Abstract
Kumru is a traditional fermented cereal food made with flour and chickpea yeast. Ten samples of kumru supplied from different manufacturers in Turkey were analysed for the first time to determine biogenic amine contents using HPLC with benzoyl derivatization. Of the 10 amines under study, putrescine, cadaverine, spermidine, spermine, and histamine were detected in all samples. Spermine was the prevailing biogenic amine. Spermine concentrations of kumru samples changed from 2.4 to 17.9 mg/kg of kumru. Total amine contents of kumru samples were between 23.9 and 42.2 mg/kg of kumru. Concentrations of biogenic amines were far below the allowable limits. The pH values of kumru samples were in the range from 5.28 to 6.40; acidities were in the range from 0.12 to 0.28 g/100 g kumru (as lactic acid); total dry matters were from 63.36 to 69.71 g/100 g kumru; and total free amino acid contents were from 0.101 to 0.251 g/100 g kumru (as leucine). Significant correlations were detected between biogenic amine concentrations and pHs, acidities, and total free amino acid contents. No significant correlations were detected between biogenic amine concentrations and total dry matter contents of kumru samples.
INTRODUCTION
Biogenic amines are organic bases with aliphatic (putrescine, cadaverine, spermine, and spermidine), aromatic (tyramine and 2-phenylethylamine), or heterocyclic (histamine and tryptamine) structures, that may be found in several foods, in which they are mainly produced by microbial decarboxylation of amino acids with the exception of physiological polyamines.[Citation1] Biogenic amines may be found in a variety of foods, beverages, and fermented food products, especially in protein-rich foods, e.g., fish and fish products, meat and meat products, cheese, fermented vegetables, fruits, nuts, chocolate, soybean products, and wine.[Citation1,Citation2]
Polyamines like putrescine, spermidine, and spermine occur universally in animals and plants, while putrescine and spermidine are also found in most bacteria.[Citation2] Polyamines are endogenous and indispensable components of living cells and are important in the cell proliferation and differentiation, regulation of nucleic acid function, protein synthesis, brain development, nerve growth, and regeneration.[Citation1,Citation3–5 Citation Citation5 In plants, they are associated with pH, thermic or osmotic stress responses, cell division, and flowering. They may function as allelochemical compounds and as components of the chemical and physical defenses against herbivores and pathogens.[Citation6] Many factors contribute to the presence and accumulation of biogenic amines, such as availability of free amino acids, pH, water activity, temperature, bacterial density, and synergistic effects between microorganisms[Citation7,Citation8] and, primarily, the presence of microorganisms possessing amino acid decarboxylase activity, such as lactobacilli, enterococci, micrococci, and many strains of Enterobacteriaceae.[Citation9–11 Citation Citation11
In the human gut, low amounts of biogenic amines are metabolised to physiologically less active degradation products. This detoxification system includes specific enzymes, such as diamine oxidases (DAO). However, when large amounts of biogenic amines are ingested, the detoxification system is unable to eliminate them sufficiently.[Citation12] Thus, they may cause distinctive pharmacological, physiological, and toxic effects.[Citation5] Genetic predisposition, gastrointestinal diseases, or inhibition of DAO activity due to medicines or alcohol may result in suppressed detoxification of amines.[Citation12] It is worthy to mention that there is also evidence of linkage between elevated polyamine levels and cancer. High polyamine concentrations were reported in breast and colon cancer cells.[Citation5]
Analysis of biogenic amines is important not only from the toxicological point of view, but also they can be used as indicators of degree of freshness or spoilage of food.[Citation13] Several methods have been developed for determination of biogenic amines in foods. The analytical methods used for quantification of biogenic amines are mainly based on chromatographic methods: thin layer chromatography, gas chromatography, capillary electrophoresis, and HPLC.[Citation14] Nout[Citation15] proposed acceptable levels for fermented foods of 50–100, 100–800, and 30 mg/kg for histamine, tyramine, and 2-phenylethylamine, respectively. Such levels could be regarded as acceptable also for non-fermented foods. Silla-Santos[Citation1] proposed an acceptable level of 1000 mg/kg for total biogenic amine content. It has been reported that 40 mg of biogenic amines per meal can be considered potentially toxic. However, not all biogenic amines are equally toxic; consequently, histamine, tyramine, and 2-phenylethylamine are of concern.[Citation16] Tailor et al.[Citation17] considered that a limit of 10 mg/l of tyramine is acceptable for alcoholic beverages. The presence of 6 mg of tyramine in one or two usual servings is thought to be sufficient to cause a mild adverse event while 10–25 mg will produce a severe adverse event in those using monoamine oxidase inhibitor-containing drugs.[Citation18]
Fermented food products are widely consumed all over the world. Cereal-based fermented foods play an important role in the diets of many people in Asia, Africa, the Middle East, and some parts of Europe.[Citation19] Kumru is a traditional Turkish fermented cereal product. It is a kind of sandwich bread. It is produced with chickpea (Cicer arietinum) yeast and is called kumru because its shape is similar to a bird, dove (kumru in Turkish).[Citation20] Kumru has been produced and consumed in Izmir (the biggest city in the Aegean region of Turkey) for 150 years. Formerly, it had been consumed cold but after the 1940s it has been consumed both like a hot sandwich with salami, sausage, and cheese and as a cold sandwich with tomato and green pepper. There has been no scientific study about chemical composition of kumru. However, it is a similar product to bread. Bread contains moisture 36.5 g/100 g bread, protein 5.71 g/100 g bread, fat 1.07 g/100 g bread, and carbohydrate 54.7 g/100 g bread as mean values.[Citation21]
During kumru production, dough is prepared with flour and chickpea yeast. After dough preparation and fermentation, shaping and cooking are realised. Chickpea yeast is prepared 1 day before production. Chickpea yeast is prepared by grinding chickpea, then mixing with hot water in a glass bottle. After sealing the bottle, fermentation is carried out for 9–10 h at 25°C. Fermentation lasts 16 h in the winter. Duration of fermentation is very important. After grinding the chickpea into particles, it is filtered. This supernatant is used as yeast. Flour, chickpea yeast, warm water, and sometimes salt are mixed. It is fermented for 2–3 h at 30°C. Then shaping is carried out and the samples are allowed to stand for 10–15 min. Cooking is performed at 200–225°C for 7–8 min. The central temperature of baked kumru is about 90°C, but it is served like bread. So kumru is consumed at about 25°C.[Citation20,Citation22] The isolates obtained from chickpea starters and sweet dough sponges were identified as Enterococcus mundtii/E. gallinarum, E. casseliflavus, Lactobacillus plantarum/L. pentosus, L. sanfrancisco, L. viridescens, L. bifermantans, Pediococcus urinea-equi, Streptococcus thermophilus, Lactococcus lactis subsp. cremoris, and Saccharomyces cerevisiae.[Citation22] Biogenic amine content of kumru has not been studied before. Since it is a fermented food containing protein and free amino acids, which might be used by decarboxylase-positive microorganisms, the formation of various biogenic amines might be expected. Consequently, the aim of the present study was to investigate biogenic amine contents of kumru samples supplied from different producers in Izmir in Turkey.
MATERIALS AND METHODS
Materials
Ten kumru samples produced in winter by different manufacturers were purchased from local markets in Izmir in Turkey. Shelf life of kumru is 3 days and all of the samples were analysed in the first day of shelf life. Cadaverine dihydrochloride, tryptamine, 2-phenylethylamine, spermidine trihydrochloride, spermine, histamine dihydrochloride, tyramine, and agmatine sulphate were obtained from Sigma (Steinheim, Germany). Methylamine hydrochloride and 1,7-diaminoheptane (internal standard [IS]) were obtained from Merck (Schuchardt, Germany). Putrescine dihydrochloride was obtained from Fluka (Steinheim, Germany). The other reagents: sodium hydroxide, benzoyl chloride, sodium chloride, anhydrous sodium sulphate, cadmium acetate dihydrate, ninhydrin, ethanol, acetic acid, trichloroacetic acid (TCA), L-leucine, and distilled water were supplied from Merck (Darmstadt, Germany); hydrochloric acid from J. T. Baker (Deventer, Holland); methanol, acetonitrile, and diethyl ether (all of them HPLC grade) from Lab-Scan (Dublin, Ireland); sodium acetate trihydrate from Riedel (Germany); and phenolphthalein from Panreac (Barcelona, Spain). Standard solution of biogenic amines and IS solution were prepared following the method of Özdestan and Üren.[Citation23]
Methods
Sample preparation
Ten grams of kumru samples were homogenized in 50 ml of 5 g/100 ml TCA solution for 15 min. The resulting mixture was centrifuged at 2150 g for 10 min. The aqueous layer was collected, filtered through a Whatman (42) filter paper, and amines were derivatized prior to analysis by HPLC.
Derivatization procedure
Derivatization was achieved following the method of Özdestan and Üren.[Citation24] Benzoyl derivatives of the amines in the kumru extract were prepared, and then the resulting derivatives were extracted with diethyl ether. Following the evaporation of diethyl ether under a current of nitrogen, the solid residue was dissolved in methanol and injected into HPLC. Chromatograms were obtained for three aliquots of the same kumru sample that underwent the whole analytical procedure.
Chromatographic conditions
Chromatographic separations of benzoyl derivatives were realized following the method of Yeğin and Üren[Citation25] using a binary gradient elution consisting of methanol and acetate buffer.
Determinations of pH, acidity, total dry matter, and total free amino acid content
All of the samples were milled before analyses. pH values of kumru samples were measured with a digital pH meter.[Citation26] Acidities (as lactic acid) of kumru samples were determined by titration with 0.1 M NaOH solution.[Citation27] Total dry matter was determined by drying the samples at 110°C to a constant weight.[Citation27] Total free amino acid contents of kumru samples were quantified (as leucine, which has an average formula weight among the amino acids) following the method of Folkertsma and Fox.[Citation28] A 10-g sample of kumru was homogenized in 50 ml of 0.1 M HCl solution. The homogenized sample was mixed for 15 min. The homogenate was held at 40°C for 1 h and then centrifuged at 2150 g for 30 min. The supernatant was collected and then filtered through a Whatman (42) filter paper. A 0.3-ml portion of the filtrate was diluted to 2 ml with distilled water and treated with 4 ml of Cd-ninhydrin reagent. The mixture was allowed to stand for 5 min at 84°C, cooled to room temperature, centrifuged at 2150 g for 5 min, and then the absorbance value of the solution was measured at 507 nm.[Citation28]
Apparatus
HPLC separations were performed by using an Agilent 1100 liquid chromatograph (Agilent, Santa Clara, CA, USA) equipped with an Agilent 1100 diode array detector, a gradient elution pump and vacuum degasser, an autosampler system, and a thermostatted column compartment. The chromatographic column was Hichrom C18 (10 μm particle size, 300 mm × 3.9 mm i.d., Hichrom Ltd., Theale, UK) thermostatted at 20°C. An HI 221 microprocessor pH meter (Hanna Instruments, Cluj-Napoca, Romania) was used for pH measurements. The determinations of total dry matter contents were realised by using an oven (Dedeoğlu, Turkey). Absorbance measurements for total free amino acid content were achieved with a Cary 50 UV-vis spectrophotometer (Varian, London, UK).
Statistical Analysis
Throughout the present study, all of the experiments were performed in triplicate. Statistical analyses were realized with the SPSS 10.0 statistics package program (IBM Corp., New York, USA). The statistical analyses of the data were achieved by using one-way analysis of variance (ANOVA), Duncan post-test, Pearson correlation test, and paired t-test. In all data analyses a value of p < 0.05 was considered as statistically significant.
RESULTS AND DISCUSSION
The standard addition method and internal standard method were used together to calculate the amine contents of kumru samples. Types and concentrations of amines detected in 10 kumru samples from different manufacturers are shown in . The chromatogram for a kumru sample is given in . To examine the method performance and the matrix effect of kumru, recovery rates of biogenic amine determinations were estimated. Recovery rates varied from 77.9 to 101.8%. Detection limits of the amines for the applied method were 0.2 mg/kg food for methylamine, putrescine, tryptamine, and spermidine; 0.3 mg/kg food for cadaverine; 0.4 mg/kg food for histamine; 0.5 mg/kg food for 2-phenylethylamine and spermine; 2.5 mg/kg food for tyramine;[Citation24] and 0.3 mg/kg food for agmatine. Linearity of the determinations for methylamine, putrescine, cadaverine, tryptamine, 2-phenylethylamine, spermidine, spermine, histamine, tyramine, and agmatine was reported by Özdestan and Üren[Citation24] as being up to 133.6, 185.7, 186.0, 265.0, 272.0, 268.4, 226.6, 995.3, 1433, and 95.32 mg/kg of food, respectively. Linearity was obtained for each amine with a good correlation coefficient.
Table 1 Amine contents (mg/kg of kumru) and standard deviation values of kumru samples.Footnote a
Figure 1 HPLC chromatogram of kumru sample (K7). Peak identification: 1: methylamine; 2: putrescine; 3: cadaverine; 6: spermidine; 7: 1,7-diaminoheptane (IS); 8: spermine; 9: histamine.
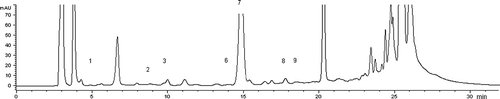
shows that each of the samples contained at least five biogenic amines. Putrescine, cadaverine, spermidine, spermine, and histamine were detected in all samples. Tryptamine, 2-phenylethylamine, and agmatine were not detected in any of the kumru samples. Tyramine was detected in six samples. Among the 10 amines that were under study, spermine was detected at the highest level. Spermine contents of samples varied from 2.4 to 17.9 mg/kg of kumru. Average spermine concentration of kumru samples was 11.1 mg/kg of kumru. Total amine contents of kumru samples were between 23.9 and 42.2 mg/kg of kumru. The concentrations of biogenic amines detected in 10 kumru samples were far below the maximum permissible limits. According to Turkish regulations, the level of histamine in fish must not exceed 200 mg/kg.[Citation29] The European Union requires that nine samples must be taken from each batch of fish species of the following families: Scombridae, Clupeidae, Engraulidae, and Coryphaenidae for histamine analysis. The mean value for histamine in fish must not exceed 100 mg/kg.[Citation30]
As shown in , significant differences were detected between spermine concentrations of different kumru samples (p < 0.05). There were also significant differences between putrescine, cadaverine, spermidine, histamine, tyramine, methylamine, and total biogenic amine concentrations of different kumru samples (p < 0.05). Among the 10 kumru samples, K7 contained the highest amount of amines and K9 contained the lowest. According to the results of the paired t-test, there were no significant differences between the K7 sample and the others (p < 0.05). The K9 sample contained a significantly less amount of amines than those in K2 and K5 (p < 0.05). Correlations between the amine contents of kumru samples were tabulated in . As is seen in this table, statistically significant positive correlations were obtained between concentrations of various amines with the exception of tyramine, which had negative correlations with methylamine, putrescine, cadaverine, and spermine (p < 0.05). Based on these powerful correlations between biogenic amines, it may be concluded that putrescine, cadaverine, and histamine were produced by the same microorganisms. Biogenic amine contents of kumru samples were found below those for many fermented foods. Short fermentation time; controlled production conditions, such as heating at 200–225°C; and low microbial count in raw material are main reasons for low biogenic amine content. Biogenic amine contents of boza samples were determined by Yeğin and Üren,[Citation25] which is a traditional, cereal-based and fermented beverage made by yeast and lactic acid fermentation. Tyramine was the prevailing biogenic amine. Total biogenic amine contents of boza samples were between 25 and 69 mg/kg of boza. Özdestan and Üren[Citation23] determined biogenic amine contents of shalgam samples from different manufacturers in Turkey. Putrescine was the prevailing biogenic amine. Total biogenic amine contents were between 26.7 and 134.3 mg/l of shalgam. Kefir is a fermented dairy product. Kefir samples were analysed for the first time to determine biogenic amine contents. Tyramine was the prevailing biogenic amine. Total biogenic amine contents changed from 2.4 to 35.2 mg/l of kefir.[Citation31] Okamoto et al.[Citation32] determined the polyamine content of wheat flour. Average putrescine, spermidine, and spermine contents of wheat flour were 1.5, 9.6, and 5.3 mg/kg of flour, respectively. Bardócz et al.[Citation33] determined the polyamine content of bread. Putrescine content of bread was between 1.5 and 1.8 mg/kg of bread, spermidine content was between 5.0 and 5.2 mg/kg of bread, and spermine content was between 3.4 and 3.8 mg/kg of bread.
Table 2 Correlations between amine contents of kumru samples (p < 0.05).Footnote a
The pHs, acidities, total dry matter contents, and total free amino acid contents of kumru samples were tabulated in . The pH values of kumru samples varied from 5.28 to 6.40. Acidities of kumru samples varied from 0.12 to 0.28 g/100 g kumru. According to the Pearson correlation test, significant correlations were detected between pHs and methylamine, cadaverine, spermine, and total biogenic amine concentrations (p < 0.05). A negative significant correlation was detected between acidities and tyramine concentrations of kumru samples (p < 0.05). No significant correlations were detected between acidities and the other biogenic amine concentrations. It has been suggested that biogenic amine formation by bacteria is a physiological mechanism to counteract the acid environment. The pH level is an important factor influencing amino acid decarboxylase activity of bacteria. In particular, amino acid decarboxylase activities were higher when pH was between 4 and 5.5. However, biogenic amine formation was found to depend on the growth activity of bacteria rather than the growth conditions.[Citation1,Citation8]
Table 3 pH, acidity, total dry matter and total free amino acid contents and standard deviation values of kumru samples.Footnote a
Since biogenic amine formation occurs by decarboxylation of amino acids, it was thought that free amino acid content of kumru could affect the biogenic amine formation. Availability of free amino acids contributes to the presence and accumulation of biogenic amines in foods. Total free amino acid contents of kumru samples varied from 0.101 to 0.251 g/100 g kumru. According to the Pearson correlation test, a significant correlation was obtained between total free amino acid contents and tyramine concentrations in kumru samples (p < 0.05). As is evident, activities of microorganisms and concentrations of biogenic amines were higher when medium contained more substrate and amino acids for lactic acid bacteria and yeasts.
Total dry matter content of samples varied from 63.36 to 69.71 g/100 g kumru. No statistically significant correlations were obtained between biogenic amine concentrations and total dry matter contents in kumru samples (p < 0.05). This result was reasonable since less than 0.5 g of amino acids was contained in 100 g of total dry matter. The remaining part was composed of protein, carbohydrate, fat, crude fibre, and salt. In the study of Yeğin and Üren,[Citation25] with boza samples, no significant correlations were detected between biogenic amine contents of boza and pH, protein contents, and total dry matter contents. Özdestan and Üren[Citation23] found significant correlations between biogenic amine concentrations of shalgam samples and pH values, acidities, total dry matter contents, and total free amino acid contents. Özdestan and Üren[Citation31] reported biogenic amine contents of kefir samples. No significant correlations were detected between biogenic amine concentrations and pHs and total dry matter contents, whereas, significant correlations were obtained between biogenic amine concentrations and acidities and total free amino acid contents of kefir samples. The production of biogenic amines is an extremely complex phenomenon, depending on several variables, such as raw materials, processing conditions, growth kinetics of microorganisms, and their proteolytic and decarboxylase activities, which interact with each other. Moreover, decarboxylating ability of bacteria is not species-dependent, but strain-dependent.[Citation8]
CONCLUSION
Kumru is a traditional fermented cereal food. The use of the standard addition technique and internal standard technique together for the quantification of biogenic amines in kumru samples provided reliable results. Using these methods, methylamine, putrescine, cadaverine, tryptamine, 2-phenylethylamine, spermidine, spermine, histamine, tyramine, and agmatine contents of 10 kumru samples supplied from different manufacturers were determined. Spermine was the prevailing biogenic amine and the maximum spermine concentration was 17.9 mg/kg of kumru. Putrescine, cadaverine, spermidine, spermine, and histamine were detected in all samples. Total biogenic amine contents of kumru samples were between 23.9 and 42.2 mg/kg of kumru. Average total biogenic amine concentration of kumru samples was 31.9 mg/kg of kumru. Maximum histamine concentration was 5.9 mg/kg of kumru. Concentrations of biogenic amines detected in Turkish kumru were far below the maximum permissible limits. Kumru consumption does not exceed 200 g per meal and this consumption level appears to be safe for individuals.
REFERENCES
- Silla Santos , M.H. 1996 . Biogenic amines: Their importance in foods . International Journal of Food Microbiology , 29 : 213 – 231 .
- Shalaby , A.R. 1996 . Significance of biogenic amines to food safety and human health . Food Research International , 29 ( 7 ) : 675 – 690 .
- Eliassen , K.A. , Reistad , R. , Risøen , U. and Rønning , H.F. 2002 . Dietary polyamines . Food Chemistry , 78 : 273 – 280 .
- Kalač , P. and Krausová , P.A. 2005 . Review of dietary polyamines: Formation, implications for growth and health and occurrence in foods . Food Chemistry , 90 : 219 – 230 .
- Tassoni , A. , Germaná , M.A. and Bagni , N. 2004 . Free and conjugated polyamine content in citrus sinensis Osbeck, cultivar Brasilino N.L. 92, a navel orange, at different maturation stages . Food Chemistry , 87 : 537 – 541 .
- Bouchereau , A. , Guénot , P. and Larher , F. 2000 . Analysis of amines in plant materials . Journal of Chromatography B , 747 : 49 – 67 .
- Stratton , J.E. , Hutkins , R.W. and Taylors , S.L. 1991 . Biogenic amines in cheese and other fermented foods: A review . Journal of Food Protection , 54 ( 6 ) : 460 – 470 .
- Gardini , F. , Martuscelli , M. , Caruso , M.C. , Galgano , F. , Crudele , M.A. , Favati , F. , Guerzoni , M.E. and Suzzi , G. 2001 . Effect of pH, temperature and NaCl concentration on the growth kinetics, proteolytic activity and biogenic amine production of Enterococcus faecalis . International Journal of Food Microbiology , 64 : 105 – 117 .
- Halasz , A. , Barath , A. , Simon-Sarkadi , L. and Holzapfel , W. 1994 . Biogenic amines and their production by microorganisms in food . Trends in Food Science and Technology , 5 : 42 – 49 .
- Martuscelli , M. , Gardini , F. , Torriani , S. , Mastrocola , D. , Serio , A. , Chaves-Lopez , C. , Schirone , M. and Suzzi , G. 2005 . Production of biogenic amines during the ripening of Pecorino Abruzzese cheese . International Dairy Journal , 15 : 571 – 578 .
- Guillén-Velasco , S. , Ponce-Alquicira , E. , Farrés-González Saravia , A. and Guerrero-Legarreta , I. 2004 . Histamine production by two Enterobacteriaceae strains isolated from tuna (Thunnus thynnus) and jack mackerel (Trachurus murphyii) . International Journal of Food Properties , 7 : 91 – 103 .
- Křížek , M. , Pavlíček , T. and Vácha , F. 2002 . Formation of selected biogenic amines in carp meat . Journal of the Science of Food and Agriculture , 82 : 1088 – 1093 .
- Erkan , N. and Özden , Ö. 2006 . Gutted and un-gutted sea bass (Dicentrarchus labrax) stored in ice: Influence on fish quality and shelf-life . International Journal of Food Properties , 9 : 331 – 345 .
- Önal , A.A. 2007 . Review: Current analytical methods for the determination of biogenic amines in foods . Food Chemistry , 103 ( 4 ) : 1475 – 1486 .
- Nout , M.J.R. 1994 . Fermented foods and food safety . Food Research International , 27 ( 3 ) : 291 – 298 .
- Chiacchierini , E. , Restuccia , D. and Vinci , G. 2006 . Evaluation of two different extraction methods for chromatographic determination of bioactive amines in tomato products . Talanta , 69 : 548 – 555 .
- Tailor , S.A.N. , Shulman , K.I. , Walker , S.E. , Moss , J. and Gardner , D. 1994 . Hypertensive episode associated with phenelzine and tap beer—A reanalysis of the role of pressor amines in beer . Journal of Clinical Psychopharmacology , 14 ( 1 ) : 5 – 14 .
- Ten Brink , B. , Damink , C. , Joosten , H.M.L.J. and Huis In't Veld , J.H.J. 1990 . Occurrence and formation of biologically active amines in foods . International Journal of Food Microbiology , 11 : 73 – 84 .
- Tamer , C.E. , Kumral , A. , Aşan , M. and Şahin , İ. 2007 . Chemical compositions of traditional tarhana having different formulations . Journal of Food Processing and Preservation , 31 ( 1 ) : 116 – 126 .
- Alpözen , E. , Güven , G. and Üren , A. Three traditional bakery foods specific to Izmir: Izmir Gevreği, Kumru and Boyoz . 2nd Traditional Foods Conference . May 27–29 . pp. 920 – 923 . [Izmir'in 3 geleneksel fırın ürünü; Izmir gevreği, kumru ve boyoz, 2. Geleneksel Gıdalar Sempozyumu Kitabı (27–29 Mayıs 2009, Van)]
- El , S.N. 1999 . Determination of glycemic index for some breads . Food Chemistry , 67 : 67 – 69 .
- Sıkılı , Ö.H . 2003 . Investigation of microbiological and flavour characteristics of chickpea sweet dough , 39 – 41 . Izmir , , Turkey : Ph. D. Thesis, Ege University, Food Engineering Department .
- Özdestan , Ö. and Üren , A. 2010 . Biogenic amine content of Shalgam (Şalgam): A traditional lactic acid fermented Turkish beverage . Journal of Agricultural and Food Chemistry , 58 ( 4 ) : 2602 – 2608 .
- Özdestan , Ö. and Üren , A. 2009 . A method for benzoyl chloride derivatization of biogenic amines for high performance liquid chromatography . Talanta , 78 ( 4–5 ) : 1321 – 1326 .
- Yeğin , S. and Üren , A. 2008 . Biogenic amine content of boza: A traditional cereal-based, fermented Turkish beverage . Food Chemistry , 111 ( 4 ) : 983 – 987 .
- Erbaş , M. , Uslu , M.K. , Erbaş , M.O. and Certel , M. 2006 . Effects of fermentation and storage on the organic and fatty acid contents of tarhana, a Turkish fermented cereal food . Journal of Food Composition and Analysis , 19 : 294 – 301 .
- İbanoğlu , Ş. , İbanoğlu , E. and Ainsworth , P. 1999 . Effect of different ingredients on the fermentation activity in tarhana . Food Chemistry , 64 : 103 – 106 .
- Folkertsma , B. and Fox , P.F. 1992 . Use of the Cd-Ninhydrin reagent to assess proteolysis in cheese during ripening . Journal of Dairy Research , 59 : 217 – 224 .
- Fishery Products Regulation . 2008 . Maximum allowable levels for organoleptic, chemical and microbiological properties of fish . Official Journal Turkey-21/9/2008–27004 ,
- EEC . 1991 . Laying down the health conditions for the production and the placing on the market of fishery products . Council Directive, EEC, 91/493/EEC , 22 July
- Özdestan , Ö. and Üren , A. 2010 . Biogenic amine content of kefir: A fermented dairy product . European Food Research and Technology , 231 ( 1 ) : 101 – 107 .
- Okamoto , A. , Sugi , E. , Koizumi , Y. , Yanadiga , F. and Udaka , S. 1997 . Polyamine content of ordinary foodstuffs and various fermented foods . Bioscience, Biotechnology and Biochemistry , 61 : 1582 – 1584 .
- Bardócz , S. , Grant , G. , Brown , D.S. , Ralph , A. and Pusztai , A. 1993 . Polyamines in food—Implications for growth and health . The Journal of Nutritional Biochemistry , 4 : 66 – 71 .