Abstract
Punica granatum L. (Family: Punicaceae), commonly known as pomegranate (Anar) has been used traditionally as medicine to treat a number of diseases and disorders. Various parts of the plant and their active constituents are known to possess diverse biological activity. However, little is known about the antioxidant potential of pomegranate fruit peel, which is otherwise considered as waste. Therefore, the concentration-dependent hydroxyl radical scavenging ability of Punica granatum seed and peel extracts (alcoholic and aqueous) using deoxyribose degradation assay were analyzed and compared. The hydroxyl radical scavenging activity at different concentrations of extract (10 to 250 μg/mL) both in presence and/or absence of ascorbic acid and ethylenediamine tetra acetic acid was determined. It was observed that a higher concentration of extract suppresses scavenging activity and lower promotes antioxidant property. Based on the observations, it may be inferred that pomegranate extract, especially from the spent/waste prt, has a strong antioxidant property as assessed by its property of scavenging hydroxyl radical formation.
INTRODUCTION
Dietary habits play an important role in human health. The human diet contains a wide variety of natural components, such as phenolic compounds, fibers, flavonoids, isoflavones, and sterols, which besides enriching our diet act as natural medicare. Dietary consumption of the natural antioxidants present in the fruits and vegetables serve as an important factor in the body's defense mechanism against various reactive oxygen species generated within the body that are responsible for an array of diseases.[Citation1]
Punica granatum Linn. (Punicaceae), commonly known as pomegranate, is a shrub or a small tree, native to the Mediterranean region used extensively as the dietary folk medicine of many cultures. The plant is known for its immense therapeutic value since time immemorial. A number of biological activities, such as antitumour, antibacterial, antidiarrhoeal, antihysteric, diuretic, carminative, astringent, antifungal, and antiulcer have been ascribed to the various parts of the plant.[Citation2–6 Gulnar, the flowers of the pomegranate plant have been extensively used in the Unani and Ayurvedic system of medicines as they possess strong astringent, antidiabetic, antidiarrhoel capacity, and their decoction stops bleeding and purging.[Citation7,Citation8] In folk medicine, pomegranate preparations, especially of the dried pericarp, but also of the roots, barks of the tree and roots are employed as per orum medication in the treatment of colic, colitis, diarrhea, dysentery, leucorrhea, menorrhagia, oxyuriasis, paralysis, and rectocele, and to the nape of the neck in mumps and headache.[Citation9] Pomegranate is now gaining attention due to its potent antioxidant capacity.[Citation10–13 Various researches on the antioxidant capability and thermal transition of pomegranate fruit juice, fruit, and peel extracts have been performed, which suggests them to be constituted of potent antioxidant agents.[Citation14,Citation15] Fresh juice of pomegranate contains a small amount of pectin, ascorbic acid, and polyphenolic flavonoids. The soluble polyphenolic content of pomegranate juice (0.2–1.0 g/100 g) includes anthocyanins, catechins, tannins, and gallic and ellagic acids, which are known for their antioxidant capacity.[Citation9,Citation16] The active constituent of pomegranate, namely, punicotannic acid, punicic acid, gallic acid, mannite, pelletierine, N-methylisopelletierine, ellagitannins, pelargonidin, punicalin, punicalagin, anthocyanins, cyanidin, and ellagic acid possess antioxidant, anti-ageing, anti-viral, anti-osteoarthritis, anti-atherosclerotic, anti-hepatotoxic, anti-tumor, anti-angiogenic, antimutagenic, and hypolipidemic properties.[Citation17–19 Various parts of the plant and their active constituents are known to possess diverse biological activity. However, little is known about the antioxidant potential of pomegranate fruit peel, which is otherwise considered as waste. The present investigation was undertaken to study and compare the concentration-dependent hydroxyl radical scavenging ability of seed and peel extract of pomegranate using deoxyribose degradation assay.
MATERIALS AND METHODS
Collection of Plant Material
The fruit of Punica granatum were either collected from the CIMAP Research Farm and/or purchased from a local market. The materials were authenticated by the taxonomist of the Institute and the herbarium specimen was deposited in Gyan Surabhi of CIMAP, Lucknow (Voucher Specimen No. 9078).
Extract Preparation
The seed and peel samples were washed properly, shade dried at 40°C. The dried samples were extracted with 100% ethanol or distilled water. The extracts were collected at least three times and were filtered through Whatman No.1 filter paper and then concentrated on a rotary evaporator (Buchi, Flavil, Switzerland) at 45°C, dried and kept at 4°C till used for the assay. The sample and solvent mass ratio was 1:2 during extraction. The extracts were dissolved/solubilised in dimethyl sulfoxide (DMSO) and diluted with sterile water to get the final concentration as per requirement.
Preparation of Buffer and Reagents for Hydroxyl Radical Scavenging (HRS) Assay
Phosphate buffer (20 mM, pH 7.4): 272.2 mg potassium dihydrogen phosphate and 356 mg disodium hydrogen phosphate dissolved in 100 ml of distilled water mixed and pH adjusted at 7.4. Reagent solutions for HRS assay was prepared as described in .[Citation20] Thiobarbituric acid (TBA, 1%) and trichloroacetic (TCA, 2%) acid solution was prepared in double distilled water.
Table 1 Reagents (A–D) for measuring hydroxyl radical scavenging activity
Deoxyribose Degradation (HRS) Assay
The method of HRS assay was performed according to the procedure described previously with minor modification.[Citation21–23 Different concentrations of the sample (10, 50, 100, 250 μg/mL) were mixed with 200 μl reaction mixture (A, B, C, and D). Then the samples were incubated at 37°C for 60 min. Additionally, 100 μl of 2% TCA and 100 μl of 1% TBA were added and the reaction mixture was heated at 100°C for 15 min. Similarly, appropriate control (DMSO) was also run parallel and the absorbance was read at 532 nm. All the determinations were performed in duplicates and values plotted were mean ± SE of three independent analyses. Percent of scavenging activity was calculated by using the formula: Control − Experimental/Control × 100. The difference in the percent scavenging activity of experimental values was found to be significant (p < 0.05) compared to control in student's t test.
RESULTS AND DISCUSSION
The concentration-dependent hydroxyl radical scavenging activity of aqueous and alcoholic extracts of seed and peel of Punica granatum was tested by performing in vitro deoxyribose degradation assay and observations are presented in a–1d). The hydroxyl radical (•OH) is an extremely reactive oxidizing radical produced when superoxide and hydrogen peroxide react together (Haber-Weiss Reaction) affecting many biomolecules, including purine and pyrimidine bases of DNA.[Citation24] It is produced in vivo by Fenton Reaction that abstract hydrogen atom from biological molecules.[Citation25–28]
Figure 1 Concentration-dependent hydroxyl radical scavenging effect of seed and peel extracts of Punica granatum L. (a) In presence of EDTA but absence of ascorbic acid; (b) In absence of both EDTA and ascorbic acid; (c) In presence of ascorbic acid but absence of EDTA; (d) In presence of both EDTA and ascorbic acid. PPW: Punica peel aqueous extract; PPE: Punica peel ethanol extract; PSE: Punica seed ethanol extract; PSW: Punica seed aqueous extract. Values are mean ± SE of three independent analysis.
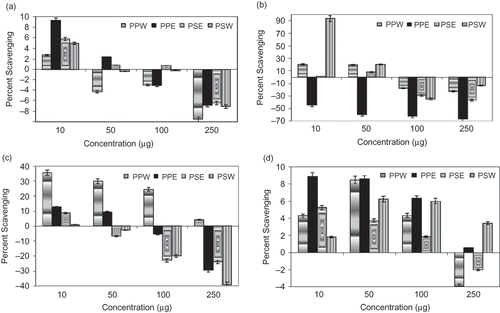
In the deoxyribose assay, a mixture of Fe+++-EDTA, H2O2, and ascorbic acid generates hydroxyl radical, which can be detected by their ability to degrade the sugar deoxyribose into fragments.[Citation29] The resulting complex mixture of products is heated under acidic conditions; malonaldehyde is formed that can be detected by its ability to react with thiobarbituric acid to form a pink chromogen. A scavenger of •OH∼ can inhibit the deoxyribose damage and a pro-oxidant can accelerate the damage.[Citation30–32 Degradation of deoxyribose by •OH radicals produced from Fenton-type reactions has been a useful measurement for studying the conditions underlying free radical initiated oxidative stress and antioxidant activity of various test compounds.[Citation33–36 In this method, the non site-specific assay measures the antioxidant efficacy of the phytochemicals based on their efficacy to compete with deoxyribose for •OH radicals that were produced free in solution from a Fe++-EDTA chelate.
The aqueous and alcoholic extracts of seed and peel of Punica granatum exhibits a potent scavenging activity for hydroxyl radical in a concentration-dependent manner (10 to 250 μg/mL). The omission of ascorbate from the reaction mixture promotes generation of hydroxyl radicals and causes degradation of deoxyribose ( and ). The use of EDTA in the reaction system is essential because inhibition of iron-dependent deoxyribose degradation in the absence of EDTA depends not only on the ability of a scavenger to react with •OH but also on its ability to form complexes with iron ( and ). The hydroxyl radical scavenging effect of aqueous and alcoholic extracts of the seed and peel of Punica granatum at different concentrations in the presence of EDTA and ascorbic acid show a strong scavenging effect (). The experiment was done under four conditions to derive two separate inferences, i.e., the role on hydroxyl trapping and role of metal chelation. These conditions are referred to as ‘non-site-specific assay’, where EDTA is added and the other is ‘site specific assay’, where no EDTA is added. In the former case, EDTA forms a complex with Fe+++, and hydroxyl radicals are generated in solution, whereas in the latter, EDTA is not available, therefore, Fe+++ can bind directly to the deoxyribose molecule and produce hydroxyl radicals itself. The inhibition of the ribose degradation by the extract(s) or phytochemical(s) in a concentration-dependent manner, in absence of EDTA, indicates a possibility of chelating the iron ion and also trapping the •OH radical as reported.[Citation20,Citation23,Citation37]
CONCLUSION
Alcoholic and aqueous extracts of Punica granatum seed and peel were tested for hydroxyl radical scavenging activity at different concentrations (10–250 μg/mL) using deoxyribose degradation assay. The activity was determined both in presence and/or absence of ascorbic acid and ethylenediamine tetra acetic acid. Our results suggest that pomegranate extract especially from the spent/waste part has a strong antioxidant property as assessed by its property of scavenging hydroxyl radical formation. From the present study, it may also be inferred that a higher concentration of extract suppresses scavenging activity and a lower concentration promotes antioxidant property.
ACKNOWLEDGMENTS
This paper was presented at the 4th Nutraceutical Summit 2008, World Trade Centre, Mumbai, India. This work was supported by the Department of Science and Technology (DST), Ministry of Science and Technology, Government of India under Fast Track Scheme for Young Scientists. The authors are thankful to the Director, CIMAP for constant encouragement, support, and providing necessary facilities; they are also grateful to Ms. Suchita Srivastava for her help during the preparation of this manuscript.
REFERENCES
- Balsano , C. and Alisi , A. 2009 . Antioxidant effects of natural bioactive compounds . Current Pharmaceutical Design , 15 ( 26 ) : 3063 – 3073 .
- Afaq , F. , Saleem , M. , Krueger , C.G. , Reed , J.D. and Mukhtar , H. 2005 . Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice . International Journal of Cancer , 113 : 423 – 433 .
- Prashanth , D. , Asha , M.K. and Amit , A. 2001 . Antibacterial activity of Punica granatum . Fitoterapia , 72 : 171 – 173 .
- Das , A.K. , Mandal , S.C. , Banerjee , S.K. , Sinha , S. , Das , J. , Saha , B.P. and Pal , M. 1999 . Studies on antidiarrhoeal activity of Punica granatum seed extract in rats . Journal of Ethnopharmacology , 68 : 205 – 208 .
- Dutta , B.K. , Rahman , I. and Das , T.K. 1998 . Antifungal activity of Indian plant extracts . Mycoses , 41 : 535 – 536 .
- Gharzouli , K. , Khennouf , S. , Amira , S. and Gharzouli , A. 1999 . Effects of aqueous extracts from Quercus ilex L. root bark, Punica granatum L. fruit peel and Artemisia herba-alba Asso leaves on ethanol-induced gastric damage in rats . Phytotherapy Research , 13 : 42 – 45 .
- Jafri , M.A. , Aslam , M. , Javed , K. and Singh , S. 2000 . Effect of Punica granatum Linn. (flowers) on blood glucose level in normal and alloxan induced diabetic rats . Journal of Etnopharmacology , 70 : 309 – 314 .
- Sivarajan , V.V. and Balachandran , I. 1994 . Ayurvedic Drugs and Their Plant Sources , Edited by: Mohan , P. 1 – 590 . New Delhi , , India : Oxford and IBH Publishing Co Ltd .
- Negi , P.S. and Jayaprakasha , G.K. 2003 . Antioxidant and antibacterial activities of Punica granatum peel extracts . Journal of Food Science , 68 : 1473 – 1477 .
- Gil , M.I. , Tomas-Barberan , F.A. , Hess-Pierce , B. , Holcroft , D.M. and Kader , A.A. 2000 . Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing . Journal of Agriculture and Food Chemistry , 48 : 4581 – 4589 .
- Noda , Y. , Kaneyuki , T. , Mori , A. and Packer , L. 2002 . Antioxidant activities of pomegranate fruit extract and its anthocyanidins: Delphinidin, cyanidin, and pelargonidin . Journal of Agriculture and Food Chemistry , 50 : 166 – 171 .
- ChidambaraMurthy , K.N. , Jayaprakasha , G.K. and Singh , R.P. 2002 . Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models . Journal of Agriculture and Food Chemistry , 50 : 4791 – 4795 .
- Singh , R.P. , ChidambaraMurthy , K.N. and Jayaprakasha , G.K. 2002 . Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models . Journal of Agriculture and Food Chemistry , 50 : 81 – 86 .
- Cam , M. , Hisil , Y. and Durmaz , G. 2009 . Characterisation of pomegranate juices from ten cultivars grown in turkey . International Journal of Food Properties , 12 : 388 – 395 .
- Hobani , A.I. and Elansari , A.M. 2004 . Thermal transitions of pomegranate extracts using modulated differential scanning calorimeter (MDSC) . International Journal of Food Properties , 7 ( 3 ) : 671 – 681 .
- Aviram , M. , Dornfeld , L. , Rosenblat , M. , Volkova , N. , Kaplan , M. , Coleman , R. , Hayek , T. , Presser , D. and Fuhrman , B. 2000 . Pomegranate juice consumption reduces oxidative stress, atherogenic modification to LDL and platelet aggregation: Studies in human and in atherosclerotic apolipoprotein deficient mice . American Journal of Clinical Nutrition , 71 : 1062 – 1076 .
- Valadares , M.C. , Pereira , E.R.T. , Benfica , P.L. and Paula , J.R. 2010 . Assessment of mutagenic and antimutagenic effects of Punica granatum in mice . Brazilian Journal of Pharmaceutical Sciences , 46 ( 1 ) : 121 – 127 .
- Li , Y.F. , Guo , C.J. , Yang , J.J. , Wei , J.Y. , Xu , J. and Cheng , S. 2006 . Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract . Food Chemistry , 96 : 254 – 260 .
- Negi , P.S. , Jayaprakasha , G.K. and Jena , B.S. 2003 . Antioxidant and antimutagenic activities of pomegranate peel extracts . Food Chemistry , 80 : 393 – 397 .
- Halliwell , B. , Gutteridge , M.C. and Aruoma , O.I. 1987 . The deoxyribose method: A simple test-tube assay for determination of rate constants for reactions of hydroxyl radicals . Analytical Biochemistry , 165 : 215 – 219 .
- Paya , M. , Halliwell , B. and Hout , J.R.S. 1999 . Interactions of a series of coumarins with reactive oxygen species . Scavenging of superoxide, hypochlorous acid and hydroxyl radicals. Biochemical Pharmacology , 44 : 205 – 214 .
- Aruoma , O.I. 1994 . Deoxyribose assay for detecting hydroxyl radicals . Methods in Enzymology , 233 : 57 – 66 .
- Luqman , S. , Kumar , R. , Srivastava , S. , Darokar , M.P. , Lal , R.K. , Bahl , J.R. and Khanuja , S.P.S. 2008 . Hydroxyl radical scavenging activity of aromatic grass ‘Khus’ (Vetiveria zizanioides) . Journal of Medicinal and Aromatic Plant Sciences , 30 : 320 – 324 .
- Cheeseman , K.H. and Slater , T.F. 1993 . An introduction to free radical biochemistry . British Medical Bulletin , 49 : 481 – 493 .
- Halliwell , B. and Gutteridge , J.M. 1992 . Biologically relevant metal ion-dependent hydroxyl radical generation . An update. FEBS Letters , 307 : 108 – 112 .
- Halliwell , B. 1999 . Antioxidant defense mechanisms: From the beginning to the end (of the beginning) . Free Radical Research , 31 : 261 – 272 .
- Puppo , A. and Halliwell , B. 1988 . Formation of hydroxyl radicals from hydrogen peroxide in the presence of iron . Is haemoglobin a biological Fenton reagent? Biochemical Journal , 249 : 185 – 190 .
- Puppo , A. and Halliwell , B. 1988 . Formation of hydroxyl radicals in biological systems. Does myoglobin stimulate hydroxyl radical formation from hydrogen peroxide? . Free Radical Research Communication , 4 : 415 – 422 .
- Li , C. and Xie , B. 2000 . Evaluation of the antioxidant and pro-oxidant effects of tea catechin oxypolymers . Journal of Agriculture and Food Chemistry , 48 : 6362 – 6366 .
- Aruoma , O.I. and Halliwell , B. 1987 . Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Are lactoferrin and transferrin promoters of hydroxyl-radical generation? . Biochemical Journal , 241 : 273 – 278 .
- Gutteridge , J.M. and Halliwell , B. 1990 . The measurement and mechanism of lipid peroxidation in biological systems . Trends in Biochemical Sciences , 15 : 129 – 135 .
- Halliwell , B. , Kaur , H. and Ingelman-Sundberg , M. 1991 . Hydroxylation of salicylate as an assay for hydroxyl radicals: A cautionary note . Free Radical Biology and Medicine , 10 : 439 – 441 .
- Asamari , A.M. , Addis , P.B. , Epley , R.J. and Krick , T.P. 1996 . Wild rice hull antioxidants . Journal of Agriculture and Food Chemistry , 44 : 126 – 130 .
- Aruoma , O.I. , Chaudhary , S.S. , Grootreld , M. and Halliwell , B. 1990 . Binding of iron (II) ions to pentose sugar 2-deoxyribose . Journal of Inorganic Biochemistry , 35 : 149 – 155 .
- Gutteridge , J.M.C. 1984 . Reactivity of hydroxyl and hydroxyl-like radical discriminated by release of thiobarbituric acid-reactive material from deoxyribose, nucleosides and benzoate . Biochemical Journal , 224 : 761 – 767 .
- Halliwell , B. , Aeschbach , R. , Loliger , J. and Aruoma , O.I. 1994 . The characteristics of antioxidants . Food and Chemical Toxicology , 32 : 671 – 683 .
- Tiwari , O.P. and Tripathi , Y.B. 2007 . Antioxidant properties of different fractions of Vitex negundo Linn . Food Chemistry , 100 : 1170 – 1176 .