Abstract
The effects of soluble solids content and temperature on thermal properties of papaya puree were studied. Density and specific heat were measured using a pycnometer and differential scanning calorimeter, respectively, while thermal conductivity was measured using a line heat source probe. Thermal diffusivity was then calculated from the experimental results of the specific heat, thermal conductivity, and density. Thermal properties of papaya puree were experimentally determined within a soluble solids content range of 10 to 25 °Brix and temperature between 40 and 80°C. The density, specific heat, thermal conductivity, and thermal diffusivity of papaya puree were found to be in the ranges of 1014.6 to 1098.9 kg/m3, 3.652 to 4.092 kJ/kg °C, 0.452 to 0.685 W/m °C, and 1.127 × 10−7 to 1.650 × 10−7 m2/s, respectively. Moreover, the empirical models for each property as a function of soluble solids content and temperature were obtained.
INTRODUCTION
Papaya (Carica papaya L.) is an important fruit of the tropical and subtropical regions of the world, including Thailand. Nutritionally, it is a good source of provitamin A, ascorbic acid, some B complex vitamins, and many phytochemicals having antioxidant properties. Papaya is consumed fresh and processed into various forms, such as dehydrated slices, chunk and slices for tropical fruit salad and cocktail, or processed into puree, which is an intermediate papaya product and is thermally processed and stored for the production of beverage, ice cream, jam, jelly, juice, and nectar.[Citation1,Citation2] Papaya is considered as one of the climacteric fruits, which can be ripened after harvest. Chemical changes, i.e., soluble solids content, pH, and acidity in papaya are indicators to identify and evaluate its degree of ripeness. The chemical changes in ripened papaya involve a decrease in acidity and an increase in soluble solids content. The degree of ripeness of papaya causes different food compositions, which consequently affects thermal properties.
Knowledge of thermal properties of food, i.e., thermal conductivity, specific heat, and thermal diffusivity, and how these properties change during processing are necessary for proper designing and development of the equipment and processes.[Citation3] The substantial changes of these properties strongly depend on the temperature, chemical composition, and the physical structure of food. General models for predicting thermal properties of each specific food material based on its basic components (fat, protein, water, carbohydrate, fiber, and ash) and temperature was proposed by Choi and Okos.[Citation4] The experimental data on thermal properties of an individual food material are needed for model development. It is expected that empirical models of thermal properties for food materials would give more specific and accurate predictions.[Citation5–8
Many literatures related to thermal properties of several food products are available.[Citation9] Kurozawa et al.[Citation10] reported the thermal conductivity and the thermal diffusivity of fresh ripe papaya in the temperature range of 20–40°C. Espinoza-Guevara et al.[Citation11] observed the thermophysical properties of pulp and rind of papaya (Carica papaya L., cv. Maradol) in the temperature range of 20–60°C. Fasina et al.[Citation12] reported thermal properties of sweet potato puree in the temperature range of 5–80°C. However, there is no information on thermal properties of papaya puree as a function of both soluble solids content and temperature. Thermal properties of papaya puree would be necessary for designing equipment and the evaporating process during the value-added utilization of papaya puree. Thus, the objectives of this research were to investigate the effects of soluble solids content within the concentration range of 10 to 25 °Brix and temperature between 40 and 80°C on thermal properties of papaya puree and to propose an empirical correlation relating each thermal property as a function of soluble solids content and temperature.
MATERIALS AND METHODS
Sample Preparation
Each fresh ripe papaya (Carica papaya L., cv. Kaekdum) from the local market was washed with tap water to remove pesticides and contaminated substances. Then, papaya was peeled, halved, deseeded, and cut into small pieces. Papaya puree was prepared from papaya flesh using a pilot plant crushing machine fitted with a screen of 1.5 mm opening size and operated at 100 rpm. The initial concentration of papaya puree was about 10 °Brix. Then, 250 g of papaya puree obtained was concentrated in a rotary evaporator (Resona Model Labo Rota S-300, Gossau, Switzerland) at a vacuum pressure of 80 kPa and water bath temperature of 70°C to various soluble solids contents of 15, 20, and 25 °Brix. The evaporation time required to concentrate papaya puree to 15, 20, and 25 °Brix were 20, 28, and 39 min, respectively. The papaya puree samples were finally packed into polyethylene bottles and stored in the refrigerator at 4°C for experimental measurement.
Determination of Physicochemical Characteristics
Proximate compositions, including fat, moisture content, protein, fiber, and ash, of the single strength papaya puree samples were first analyzed according to the Association of Official Analytical Chemists method.[Citation13] The moisture content was determined by the oven drying method at 70°C for 24 h. The protein content, the fat content, and the fiber content were determined by the Kjeldahl method, the Soxhlet method, and acid and alkali digestion, respectively. For ash content, the sample was first dried in an oven at 105°C before being transferred to a muffle furnace at 550°C until a white or light gray ash was obtained. In addition, soluble solids content, pH, and acidity were measured by a refractometer (Atago, Tokyo, Japan), pH meter (Schott Gerate, Model CG841, Hofheim, West Germany), and titration, respectively. For soluble solids content determination, 30 g of sample was centrifuged at 20,000 rpm for 30 min using a refrigerated centrifuger (Hitachi, model CR21, Tokyo, Japan). After centrifugation, the supernatant was poured off. The obtained supernatant was used to find soluble solids content by a refractometer.
Density Measurement
Papaya puree was filled into a known weight volumetric pycnometer using a syringe. The pycnometer of 25-ml capacity had previously been calibrated with distilled water. The reference values of water density at 40, 50, 60, 70, and 80°C are 992.2, 988.1, 983.2, 977.8, and 971.8 kg/m3, respectively.[Citation9] The temperature was controlled by a thermostatic water bath (Heto-Holten A/S Type AT 110, Gydevang, Denmark). Density was then calculated from the ratio of weight of the sample in the pycnometer to the volume of pycnometer.
Specific Heat Measurement
Specific heat of papaya puree was measured using a differential scanning calorimeter (DSC), model Pyris1 (Perkin Elmer, Weltham, MA, USA). The DSC was calibrated prior to the experiments with indium (temperature and enthalpy) as described in the DSC operating manual. Papaya puree samples (10–15 mg) with different soluble solids contents were sealed in aluminum pans and were scanned from 40 to 80°C at a heating rate of 5°C/min using an empty pan as the reference. Thermograms of sample, baseline, and sapphire, as a standard with a known specific heat value, were used to determine the specific heat of the sample as expressed in EquationEq. (1):[Citation3]
Thermal Conductivity Measurement
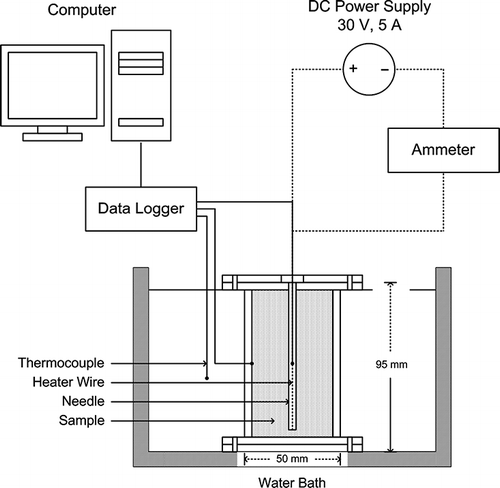
Thermal conductivity was determined by the line-heat source probe method. A schematic diagram of the apparatus used in this research is shown in . The thermal conductivity probe was constructed according to the research of Nithatkusol.[Citation14] To test a sample, a cylinder containing papaya puree was placed in a controlled temperature water bath (Heto-Holten A/S Type AT 110, Gydevang, Denmark). The sample was then allowed to equilibrate to the desired temperature. After equilibration, the thermal conductivity probe was inserted into the sample and the power for the heater wire in the probe was turned on, resulting in a constant electric current through the heater wire. A digital multi-meter was used to monitor the current. Power levels of 9–10.4 W/m were used to raise the temperature of the probe to about 5.8–10.1°C. Time and temperature data were recorded by a datalogger (Hybrid Temperature Recorder, Yokogawa DR230, Tokyo, Japan) every 0.5 s for a total duration of 1 min. The datalogger contains an accuracy of ±(0.05% of reading + 2 digits) and a resolution of 0.1°C. Thermal conductivity was calculated using EquationEq. (2):[Citation3]
Before measuring the samples, the probe was calibrated with 0.5% agar gel and glycerine. The expected values of 0.5% agar gel and glycerine at 30°C was 0.628 and 0.289 W/m°C, respectively.[Citation15] The expected values of glycerine at 80°C was 0.285 W/m °C.[Citation16] The measured average thermal conductivity (three replications) of 0.5% agar gel at 30°C was 0.625 ± 0.007 W/m°C, an approximately 0.48% deviation from the expected value. The measured average thermal conductivity (three replications) of glycerine at 30°C was 0.287 ± 0.008 W/m°C, an approximately 0.69% deviation from the expected value. The measured average thermal conductivity (three replications) of glycerine at 80°C was 0.281 ± 0.008 W/m°C, an approximately 1.1% deviation from the expected value.
Determination of Thermal Diffusivity
Thermal diffusivity was calculated from the experimental results of density, specific heat, and thermal conductivity of papaya puree, using the relationship given in EquationEq. (3):
Experimental Design and Data Analysis
The experiments were performed at five levels of temperature (40, 50, 60, 70, and 80°C) and four levels of soluble solids content (10, 15, 20, and 25 °Brix), which are common conditions applied during evaporation processes of papaya puree. A 2-factor factorial design was used in scheduling of the experiments with three replications in each case. The results were reported as an average of three replicates. Analysis of variance of the two factors and interactions were applied to the different sets of data. Least significant differences were calculated by the Fisher test (α = 0.05). The analysis was performed using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA).
RESULTS AND DISCUSSION
The physicochemical characteristics of single strength papaya puree are given in . Among the physicochemical determinants, pH, acidity, and soluble solids are very good quantifiers of ripening of the papaya fruit. In addition, the corresponding values of moisture content of papaya puree with 15, 20, and 25 °Brix were 84.3, 80.0, and 75.8 g/100 g sample, respectively.
Table 1 Physicochemical characteristics of the single strength papaya puree
Density
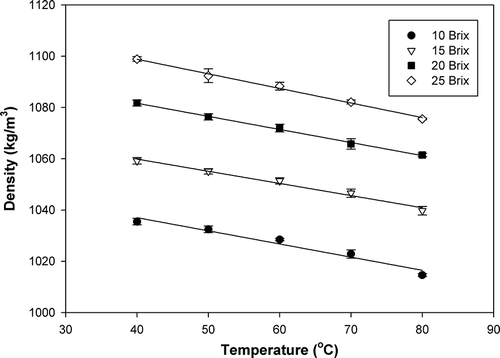
The average density values of papaya puree with various soluble solids contents at different temperatures varied in the range of 1014.61 to 1098.86 kg/m3. It was observed from and that density of papaya puree decreased with increasing temperature but increased with increasing soluble solids content. A similar response has been reported for banana puree,[Citation17] Brazilian orange juice,[Citation18] and apple and quince puree.[Citation19] The density of papaya puree decreasing with increasing temperature is due to thermal expansion, while the density of papaya puree increased with increasing soluble solids content (decreasing moisture content) because the density of water is lower than that of all other components except for fat.[Citation20] From the statistical analysis, both soluble solids content and temperature were found to have significant effects on density of papaya puree at a 95% confidence level. Multiple regression of density as a function of soluble solids content (B) and temperature (T) was developed as shown in EquationEq. (4). The suitability of the fitted model was evaluated by the high coefficient of determination (R 2) and low standard error of prediction (SE), which is the standard deviation of the residuals.
Table 2 Density of papaya puree at different soluble solids contents and temperatures
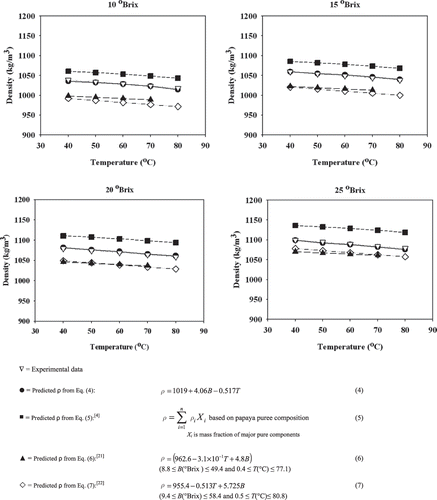
The calculated density values of papaya puree based on EquationEqs. (5),[Citation4] (6),[Citation21] and (7)[Citation22] were compared with the experimental data and those from the regression model based on EquationEq. (4) as shown in . Within the range of the study, all predictive equations had similar relationships that increasing soluble solids content resulted in an increase in density whereas increasing temperature caused a decrease in density. The different values obtained from those predictive equations were variability in the magnitude because those equations were developed from the different types of food products.
Specific Heat
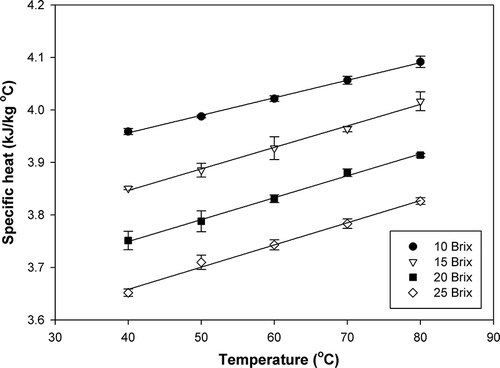
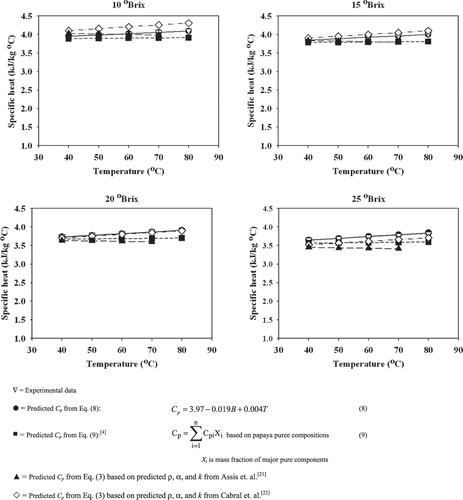
The average specific heat values of papaya puree with various soluble solids contents at different temperatures varied in the range of 3.652 to 4.092 kJ/kg°C . The experimental data obtained for the specific heat of papaya puree at the studied soluble solids contents and temperatures are shown in and . Specific heat of papaya puree was found to decrease with increasing soluble solids contents but increased with increasing temperature. A similar response has been reported for Brazilian orange juice.[Citation18] An increase in soluble solids content means a decrease in moisture content, which in turn decreases the specific heat of papaya puree due to the relatively high specific heat of water.[Citation3] Both soluble solids content and temperature were found to have significant effects on specific heat of papaya puree at a 95% confidence level. Multiple regression of specific heat as a function of soluble solids content (B) and temperature (T) was obtained as shown in EquationEq. (8):
Table 3 Specific heat of papaya puree at different soluble solids contents and temperatures
The calculated specific heat values of papaya puree based on Eqs. (9)[Citation4] and Equation(3),[Citation21,Citation22] and the regression model based on Eq. (8) were compared with the experimental data as shown in . All predictive equations had a similar trend that the specific heat decreased with soluble solids content and increased with temperature.
THERMAL CONDUCTIVITY
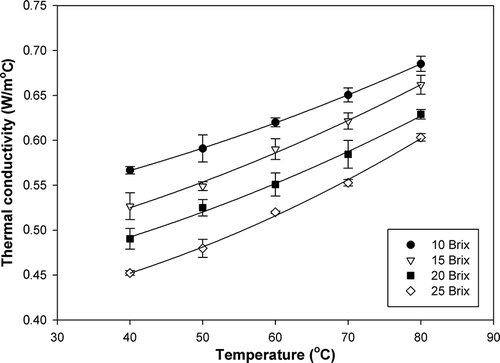
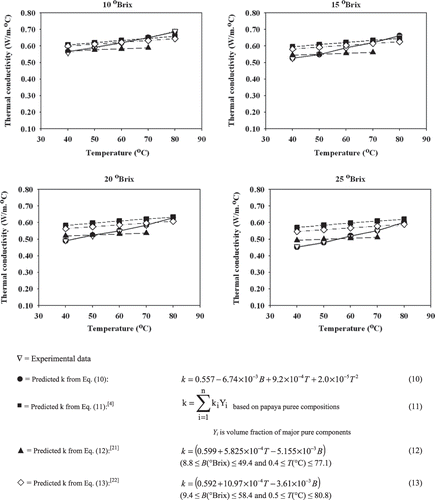
The thermal conductivity of papaya puree with various soluble solids contents at different temperatures ranged from 0.452 to 0.685 W/m °C as shown in and . An increase in temperature resulted in an increase in thermal conductivity, whereas an increase in soluble solids content resulted in a decrease in thermal conductivity. A similar response has been reported for Brazilian orange juice.[Citation18] Among all basic components, i.e., water, protein, fat, carbohydrate, fiber and ash, the thermal conductivity of water is the highest.[Citation9] Therefore, as the moisture content decreased due to concentration, the thermal conductivity decreased accordingly. In addition, the thermal conductivity of water is higher at a higher temperature.[Citation9] Thus, as the temperature increased, the thermal conductivity of papaya puree increased. Both the soluble solids content and temperature were found to have significant effects on thermal conductivity of papaya puree at a 95% confidence level. Multiple regression of thermal conductivity as a function of soluble solids content (B) and temperature (T) was obtained as shown in EquationEq. (10):
Table 4 Thermal conductivity of papaya puree at different soluble solids contents and temperatures
The calculated thermal conductivity values of papaya puree based on Eqs. (11),[Citation4] (12),[Citation21] and (13)[Citation22] were compared with the experimental data and those from the regression model based on EquationEq. (6) as shown in . Within the range of the study, all predictive equations had a similar trend that increasing temperature and decreasing soluble solids content resulted in an increase in thermal conductivity. The different values obtained from those predictive equations were variability in the magnitude because those equations were developed from the different types of food products.
Figure 7 Experimental data and predicted thermal conductivity of 10–25 °Brix papaya puree in the temperature range of 40 to 80°C.
Thermal Diffusivity
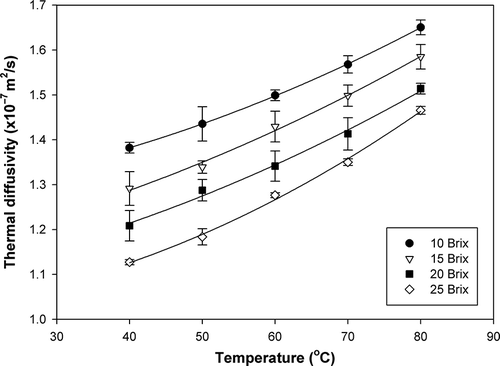
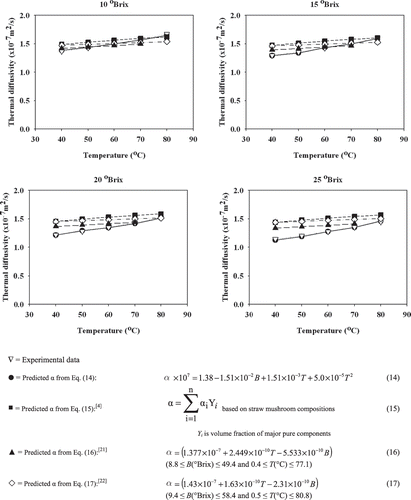
The calculated thermal diffusivity values of papaya puree at different soluble solids contents and temperatures are illustrated in and . An increase in temperature resulted in an increase in thermal diffusivity, whereas an increase in soluble solids content resulted in a decrease in thermal diffusivity. A similar response has been reported for Brazilian orange juice.[Citation18] Among all basic components, i.e., water, protein, fat, carbohydrate, fiber, and ash, the thermal diffusivity of water is the highest.[Citation9] Therefore, as the moisture content decreased due to concentration, the thermal diffusivity decreased accordingly. In addition, the thermal diffusivity of water is higher at a higher temperature.[Citation9] Thus, as the temperature increased, the thermal diffusivity of papaya puree increased. The thermal diffusivity values of papaya puree samples varied from 1.127 × 10−7 to 1.650 × 10−7 m2/s. Both the soluble solids content and temperature were found to have significant effects on thermal diffusivity of papaya puree at a 95% confidence level. Multiple regression of thermal diffusivity as a function of soluble solids content (B) and temperature (T) was obtained as given in Eq. (14):
Table 5 Thermal diffusivity of papaya puree at different soluble solids contents and temperatures
The calculated bulk thermal diffusivity values of papaya puree based on Eqs. (15),[Citation4] (16),[Citation21] and (17),[Citation22] and the regression model based on Eq. (14) were compared with the experimental data as shown in . All predictive equations had a similar trend that an increase in temperature increased in thermal diffusivity for all soluble solids contents considered.
CONCLUSIONS
The effects of soluble solids content in the range of 10–25 °Brix and temperature in the range of 40–80°C on the thermophysical properties, i.e., density, thermal conductivity, specific heat, and thermal diffusivity of papaya puree were determined in this research. Density, specific heat, and thermal conductivity were determined through experiments, while thermal diffusivity was calculated afterwards. For the range of soluble solids content and temperature used in this study, it was concluded that an increase in temperature decreased the density but increased the specific heat and thermal conductivity. While an increase in soluble solids content increased the density but decreased the specific heat and thermal conductivity. These two effects (i.e., temperature and soluble solids content) on density, specific heat, and thermal conductivity could not compensate each other in calculation of thermal diffusivity. Thus, an increase in temperature resulted in an increase in thermal diffusivity, whereas an increase in soluble solid contents resulted in a decrease in thermal diffusivity. In addition, empirical models for these thermal properties of papaya puree were also proposed and compared with other models.
ACKNOWLEDGMENTS
This work was supported by the Thailand Research Fund (TRF) and the Commission on Higher Education (CHE) under the TRF-CHE Research Grant for Mid-Career University Faculty.
REFERENCES
- Nakasone , H.Y. and Paull , R.E. 1998 . “ Papaya ” . In Tropical Fruits , Edited by: Nakasone , H.Y. and Paull , R.E. 445 New York : CAB International .
- Ahmed , J. , Shivhare , U. and S; Sandhu , K.S. 2002 . Thermal degradation kinetics of carotenoids and visual color of papaya puree . Journal of Food Science , 67 ( 7 ) : 2692 – 2695 .
- Mohsenin , N.N. 1980 . Thermal Properties of Foods and Agricultural Materials , 407 New York : Gordon and Breach .
- Choi , Y. and Okos , M.R. 1986 . “ Effects of temperature and composition on the thermal properties of foods ” . In Food Engineering and Process Applications , Edited by: Maguer , M.L. and Jelen , P. Vol. 1 , 93 – 101 . London : Elsevier .
- Sweat , V.E. 1995 . “ Thermal properties of foods ” . In Engineering Properties of Foods , 2nd , Edited by: Rao , M.A. and Rizvi , S.S.H. 126 New York : Marcel Dekker .
- Heldman , D.R. and Singh , R.P. 1981 . Food Process Engineering , 2nd , Westport , Connecticut : AVI Publishing Company .
- Tansakul , A. and Chaisawang , P. 2006 . Thermophysical properties of coconut milk . Journal of Food Engineering , 73 : 276 – 280 .
- Tansakul , A. and Lumyong , R. 2008 . Thermal properties of straw mushroom . Journal of Food Engineering , 87 : 91 – 98 .
- Rahman , S. 1995 . Food Properties Handbook , 500 Boca Raton : CRC Press .
- Kurozawa , L.E. , El-Aouar , A.A. , Simões , M.R. , Azoubel , P.M. and Murr , F.E.X. Determination of thermal conductivity and thermal diffusivity of papaya (Carica papaya L.) as a function of temperature . Proceedings of the 2nd Mercosur Congress on Chemical Engineering and the 4th Congress on Process Systems Engineering . August , Rio de Janeiro , Brazil. pp. 14 – 18 . Rio das Pedras
- Espinoza-Guevara , R. , Caro-Corrales , J. , Ordorica-Falomir , C. , Zazueta-Morales , J. , Vega-Carcia , M. and Crnin , K. 2010 . Thermophysical properties of pulp and rind of papaya cv . Maradol. International Journal of Food Properties , 13 : 65 – 74 .
- Fasina , O.O. , Farkas , B.E. and Fleming , H.P. 2003 . Thermal and dielectric properties of sweet potato puree . International Journal of Food Properties , 6 : 461 – 472 .
- AOAC . 1995 . Official Methods of Analysis , 15th , Gaithersburg , MD : The Association of Official Analytical Chemists .
- Nithatkusol , A. 1998 . The development of apparatus for determination of thermal conductivities of nonfrozen food by the line heat source method , 116 Bangkok , Thailand : Master's Degree Thesis, King Mongkut's University of Technology Thonburi .
- Sweat , V.E. and Haugh , C.G. 1974 . Thermal conductivity probe for small food samples . Transactions of the American Society of Agricultural Engineers , 17 ( 1 ) : 56 – 58 .
- Bates , O.K. 1936 . Binary mixtures of water and glycerol . Industrial and Engineering Chemistry , 28 : 495 – 498 .
- Tsen , J.-H. and King , V.A.-E. 2002 . Density of banana puree as a function of soluble solids concentration and temperature . Journal of Food Engineering , 55 : 305 – 308 .
- Telis-Romero , J. , Telis , V.R.N. , Gabas , A.L. and Yamashita , F. 1998 . Thermophysical properties of Brazilian orange juice as affected by temperature and water content . Journal of Food Engineering , 38 : 27 – 40 .
- Ramos , A.M. and Ibarz , M. 1998 . Density of juice and fruit puree as a function of soluble solids content and temperature . Journal of Food Engineering , 35 : 57 – 63 .
- AbuDagga , Y. and Kolbe , E. 1997 . Thermophysical properties of surimi paste at cooking temperature . Journal of Food Engineering , 32 : 325 – 337 .
- Assis , M.M.M. , Lannes , S.C.S. , Tadini , C.C. , Telis , V.R.N. and Telis-Romero , J. 2006 . Influence of temperature and concentration on thermophysical properties of yellow mombin (Spondias mombin, L.) . European Food Research and Technology , 233 : 585 – 593 .
- Cabral , R.A.F. , Orrego-Alzate , C.E. , Gabas , A.L. and Telis-Romero , J. 2007 . Rheological and thermophysical properties of blackberry juice . Ciência e Tecnologia de Alimentos , 27 : 589 – 596 .