Abstract
Storage stability of protein hydrolysate from yellow stripe trevally prepared using Flavourzyme with a degree of hydrolysis of 25% (HF25) was determined. During storage, slight increases in water activity and moisture content and a slight decrease in lightness of HF25 and Bacto Peptone were observed. Generally, browning intensity, A294, and fluorescent intensity of HF25 and Bacto Peptone increased. Both samples had an excellent solubility at pH 5 and 7. Thiobarbituric acid reactive substances in HF25 and Bacto Peptone increased (p < 0.05). The efficacy of HF25 as media for bacteria was equivalent to that of Bacto Peptone and was quite stable during storage.
INTRODUCTION
Hydrolysis processes have been used to convert proteinaceous materials, including byproducts from fish processing plants. Protein hydrolysates generally exhibited the improved functional properties associated with the increased solubility. Protein hydrolysates from many sources, such as fish,Citation[1, Citation2] soy,Citation[3] ram horn,Citation[4] and banana peelCitation[5] have been used as functional ingredients and microbial media and their performances were comparable to that of commercial peptone.Citation[1,Citation4] As microbial media, protein hydrolysate should be clear or only slightly colored and free from precipitates.Citation[4]
Due to its hydroscopicity in nature, the physical and chemical changes take place rapidly when the storage condition is not appropriate. The color of freeze-dried protein hydrolysate from round scad was stable for 6 weeks when stored at 4°C, and the changes in color were more pronounced when the storage temperature increased. The solubility of round scad protein hydrolysate decreased during 6 weeks of storage due to the aggregation of those peptides.Citation[6] The available lysine content of dried whey concentrate decreased to the highest extent when stored at water activity (aw) of 0.41 and 40°C. The loss by 23% was obtained after 3 months of storage.Citation[7] Losses in lysine via Maillard browning reaction were influenced more by temperature than by aw. However, methionine and tryptophan contents were not changed significantly during storage at any of the conditions tested.Citation[7] The decrease in lipid content reduced lipid oxidation and enhanced the storage stability of capelin hydrolysate.Citation[8]
Recently, protein hydrolysates from yellow stripe trevally have been produced successfully.Citation[9,Citation10] Protein hydrolysate from yellow stripe trevally prepared using Flavourzyme with 25% degree of hydrolysis (HF25) can be used as a nitrogen source in culture media and exhibited the comparable performance to Bacto Peptone.Citation[11] However, the information regarding storage stability of yellow stripe trevally protein hydrolysate has not been reported. Therefore, the objective of this study was to investigate the stability during storage of HF25 in comparison with commercial Bacto Peptone.
MATERIALS AND METHODS
Enzyme and Cultivation Medium
Flavourzyme was obtained from East Asiatic Company Ltd., (Bangkok, Thailand). Bacto Peptone was purchased from Difco Laboratories (Sparks, MD, USA).
Collection and Preparation of Fish Sample
Yellow stripe trevally (Selaroides leptolepis), caught along the coast of the Andaman Sea with the size of 65 g/fish, off-loaded approximately 24–36 h after capture, were obtained from the fishing port in Satul Province, Thailand. Fish were placed in ice with a fish/ice ratio of 1:2 (w/w) and transported to the Department of Food Technology, Prince of Songkla University within 2 h. Upon arrival, the fish were washed and the meat was separated manually. The meat was minced using a mincer with 0.4-cm diameter holes. The mince was used for hydrolysate preparation.
Production of Protein Hydrolysates
Protein hydrolysate was prepared as per the method of Klompong et al.Citation[9] Mince (60 g) was suspended in 240 ml of distilled water. The mixture was homogenized using a homogenizer (IKA Labortechnik, Selangor, Malaysia) at a speed of 11,000 rpm for 1 min. The homogenate was adjusted to pH 7.0 and preincubated at 50°C for 20 min prior to hydrolysis using Flavourzyme. The hydrolytic reaction was started by the addition of 9.77% Flavourzyme (w/w) based on the protein content of the mince.Citation[9] The reaction was conducted at pH 7.0 using the pH-stat methodCitation[12] for 20 min to obtain the degree of hydrolysis (DH) of 25% as described by Klompong et al.Citation[9]
To terminate the enzymatic reaction, the reaction mixture was heated in a water bath at 90°C for 15 min with an occasional agitation. The sample was cooled and the pH of the sample was then adjusted to 7.0 with 1 M HCl or 1 M NaOH. The hydrolysate was centrifuged at 2000× g for 10 min at 25°C using a centrifuge (RC 5B plus; Sorvall, Norwalk, CT, USA). The supernatant obtained was subjected to spray-drying using a spray dryer (Niro A/S, 305 DK-2860; Gladsaxevej, Soeborg, Denmark). The resulting powder, referred to as ‘HF25’ (approximately 100 g), was placed in an airtight plastic container and the lid closed tightly. Bacto Peptone was kept under the same condition at room temperature. At weeks 0, 1, 2, 4, 6, 8, 10, and 12, the samples were taken randomly for physicochemical determination and microbiological analyses as microbial medium.
Measurement of aw, Moisture Contents, 2-Thiobarbituric Acid Reactive Substances (TBARS), and Turbidity
The moisture contents of HF25 and Bacto Peptone were measured according to the method of AOACCitation[13] (Method No. 950.46), and aw was determined using a water activity measuring system (Thermoconstanter; Novasina, Zurich, Switzerland). TBARS values in the samples were determined by the method of Buege and Aust.Citation[14] The solutions of HF25 and Bacto Peptone at a concentration of 1% (w/v) were prepared. To 0.5 ml of the sample solution, 4.5 ml of a solution containing 0.0375% thiobarbituric acid, 15% trichloroacetic acid, and 0.25 M HCl was added. The mixtures were heated in boiling water for 10 min, followed by cooling with running water. The absorbance of resulting mixtures was read at 532 nm using a spectrophotometer (UV-1601; Shimadzu, Kyoto, Japan). TBARS was calculated from the standard curve of molonaldehyde (MDA) with the concentration range of 0–3 mg/kg sample and expressed as mg MDA/kg sample. Turbidity of HF25 and Bacto Peptone solutions (0.5% (w/v) in distilled water) before and after heating at 121°C for 15 min using an autoclave (SS-325; Tomy, Tokyo, Japan) was determined by reading the absorbance at 660 nm using a spectrophotometer (UV-1601; Shimadzu, Kyoto, Japan).
Measurement of Color and Browning Index
The color of HF25 and Bacto Peptone was measured by colorimeter (Hunter Lab, Color Flex, Reston, VA, USA) and reported in a CIE system. L*, a*, and b* indicating lightness, redness/greenness, and yellowness/blueness, respectively, were recorded. Browning intensity of HF25 and Bacto Peptone was measured according to the method of Ajandouz et al.Citation[15] HF25 and Bacto Peptone solutions (1%, w/v) were prepared and the absorbance was measured at 420 nm using a spectrophotometer (UV-1601; Shimadzu, Kyoto, Japan). Intermediate products from the Maillard reaction of HF25 and Bacto Peptone solutions were monitored. Solutions (1%, w/v) were prepared and the fluorescence intensity was measured at an excitation wavelength of 347 nm and emission wavelength of 415 nm using a spectrofluorophotometer (RF-1501; Shimadzu, Kyoto, Japan).Citation[16] Additionally, the absorbance at 294 nm was measured and used as an indicator for the UV-absorbance intermediate.Citation[16]
Measurement of pH and Solubility
The pH of HF25 and Bacto Peptone solutions (1% (w/v) in distilled water) was measured by a pH meter (PB 10; Sartorious, Goettingen, Germany). To determine protein solubility, HF25 and Bacto Peptone (200 mg) were dispersed in 20 ml of deionized water and pHs of the mixtures were adjusted to 5 and 7 with 1 M HCl or 1 M NaOH. The mixtures were stirred at room temperature for 10 min, followed by centrifugation at 2000× g for 15 min using a centrifuge (Mikro 20; Hettich, Zentrifugen, Germany). Protein contents in the supernatant were determined by the Biuret method.Citation[17] Total protein contents in the samples were determined after solubilization of the samples in 0.5 M NaOH. Protein solubility was calculated as follows:
Determination of the Ability as Nutrients for Microbial Growth
Preparation of media containing HF25
Nutrient broth (NB) and nutrient agar (NA) were prepared for culturing bacteria. NB consisted of 3 g of beef extract and 5 g of HF25 or Bacto Peptone. For NA, 15 g of agar was included in the NB. The mixture was added with distilled water. The final volume was adjusted to 1 l with distilled water. Thereafter, the solution obtained was adjusted to pH 6.8 ± 0.2 and autoclaved at 121°C for 15 min using an autoclave (SS-325; Tomy, Tokyo, Japan).
Preparation of bacteria and inocula
Staphylococcus aureus (S. aureus) TISTR 118 and Escherichia coli (E. coli) TISTR 780 were obtained from the Department of Food Technology, Prince of Songkla University, Hat Yai, Thailand. Bacteria were kept on NA slants at 4°C until use. To activate bacteria before culturing, inocula were prepared. One loopful of each bacterium cultured on NA slants containing HF25 or Bacto Peptone for 15 h was inoculated in NB containing HF25 or Bacto Peptone. The culture broths were then incubated at 37°C for 15 h. Thereafter, 1.5 ml of culture broths were inoculated in 50 ml NB containing HF25 or Bacto Peptone and incubated at 37°C for 15 h using a shaking incubator (VS-8480SR-L; LMS, Hat Yai, Thailand) at 120 rpm. Culture broths were adjusted to obtain A660 of 0.02 with corresponding media. The obtained cell suspension was used as late log phase inocula.
Determination of Bacterial Growth
Growths were determined by submerged cultivation. To 50 ml of NB containing HF25 or Bacto Peptone, 1.5 ml of inocula with A660 of 0.02 was added. Incubation was carried out at 37°C for 15 h with continuous shaking at 120 rpm using a shaking incubator (VS-8480SR-L; LMS, Hat Yai, Thailand). Culture broths obtained were subjected to measurements of turbidity (A660) and total viable count. Serial dilutions were prepared from culture broths of S. aureus and E. coli with sterile normal saline. Thereafter, aliquots (0.1 ml) of appropriate dilutions were pipetted onto NA containing HF25 or Bacto Peptone and then spread using a sterile spreader. The plates were upside-down incubated at 37°C for 24 h before counting.
Statistical Analysis
Data were subjected to analysis of variance (ANOVA). Mean comparison was performed using Duncan's multiple range test. For pair comparison, independence-samples t-test were used.Citation[18] Statistical analysis was carried out using SPSS statistic program (Version 11.0) for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS AND DISCUSSION
Changes in aw, Moisture Content, TBARS, and Turbidity during Storage
Changes in aw and moisture content of spray-dried yellow stripe trevally protein hydrolysate (HF25) and Bacto Peptone during 12 weeks of storage are depicted in and Slight increases in aw and moisture content of both samples were observed throughout the storage (p < 0.05). At week 0, HF25 showed the higher aw and moisture content than did Bacto Peptone (p < 0.05). After 12 weeks of storage, HF25 and Bacto Peptone had aw of 0.27 and 0.26, while the moisture contents were 5.49 and 4.10% for HF25 and Bacto Peptone, respectively. Changes in physical, chemical, or microbiological properties of a product can be considered as loss of stability.Citation[19] aw is one of several important parameters affecting stability of products.Citation[19] aw is a measure of the free moisture that is available to participate in physical, chemical, and biological reactions, while moisture content is the combination of free and bound moisture.Citation[20] aw plays a role in the microbial stability. Bacteria, molds, and yeasts require water for growth and every microorganism has a minimum aw below which it will not grow.Citation[20] Due to the low level of aw (<0.70), HF25 and Bacto Peptone were most likely stable to microbial spoilage. However, non-enzymatic browning might take place at very low aw.Citation[20] During the extended storage, dry and hygroscopic HF25 could absorb the moisture in the environment until it reached the equilibrium level. If aw is above the critical limit for hydrolysate, it will begin to cake, which is unacceptable as microbial media. To lower any change of HF25, the selected packaging exhibiting the excellent water vapor barrier property was required. The transfer of water from an environment with higher aw to HF25 with a lower aw caused HF25 to reach its critical limit, initiating caking. Storage factors, such as temperature, can change the rate of water transfer and caking. Lower temperatures slow the water transfer, while higher temperatures speed the water transfer rate.Citation[19] Therefore, aw could be an important factor determining the chemical and microbiological stability of protein hydrolysate. TrollerCitation[21] found that at low aw levels, the total number and growth rate of S. aureus were diminished and generation times were increased. Additionally, enterotoxin synthesis was extremely sensitive to lowered aw. Tsoraeva and ZhurbenkoCitation[22] reported that the moisture content of protein hydrolysate should be lower than 7% since the higher levels of moisture content will reduce the stability and shelf-life of protein hydrolysate, causing color changes and falling pH values.
Figure 1 Changes in (a) aw, (b) moisture content, and (c) TBARS of yellow stripe trevally protein hydrolysate (HF25) (•) and Bacto Peptone (○) and (d) turbidity of HF25 before (•) and after (▲) autoclaving and of Bacto Peptone before (○) and after (∆) autoclaving during 12 weeks of storage at room temperature. Bars represent the standard deviation from triplicate determinations.
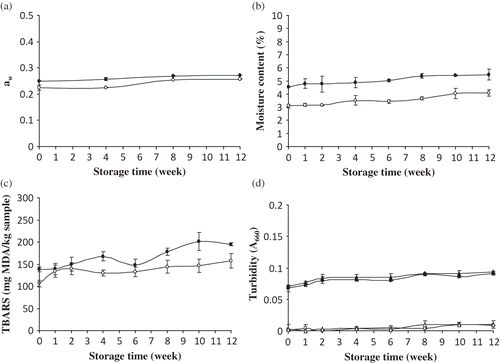
TBARS values of HF25 and Bacto Peptone during storage for 12 weeks are shown in As the storage time increased, TBARS of HF25 increased, up to week 10 (p < 0.05). It was noted that there was a non-significant decrease in TBARS at week 6 (p > 0.05). However, no change was found during week 10 and 12 (p > 0.05). For Bacto peptone, the increase in TBARS was observed within the first week (p < 0.05) and remained constant up to week 12 (p > 0.05). The increase in TBARS of HF25 was higher than that of Bacto Peptone throughout the storage (p < 0.05). The increase in TBARS of HF25 and Bacto Peptone during storage indicated the occurrence of lipid oxidation. Lipid in HF25 and Bacto Peptone might be vulnerable to oxidation, resulting in production of TBARS.Citation[23] At low aw (0.22–0.27), lipid oxidation of HF25 and Bacto Peptone could be enhanced since the lipids were more exposed to oxygen at a low water level. As a consequence, the oxidation proceeded rapidly in both dried samples. Lipid oxidation during storage occurred relatively rapidly at both low (0.05–0.15) and high (0.5–0.8) aw.Citation[20]
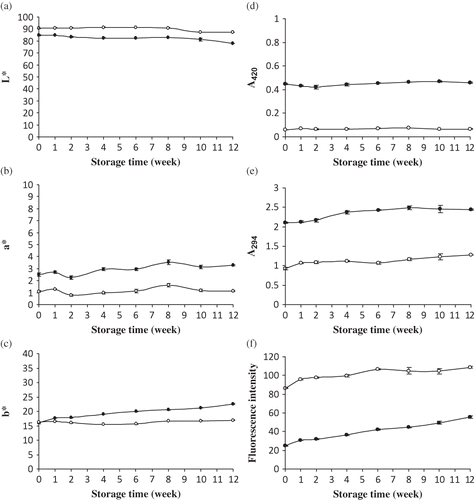
Figure 2 Changes in color (a) L*, (b) a*, and (c) b* values and browning index (d) A420, (e) A294, and (f) fluorescence intensity of yellow stripe trevally protein hydrolysate (HF25) (•) and Bacto Peptone (○) during 12 weeks of storage at room temperature. Bars represent the standard deviation from triplicate determinations.
Turbidity before and after autoclaving of HF25 and Bacto Peptone stored for different times is shown in As the storage time increased, the turbidity of HF25 and Bacto Peptone, either before or after autoclaving, slightly increased. However, no difference in turbidity of both samples before and after autoclaving was observed (p > 0.05). HF25 contained the small MW peptides, which were not prone to coagulation or precipitation after being subjected to heating at 121°C for 15 min. At the same storage time, turbidity of HF25 was higher than that of Bacto peptone (p < 0.05). The difference in turbidity between HF25 and Bacto Peptone might be associated with the differences in raw material pigments, pretreatment, hydrolysis condition, and drying process. Additionally, the higher residual lipid content in HF25 Citation[11] might result in the higher turbidity, in comparison with Bacto Peptone, which had the lower lipid content. Fish lipid underwent oxidation easily, in which the oxidation products could be formed and acted as protein/peptide cross-linker. The larger aggregate formed probably affected the turbidity of HF25 solution. Gildberg et al.Citation[24] reported that freeze-dried capelin hydrolysate solution was slightly turbid due to some residual lipids.
Changes in Color and Browning Index during Storage
During the storage, the changes in color of HF25 and Bacto Peptone are shown in – At week 0, HF25 had the higher a* value, but lower L* value than did Bacto Peptone (p < 0.05). However, no difference in b* value was found (p > 0.05). This suggested that HF25 was slightly darker and more reddish. L* value of both HF25 and Bacto Peptone slightly decreased after 8 weeks of storage (p < 0.05). a* and b* values of HF25 gradually increased up to 12 weeks of storage (p < 0.05). Conversely, no significant change in a* and b* values of Bacto Peptone was noticeably observed during storage (p > 0.05). The result indicated a slight decrease in lightness of HF25 and Bacto Peptone after the extended storage. Redness and yellowness of HF25 gradually increased during storage (p < 0.05). The decrease in lightness and the increases in redness and yellowness of HF25 during storage might be associated with non-enzymatic browning, known as the Maillard reaction. Maillard reaction is a chemical reaction between simple sugars and amino acids and occurs between aw of 0.2 and 0.8.Citation[20] Klompong et al.Citation[11] reported that HF25 contained fermentable carbohydrates, while it was not found in Bacto Peptone. Those fermentable carbohydrates might consist of reducing sugars, which underwent Maillard reaction readily under the storage condition. Additionally, the decrease in lightness was probably due to the oxidation of myoglobin and the melanin pigment present in HF25. Citation[25] The result was in agreement with Hoyle and MerrittCitation[26] who found that hydrolysates from herring had decreases in lightness and increases in yellowness, which indicated the darkening during storage.
As measured by A420, a slight increase in browning of HF25 was observed as the storage time increased (p < 0.05), while no change was noticeable in Bacto Peptone (p > 0.05) (). Furthermore, HF25 possessed the higher browning intensity than did Bacto Peptone throughout the storage time (p < 0.05). The increase in browning in HF25 might be associated with the presence of reducing sugars or other carbohydrate compounds in HF25. As a consequence, the amino acid-sugar complex could be formed via Maillard reaction. The increase in browning intensity was in accordance with the decrease in L* value and the increases in a* and b* values (). A continuous increase in A294 of HF25 was observed within the first 4 weeks of storage (p < 0.05). Thereafter, A294 was quite constant (p > 0.05). The gradual increase in A294 was observed in Bacto Peptone as the storage time increased (p < 0.05) (). HF25 showed the higher A294 than did Bacto Peptone throughout the storage (p < 0.05). A294 was used to determine the intermediate compounds of the Maillard reaction.Citation[15] The result suggested that the formation of an uncolored compound in HF25 was more intense than Bacto Peptone. Fluorescence intensity of HF25 increased as the storage time increased (p < 0.05). For Bacto Peptone, fluorescence intensity increased up to 6 weeks of storage. No differences in fluorescence intensity were obtained during 6–10 weeks of storage. However, the increase in fluorescence intensity was noticeable at the end of storage (week 12) (p < 0.05) (). Bacto Peptone possessed the greater fluorescence intensity than did HF25 throughout the storage time (p < 0.05). Development of fluorescent compounds occurs in the Maillard reaction prior to the generation of brown pigments.Citation[16] Therefore, the increase in fluorescence intensity suggested the increased formation of fluorescent intermediates for the development of browning. It was noted that the rate of the increase in fluorescence intensity of HF25 was slightly greater than Bacto Peptone. The differences in fluorescence intensity and UV-absorbance (A294) of HF25 and Bacto Peptone suggested that different types of intermediate products, either fluorescent or non-fluorescent compounds, were formed and underwent the final stage of reaction at different rates. The differences in browning intensity, UV-absorbance, and fluorescence intensity between HF25 and Bacto Peptone might be due to the differences in amino acid compositions and other substances. Benjakul et al.Citation[27] reported that both concentration and type of sugar influenced the browning of porcine plasma protein-sugar Maillard reaction products and the formation of intermediate compounds was varied, depending on the type of sugar. Hoyle and MerrittCitation[26] concluded that non-enzymatic browning reaction resulted in the darkening of protein hydrolysate from herring during 3 months of storage.
Changes in pH and Solubility during Storage
pH of HF25 and Bacto Peptone ranged from 6.83 to 7.02 (data not shown). However, no difference in pH between HF25 and Bacto Peptone was observed during storage of 12 weeks (p > 0.05). The pH of both samples was quite stable throughout the storage. In general, media preparation for bacteria requires the pH of 6.8 ± 0.2.Citation[28] The decrease in pH values of hydrolysate indicated the decrease in stability and shelf-life of protein hydrolysate.Citation[22] The changes in pH during storage could be used as a factor determining the stability of protein hydrolysate during storage.
Solubility of HF25 at pHs of 5 and 7 was lower than that of Bacto Peptone (p < 0.05) (data not shown). However, HF25 and Bacto Peptone were soluble at both pHs with the solubility being greater than 93%. No difference in solubility between both pHs was observed in HF25 and Bacto Peptone (p > 0.05). The high solubility of HF25 was mainly due to the existence of low molecular weight peptides (lower than 7 kDa).[10] Additionally, HF25 might possess proportionally more polar residues with the ability to form hydrogen bonds with water.Citation[29] Gildberg et al.Citation[24] reported that freeze-dried powders of capelin and blue whiting hydrolysates were easily solubilized in distilled water with the aid of heating. With high solubility at pHs 5 and 7 throughout the storage time, HF25 could be served as a readily available nutrient that could be uptaken efficiently by molds, yeasts, and bacteria cultured in the media with the pHs of 5 and 7, respectively. Generally, it is a common practice to culture molds at a pH of approximately 5, whereas the bacteria and yeasts are cultivated at a pH of approximately 6–7.Citation[28]
Changes in Efficacy as Microbial Media during Storage
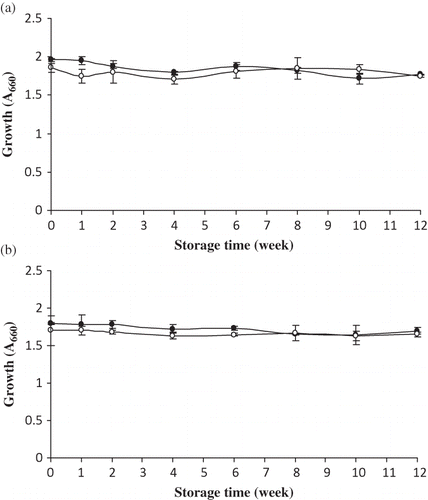
Efficacy of HF25 and Bacto Peptone as microbial media during storage is depicted in As measured by A660, a slight decrease in growth of S. aureus cultured in NB containing HF25 was observed as the storage time increased (p < 0.05), while no difference was found in that cultured in NB containing Bacto Peptone () (p > 0.05). However, NB containing HF25 yielded a higher growth of S. aureus than did Bacto Peptone within the first 2 weeks of storage (p < 0.05). Thereafter, no difference was observed (p > 0.05). The decrease in growth of S. aureus during storage might be associated with the loss of some amino acids required for growth of S. aureus during storage, possibly via Maillard reaction or lipid oxidation. The rate of Maillard browning was related to the loss in protein nutrition value.Citation[7] Nielsen et al.Citation[30] found that whey proteins stored in the presence of oxidized lipids had the losses of lysine and extensive methionine oxidation but only minor losses of tryptophan during storage. The losses in nutritive value were due to interaction of protein with oxidized lipids. The decrease in growth of S. aureus culturing in HF25 during storage was in accordance with the increase in aw, moisture content, dark color, Maillard reaction, and lipid oxidation. When E. coli was cultured in NB containing HF25 or Bacto Peptone stored for different times, no different growth was observed throughout the storage () (p > 0.05). The growth of E. coli culturing in NB containing HF25 was higher than that observed in NB containing Bacto Peptone within the first 6 weeks of storage (p < 0.05). Thereafter, no difference was observed (p > 0.05). Klompong et al.Citation[11] reported that HF25 contained a readily available nitrogen source required for growth of S. aureus and E. coli. Regardless of storage time, the efficacy of HF25 as microbial media was equivalent to Bacto Peptone when tested for the growth of S. aureus and E. coli. To elucidate the possible impact of medium turbidity on A660 measurement used as an indicator for bacterial growth, total viable count was determined on media containing freshly prepared HF25. The numbers (log CFU/ml) of S. aureus cultured on NA containing HF25 or Bacto Peptone were 6.99 ± 0.03 and 6.94 ± 0.02, respectively. For E. coli, the numbers were 7.80 ± 0.06 and 7.71 ± 0.05, when cultured on NA containing HF25 or Bacto Peptone, respectively. The numbers of both organisms cultured on NA containing HF25 were greater than those cultured on NA containing Bacto Peptone, which were in accordance with the A660 measurement (). The result suggested that the medium turbidity had no interfering effect on the A660 measurement used to determine bacterial growth.
CONCLUSIONS
Protein hydrolysate from yellow stripe trevally prepared using Flavourzyme with 25% DH was quite stable during storage. No marked changes in pH and solubility were observed throughout the storage of 12 weeks at room temperature. Nevertheless, slight increases in water activity, moisture content, turbidity, browning, and TBARS formation were obtained with increasing storage time. The efficacy for culturing bacteria was equivalent to commercial Bacto Peptone and generally remained unchanged throughout 12 weeks of storage at room temperature. Therefore, protein hydrolysate from yellow stripe trevally could be used as alternative microbial media or as a food ingredient and could be stored for a long time.
ACKNOWLEDGMENTS
This research was supported by the Staff Development Project from the Ministry of Education, a grant for dissertation from Graduate School, Prince of Songkla University, Thailand, and Thailand Research Fund Senior Research Scholar program. The authors thank East Asiatic Company Ltd., Thailand, for donating Flavourzyme.
REFERENCES
- De la Broise , D. , Dauer , G. , Gildberg , A. and Guerard , F. 1998 . Evidence of positive effect of peptone hydrolysis rate on Escherichia coli culture kinetics . Journal of Marine Biotechnology , 6 : 111 – 115 .
- Wasswa , J. , Tang , J. and Gu , X. 2008 . Functional properties of grass carp (Ctenopharyngodon Idella), Nile perch (Lates Niloticus) and Nile tilapia (Oreochromis Niloticus) skin hydrolysates . International Journal of Food Properties , 11 : 339 – 350 .
- Khuwijitjaru , P. , Anantanasuwong , S. and Adachi , S. 2010 . “ Emulsifying and foaming properties of defatted soy meal extracts obtained by subcritical water treatment ” . In International Journal of Food Properties , published online .
- Kurbanoglu , E.B. and Algur , O.F. 2002 . Use of ram horn hydrolysate as peptone for bacterial growth . Turkish Journal of Biology , 26 : 115 – 123 .
- Essien , J.P. , Akpan , E.J. and Essien , E.P. 2005 . Studies on mould growth and biomass production using waste banana peel . Bioresource Technology , 96 : 1451 – 1456 .
- Thiansilakul , Y. , Benjakul , S. and Shahidi , F. 2007 . Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (Decapterus maruadsi) . Food Chemistry , 103 : 1385 – 1394 .
- Lindemann-Schneider , U. and Fennema , O. 1989 . Stability of lysine, methionine, and tryptophan in dried whey concentrate during storage . Journal of Dairy Science , 72 : 1740 – 1747 .
- Shahidi , F. , Han , X.Q. and Synowiecki , J. 1995 . Production and characteristics of protein hydrolysates from capelin (Mallotus villosus) . Food Chemistry , 53 : 285 – 293 .
- Klompong , V. , Benjakul , S. , Kantachote , D. and Shahidi , F. 2007 . Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type . Food Chemistry , 102 : 1317 – 1327 .
- Klompong , V. , Benjakul , S. , Kantachote , D. , Hayes , K.D. and Shahidi , F. 2007 . Comparative study on antioxidative activity of yellow stripe trevally protein hydrolysate produced from Alcalase and Flavourzyme . International Journal of Food Science and Technology , 43 : 1019 – 1026 .
- Klompong , V. , Benjakul , S. , Kantachote , D. and Shahidi , F. 2009 . Characteristics and use of yellow stripe trevally hydrolysate as culture media . Journal of Food Science , 74 : 219 – 225 .
- Adler-Nissen , J. 1986 . Enzymic Hydrolysis of Food Proteins , New York : Elsevier Applied Science Publishers .
- AOAC . 2000 . Official Methods of AnalysisAssociation of Agricultural Chemists Edited by: Seventeenth . Gaithersberg , MD
- Buege , J.A. and Aust , S.D. 1978 . Microsomal lipid peroxidation . Methods in Enzymology , 52 : 302 – 310 .
- Ajandouz , E.H. , Tchiakpe , L.S. , Ore , F.D. , Benajibas , A. and Puigserver , A. 2001 . Effect of pH on caramelisation and Maillard reaction kinetics in fructose-lysine model systems . Journal of Food Science , 66 : 926 – 932 .
- Morales , F.J. and Jimennez-Perez , S. 2001 . Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence . Food Chemistry , 72 : 119 – 125 .
- Robinson , H.W. and Hodgen , C.G. 1940 . The biuret reaction in the determination of serum protein I. A study of the condition necessary for the production of the stable color which bears a quantitative relationship to the protein concentration . Journal of Biological Chemistry , 135 : 707 – 725 .
- Steel , R.G.D. and Torrie , J.H. 1980 . Principle and Procedure of Statistics , New York : MacGraw-Hill .
- Labuza , T.P. 1984 . Moisture Sorption: Practical Aspects of Isotherm Measurement and Use , St. Paul , MN : American Association of Cereal Chemists .
- Fennema , O.R. “ Water and ice ” . In Principles of Food Science; Fennema, O.R , 13 – 39 . New York , 1976 : Marcel Dekker .
- Troller , J.A. 1971 . Effect of water activity on enterotoxin B production and growth of Staphylococcus aureus . Applied Microbiology , 21 : 435 – 439 .
- Tsoraeva , A. and Zhurbenko , R. 2000 . Development and characterization of a mixed nutrient base for the culture of a wide range of microorganisms . Revista Latinoamericana de Microbiologia , 42 : 155 – 161 .
- Pena-Ramos , E.A. and Xiong , Y.L. 2003 . Whey and soy protein hydrolysates inhibit lipid oxidation in cooked pork patties . Meat Science , 64 : 259 – 263 .
- Gildberg , A. , Batista , I. and Strom , E. 1989 . Preparation and characterization of peptones obtained by a two-step enzymatic hydrolysis of whole fish . Biotechnology and Applied Biochemistry , 11 : 413 – 423 .
- Benjakul , S. and Morrissey , M.T. 1997 . Protein hydrolysates from Pacific whiting solid wastes . Journal of Agricultural and Food Chemistry , 45 : 3423 – 3430 .
- Hoyle , N.T. and Merritt , J.H. 1994 . Quality of fish protein hydrolysate from herring (Clupea harengus) . Journal of Food Science , 59 : 76 – 80 .
- Benjakul , S. , Lertittikul , W. and Bauer , F. 2005 . Antioxidant activity of Maillard reaction products from a porcine plasma protein-sugar model system . Food Chemistry , 93 : 189 – 196 .
- Downes , F.P. and Ito , K. 2001 . Compendium of Methods for the Microbiological Examination of Foods , Edited by: Fourth . Washington : American Public Health Association .
- Gbogouri , G.A. , Linder , M. , Fanni , J. and Parmentier , M. 2004 . Influence of hydrolysis degree on the functional properties of salmon byproduct hydrolysates . Journal of Food Science , 69 : 615 – 622 .
- Nielsen , H.K. , De Weck , D. , Finot , P.A. , Liardon , R. and Hurrellt , R.F. 1985 . Stability of tryptophan during food processing and storage . British Journal of Nutrition , 53 : 281 – 292 .