophoresis . European Food Research and Technology , 212 : 511 – 517 .
- ICH Guidance for Industry . Q2B Validation of Analytical Procedures: Methodology, U.S. Department of Health and Human Services, Food and Drug Administration , Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) . November 1996, ICH
- Zgoła-Grześkowiak , A. , Grześkowiak , T. , Zembrzuska , J. , Frańska , M. , Frański , R. and Łukaszewski , Z. 2005 . Isotachophoretic determination of carboxylic acids in biodegradation samples . Journal of Chromatography A , 1068 : 327 – 333 .
- Petritis , K. , Elfakir , C. and Dreux , M. 2002 . A comparative study of commercial liquid chromatographic detectors for the analysis of underivatized amino acids . Journal of Chromatography A , 961 : 9 – 21 .
- Sorrequieta , A. , Ferraro , G. , Boggio , S.B. and Valle , E.M. 2010 . Free amino acid production during tomato fruit ripening: A focus on L-glutamate . Amino Acids , 38 : 1523 – 1532 .
- Mahdi , A.A. , Rice , A.C. and Weckel , K.G. 1959 . Off-flavours in foods, formation of pyrrolidonecarboxylic acid in processed fruit and vegetable products . Journal of Agricultural and Food Chemistry , 7 : 712 – 714 .
- Yoshimura , M. , Toyoshi , T. , Sano , A. , Izumi , T. , Fujii , T. , Konishi , C. , Inai , S. , Matsukura , C. , Fukuda , N. , Ezura , H. and Obata , A. 2010 . Antihypertensive effect of a γ-aminobutyric acid rich tomato cultivar ‘DG03-9’ in spontaneously hypertensive rats . Journal of Agricultural and Food Chemistry , 58 : 615 – 619 .