Abstract
In the present study, the antioxidant and free radical scavenging activities of five major polyphenolic compounds (resveratrol, quercetin, myricetin, catechin, gallic acid) were compared by performing FRAP and DPPH radical scavenging assays followed by their reaction kinetics. Results obtained for the conditions used in this study demonstrated that the quercetin showed the highest ferric reducing ability, while gallic acid was the strongest radical scavenger. Resveratrol exhibited the lowest antioxidant and radical scavenger among the polyphenols assayed. To the best of our knowledge, this is the first comparative report on these polyphenols.
Keywords:
INTRODUCTION
Oxidative stress is an unavoidable consequence of life in an oxygen-rich atmosphere. Reactive oxygen species (ROS) are generated as a by-product of aerobic metabolism and exposure to various natural, as well as synthetic, toxicants. A certain amount of oxidative damage takes place even under normal conditions; however, the rate of this damage increases during the aging and other pathological events, as the efficiency of antioxidative and repair mechanisms decreases, leading to the condition of oxidative stress.[Citation1,Citation2] Researches in recent years have confirmed the implication of oxidative stress in degenerative diseases, such as cancer, cardiovascular diseases, and neurodisorders.[Citation2,Citation3]
There is overwhelming evidence to suggest that nutritional sources of antioxidants, such as fruits, vegetables, tea, and wine, may attenuate tissue damage caused by oxidative challenges.[Citation4] Increased intake of dietary antioxidants may help to maintain an adequate antioxidant status, defined as the balance between antioxidants and oxidants in living organisms.[Citation5] Epidemiological studies support this protective effect of dietary antioxidants: increased intakes of fruit and vegetables have been related to a reduction of the risk of cardiovascular diseases and cancer.[Citation6,Citation7] Polyphenolic compounds, abundant in these nutritional sources, have been shown to be a major dietary factor responsible for such protective effects.[Citation8,Citation9] These effects are due to the property of polyphenols to act as reducing agents by donating hydrogen, by quenching singlet oxygen, and/or by acting as chelators and by trapping the free radicals.
In the past few years there has been renewed interest in evaluating the antioxidant properties of dietary plant polyphenols and for their possible development as nutraceuticals. Among more than 8000 investigated polyphenolic compounds, quercetin, myricetin, catechins, gallic acid, and resveratrol are the most common polyphenolic compounds of biological interest, abundantly present in grapes, onions, and other food sources including red wine.[Citation9]
Although these polyphenols are proven to have important biological, pharmacological, and medicinal properties,[Citation10] the mechanism(s) involved in eliciting these beneficial effects is still not clear. There are only a few reports on the comparative study of these secondary plant products in terms of antioxidant/free radical scavenging activities. The knowledge of kinetics of atom transfer is important because free radicals in vivo are short-lived species, implying that the impact of a substance as an antioxidant depends on its fast reactivity towards free radicals. The present study was conducted to investigate and to compare the efficiency of these five polyphenolic compounds towards their health protective effects in terms of their antioxidant activity and free radical scavenging capacity by using the ferric reducing ability/power (FRAP) and 1,1-diphenyl-2-picrylhydrazyl free radical (DPPH•) scavenging assays along with the reaction kinetics of FRAP and DPPH• with these polyphenols. The present study also explored the possible relationship between the chemical structures of polyphenolic compounds and their antioxidant activities. This knowledge may be helpful to better understand the in vivo behavior of polyphenols.
MATERIALS AND METHODS
FRAP Assay
The antioxidant capacity of each polyphenol was determined by FRAP assay, following the method of Benzie and Strain.[Citation11] Working FRAP reagent was prepared by mixing acetate buffer (300 mM, pH 3.6), 2, 4, 6-tri [2-pyridyl]-s-triazine (10 mM in 40 mM HCl) solution, and FeCl3.6H2O (20 mmol/L) solution in 10:1:1 ratio, respectively. Three milliliters of FRAP reagent was mixed with the 10 μM of each polyphenolic solution and the content was mixed vigorously. The absorbance was read at 593 nm at the interval of 30 s for 4 min. An aqueous solution of known Fe2+ concentration in the range of 100–1000 μmol/L was used for calibration. Using the regression equation, the FRAP values (μmol Fe (II)/L) of the each polyphenolic solution were calculated.
DPPH• Assay
Anti-radical activity of polyphenols was estimated by the procedure described by Miliauskas et al.[Citation12] Briefly, 0.1 mL of each polyphenolic compound was incubated in the methanolic solution of DPPH• (0.1 mM). Absorbance at 517 nm was measured after 30 min of incubation with vigorous shaking. Methanol was used as blank reference. All the measurements are performed in triplicate. The free radical DPPH• scavenging (i.e., reduction) activity was calculated from the equation: Activity [% of DPPH reduction] = [(A-Ax)/A] × 100%, where A is absorbance of DPPH• solution with methanol and Ax is absorbance of DPPH• solution with polyphenols.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 4.00 for Windows, (GraphPad Software, San Diego, California, USA). The results are reported as means ± SD.
RESULTS AND DISCUSSION
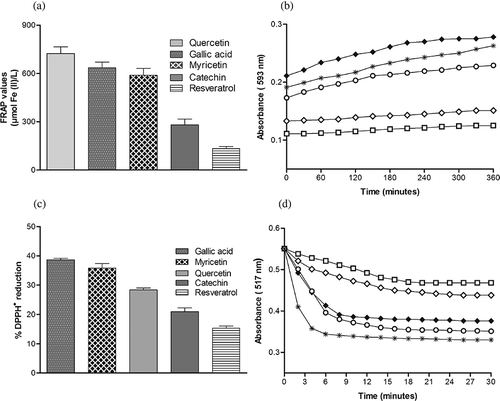
All five polyphenols showed the ferric reducing ability when assayed with FRAP reagent, however, their capacities were observed differently. Quercetin showed the highest while resveratrol showed the lowest reducing ability among all of the polyphenoilc compounds assayed. Their antioxidant capacities decreased in terms of FRAP in the order: quercetin > gallic acid > myricetin > catechin > resveratrol (). shows the reaction kinetics of all five polyphenols with FRAP reagent. All of the assayed polyphenolic compounds inhibited the DPPH• radical but at different rates. Interestingly, gallic acid showed the highest radical scavenging ability/antiradical power followed by myricetin and then quercetin, while catechin and resveratrol were in the same order as in the case of FRAP assay (). However, the difference between the reducing ability of myricetin and gallic acid was not significant. Reaction kinetics of DPPH• scavenging activity of polyphenols is given in . The reaction between DPPH• and most of the assayed polyphenols reached the steady state within 30 min.
Figure 1 (a) Comparative FRAP values of assayed polyphenolic compounds at concentration 10 μM. Values are mean ± SD of 10–12 independent experiments. (b) FRAP reaction kinetics of assayed polyphenols (10 μM): ♦, quercetin; *, gallic caid; ○,,myricetin; ◊, catechin, and □, resveratrol. (c) Comparative %DPPH• reducing activity of polyphenolic compounds at the concentration of 10 μM. Values are mean ± SD of 10–12 independent experiments. (d) Reaction kinetics of DPPH• with polyphenols (10 μM): *, gallic caid; ○, myricetin; ♦, quercetin; ◊, catechin; and □, resveratrol.
A number of methods have been developed to measure the efficiency of dietary polyphenols, either as pure compounds or in food extracts in vitro. These methods focused on different mechanisms of the antioxidant defense system, i.e., scavenging of oxygen and hydroxyl radicals, reduction of lipid peroxyl radicals, inhibition of lipid peroxidation, or chelation of metal ions.[Citation13] Some methods determine the ability of antioxidants to scavenge free radicals generated in the reaction medium, such as the TEAC (trolox equivalent antioxidant capacity), ORAC (oxygen radical absorbance capacity), or TRAP (total reducing ability of plasma) assays, and some, such as xanthine/xanthine oxidase assay, determine the efficiency of antioxidants quenching singlet oxygen generated by the enzymatic system.[Citation13]
In most of the methods, irrespective of the stage in the oxidative chain in which the antioxidant action is assessed, a common mechanism involving a redox reaction takes place. FRAP assay developed by Benzie and Strain[Citation11] based on ferric reducing ability of antioxidant gets superiority because it is not dependent on the enzymatic/non-enzymatic method to generate free radical prior to evaluate the anti-radical activity of antioxidants. Initially designed to determine the antioxidant activity of plasma, it was also applied to other substrates, such as tea and wine, and renamed as the ferric reducing/antioxidant power assay.[Citation14,Citation15]
Ability to reduce the ferric ion by all five polyphenols in different capacities confirms their antioxidant property accordingly. The five polyphenolic compounds used in this study belong to the different classes of polyphenols. Gallic acid, known as 3, 4, 5-hydroxybenzoic acid, is a phenolic acid which belongs to the subclass hydroxybenzoic acid. Quercetin, myricetin, and catechin are flavonoids, while resveratrol belongs to the stilbene class of the polyphenolic compounds. Onions, grapes, tea, and red wine are the major sources of these active compounds and have frequently been attributed for their health protective effects.[Citation16–18]
Estimation of free radical scavenging activity of polyphenols via the DPPH• method present the advantage of using a stable and commercially available free radical; this method has been extensively applied to the study of antioxidant activity of food items, such as olive oil, fruits, juices, and wines.[Citation19–21] Easy to perform and high reproducibility adds an extra advantage over other methods. Our result is strongly supported by the studies of Sanchez-Moreno et al.,[Citation22] Villano et al.,[Citation23] and Iacopini et al.,[Citation24] in which they have reported that myricetin and gallic acid were the most effective polyphenols towards scavenging the DPPH• radical followed by quercetin and resveratrol.
Several factors played a role in determining the antioxidant, as well as radical scavenging, capacity of polyphenols. The antioxidant activity of monomeric phenolics depended on the degree of hydroxylation and extent of conjugation.[Citation25] While the presence of a 3′, 4′-dihydroxy structure in the B ring, the presence of a 2, 3-double bond in conjunction with the 4-oxo group in the heterocycle, allowing for conjugation between the A and B rings and presence of 3- and 5-hydroxyl groups in the A ring together with a 4-oxo function in the A and C rings, determine the radical scavenging ability of flavonoids.[Citation13,Citation26] It is known that monophenols are less efficient than polyphenols, but in gallic acid the inductive effect of the three hydroxyl groups is an important factor that may enhance the activity. The highest activities among flavonols correspond to those with an ortho-dyhydroxy structure on B ring and a –OH group at position 3, as occurs with quercetin. Since myricetin has an additional hydroxyl group, this may explain the slightly increased radical scavenging activity. In the FRAP assay, ortho-dihydroxy substitution in the B ring plays an important role in the ferric reducing activity of quercetin, resulting in increased FRAP value.
On the other hand, quercetin and myricetin (flavonols) and (+)-catechin (flavanol) share the same substitution pattern on the B ring but quercetin or myricetin have a 2,3 double bond and a 4-oxo function on the C ring, which increases electron delocalization and, therefore, greater ferric reducing/radical scavenging capacity. The stilbene, resveratrol, although having three hydroxyl groups and conjugation between both aromatic rings, has a structure different from the diphenylpropane skeleton of flavonoids, and no o-dihydroxy phenolic structure in the B ring. This structural arrangement may account for the low reducing power of resveratrol.
Study of reaction kinetics of DPPH• scavenging activity of polyphenols showed that the gallic acid elicited fast DPPH• inhibition in comparison to quercetin and myricetin. Catechin and resveratrol showed a slow free radical scavenging effect. In the study of kinetics of polyphenols with FRAP reagent, it was found that the reduction of the ferric-TPTZ complex continued beyond 4 min except for resveratrol and catechin. Conditions for the determination of the ferric reducing ability of antioxidants in the original method established a 4 min interval as suitable for such measurements, since the absorbance of the reduced ferrous-TPTZ complex was stable at this time.[Citation11] The time-dependent increase in the ferric reducing ability of polyphenols might imply an ability to maintain their antioxidant activity for longer times, helping to maintain an adequate antioxidant status in vivo. When these polyphenols are present in foods naturally or as food ingredients, a longer reducing ability with time might signify a longer protecting effect of polyphenols against oxidative damage of the food materials.[Citation13]
CONCLUSION
Since all the physiological conditions cannot be fulfilled by any available methods to measure antioxidant as well as anti-radical capacity of polyphenpls in vitro, the results obtained from our experiments cannot be correlated with the relative biological effectiveness of these polyphenols in vivo. The absolute values of antioxidant activity may vary from one study to another, even when the same test is used. The order of antioxidant activities may also differ, since different methods fallow different mechanisms. However, from the results obtained here and from the results reported by other authors, it is apparent that the degree of hydroxylation and extent of conjugation seems to be the ruling criteria for the reducing, as well as radical, scavenging power of dietary polyphenols. Our results on the five polyphenolics explain the active roles of these polyphenols, present in significant amount in most of the fruits, vegetables, and beverages as antioxidants to convert potentially toxic ROS into non-toxic species. This property confirms the use of these polyphenols in prevention of several oxidative stress-mediated degenerative diseases.
ACKNOWLEDGMENTS
Kanti Bhooshan Pandey is thankful to the Council of Scientific and Industrial Research (CSIR), New Delhi, India for the financial support as senior research fellowship.
REFERENCES
- Gil , L. , Siems , W. , Mazurek , B. , Gross , J. , Schroeder , P. , Voss , P. and Grune , T. 2006 . Age associated analysis of oxidative stress parameters in human plasma and erythrocytes . Free Radical Research , 40 : 405 – 505 .
- Halliwell , B. and Gutteridge , J.M.C. 2006 . Free Radicals in Biology and Medicine , 4th , Oxford : Clarendon Press .
- Droge , W. 2002 . Free radicals in the physiological control of cell function . Physiological Reviews , 82 : 47 – 95 .
- Cao , G. , Booth , S.L. , Sadowski , J.A. and Prior , R.L. 1998 . Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables . American Journal of Clinical Nutrition , 68 : 1081 – 1087 .
- Halliwell , B. , Murcia , M.A. , Chirico , S. and Aruoma , O.I. 1995 . Free radicals and antioxidants in food and in vivo: What they do and how they work . Critical Reviews in Food Science and Nutrition , 35 : 7 – 20 .
- Steinmetz , K.A. and Potter , J.D. 1996 . Vegetables, fruit, and cancer prevention: A review . Journal of the American Dietetic Association , 96 : 1027 – 1039 .
- Ness , A.R. and Powles , J.W. 1997 . Fruit and vegetables, and cardiovascular disease: A review . International Journal of Epidemiology , 26 : 1 – 13 .
- Hertog , M.G.L. 1996 . Epidemiological evidence on potential health properties of flavonoids . Proceedings of the Nutrition Society , 55 : 385 – 397 .
- Scalbert , A. , Manach , C. , Morand , C. and Remesy , C. 2005 . Dietary polyphenols and the prevention of diseases . Critical Reviews in Food Science and Nutrition , 45 : 287 – 306 .
- Pandey , K.B. and Rizvi , S.I. 2009 . Plant polyphenols as dietary antioxidants in human health and disease . Oxidative Medicine and Cellular Longevity , 2 : 270 – 278 .
- Benzie , I.F. and Strain , J.J. 1996 . The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay . Analytical Biochemistry , 239 : 70 – 76 .
- Miliauskas , G. , Venskutonis , P.R. and van Beek , T.A. 2004 . Screening of radical scavenging activity of some medicinal and aromatic plant extracts . Food Chemistry , 85 : 231 – 237 .
- Pulido , R. , Bravo , L. and Saura-Calixto , F. 2000 . Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay . Journal of Agricultural and Food Chemistry , 48 : 3396 – 3402 .
- Benzie , I.F. and Strain , J.J. 1999 . Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration . Methods in Enzymology , 299 : 15 – 27 .
- Benzie , I.F. and Szeto , Y.T. 1999 . Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay . Journal of Agricultural and Food Chemistry , 47 : 633 – 636 .
- Pandey , K.B. , Mishra , N. and Rizvi , S.I. 2009 . Protective role of myricetin on markers of oxidative stress in human erythrocytes subjected to oxidative stress . Natural Products Communications , 4 : 221 – 226 .
- Pandey , K.B. and Rizvi , S.I. 2010 . Protection of protein carbonyl formation by quercetin in erythrocytes subjected to oxidative stress . Medicinal Chemistry Reseach , 19 : 186 – 192 .
- Pandey , K.B. and Rizvi , S.I. 2010 . Protective effect of resveratrol on markers of oxidative stress in human erythrocytes subjected to in vitro oxidative insult . Phytotherapy Research , 24 : S11 – S14 .
- Ding , H. , Gao , Y. , Lei , H. , Luo , L. , Chao , H. and Ruan , R. 2010 . In vitro antioxidant effects of flavonoids of sweet potato vines . International Journal of Food Properties , 13 : 360 – 368 .
- Sanchez-Moreno , C. , Plaza , L. , De Ancos , B. and Cano , M.P. 2003 . Quantitative bioactive compounds assessment and their relative contribution to the antioxidant capacity of commercial orange juices . Journal of the Science of Food and Agriculture , 83 : 430 – 439 .
- Mandic , A.I. , Dilas , S.M. , Cetkovi , G.S. , Canadanovi-Brunet , J.M. and Tumbas , V.T. 2008 . Polyphenolic composition and antioxidant activities of grape seed extract . International Journal of Food Properties , 11 : 713 – 726 .
- Sánchez-Moreno , C. , Larrauri , J.A. and Saura-Calixto , F. 1998 . A procedure to measure the antiradical efficiency of polyphenols . Journal of the Science of Food and Agriculture , 76 : 270 – 276 .
- Villano , D. , Fernández-Pachón , M.S. , Moyá , M.L. , Troncoso , A.M. and García-Parrilla , M.C. 2007 . Radical scavenging ability of polyphenolic compounds towards DPPH free radical . Talanta , 71 : 230 – 235 .
- Iacopini , P. , Baldi , M. , Storchi , P. and Sebastiani , L. 2008 . Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions . Journal of Food Composition and Analysis , 21 : 589 – 598 .
- Hodnick , W.F. , Milosavljević , E.B. , Nelson , J.H. and Pardini , R.S. 1988 . Electrochemistry of flavonoids. Relationships between redox potentials, inhibition of mitochondrial respiration, and production of oxygen radicals by flavonoids . Biochemical Pharmacology , 37 : 2607 – 2611 .
- Rice-Evans , C.A. , Miller , N.J. and Paganga , G. 1996 . Structure-antioxidant activity relationships of flavonoids and phenolic acids . Free Radical Biology and Medicine , 20 : 933 – 956 .