Abstract
Microstructure, texture, and syneresis of Streblus asper leaf extract, calf rennet, and Fromase made milk coagula were investigated. Microstructure of the milk coagulum was assessed by scanning and transmission electron microscopy. Milk coagulum produced with Streblus asper showed a sponge-like structural network and a denser casein network compared with calf rennet and Fromase produced coagula. Streblus asper made coagulum displayed low porosity, low strength, and low syneresis than those of calf rennet and Fromase made coagula. The spong-like structure, low porosity, and low syneresis were attributed to the high proteolytic nature of Streblus asper leaf extact and to interactions of its phenolic compounds.
INTRODUCTION
The milk coagulant traditionally used for cheese making is calf rennet, which is extracted from the fourth stomach of milk-fed calves. A decrease in supply of calf rennet has led to an intensive search for suitable rennet substitutes.[Citation1–3] Several plant rennets were investigated recently and some were suggested to be suitable milk coagulants for cheese making.[Citation4–7] The most obvious function of a cheese making coagulant is the conversion of liquid milk to a coagulum or gel.[Citation8] Properties of the resulting milk coagulum, which are important factors in determining cheese quality, are largely affected by the type of the coagulant used. Plant coagulants are more proteolytic than calf rennet, and it has been suggested that their increased proteolytic activity could be beneficial in shortening ripening time of cheese compared to calf rennet.[Citation9] Texture, which is one of the most important characteristics of cheese, is influenced to a great extent by the type of the coagulant.[Citation10] Study of microstructure of curd could be useful in evaluation of the potential suitability of a milk coagulating protease as a rennet substitute. Microstructure of many dairy products has been examined by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) and evaluated using different methods.[Citation10–13] However, few investigations of microstructure of curd relative to the type of milk coagulant used have been reported. Microstructure of milk gels made from some plant coagulants and chymosin were studied using confocal scanning laser microscopy.[Citation14] Leaf extract of Streblus asper, a tree known in Malaysia as Kesinai, has been reported to contain a milk coagulating protease that could be useful in cheese making.[Citation15–17] However, microstructure of its milk coagulum has not been studied. This article reports on a comparative evaluation of microstructure (using SEM and TEM), texture, and syneresis of Streblus asper leaf extract, calf rennet, and Fromase milk coagulum.
MATERIALS AND METHODS
Preparation of Enzyme Samples
Streblus asper milk coagulant was extracted from the plant leaves in 10 mM Tris-HCl, buffer pH 7.2 containing 2 mM sodium metabisulphite, according to the method described by Idris et al.[Citation18] Calf rennet (Sigma Chemicals Co., St. Louis, MO, USA) and Fromase (Gist-brocades, Seclin, France) were dissolved in distilled water to the desired protein content (1.3 mg protein/ml) and stored at −20°C till used.
Preparation of the Milk Coagulum
Milk coagulum was prepared from fresh cow's milk and calcium chloride was added to the milk to a concentration of 0.2 mM. One hundred milliliters of milk in a glass beaker was equilibrated at 37°C in a water bath. An aliquot (2.0 ml) of the respective rennet or extract was added and the mixture stirred with a glass rod for 15 s. The mixture was incubated in a water bath set at 37°C and left undisturbed till a coagulum was formed.
Preparation of Samples for SEM and TEM Examination
Milk coagulum was prepared in triplicate, with each of Streblus asper leaf extract, Fromase, and calf rennet. Samples for SEM were prepared according to the procedure described by Kalab[Citation19] with little modification. SEM samples were transferred into specimen baskets and critical point dried for 30 min in a critical point drier (CPD 030; BAL-TEC, Witten/Ruhr, Germany) with CO2 as the drying agent. The dried pieces were mounted on specimen stubs using high conductivity fast drying silver suspension (Acheson Colloids, Prince Rock, England), and were double gold coated (2 min each time) in SEM coating unit E5100 (Polaron Equipment LTD, Watford, England). The samples were examined (7000×) in JEM 6400 scanning electron microscope (JEOL, Eching, Germany). Dehydrated TEM specimens were infiltrated with 1:1 acetone and resin mixture for 1 h, followed by 1:3 acetone and resin mixture for 2 h and then infiltrated with 100% resin overnight followed by 100% resin for 2 h. The resin infiltrated specimens were embedded by placing into beam capsules and the capsules were filled up with resin. The embedded specimens were polymerized at 60°C for 48 h. Specimens were then cut into 1-μm thick sections using a glass knife and ultramicrotome and were stained with toluidine blue (AGAR Scientific LTD, Stansted, Essex, UK). Ultra-thin sections (70 nm) were cut with a diamond knife. The sections were stained with saturated alcoholic urnyl acetate (BDH Chemicals Ltd., Poole, England) for 10 min, and subsequently washed with 50% filtered ethanol. Sections were lead stained, washed with double distilled water, and viewed at 30,000× using a Hitachi H-8000 transmission electron microscope (Tokyo, Japan). Porosity of the milk coagulum was determined by quantification of pores fractional area (AA) of SEM and TEM micrographs using a 24 × 16 line rectangular test grid with 384 intersection points according to the method described by Gundersen et al.[Citation20] The relative values were transformed to Arcsine values for statistical analysis.[Citation21]
Coagulum strength was determined in four replicates by pushing a 10-mm diameter cylindrical probe 25 mm (approximately halfway) into the milk coagulum at a speed of 5 mm/min using the Texture Analyser (SMS, Godalming, UK). Load cell was 2.0 Kg and trigger was 1.0 g with a pre-speed of 2.0 mm/min and a post speed of 2.0 mm/min. Coagulum strength was considered to be the maximum force (g) required to push the probe halfway into the milk coagulum. Measurements were made at room temperature (∼26°C) approximately 1 h after coagulation.
The extent of syneresis was determined by measuring milk sample volume and that of the whey separated from the coagulum by filtration. A funnel containing Whatman filter paper (No. 4, Whatman Corp., Piscataway, NJ, USA) was placed mouth down on top of the beaker containing the sample. The funnel and beaker were then inverted and the whey was allowed to drain into a 100-mL measuring cylinder. The volume of the collected whey was compared to the original volume of the milk sample to determine the extent of syneresis.[Citation22] Data on coagulum porosity, coagulum strength, and whey volume was analyzed for significance of difference by the t-test using SAS.[Citation23]
RESULTS AND DISCUSSION
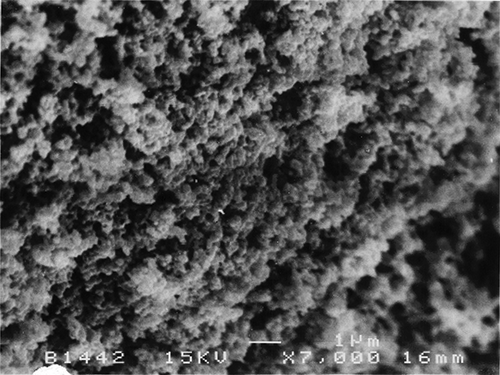
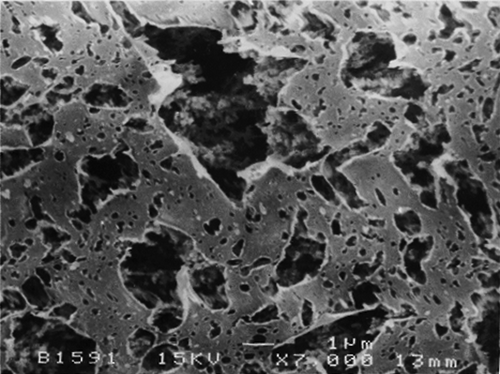
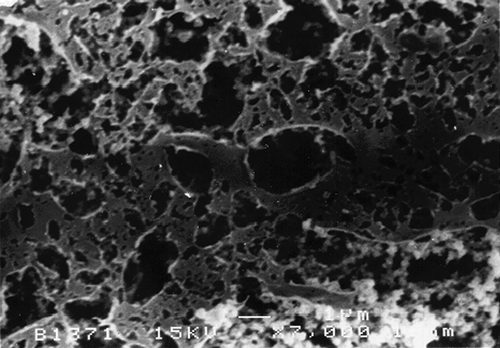
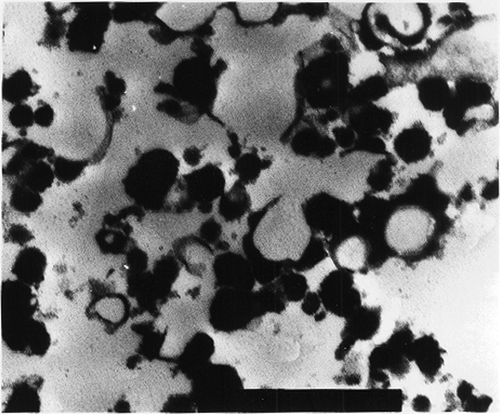
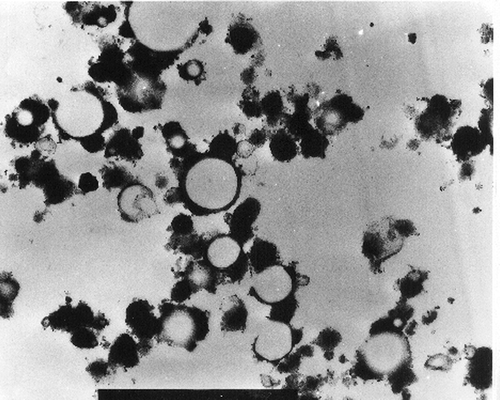
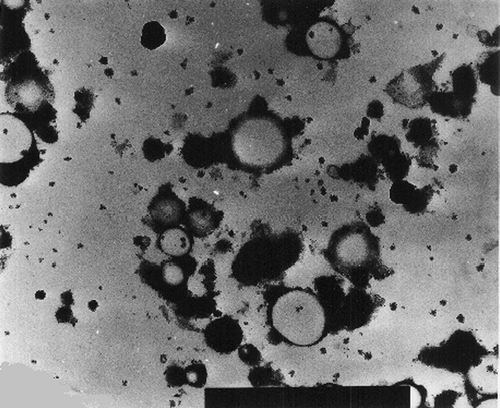
The SEM micrographs of Streblus asper, calf rennet, and Fromase coagulum are shown in Figs. , respectively. TEM micrographs of Streblus asper, calf rennet, and Fromase coagulum are shown in Figs. , respectively. The SEM micrographs showed that protein aggregates in curd formed with Streblus asper extract () appeared as a sponge-like structural network, whereas the protein material in curd produced with calf rennet () and Fromase () appeared as a fibrous framework. Pores in Streblus asper milk coagulum appeared smaller than those of calf rennet and Fromase coagulum.
The formation of a sponge-like structural network by Streblus asper milk coagulum could be due to the difference in the nature and proteolytic specificity of Streblus asper protease as compared to calf rennet and Fromase. It has been established that calf rennet cleaves phenylalanine105–methionine106 peptide bond of κ-casein, and the splitting of this particular bond has been claimed to be responsible for the primary phase of milk coagulation by rennet.[Citation24] Possibly, Streblus asper protease attacks other bonds and/or casein fractions, thus causing a different interlinkage mechanism. The leaf extract of Streblus asper is rich in phenolic compounds.[Citation16] The presence of polyphenols in Streblus asper leaf extract could be responsible, at least in part, for the characteristic microstructure of its milk coagulum, where the effect could be through new cross-linkages between caseins molecules by the multidentate polyphenols. It has been reported[Citation25,Citation26] that phenolic compounds interact with proteins, and that hydrogen and hydrophobic bonds are the predominant interactive forces;[Citation27] however, covalent interactions between phenolic compounds and proteins have also been suggested.[Citation26]
Quantification of coagulum porosity using both SEM and TEM micrographs showed that Streblus asper coagulum had a lower porosity compared with those of calf rennet and Fromase (). Results of quantification of porosity using SEM micrographs showed that surface porosity of milk coagulum produced with Streblus asper leaf extract was significantly lower than those produced with calf rennet and Fromase (P ≤ 0.05). Results of quantification of coagulum interior porosity using TEM micrographs revealed that Streblus asper extract-produced coagulum had significantly lower porosity (P ≤ 0.05) than those produced with calf rennet and Fromase. Furthermore, calf rennet produced coagulum had significantly lower interior porosity than that produced with Fromase (P ≤ 0.05). These results indicate clearly that the surface and interior porosity of Streblus asper coagulum were consistently lower than those of the two other coagulum types. Furthermore, the results showed that of the three coagula Streblus asper coagulum had the most dense structure followed in descending order by Fromase and calf rennet produced ones.
Table 1 Porosity of Streblus asper, calf rennet, and Fromase milk coagulum using scanning electron microscopy (SEM) and transmission electron microscopy (TEM) micrographs
Strength of Streblus asper coagulum was significantly lower P ≤ 0.05) than that of calf rennet and Fromase coagula (). Fromase coagulum had significantly higher (P ≤ 0.05) strength than that produced with calf rennet. The extent of syneresis of Streblus asper coagulum expressed as percent of whey volume to original milk volume, was lower than those produced with calf rennet and Fromase. Syneresis of calf rennet and Fromase produced coagula were, respectively, 12 and 14% higher than that produced with Streblus asper leaf extract.
Table 2 Assessment of texture and whey volume of Streblus asper, calf rennet, and Fromase milk coagulum
Streblus asper coagulum, albeit denser than those produced by calf rennet and Fromase, had lower strength. The low coagulum strength, an indicative of soft texture, could be due to incomplete curd fusion. Cheeses with incomplete curd fusion were reported to have lower texture scores and discontinuous protein matrix.[Citation28] The low strength of Streblus asper milk coagulum could also be partially attributed to the high concentration of phenolic compounds in the leaf extract. Plant extracts rich in phenolic compounds were reported[Citation29] to increase rennet coagulation time of milk and the effect was thought to be on the aggregation of the enzymatically modified micelles rather than on the primary phase of milk coagulation. Polyphenols are highly reactive compounds with a multiplicity of potential binding sites, i.e., phenolic groups,[25,27] and as such they could in one way or another interfere with the secondary phase of enzymatic coagulation of milk, thus, producing a weak milk coagulum. The ability of phenolic compounds to chelate metal ions has been established,[Citation30] and chelation of Ca2+ by phenolic compounds could interfere with the rennet coagulation of milk, which would result in a soft milk coagulum.
The results showed that Streblus asper coagulum expelled the least whey, while those of calf rennet and Fromase are comparable to each other. The low whey volume expelled by Streblus asper coagulum could be due to low curd shrinkage, which is the driving force for curd firmness and syneresis. Syneresis is an important step in the reduction of moisture content of cheese. The tendency of a gel network to shrink can be expressed as the pressure exerted by the network on the entrapped moisture, and the moisture, therefore, tends to flow out but the flow is hindered by the viscous resistance exerted by the gel network on the liquid streaming through the pores.[Citation31] Low syneresis of Streblus asper coagulum could be partly due to incomplete transformation of casein into paracasein, thus, resulting in lower dehydration compared to calf rennet and Fromase coagulum.
Curd structure had a direct influence on the structure and texture of cheese.[Citation13] The nature of the coagulant may affect the structure and properties of the curd and cheese. The main action is on the extent of proteolysis during manufacturing and ripening. The more proteolytic enzymes appear to cause the curd to form slowly. Curd and cheese formed with bovine pepsin have more open, looser structure than those formed with calf rennet.[Citation10] Fat losses during cheese making were higher with pepsin, indicating that the curd with the more open structure retained less fat.
CONCLUSION
Streblus asper leaf extract was found to produce a milk coagulum with a sponge-like microstructure and a denser casein network, compared with calf rennet and Fromase produced coagula. Milk coagulum obtained using Streblus asper extract was very soft and expelled less whey compared to commercial rennet preparations. These properties suggest that the milk coagulating enzyme in Streblus asper leaf extract was highly proteolytic.
REFERENCES
- Richardson , G.H. , Nelson , J.H. , Lubnov , R.E. and Schwarberg , R.L. 1967 . Rennin-like enzyme from Mucor pusillus for cheese manufacture . Journal of Food Science , 50 : 1066 – 1072 .
- Ogundiwin , J.O. and Oke , O.L. 1983 . Factors affecting the processing of wara-a Nigerian cheese . Food Chemistry , 11 : 1 – 13 .
- Campos , R. , Guerra , R. , Aguilar , M. , Ventura , O. and Camacho , L. 1990 . Chemical characterization of proteases extracted from wild thistle (Cynara cardunculus) . Food Chemistry , 35 : 89 – 97 .
- Hou-Pin , S. , Mei-Ju , H. and Han-Tsung , W. 2009 . Characterization of ginger proteases and their potential as a rennin replacement . Journal of the Science of Food and Agriculture , 89 ( 7 ) : 1178 – 1185 .
- Bruno , M. , Lazza , C.M. , Errasti , M.E. , Lopez , L.M.I. , Caffini , N.O. and Pardo , M.F. 2010 . Milk clotting and proteolytic activity of an enzyme preparation from Bromelia hieronymi fruits . LWT—Food Science and Technology , 43 ( 4 ) : 695 – 701 .
- Ahmed , I.A.M. , Babiker , E.E. and Mori , N. 2010 . pH stability and influence of salt on activity of a milk clotting enzyme from Solanum dubium seeds and its enzymatic action on bovine caseins . LWT—Food Science and Technology , 43 ( 5 ) : 759 – 764 .
- Bornaz , S. , Guizani , N. , Fellah , N. , Sahli , A. , Ben Slama , M. and Attia , H. 2010 . Effect of plant originated coagulants and chymosin on ovine milk coagulation . International Journal of Food Properties , 13 ( 1 ) : 10 – 22 .
- Green , M.L. 1977 . Review of the Progress of Dairy Science: Milk coagulants . Journal of Dairy Research , 44 : 159 – 188 .
- Galan , E. , Prados , F. , Tejada , L. and Fernandez-Salguero , J. 2008 . Influence of different amounts of vegetable coagulant from cardoon Cynara cardunculus and calf rennet on the proteolysis and sensory characteristics of cheese made with sheep milk . International Dairy Journal , 18 ( 1 ) : 93 – 98 .
- Eino , M.F. , Biggs , D.A. , Irvine , D.M. and Stanley , D.W. 1976 . A comparison of microstructure of cheddar cheese curd manufactured with calf rennet, bovine pepsin, and porcine pepsin . Journal of Dairy Research , 43 : 113 – 115 .
- Kalab , M. and Harwalker , V.R. 1973 . Milk gel structure. I. Application of scanning electron microscopy to milk and other food gels . Journal of Dairy Science , 56 : 835 – 842 .
- Kalab , M. and Harwalker , V.R. 1974 . Milk gel structure. II. Relations between firmness and ultrastructure of heat-induced skim-milk gels containing 40–60% total solids . Journal of Dairy Research , 41 : 131 – 135 .
- Green , M.L. , Turvey , A. and Hobbs , D.G. 1981 . Development of structure and texture of cheddar cheese . Journal of Dairy Research , 48 : 343 – 355 .
- Esteves , C.L.C. , Lucey , J.A. , Wang , T. and Pires , E.M.V. 2003 . Effect of pH on the gelation properties of skim milk gels made from plant coagulants and chymosin . Journal of Dairy Science , 86 : 2558 – 2567 .
- Manap , M.Y. , Sipat , A. , Lajis , M.N.H. and Ibrahim , F.H. 1992 . Coagulation of milk using plant (Streblus asper) extract . Scienza Etecnica Lattiero-Casearia , 3 : 37 – 43 .
- Idris , Y.M.A. , Yazid , A.M. and Sipat , A.B. 1998 . Decolourisation of Streblus asper (Kesinai) leaf extract . Asia Pacific Journal of Molecular Biology and Biotechnology , 6 : 187 – 190 .
- Ishak , R. , Idris , Y.M.A. , Mustafa , S. , Sipat , A. , Syed Muhammed , S.K. and Abd Manap , M.Y. 2006 . Factors affecting milk coagulating activities of Kesinai (Streblus asper) extract . International Journal of Dairy Science , 1 ( 2 ) : 131 – 135 .
- Idris , Y.M.A. , Sipat , A.B. , Shuhaimi , M. and Yazid , A.M. 1999 . Inhibition of enzymatic browning during extraction of a milk coagulating protease from Streblus asper (Kesinai) . Pakistan Journal of Biological Sciences , 2 ( 2 ) : 378 – 381 .
- Kalab , M. 1981 . Electron microscopy of milk products: A review of techniques . Scanning Electron Microscopy , 3 : 453 – 472 .
- Gundersen , H.J.G. , Bendtsen , T.F. , Korbo , L. , Marcussen , N. , Moller , A. , Nielsen , K. , Nyengaard , J.R. , Pakkenberg , B. , Sorensen , F.B. , Vesterby , A. and West , M.J. 1988 . Some new, simple and efficient stereological methods and their use in pathological research and diagnosis . Acta Pathologica, Microbiologica Et Immunologica Scandinavica , 96 : 379 – 394 .
- Gomez , K.A. and Gomez , A.A. 1984 . Statistical Procedures for Agricultural Research; , 307 New York : John Wiley and Sons .
- Smith , M. and McMahon , D.J. 1996 . Aseptic rennet coagulation of ultra-high temperature processed milk concentrates . Journal of Dairy Science , 79 : 1513 – 1520 .
- SAS . 1990 . SAS/STAT User's Guide, Version 6 , Fourth , Vol. 2 , Cary , NC : SAS Institute Inc .
- Dalgleish , D.G. 1979 . Proteolysis and aggregation of casein micelles treated with immobilized or soluble chymosin . Journal of Dairy Research , 46 : 653 – 661 .
- Spencer , C.M. , Cia , Y. , Martin , R. , Gaffney , S.H. , Goulding , P.N. , Magnalato , D. , Lilley , T.H. and Haslam , E. 1988 . Polyphenol complexation—Some thoughts and observations . Phytochemistry , 27 : 2397 – 2409 .
- Meek , K.M. and Weiss , J.B. 1979 . Differential fixation of poly (L-arginine) and poly (L-lysine) by tannic acid and its application to the fixation of collagen in electron microscopy . Biochemica Et Biophysica Acta , 587 : 112 – 120 .
- Haslam , E. and Lilley , T.H. 1988 . Natural astringency in foodstuffs—A molecular interpretation . CRC Critical Reviews in Food Science and Nutrition , 27 : 1 – 40 .
- Anderson , D.L. and Mistry , V.V. 1994 . Reduced fat cheddar cheese from condensed milk: Microstructure . Journal of Dairy Science , 77 : 7 – 15 .
- O'Connell , J.E. and Fox , P.F. 1999 . Effects of extracts of oak (Quercus petraea) bark, oak leaves, aloevera (Curacao aloe), coconut shell and wine on the colloidal stability of milk and concentrated milk . Food Chemistry , 66 : 93 – 96 .
- McDonald , M. , Mila , I. and Scalbert , A. 1996 . Precipitation of metal ions by plant polyphenols: Optimal conditions and origin of precipitation . Journal of Agricultural and Food Chemistry , 44 : 599 – 606 .
- Walstra , P. , van Dijk , H.J.M and Geurts , T.J. 1987 . “ Syneresis of curd ” . In Cheese: Chemistry, Physics and Microbiology , Edited by: Fox , P.F . 135 – 177 . London , , New York : Elsevier Applied Science .