Abstract
Photopyroelectric (PPE) methods belong to the class of photothermal techniques and provide the means for determining some thermal properties of foods in a relatively fast and simple way. In particular, the inverse variant of the photopyroelectric method, abbreviated IPPE, was used here to determine thermal effusivity (also called heat penetration coefficient) of the sour cream and mayonnaise as a function of their fat content. In the sour cream the latter varied from 12 to 31 g/100 g as compared to 27 to 80 g/100 g range in mayonnaise; for both samples the effusivity decreased linearly with the increasing fat content. Each additional gram of fat in 100 g sour cream or mayonnaise resulted in 11.13 and 12.11 Ws1/2m−2K−1 drop in effusivity. Good agreement between the experimentally obtained data and the calculated effusivity was observed if both, the composition and the thermal properties of individual constituents of sour cream were known.
INTRODUCTION
In addition to the taste, nutritional value and a colour, food constituents also affect physical and chemical properties of foods including the thermo physical ones.[Citation1] The availability of food's thermal parameters is of a substantial relevance. As an example, the quality of the sour cream, a product derived from milk, is affected by different technological processes such as the heat treatment, deep freezing, etc. One of important quality parameters of sour cream is the content of fat. In general, there exist two major kinds of sour cream: (i) the ”half fatty” sour cream with a fat content varying between 10–20 g/100 g, and (ii) another kind of sour cream with the fat content exceeding 20 g/100 g.[Citation2]
The fat content of the milk products in the sour cream is important for large energy (39 kJ/g), the essential amino acids (omega-3, omega-6) and the vitamin content.[Citation3] It is the content of fat that affects the consistency of the sour cream. In milk and milk products, the lipids, mainly (98–99%) triglycerides, are present in fat droplets; their digestibility exceeds 95%. The fat content of mayonnaise (stable oil-in-water emulsion) as well as of other foods influences the consistency and thermal properties. Traditionally, mayonnaise is prepared by carefully mixing egg yolk, vinegar, oil and spices. However, the emulsion is stable only if the viscosity of the water phase is rather high, precluding the aggregation of oil droplets.[Citation4] The oil phase and the amount of oil droplets determine the rheological and thermal properties of the product. The optimal result is obtained with the fraction of oil droplets constituting 74% of the total oil content.[Citation5] In traditional mayonnaise, fat content varies between 70 and 80%. Nowadays, low fat content mayonnaises with rheological and thermal properties differing from those of traditional ones are available and attract the public's eye.[Citation6] On the other hand, it is the poor storage life of such products that causes a loss of quality upon long storage periods.[Citation7] The amount of fat in mayonnaise offers the possibility to produce mayonnaise with different aroma; this is because the fat content influences the flavour.[Citation8]
Techniques used to assess the content of fat in foods[Citation9] include gravimetry,[Citation10] densitometry,[Citation11] variety of spectrophotometric methods,[Citation12–14] van Gulik method[Citation15,Citation16] and Gerber's approach,[Citation17] differential scanning calorimetry (DSC),[Citation1] etc. Next to these well established methods, the inverse photopyroelectric method (IPPE) was successfully used in the past to determine the thermal effusivity of margarine, custard and yoghurt.[Citation18] The outcome of that study revealed very high sensitivity of IPPE to the fat/water ratio in the above stated products. Dadarlat et al.[Citation19] combined IPPE and the standard (SPPE) and photopyroelectric method to access four thermal parameters (thermal effusivity, diffusivity, conductivity and volume specific heat) of saturated and monoenoic unsaturated fatty acids. The problems related to the opacity of the sample and the control of sample thickness are of no relevance in such a combined SPPE-IPPE approach. Thermal effusivity of several unsaturated and saturated straight chain fatty acids and of some triglycerides was also measured by means of the inverse photopyroelectric (IPPE) method.[Citation20] An improved IPPE cell, the application of which was not limited by the calibration range, was designed and its versatility demonstrated by obtaining dynamic thermo-physical parameters (thermal conductivity and thermal diffusivity) for the above mentioned samples assuming known volume specific heats. Dadarlat et al.[Citation21] used the IPPE method to determine thermal effusivity of different vegetable oils (soya, sunflower, olive and pumpkin) and various colloidal type foods like ketchup, mustard and sour cream. In an attempt to detect the extent of adulteration in the flax oil contaminated with the sunflower oil, Streza et al.[Citation22] recently combined the photopyroelectric method in thermal-wave-resonator-cavity configuration with gas chromatography. The correlation between the magnitude of the PPE signal and the composition of pure and adulterated vegetable oils supports data obtained previously from fresh and spoiled vegetable oils. It further suggests that thermal diffusivity is a suitable parameter for assessing both the quality of the oil and the early stage of spoilage and adulteration.
The objective of the study described in this paper was to explore the potential of the IPPE method to determine thermal effusivity of sour cream and mayonnaise containing varying proportion of fat.
THEORETICAL BACKGROUND
Heat generated due to the sample's absorption of modulated laser beam produces the thermal wave field T (x, y, z, t) in the photopyroelectric sensor:
In EquationEq. (1) T0 is the ambient temperature, Tdc is the steady-state (dc) component of the temperature (depending on the modulation frequency and the geometry of the sensor), Tac is the oscillating component of the temperature field and x, y, z and t are the spatial coordinates and time, respectively. The current Ip generated in the photopyroelectric sensor due to the temperature variation (relative to the ambient temperature) is[Citation23–25]:
where P is the pyroelectric coefficient, A is the area of the sensor and τ(t) is the averaged temperature variation (relative to the ambient's temperature). For the one-dimensional case τ(t) can be described by:
where L is the thickness of the pyroelectric sensor. The phase sensitive detection (lock-in amplifier) enables one to measure Tac at different modulation frequencies f. The obtained signal V(ω) depends on the impedance of the sensor and the electronic equipment. Assuming an ideal current source V(ω) can be written as:
where ω = 2πf is the angular modulation frequency, while τe and R are the time constant and the resultant resistance of the electronics, i is the imaginary radical unit and
is the average temperature of the pyroelectric sensor. EquationEquation (4) gives a magnitude of the photopyroelectric signal as a function of sample's thermal and optical parameters. The complexity of this expression depends on the actual experimental conditions. Test samples can be optically transparent or opaque as well as thermally thin or thick. In the IPPE experiments described here, all samples were optically opaque and thermally thick. For a sample which is simultaneously optically opaque (the absorption penetration depth is shorter than the sample thickness) and thermally thick (the thermal diffusion length is shorter than the sample thickness), the amplitude V(ω) and the phase φ(ω) of the output signal are:
In EquationEq. (6) I0 is the intensity of incident radiation at the sample's surface, ηs is the efficiency of the non-radiative conversion, ρp is the density of the sensor while es, ep, and em refer to the thermal effusivities of the sample, sensor and the contacting gas. Furthermore, cp is the specific heat capacity of the sensor, whilst Ls and Ds are sample's thickness and thermal diffusivity respectively.
As shown above, the pyroelectric sensor produces the output signal V(ω) the magnitude of which is proportional to τp. For a given experimental arrangement this implies that the ratio of Vsample (i.e., signal obtained from the sample being studied) and Vreference (signal acquired from a reference sample with for which thermo physical parameters are well known) is solely a function of their effusivities, i.e.,[Citation26]
Clearly, by normalizing measured Vsample to Vreference obtained under same experimental conditions from a reference specimen (usually water), one can determine the thermal effusivity of the unknown sample using EquationEq. (8).
MATERIALS AND METHODS
The fat content of commercially available sour creams usually varies between 12 and 20 g/100 g. For the purpose of our experiments the Hungarian Dairy Research Institute from Mosonmagyaróvár, produced sour cream containing significantly more fat (31.4 g/100 g). The three remaining sour cream samples used in this study were prepared by mixing this product with the commercially available samples. Overall, six samples with different fat content have been studied (). As to mayonnaise, all samples were purchased in Hungarian supermarkets. The mayonnaise termed M6 had the highest fat content (80 g/100 g) while the fat content that of mayonnaise M1 was lowest, i.e., 27 g/100 g. The products M2 to M5 were produced by mixing various proportions of M1 and M6; their corresponding fat content is reported in .
Table 1 The content (in grams) of different constituents per 100 g sour cream and mayonnaise
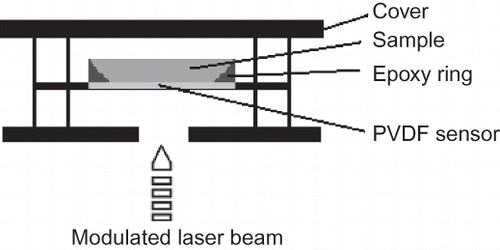
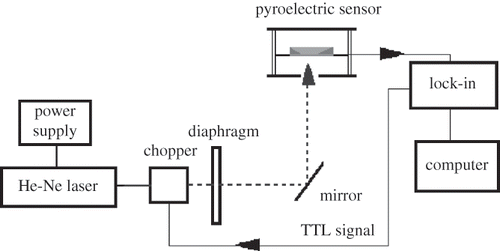
In the IPPE configuration () the periodically modulated radiation (from a moderately strong laser) impinges on a pyroelectric sensor, a 20 microns thick metalized polyvinylidenedifluoride (PVDF) foil. The side of the foil that “sees” the incident radiation was painted black to act as an ideal universal absorber independently of the wavelength of the laser.[Citation23] Due to good thermal contact between the sensor and the sample, the heat was extracted from the IPPE sensor; the amount of extracted heat depends on the sample's properties, such as thermal conductivity κ, specific heat c and density ρ that are all combined in thermal effusivity as e = (κρc)1/2.
The apparatus built in house used to measure thermal effusivity is shown in . Melles Griott He-Ne laser at 632.8 nm (Type: 05-LHP-141, Carlsbad, CA, USA) served as the radiation source. The diameter of the laser beam was about 1.5 mm and the beam divergence was about 2 mrad. The power of the unmodulated beam was 3.6 mW and the degree of polarization was typically 1000:1. The intensity of the laser beam was periodically varied using a homemade acousto-optical modulator driven by the internal oscillator of the lock-in amplifier. The laser energy absorbed at the blackened PVDF foil generated the IPPE signal that was fed into the lock-in amplifier, the output signal of which was processed by the computer. One data point represents an average of 256 successive readouts of the lock-in amplifier. All measurements were performed at 0.5 Hz and in triplicate.
RESULTS
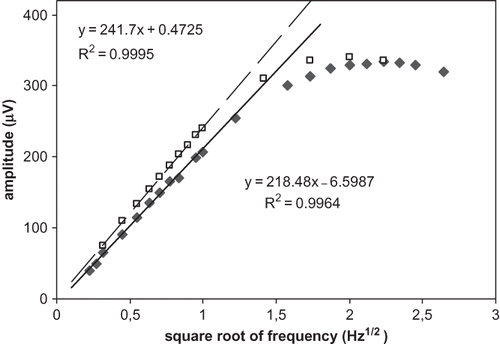
Distilled water of known effusivity (ereference = 1580 Ws1/2m−2K−1) was used first to check the dependence of the amplitude of IPPE signal on the modulation frequency f in the frequency range from 0.1 to 7 Hz. displays the amplitude of the measured signal (symbol □) from water plotted versus f1/2. At frequencies below 5 Hz the amplitude of the signal depended linearly (R2 = 0.9995) on f1/2. The effect of saturation became obvious at frequencies higher than 5 Hz. Symbol (♦) in refers to the amplitude of a sour cream with 12 g/100 g fat content. The trend at frequencies below 5 Hz was the same as observed previously with water, i.e., the signal correlates with the fat content (linearity R2 = 0.9964). Very small difference between the measured signals from water and sour cream containing 12 g/100 g fat is not surprising if one realizes that sour cream is for more than 80% water. The 0.5 Hz was selected as the modulation frequency and used consistently in all remaining measurements.
Figure 3 The amplitude of the IPPE signal obtained from distilled water (□) and the sour cream S1 (♦) plotted versus the square root of the modulation frequency.
Each sour cream was investigated in triplicate and its thermal effusivity calculated using EquationEq. (8). Water and alcohol were used to clean the pyroelectric sensor between the two successive measurements. displays the effusivity of sour creams and mayonnaise samples with the corresponding standard deviations. Based on the outcome of IPPE study, one concludes that the increasing fat content lowers the thermal effusivity. The linear correlation is high: R2 = 0.9824 for sour creams as compared to R2 = 0.9706 for samples of mayonnaise. Such a statement is true for fat content covering a wide range (from 27 g/100 g to 80 g/100 g).
Table 2 Experimentally obtained effusivity for sour creams (S) and mayonnaise (M) with a varying fat content
An attempt was made to calculate thermal effusivity e = (κρc)1/2 of investigated samples using known values for thermal conductivity, heat capacity and density of all sample's constituents. It was furthermore assumed that the effusivity esample of a composite sample is the sum of effusivities ei of all individual constituents
where ai is the mass fraction of a specific constituent in a mixture. , the result of such calculation, shows that for sour creams the discrepancy between calculated and experimental effusivity data does not exceed 8%. In the case of mayonnaise samples the discrepancy is not only significantly higher but also increases with higher concentration of unknown components. Most likely this is due to the uncertainty in the sample's composition (concentration of unknown components between 2.2 and 33.8 g/100 g as shown in ).
Table 3 Specific heat, thermal conductivity and density of various food constituents and thermal effusivity values calculated from the literature data
CONCLUSIONS
The IPPE method was shown capable of simply and rapidly detecting differences in thermal effusivity of sour cream and mayonnaise characterized by a varying content of fat. The observed relationship between the effusivity and the fat content is linear. The gradient of thermal effusivity (expressed as a change in effusivity for a 1% change in fat content of the sample) was 11.13 Ws1/2m−2K−1 and 12.11 Ws1/2m−2K−1 for sour cream and mayonnaise, respectively. These values indicate that thermal effusivity is very sensitive to the content of fat in test samples. However, it should be emphasized that despite its speed and the non-destructive character, the IPPE is not capable of discriminating between various lipids. Thermal effusivities of sour creams are close to the thermal effusivity of water. An attempt was also made to calculate e = (κρc)1/2 for a specific product using the known data for abundance and thermal effusivity of each individual constituent. Very good agreement between measured and predicted effusivity (based on the additive property of this quantity) was obtained for sour cream. The insufficient knowledge about the composition of mayonnaise samples is the likely reason for the observed poor agreement between experimental and calculated effusivity data for this class of sample.
REFERENCES
- Hu , J. , Sari , O. , Eicher , S. and Rakotozanakajy , R.A. 2009 . Determination of specific heat of milk at different fat content between 1°C and 59°C using micro DSC . Journal of Food Engineering. , 90 ( 3 ) : 395 – 399 .
- Codex Alimentarius Hungaricus 1-3/51-1, 2008.
- Thorsdottir , I. , Hill , J. and Ramel , A. 2004 . Omega-3 fatty acid supply from milk associates with lower type 2 diabetes in men and coronary heart disease in women . Preventive Medicine. , 39 ( 3 ) : 630 – 634 .
- Powrie , W. and Nakai , S. 1985 . “ Characteristics of edible fluid animal origin: eggs ” . In Food Chemistry; Fennema, O.R , 829 – 855 . New York : Marcel Dekker .
- Pons , M. , Galotto , M.J. and Subirats , S. 1994 . Comparison of the steady rheological characterization of normal and light mayonnaise . Food Hydrocolloids. , 8 ( 3–4 ) : 389 – 400 .
- Worrasinchai , S. , Suphantharika , M. , Pinjai , S. and Jamnong , P. 2006 . β-Glucan prepared from spent brewer's yeast as a fat replacer in mayonnaise . Food Hydrocolloids. , 20 ( 1 ) : 68 – 78 .
- Peressini , D. , Sensidoni , A. and de Cindio , B. 1998 . Rheological Characterization of Traditional and Light Mayonnaises . Journal of Food Engineering. , 35 ( 4 ) : 409 – 417 .
- Wendin , K. , Aabyl , K. , Edris , A. , Risberg-Ellekjaerl , M. , Albin , R. , Bergenstahl , B. , Johansson , L. , Pilman-WiIlers , E. and Solheim , R. 1997 . Low-fat mayonnaise: influences of fat content, aroma compounds and thickeners . Food Hydrocolloids. , 11 ( 1 ) : 87 – 99 .
- Firestone , D. and Mossoba , M.M. 1997 . “ Newer Methods for fat analysis in foods ” . In New techniques and applications in lipid analysis , Edited by: McDonald , R.E. and Mossoba , M.M. 1 – 34 . AOCS Press .
- Evers , M.J. , Crawford , A.R. , Wightman , M.L. , Beutick , J.G. , Contarini , G. and Farrington , S.D. 1999 . An accurate and rapid method for the direct determination of fat in butter, butter-margarine blends and milkfat spreads . International Dairy Journal. , 9 ( 10 ) : 675 – 682 .
- Badertscher , R. , Berger , T. and Kuhn , R. 2007 . Densitometric determination of the fat content of milk and milk products . International Dairy Journal. , 17 ( 1 ) : 20 – 23 .
- Almendingen , K. , Meltzer , M.H. , Pedersen , J.I. , Nilsen , B.N. and Ellekjær , M. 2000 . Near infrared spectroscopy - a potentially useful method for rapid determination of fat and protein content in homogenized diets . European Journal of Clinical Nutrition. , 54 ( 1 ) : 20 – 23 .
- Forcato , J.D.O. , Carmine , M.P. , Echeverrı , G.E. , Pécora , R.P. and Kivatinitz , S.C. 2005 . Milk Fat Content Measurement by a Simple UV Spectrophotometric Method: An Alternative Screening Method . Journal of Dairy Science. , 88 : 478 – 481 .
- Chippie , A.L. , Jamieson , P.R. , Golt , C.M. , Hsu , C.-H. and Lo , Y.M. 2002 . Quantitative analysis of fat and moisture in mayonnaise using Fourier Transform Infrared spectrometer . International Journal of Food Properties , 5 ( 3 ) : 655 – 665 .
- Koca , N. and Metin , M. 2004 . Textural, melting and sensory properties of low-fat fresh kashar cheeses produced by using fat replacers . International Dairy Journal. , 14 ( 4 ) : 365 – 373 .
- Guizani , N. , Kasapis , N. , Al-Attabi , Z.H. and Al-Ruzeiki , M.H. 2002 . Microbiological, physicochemical, and biochemical changes during ripening of camembert cheese made of pasteurized cow's milk . International Journal of Food Properties. , 5 ( 3 ) : 483 – 494 .
- Ceirwyn , S.J. 1994 . Analytical Chemistry of Foods , 50 – 51 . AN ASPEN Publication .
- Bicanic , D. , Chirtoc , M. , Dadarlat , D. , Chirtok , I. , Van Loon , W. and Bot , G. 1994 . Measurement of the penetration coefficient (κρc)1/2 in Margarine and Cultured Milk Products by the Inverse Photopyroelectric Technique . International Dairy Journal. , 4 ( 6 ) : 555 – 565 .
- Dadarlat , D. , Visser , H. , Bicanic , D. , Frandas , A. and Van Asselt , K. 1994 . Combined standard (SPPE) and inverse (IPPE) photopyroelectric configurations for measurement of dynamic thermal parameters of saturated and unsaturated fatty acids . Journal de Physique IV. , 4 ( 7 ) : 483 – 486 .
- Dadarlat , D. , Visser , H. and Bicanic , D. 1995 . An improved inverse photopyroelectric cell for measurement of thermal effusivity: application to fatty acids and triglycerides . Measurements Science and Technology. , 6 ( 8 ) : 1215 – 1219 .
- Dadarlat , D. , Bicanic , D. , Gibkes , J. , Kloek , W. , von den Dries , Y. and Gerkema , E. 1996 . Study of melting processes in fatty acids and oils mixtures. A comparison of photopyroelectric (PPE) and differential scanning calorimetry (DSC) . Chemistry and Physics of Lipids. , 82 ( 1 ) : 15 – 23 .
- Streza , M. , Dadarlat , D. , Socaciu , C. , Bele , C. and Dulf , F. 2009 . Photopyroelectric Detection of Vegetable Oils’ Adulteration . Food Biophysics. , 4 ( 3 ) : 147 – 150 .
- Mandelis , A. and Zver , M.M. 1985 . Theory of photopyroelectric spectroscopy of solids . Journal Applied Physics. , 57 ( 9 ) : 4421 – 4430 .
- Coufal , H. 1984 . Photothermal spectroscopy using a pyroelectric thin film detector . Applied Physics Letters. , 44 ( 1 ) : 59 – 61 .
- Chirtoc , M. and Mihailescu , G. 1989 . Theory of the photopyroelectric method for investigation of optical and thermal materials properties . Physical Review B. , 40 ( 14 ) : 9606 – 9617 .
- Bicanic , D. , Chirtoc , M. , Dadarlat , D. , van Bovenkamp , P. and van Schayk , H. 1992 . Direct determination of thermophysical parameter (κρc)1/2 in mayonnaise, shortening, and edible oil . Applied Spectroscopy. , 46 ( 4 ) : 602 – 605 .